Abstract
Background:
One out of 4 patients who sustains a mild traumatic brain injury (mTBI) experiences persistent complaints, despite the absence of structural brain damage on conventional neuroimaging. Susceptibility to develop post concussive symptoms (PCS) is thought to originate from occult brain dysfunction. However, the influence of such neural changes on the development of persistent PCS is poorly characterized.
Methods:
In this article, we aim to integrate findings from longitudinal studies that investigated across the spectrum of neuroimaging modalities the changes within the first twelve months following a mTBI, with the goal of identifying possible predictors or biomarkers of persistent PCS.
Results:
Nine studies met inclusion criteria: 5 that used resting state functional MRI, 2 that used Diffusion Weighted Imaging, and 2 that used 1H-MR Spectroscopy. All studies indicate significant structural, functional and/or metabolic aberrations that occur in the acute and early subacute phases following a mTBI. However, in patients with persistent PCS, these mTBI-induced damages linger and relate to the severity of PCS. These biomarkers include: decreased diffusion along white matter fiber tracts, alteration of perfusion, disrupted metabolism, and reduced connectivity within several resting state networks. Additionally, in PCS patients, disruptions of brain function can manifest exclusively in the chronic phase.
Conclusion:
This review support the ongoing use of neuroimaging modalities to understand the brain changes that occur throughout the time course of mTBI. Based on the complexity of mTBI, however, more work is required to characterize injury and recovery mechanisms that could impact the emergence and persistence of PCS.
Introduction
The American Congress of Rehabilitation Medicine defines mild TBI (considered synonymous with the term “concussion” for this review) as a traumatic brain injury that encompasses the following features: 1) loss of consciousness for up to 30 minutes, 2) alteration of consciousness for less than 24 hours, 3) posttraumatic amnesia for less than 24 hours, and 4) a Glasgow Coma Scale score of 13–15 at 30 minutes after injury (Menon et al., 2010).
Symptoms emerging after concussion typically fall into one of four categories: vestibular (e.g., imbalance, nausea, dizziness), sensory (e.g., blurry vision, migraines, tinnitus, photo/phonophobia), cognitive (e.g., difficulty focusing, forgetfulness, attention, memory, and judgment), and emotional (e.g., fatigue, insomnia, irritability, depression, CDC, 2015). According to current clinical guidelines, these somatic, cognitive and emotional symptoms improve by 2 to 4 weeks in most cases of mTBI (McCrea et al., 2003), and resolve completely by 3 months (DSM-V).
The term post-concussion symptoms (PCS), also known as post-concussion disorder (PCD) is used to describe the constellation of symptoms beyond the usual recovery period after a concussion. However, the definition of PCS is plagued by several factors including poor reliability of diagnostic criteria (PCS is seen in between 15% (DSM-IV) to 50% (WHO-ICD-10) and lack of specificity (i.e., PCS symptoms as distinct from symptoms that occur in the absence of head injury and/or in conjunction with other psychiatric conditions). Most notable, there is ample evidence that PCS symptoms may last for over six months, with some evidence that ~5% of all individuals with an initial mTBI show persistent difficulties at 12 months (Iverson, 2007). To date, there is no universal agreement on the time-period in which PCS may be classified as “prolonged.” While some epidemiological studies show that prolonged PCS completely resolves by three months (Ponsford et al., 2012), others demonstrate that recovery may require up to one year (Carroll et al., 2014). Identifying predictors of persistent PCS and understanding the relationship between risk factors and long-term outcomes is necessary to enhance assessment and treatment. Research on PCS has demonstrated that risks include i) pre-existing factors that may predispose an individual to worse outcomes following a concussion/mTBI (pre-injury factors such as psychiatric history, ADHD/learning disorder, substance use); ii) peri-injury factors (e.g., ≥1 contusion on MRI, ≥4 shear foci on MRI, assault/military); and iii) post-injury factors (e.g., PTSD, cognitive biases, anxiety, depression, migraines) –for a review see (Quinn et al., 2018). Current clinical practice lacks a systematic approach to follow-ups of mild TBI beyond the acute period, with physicians treating a majority of cases with non steroidal analgesics or antidepressants, and referring about 40$ for psychological evaluation (Mittenberg et al., 2001). Concomitantly, growing evidence from neuroimaging and neurophysiological studies indicates occult brain dysfunction underlying prolonged symptoms (Mayer et al., 2015; Rapp et al., 2015). Despite this evidence, follow-ups rely on single assays of brain integrity (e.g., evidence of brain damage as measured by structural neuroimaging on CT and T1- and T2-weighted MRI immediately after the injury) that can invoke Type II errors, especially given that some biomarkers only emerge later in the course of recovery. Because of these errors, clinicians often are at risk of making misjudgments and ascribing cognitive and emotional PCS-related symptoms that emerge months later to other neuropsychiatric conditions.
To our knowledge, this is the first attempt to review the accumulating evidence on neuroimaging techniques that can be used to monitor brain biomarkers in persistent PCS. To this purpose, we examine studies that have repeatedly collected brain biomarkers within the first twelve months following a mTBI, and attempt to determine whether sensitive predictors or biomarkers of PCS persistence exist and correlate with behavioral changes.
Methods
1. Search Strategy
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009). Peer-reviewed, English-language research articles were selected for the review; non-human, review and meta-analytic reports were excluded. We identified studies for inclusion through searching the electronic databases PubMed, PsycINFO, and EMBASE. We restricted our search to the timeframe of January 2009 to February 2019.
Three sets of keyword search algorithms were used, linked with the Boolean operator AND. The first was related to diagnosis: “mTBI” OR “mild traumatic brain injury” OR “concussion”. The second was related to PCS and included “post-concus*” OR “postconcuss*” OR “PCS” OR “PPCS”. The third set of search terms was related to brain imaging techniques: “neuroimaging” OR “CT” OR “MRI” OR “FMRI” OR “glucose” OR “PET” OR “barrier” OR “diffusion tensor imaging” OR “diffusion tractography”. Using these criteria, all authors screened title and abstract of search results. During this screening phase, we excluded study protocols that clearly failed to meet inclusion criteria (below). Whenever at least one author raised concerns about study inclusion, the full text was inspected and all authors discussed until a consensus was reached. For all search results that passed the first screening, we retrieved and reviewed the full texts. Additionally, at this stage we cross-referenced lists of included studies to gather any papers that the search terms had not identified.
2. Eligibility Criteria and Study Selection
Studies were included if they: 1) were peer-reviewed English language original articles published within the specified date range above; 2) exclusively recruited clinical subjects with a diagnosis of mTBI; 3) included at least 2 neuroimaging scans or assays collected over multiple time points. Studies were excluded if: 1) they solely examined repetitive TBI, chronic TBI, moderate or severe TBI, and chronic traumatic encephalopathy, or mixed diagnostic samples; 2) were conducted on pediatric or adolescent samples; 3) were case studies or case reports; 4) had a single neuroimaging scan or assay; 5) did not indicate time elapsed between TBI and scans; 6) did not use a standardized and validated assessment specifically designed for PCS; and 7) were intervention studies. Studies were not excluded if they were secondary analyses or extended follow-up studies of original trials previously reported.
For articles that were not rated as eligible by all authors, we held a discussion meeting where we analyzed any disagreements until a consensus about study inclusion was reached. All articles matching our eligibility criteria were reviewed in full by the authors.
From each included paper, we extracted study design, demographics, clinical characteristics (diagnosis, age of onset), measures collected, as well as neuroimaging, biological and cognitive findings. Whenever risk of bias was deemed not low, and/or for every mismatch in extracted data, all authors discussed until a consensus was reached. Given the heterogeneity of study designs and samples, we did not code variables related to treatment.
Results
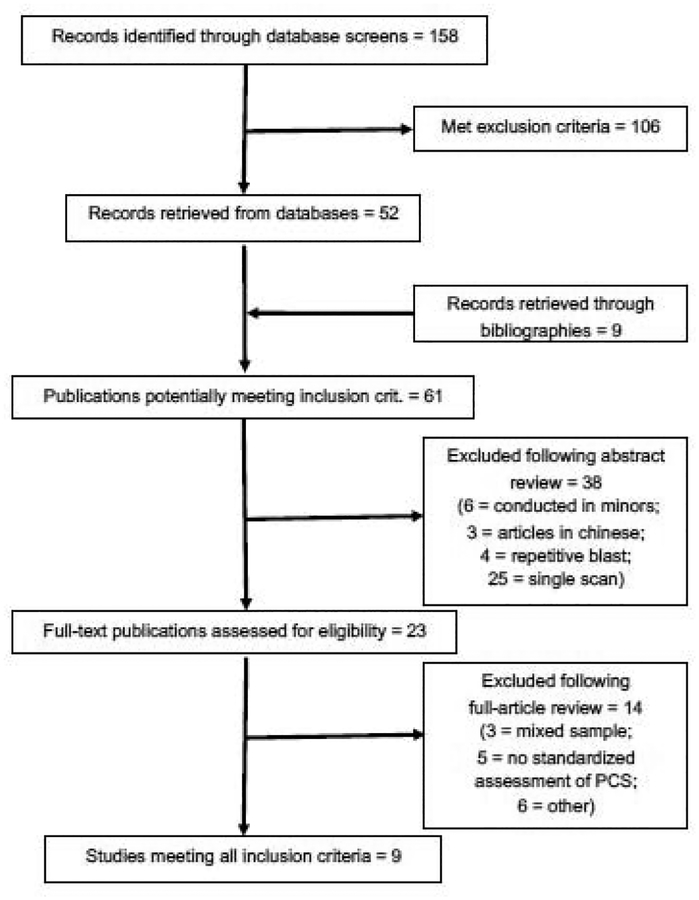
We conducted full database searches in February 2019, with the inclusion and exclusion criteria identified prior to the collection period. Figure 1 shows a PRISMA flowchart of each stage of the search process. The search strategy returned 158 unique results after duplicates were removed. Of these, 61 articles were examined further by reviewing the abstract. Of the 23 full-text publications reviewed in full, 14 were excluded as not meeting criteria (10 not rated as eligible by all authors, 4 rated as non-eligible after a discussion meeting). Thus, 9 studies were included in the review: 5 studies that used resting state functional MRI (rsfMRI), 2 studies that used Diffusion Weighted Imaging (DWI), and 2 studies that used 1H-MR Spectroscopy Imaging (1H-MRSI). They are presented below in chronological order.
Figure 1.
Flow Diagram
Henry and colleagues investigated the effects of mTBI on brain metabolism using 1H-MRSI (Henry et al., 2011). In this study, the authors compared a group of 10 athletes with mTBI with an age- and education-matched control group of 10 non-concussed athletes during the acute (1–6 days) and chronic (6-months) post-injury phases. Severity of PCS was assessed at each time point using the Post Concussion Symptom Scale (PCSS), a 22-item self-report. Symptoms endorsed by athletes with mTBI were significantly worse than controls in the acute post-injury phase, but not 6-months later. Nonetheless, athletes displayed continued metabolic disruptions despite their self-reported clinical recovery. In particular, NAA:Cr levels in the DLPFC and M1 remained lower in the concussed group across time relative to controls. In M1, mTBI athletes demonstrated a recovery of Glu:Cr levels across time, whereas levels of m-I/Cr that were unimpaired in the acute post-injury phase, were significantly decreased in the chronic phase. Importantly, none of these changes in brain biomarkers correlated with improvements in self-report measures. Besides confirming the presence of cortical neurometabolic changes in the acute phase of recovery and normalization of some biomarkers during the chronic phase, these findings also suggest cortical metabolic disruptions that emerge over time. Thus, neurometabolites may have distinct roles depending on the time post-injury.
Messé and colleagues recruited 53 mTBI patients and 40 matched healthy controls (HCs), performed DWI at the subacute (8–21 days post-concussion) and late (6 months) phases after mTBI, and detected PCS in the subacute phase using ICD-10 criteria (Messé et al., 2012). Compared to HCs, mTBI patients showed decreased fractional anisotropy values in association, commissural and projection white matter fiber tracts during the subacute phase that partially resolved over time. mTBI patients with PCS (n=22) had greater and wider impairments than patients without PCS during the subacute phase. Finally, these alterations persisted for PCS patients, while mTBI patients without PCS partly recovered over time. These results support the hypothesis that TBI-induced lasting impairments in white matter fiber bundles contribute to the pathological substrate of PCS in mTBI.
In a separate study, Messé and colleagues recruited 55 patients with mTBI and 34 HCs (Messé et al., 2013). All subjects underwent rsfMRI and graph theory examinations at the subacute (1–3 weeks) and late (6 months) phases after injury. PCS was established at the late phase based on the DSM-IV criteria, subdividing the mTBI group in 17 patients with persistent PCS and 38 PCS− patients. Overall, mTBI patients showed increased connectivity in the limbic system after mTBI, whereas PCS+ patients (compared to PCS−) showed unique early thalamic and temporal lobe changes during the subacute phase, and decreased graph properties in frontal regions at the late phase. Importantly, these changes were functionally significant in PCS+ patients, as they correlated with symptom severity. These findings highlight the relationship between functional brain network integrity and PCS.
George and colleagues recruited 43 mTBI patients who underwent 1H-MRSI at the early subacute (1 week), late subacute (1 month) and chronic (6 months) phases, as well as 21 neurologically intact subjects who were used as HCs (George et al., 2014). The Rivermead Post-Concussion Symptoms Questionnaire (RPQ, King et al., 1995) was used to assess the severity of PCS, and the Automated Neuropsychological Assessment Metrics (ANAM) was used during the chronic phase to assess cognitive performance (Kane et al., 2007). Decreased choline-to-creatine ratio was observed in the thalamus and centrum semiovale (CSV) during the late subacute phase of mTBI. Additionally, early subacute creatine measurements in the CSV predicted acquisition and recall abilities six months later. No significant correlations were reported between the severity of PCS and neurometabolic markers.
Wäljas and colleagues reported on a large sample of mTBI patients (n=126), who underwent structural MRI and DWI three weeks post-injury (n=71) and completed questionnaires and cognitive testing one month (n=126) and one year (n=103) post-injury (Wäljas et al., 2015). 59% of the mTBI sample met ICD-10 PCS criteria at one month, and 38% at one year. While multifocal areas of unusual white matter were present in 50.7% of mTBI participants, compared with 12.4% of controls, these micro microstructural white matter findings were not significantly associated with PCS.
Sours and colleagues recruited 28 mTBI patients who received resting state fMRI and a resting state perfusion scan –using the pulsed arterial spin labeling technique (PASL) – at the acute (1 week), subacute (1 month) and chronic (6 months) phases of recovery (Sours et al., 2015b). Based on self-reported symptoms collected at the chronic stage via the RPQ, mTBI patients were further subdivided into two cohorts: those with PCS (n=12) and those without (n=16). Additionally, 28 HCs received rsfMRI and PASL scans at a single time point. For all subjects, functional connectivity (FC) and cerebral blood flow (CBF) were assessed within the nodes of the Default Mode Network (DMN) and Task Positive Network (TPN). Findings from the study indicated that: 1) all mTBI patients exhibited reduced strength of rs-FC within the DMN at the acute phase and increased rs-FC between the DMN and TPN at the chronic phase; 2) compared to HCs, mTBI patients in the chronic stage failed to maintain the expected balance of resting state perfusion between the DMN and TPN; and 3) mTBI patients that were PCS+ at 6 months after injury had alterations in network perfusion patterns across all three stages of injury, while PCS− participants maintained a similar pattern of network perfusion as the HCs. In particular, the TPN nodes showed greater perfusion compared to the DMN in PCS+ participants during the chronic stage. These results are novel in that they demonstrate that, while alterations in network perfusion are present in all mTBI patients in the chronic stage, mTBI patients who suffer from persistent PCS show these signs even during the acute and sub-acute stages, suggestive of distinctive courses of functional recovery in those with and without persistent PCS.
In a separate study, Sours and colleagues reported on a sample of 32 mTBI patients (15 PCS+ and 17 PCS−, as indexed by the self-report RPQ collected 6 months after injury) who received rsfMRI at the acute (within 1 week of injury) and chronic (6 months later) post-injury phases, along with 31 HCs who received rsfMRI at a single time point (Sours et al., 2015a). Discrete wavelet decomposition was used to analyze resting-state BOLD data with the goal of determining frequency-specific alterations in connectivity. The study found that the strength of DMN connectivity was reduced across the 0.125–0.250 Hz frequency range in mTBI patients who were suffering from persistent PCS compared with those with a more complete recovery. Additionally, the strength of DMN connectivity was greater in PCS− patients compared with the control population for both time points, which could signal compensatory or protective mechanisms. These findings suggest that DMN connectivity progressively changes during the first 6 months following injury, which is suggestive of either compensatory reorganization (in the case of PCS− patients) or disrupted network communication due to potential structural damage (in the case of PCS+ patients). These results highlight the need for further investigation of resting-state connectivity within multiple frequency ranges.
Banks and colleagues used rsFMRI in a sample of 13 mTBI patients to examine FC between the thalamus and resting-state networks during the early recovery period, and the relationship to patient-reported outcomes (Banks et al., 2016). Assessments, which included the administration of the RPQ, occurred at 6 weeks and 4 months post injury for mTBI patients and at a single visit for 11 HCs. Findings indicated that thalamic FC, especially within the dorsal attention network was decreased after mTBI compared to HCs. Importantly, improvements in PCS after 4 months were associated with normalization of thalamic FC over time, implicating FC alterations as a factor underlying mTBI recovery.
Palacios and colleagues recruited 75 mTBI patients and 47 HCs to assess semiacute alterations in brain connectivity and its relationship with cognitive and behavioral performance at 6 months post-injury (Palacios et al., 2017). Patients underwent conventional CT/MRI scans, completed the RPQ, and received rsfMRI within two weeks of injury. Alterations were found in the spatial maps of the resting state networks (RSNs) between mTBI patients and HCs in the DMN, executive control network, the frontoparietal network, the dorsal attentional network, the orbitofrontal network, and the visual network. However, mTBI patients with and without evidence of lesions at CT/MRI presented different patterns of network interaction alterations. In particular, negative correlations between semiacute connectivity and RPQ score at 6 months were found within the posterior regions of several RSNs in the CT/MRI negative group (such that patients with decreased connectivity presented more PCS), but not in those with CT/MRI positive scans.
Discussion
The goal of this review was to summarize and integrate findings from studies that longitudinally investigated brain changes underlying the emergence and persistence of PCS after a mTBI. The existing human neuroimaging literature consists of both small and large studies spanning acute to chronic time points that have examined both structural and functional changes with mTBI, using many of the current and available medical imaging modalities. All studies reviewed here indicate significant structural, functional and/or metabolic aberrations that occur in the acute and early subacute phases following a mTBI. Importantly, patients with mTBI who will later develop persistent PCS can show damage to structural integrity and disrupted network communication or metabolism both in the acute (Sours et al., 2015a, 2015b) and subacute (Messé et al., 2013, 2012) phases, compared to patients without persistent PCS. Distinctions in brain dysfunction between PCS+ and PCS− patients during these stages can be quantitative (i.e., both groups are impaired, yet PCS+ significantly more so – see Messé et al., 2012) or qualitative (i.e. PCS+ show unique biomarkers of brain dysfunction that PCS− patients do not show– see Sours et al., 2015b). Interestingly, disruptions of brain function can emerge over time and manifest exclusively in the chronic phase (Henry et al., 2011; Messé et al., 2013; Sours et al., 2015b). Acute and subacute brain dysfunction appears to take distinctive courses of functional recovery: some patients show signs of compensatory reorganization over time which lead to normalization of brain signals and partial or complete recovery of neural integrity. These patients are consistently found to be without PCS at six months post injury. Conversely, in other patients these acute and subacute structural, functional, and metabolic mTBI-induced impairments linger and contribute to the pathological substrate of persistent PCS. These biomarkers include: decreased fractional anisotropy values in association, commissural and projection white matter fiber tracts (Messé et al., 2012); lower graph properties in bilateral frontal gyri (Messé et al., 2013), greater perfusion in the TPN nodes compared to the DMN (Sours et al., 2015b), reduced strength of DMN connectivity (Sours et al., 2015a), decreased thalamic FC, especially within the dorsal attention network (Banks et al., 2016), and decreased connectivity within the posterior regions of several RSNs (Palacios et al., 2017). Finally, three studies found no association between brain biomarkers of mTBI and severity of PCS (George et al., 2014; Henry et al., 2011; Wäljas et al., 2015). First, in the Henry et al. study, participants’ performance on a measure of PCS (PCSS) revealed no differences between mTBI and HC at approximately six months post-injury. Thus, examining associations between biomarkers and PCS was not feasible; further, it is unclear if mTBI participants ever exhibited persistent PCS. Finally, given the variety of neuronal processes affected in mTBI (Giza and Hovda, 2001) and the currently limited knowledge regarding how these processes evolve and interrelate, predictions may be specious. As the authors noted, future studies should include larger samples and more time intervals to begin to chart the metabolic recovery and stability of those metabolites assayed, as well as those that were not (e.g., GABA and choline containing compounds). In the George et al. study, while there was no significant correlation between the metabolites assayed and severity of PCS, early subacute creatine measurements in the CSV did predict cognitive status six months post-injury (e.g., memory acquisition and recall abilities). Finally, in the Wäljas et al. study, multifocal areas of unusual white matter were found in both HC and mTBI; thus, this biomarker may not be specific to concussion or may be too weak of a signal to serve as a predictor of PCS.
Limitations
The present review has some limitations that should be noted. First, we reviewed studies that involved a repeated physiological assay across groups. Therefore, we excluded many studies that included analyses conducted at a single time-point post-injury or only involved cases studies. At the same time, the dearth of studies repeatedly collecting brain biomarkers could be ascribed to the challenges of retaining mTBI patients in longitudinal studies, and the resources necessary to support large clinical outcomes trials. Additionally, given the heterogeneity in assessments used to characterize PCS and the variation in evaluation time-points post-injury, we were not able to compare findings outcomes across studies. Third, because medication regimens were reported inconsistently across studies, we were not able to elucidate the role played by pharmacological therapies on PCS. Future studies should rigorously report medications, involvement in other therapies (e.g., cognitive rehabilitation; speech therapy) and model the role that these factors play in the evolution of persistent PCS. Other potentially relevant limitations include the variation in methods employed. For example, structural scans that only include ROIs may have missed critical zones affected by PCS. In addition, not all studies reviewed included objective measures of cognitive ability (e.g., ANAM); most relied on self-report questionnaires related to PCS. Objective measures of disability may help to better compare patients’ perception of difficulties relative to their performance on measures that include a reference sample (i.e., norms).
Conclusions
To date, PCS has been largely associated with psychosocial factors, perhaps due to: a) lack of high-level neuroimaging protocols in routine clinical practice; b) reliance on gross measures of brain integrity (e.g., negative CTs that contribute to potential Type II errors); or c) reliance on self-report rather than objective indices of cognitive status. From the studies reviewed here, it appears that occult brain changes contribute to the persistence of symptoms for some patients. We believe that a better characterization of the brain changes occurring after mTBI is likely to improve the clinical management of persistent PCS. In fact, neurobiomarkers reviewed in these studies may serve to mitigate otherwise categorical diagnoses of adjustment or mood disorders that now dominate the clinical conceptualization of recovery from mTBI. The results of the current review also highlight what may be considered a flaw in the current state of clinical practice: on the basis of false negative determinations of brain alterations from singular and conventional CT scans administered in the acute period of injury, clinicians often prescribe as a front-line approach medications to treat mood disruptions, ignoring the underlying neuropathology. fundamental alterations in brain function, connectivity or activity (e.g. metabolism), as show by this review, may better account for - or contribute to a broader understanding of - the presentation of patients at various stages of recovery. Thus, treatment for recovery from mTBI should consider also directly addressing PCS related cognitive disruptions via other forms of treatment, including cognitive training, cognitive behavioral therapy, and occupational therapy(Han et al., 2019; Janak et al., 2017; Minen et al., 2019).
If we are to advance the understanding of the physiological underpinnings of persistent PCS, the following lines of research should be pursued. First, repeated assays for those individuals who continue to report deficits beyond the acute period and do not recover as expected should be routinely implemented. One low-impact option is resting-state functional connectivity MR (Banks et al., 2016; Messé et al., 2013; Palacios et al., 2017; Sours et al., 2015b): this approach is generally tolerable for the patient and potentially low-cost for the primary care institutions, as acquisition of rsfMRI is relatively time-limited. In addition, studies should consistently adopt the same set of behavioral assessments (good examples include, the RPQ and the ANAM) and repeat such tests at similar time points post-injury (e.g., first week and 3- or 6-months post), in order to facilitate comparisons across datasets. Finally, it is important to better control for the contribution of psychosocial factors and other medical conditions that may obscure proper diagnosis.
Table 1.
Summary of the included studies.
| Author | Year | Assessment of PCS | Samples | Neuroimaging modality | Assessment time points | Main Finding |
|---|---|---|---|---|---|---|
| Henry | 2011 | PCSS | mTBI vs HC | 1H-MRSI | 1–6 days post-concussion, 6 months later | Neurometabolic alterations |
| Messe | 2012 | ICD-10 | PCS+ vs PCS− vs HC | DWI | 8–21 days post concussion, 6 months later | White matter alterations |
| Messe | 2013 | DSM-IV | PCS+ vs PCS− vs HC | rsfMRI | 8–21 days post concussion, 6 months later | Functional connectivity alterations |
| George | 2014 | RPQ | mTBI vs HC | 1H-MRSI | 1 week post concussion, 1 month later, 6 months later | Neurometabolic alterations |
| Waljas | 2015 | ICD-10 | No group comparisons | DWI | 3 weeks post concussion | White matter alterations |
| Sours | 2015a | RPQ | PCS+ vs PCS− vs HC | rsfMRI; PASL | 1 week post concussion, 1 month later, 6 months later | Functional connectivity and perfusion alterations |
| Sours | 2015b | RPQ | PCS+ vs PCS− vs HC | rsfMRI | 1 week post concussion, 6 months later | Functional connectivity alterations |
| Banks | 2016 | RPQ | mTBI vs HC | rsfMRI | 6 weeks post concussion, 4 months later | Functional connectivity alterations |
| Palacios | 2017 | RPQ | mTBI with CT/MRI positive scan vs mTBI with CT/MRI negative scan vs HC | rsfMRI | 2 weeks post concussion, 6 months later | Functional connectivity alterations |
Post Concussion Symptom Scale (PCSS), modified Rivermead Post-Concussion Symptoms Questionnaire (RPQ), Post Concussive Syndrome (PCS), Healhty Controls (HC), resting state functional MRI (rsfMRI), Diffusion Weighted Imaging (DWI), 1H-MR Spectroscopy Imaging (1H-MRSI).
HIGHLIGHTS.
Susceptibility to develop post concussive symptoms (PCS) after a mild traumatic brain injury (mTBI) is thought to originate from occult brain dysfunction
significant structural, functional and/or metabolic aberrations occur in the acute and/or early subacute phases following a mTBI. However, in patients with persistent PCS, these mTBI-induced damages linger and relate to the severity of PCS
Additionally, in PCS patients, disruptions of brain function can manifest exclusively in the chronic phase
Brain dysfunctions may or may not be directly related to the cognitive symptoms associated with PCS
indirect, non-correlated alterations in brain function may nonetheless contribute to the persistent nature of PCS in some patients
Brain dysfunction following mTBI can be captured and characterized with various forms of neuroimaging- or neuroassay-protocols that are not current standards in clinical practice
the use of neuroimaging modalities to understand the brain changes that occur throughout the time course of mTBI is necessary to prevent the onset and consolidation of PCS
Role of the Funding source
BB and TVV are supported through grants from the National Institute of Mental Health. PB and NS are partially supported by grants from the Italian Ministry of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
BB and TVV are Senior Scientists at Posit Science, a company that produces cognitive training software. The other authors report no conflict of interest.
References
- Banks SD, Coronado RA, Clemons LR, Abraham CM, Pruthi S, Conrad BN, Morgan VL, Guillamondegui OD, Archer KR, 2016. Thalamic Functional Connectivity in Mild Traumatic Brain Injury: Longitudinal Associations With Patient-Reported Outcomes and Neuropsychological Tests. Arch. Phys. Med. Rehabil 97, 1254–1261. 10.1016/j.apmr.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cancelliere C, Côté P, Hincapié CA, Kristman VL, Holm LW, Borg J, Nygren-de Boussard C, Hartvigsen J, 2014. Systematic Review of the Prognosis After Mild Traumatic Brain Injury in Adults: Cognitive, Psychiatric, and Mortality Outcomes: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil 95, S152–S173. 10.1016/j.apmr.2013.08.300 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC): Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation (Report to Congress). Atlanta, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, 2015, n.d. [Google Scholar]
- George EO, Roys S, Sours C, Rosenberg J, Zhuo J, Shanmuganathan K, Gullapalli RP, 2014. Longitudinal and Prognostic Evaluation of Mild Traumatic Brain Injury: A 1H-Magnetic Resonance Spectroscopy Study. J. Neurotrauma 31, 1018–1028. 10.1089/neu.2013.3224 [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA, 2001. The Neurometabolic Cascade of Concussion. J. Athl. Train 36, 228–235. [PMC free article] [PubMed] [Google Scholar]
- Han K, Chapman SB, Krawczyk DC, 2019. Cognitive Training Reorganizes Network Modularity in Traumatic Brain Injury. Neurorehabil. Neural Repair 154596831986871. 10.1177/1545968319868710 [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, Lassonde M, 2011. Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 11 10.1186/1471-2377-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G, 2007. Predicting slow recovery from sport-related concussion: the new simple-complex distinction. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 17, 31–37. 10.1097/JSM.0b013e3180305e4d [DOI] [PubMed] [Google Scholar]
- Janak JC, Cooper DB, Bowles AO, Alamgir AH, Cooper SP, Gabriel KP, Pérez A, Orman JA, 2017. Completion of Multidisciplinary Treatment for Persistent Postconcussive Symptoms Is Associated With Reduced Symptom Burden: J. Head Trauma Rehabil. 32, 1–15. 10.1097/HTR.0000000000000202 [DOI] [PubMed] [Google Scholar]
- Kane R, Roebuckspencer T, Short P, Kabat M, Wilken J, 2007. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch. Clin. Neuropsychol 22, 115–126. 10.1016/j.acn.2006.10.006 [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT, 1995. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol 242, 587–592. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Bellgowan PSF, Hanlon FM, 2015. Functional magnetic resonance imaging of mild traumatic brain injury. Neurosci. Biobehav. Rev 49, 8–18. 10.1016/j.neubiorev.2014.11.016 [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, Kelly JP, 2003. Acute Effects and Recovery Time Following Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA 290, 2556 10.1001/jama.290.19.2556 [DOI] [PubMed] [Google Scholar]
- Menon DK, Schwab K, Wright DW, Maas AI, 2010. Position Statement: Definition of Traumatic Brain Injury. Arch. Phys. Med. Rehabil 91, 1637–1640. 10.1016/j.apmr.2010.05.017 [DOI] [PubMed] [Google Scholar]
- Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Lévy R, Aghakhani N, Montreuil M, Benali H, Lehéricy S, 2013. Specific and Evolving Resting-State Network Alterations in Post-Concussion Syndrome Following Mild Traumatic Brain Injury. PLoS ONE 8, e65470 10.1371/journal.pone.0065470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Montreuil M, Lévy R, Lehéricy S, Benali H, 2012. Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav. 6, 283–292. 10.1007/s11682-012-9159-2 [DOI] [PubMed] [Google Scholar]
- Minen M, Jinich S, Vallespir Ellett G, 2019. Behavioral Therapies and Mind-Body Interventions for Posttraumatic Headache and Post-Concussive Symptoms: A Systematic Review: Headache. Headache J. Head Face Pain 59, 151–163. 10.1111/head.13455 [DOI] [PubMed] [Google Scholar]
- Mittenberg W, Canyock EM, Condit D, Patton C, 2001. Treatment of Post-Concussion Syndrome Following Mild Head Injury. J. Clin. Exp. Neuropsychol 23, 829–836. 10.1076/jcen.23.6.829.1022 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535–b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EM, Yuh EL, Chang Y-S, Yue JK, Schnyer DM, Okonkwo DO, Valadka AB, Gordon WA, Maas AIR, Vassar M, Manley GT, Mukherjee P, 2017. Resting-State Functional Connectivity Alterations Associated with Six-Month Outcomes in Mild Traumatic Brain Injury. J. Neurotrauma 34, 1546–1557. 10.1089/neu.2016.4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A, Schönberger M, 2012. Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology 26, 304–313. 10.1037/a0027888 [DOI] [PubMed] [Google Scholar]
- Quinn DK, Mayer AR, Master CL, Fann JR, 2018. Prolonged Postconcussive Symptoms. Am. J. Psychiatry 175, 103–111. 10.1176/appi.ajp.2017.17020235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PE, Keyser DO, Albano A, Hernandez R, Gibson DB, Zambon RA, Hairston WD, Hughes JD, Krystal A, Nichols AS, 2015. Traumatic Brain Injury Detection Using Electrophysiological Methods. Front. Hum. Neurosci 9 10.3389/fnhum.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Chen H, Roys S, Zhuo J, Varshney A, Gullapalli RP, 2015a. Investigation of Multiple Frequency Ranges Using Discrete Wavelet Decomposition of Resting-State Functional Connectivity in Mild Traumatic Brain Injury Patients. Brain Connect. 5, 442–450. 10.1089/brain.2014.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C, Zhuo J, Roys S, Shanmuganathan K, Gullapalli RP, 2015b. Disruptions in Resting State Functional Connectivity and Cerebral Blood Flow in Mild Traumatic Brain Injury Patients. PLOS ONE 10, e0134019 10.1371/journal.pone.0134019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäljas M, Iverson GL, Lange RT, Hakulinen U, Dastidar P, Huhtala H, Liimatainen S, Hartikainen K, Öhman J, 2015. A Prospective Biopsychosocial Study of the Persistent Post-Concussion Symptoms following Mild Traumatic Brain Injury. J. Neurotrauma 32, 534–547. 10.1089/neu.2014.3339 [DOI] [PubMed] [Google Scholar]