Abstract
Household air pollution (HAP) is estimated to be an important risk factor for cardiovascular disease, but little clinical evidence exists and collecting biomarkers of disease risk is difficult in low-resource settings. Among 54 Nicaraguan women with wood-burning cookstoves, we evaluated cross-sectional associations between 48-hour measures of HAP (e.g., fine particulate matter, PM2.5) and C-reactive protein (CRP) via dried blood spots; secondary analyses included seven additional systemic injury and inflammatory biomarkers. We conducted sub-studies to calculate the intraclass correlation coefficient (ICC) in biomarkers collected over four consecutive days in Nicaragua and to assess the validity of measuring biomarkers in dried blood by calculating the correlation with paired venous-drawn samples in Colorado. Measures of HAP were associated with CRP (e.g., a 25% increase in indoor PM2.5 was associated with a 7.4% increase in CRP [95% confidence interval: 0.7, 14.5]). Most of the variability in CRP concentrations over the 4-day period was between-person (ICC: 0.88), and CRP was highly correlated between paired dried blood and venous-drawn serum (Spearman rho=0.96). Results for secondary biomarkers were primarily consistent with null associations and the sub-study ICCs and correlations were lower. Assessing CRP via dried blood spots provides a feasible approach to elucidate the association between HAP and cardiovascular disease risk.
Keywords: biomass fuel, fine particulate matter, carbon monoxide, C-reactive protein, cardiovascular health, biomarkers
INTRODUCTION
Approximately 40% of the world’s population relies on solid fuels for household cooking, primarily in low-resource settings.[1] Exposure to household air pollution (HAP) from the combustion of fuels, such as wood and coal, is a leading risk factor for global morbidity and premature mortality, including a substantial portion due to cardiovascular disease.[2] Oxidative stress and systemic inflammation are thought to be key mechanisms by which air pollution exposure, especially particulate matter less than 2.5 micrometers in diameter (PM2.5), leads to cardiovascular disease effects.[3] Obtaining measurements of clinical disease for epidemiologic studies in rural, low-resource settings is time- and cost-prohibitive and logistically challenging; consequently, focus has been placed on subclinical indicators of disease risk that are feasible to measure in field settings. Serum from venous-drawn blood is the standard tissue used in clinical settings to measure biomarkers of systemic injury and inflammation, but this approach can be difficult in the field due to a lack of onsite medical staff and post-collection processing facilities. Dried blood spots collected with a finger-stick by trained non-medical personnel are less invasive and eliminate the logistical difficulties associated with collecting, transporting, and storing blood.[4, 5] Furthermore, the use of dried blood spots has been valuable for many types of population-based epidemiologic studies.[4, 6]
In general, the choice of a biomarker to more fully understand the association between HAP and cardiovascular disease risk should be driven by: (1) how informative the marker is regarding disease prediction – with insight into mechanism being secondary; (2) the stability of the marker (i.e., low within-person variability) as it relates to the hypothesized time-course of the modifiable effect (i.e., ability to observe changes in the marker over the course of months to years); and (3) the feasibility to measure the marker in a low-resource setting.[7] Evaluating C-reactive protein (CRP) via dried blood spots meets these considerations.[5, 7, 8] CRP is produced by the liver in a nonspecific response to inflammation, infection, and tissue damage. Elevated CRP values can be a strong predictor of future coronary events, such as heart attacks and stroke. CRP may be especially useful in field settings as it has no diurnal pattern and is not impacted by food consumption, so fasting is not required.[9] CRP provides a promising approach for investigating the association between HAP and indicators of cardiovascular disease risk in the rural field settings typical of many HAP studies.[10, 11] Therefore, for our primary objective we investigated the cross-sectional association between 48-hour measures of HAP exposure and CRP in dried blood spots among 54 women using traditional wood-burning cookstoves in Nicaragua. A secondary objective was to evaluate the association between HAP and seven additional biomarkers of systemic injury and inflammation thought to be less responsive (as compared to CRP) to the considerations outlined above. To evaluate confidence in the dried blood spot measurement techniques, we evaluated associations between age and body mass index (BMI) and the biomarkers, given expected age- and obesity-related systemic inflammation as demonstrated in the literature with venous-drawn blood samples.[8]
Further, we conducted two sub-studies to support our primary and secondary objectives. For sub-study 1, we calculated the within- and between-person variability in eight biomarkers of systemic injury and inflammation measured over four consecutive days among members of the same community in Nicaragua. For sub-study 2, we evaluated the validity of dried blood spots to measure the eight biomarkers by calculating the correlation with paired serum from venous-drawn blood collected from participants at Colorado State University (CSU).
MATERIAL AND METHODS
Study sample
In May through July 2008, we selected a convenience sample of 124 households from a rural neighborhood, El Fortin, outside of Granada, Nicaragua. El Fortin was a residential neighborhood with two lightly trafficked dirt roads. Participants were nonsmoking women, aged 11–80 years, using traditional wood-burning cookstoves, recruited through community volunteers. Complete methodologic details have been published previously.[12] Study protocols were approved by the CSU Institutional Review Board and the Nicaraguan Ministry of Health, and participants gave informed consent prior to data collection.
Exposures, biomarkers, and covariates
To estimate HAP exposure, air pollutants were sampled via the UCB Particle Monitor (Berkeley Air Monitoring Group, USA) for indoor PM2.5 (µg/m3) concentrations, and the Draeger Pac 7000 (SKC, Inc, USA) for indoor and personal carbon monoxide concentrations (parts per million, ppm), previously described in detail.[12] The PM2.5 and carbon monoxide monitors measured and logged pollutant concentrations continuously over 48 hours and calculated average concentrations. Indoor sampling monitors for PM2.5 and carbon monoxide were collocated inside the kitchen at a height representative of the participant’s breathing zone and not in front of a window/door to avoid being placed within a plume of smoke from the cookstove. The personal carbon monoxide monitor was attached to the participant’s clothing near her breathing zone.
Via questionnaire, we obtained information on age, years of education, location/style of the kitchen, ownership of a pig (as a measure of wealth), secondhand-smoke exposure, current illnesses, and medication use. We measured height and weight to calculate body mass index (BMI, kg/m2).
Following the 48-hour exposure assessment, trained personnel collected blood spots with a finger-stick using a lancet and standardized filter paper (Whatman 903 Protein Saver Card, Schleicher & Schuell, USA). The procedures for collecting, shipping, and analyzing biomarkers in dried blood spots have been previously described in detail.[13–15] In brief, the participant’s arm and hand were massaged to warm up the fingers, and the site was disinfected with a 70% alcohol swab and allowed to dry completely. The middle or ring finger was pricked using a sterile, disposable, single-use, retractable lancet on the side of the ball of the finger perpendicular to the lines of the fingerprints. The first drop of blood was wiped off with a sterile gauze, because this first drop may be contaminated with tissue fluid or sloughing skin. The personnel did not squeeze the finger or “milk” the finger because this can potentially dilute the specimen with tissue fluid. After allowing the blood to drop onto the filter paper card for up to 5 spots, firm pressure was applied to the site to stop the bleeding. The filter paper cards were transported in a horizontal position and allowed to air dry for 24-hours at room temperature on a clean paper towel at the field house. For storage, the top flap of the filter paper card was folded over the spot to protect it, and the card was stored in a low gas-permeable plastic bag with 2–4 desiccant sachets to protect the specimens from moisture, and a humidity indicator card. The bags were stored in a −20-degree Celsius freezer in Nicaragua and transported to Colorado (at ambient temperature for no more than 24-hours during transport), where samples were then stored in a −80-degree Celsius freezer. DBS specimens were overnight shipped to the U.S. EPA with dry ice. Analyses were performed on the Meso Scale Discovery multiplex assay system (Meso Scale Diagnostics, LLC, Rockville, MD) in 2 separate kits, one for vascular injury: CRP, serum amyloid A (SAA), soluble intercellular adhesion molecule (sICAM), soluble vascular cell adhesion molecule (sVCAM) and another for pro-inflammatory mediators: interleukin 1-beta (IL-1β), IL-6, IL-8, and tumor necrosis factor-alpha (TNF-α). At the laboratory, a device with a 6 mm diameter was used to punch out one circle from one spot on each card. Punches were transferred to 96-well plates (each punch in a well) and a 200 ul extraction buffer (PBS with 0.5% tween-20) was added to each well. Plates were sealed and shaken overnight in a 4-degree Celsius refrigerator. Plates were then stored in a −80-degree Celsius freezer before analysis. For analysis, the extraction liquids were measured in 1:200 dilution factor for the vascular injury kit and without any dilution for the pro-inflammatory mediators kit. Data were output directly from the QuickPlex 120 instrument. Reliability (intra-plate variability) and reproducibility (inter-plate variability) were tested for all assays and the coefficient of variation was below 10%.
Statistical analysis
For the purpose of this study, the laboratory analysis was limited to a sub-sample of 54 participants that did not report using anti-inflammatory medications. All data analyses were conducted using SAS 9.4 (SAS Institute, USA). We calculated descriptive statistics for socio-demographic, exposure, and health measures. For the 8 biomarkers, we calculated Spearman correlation coefficients (rho). We compared mean biomarker levels across categories of age (11–25, 26–39, 40 or more years) and obesity (BMI <30.0, 30.0 or higher) via analysis of variance. To satisfy model assumptions, we natural-log transformed all air pollution variables and biomarkers of systemic injury and inflammation, except for sVCAM. We evaluated the mean difference in the biomarker concentration per 25% increase in 48-hour mean pollutant concentration in separate linear regression models for each biomarker and each exposure (indoor PM2.5, indoor carbon monoxide, and personal carbon monoxide). In these models, we adjusted for 3 potential confounders (selected a priori): age (continuous), BMI (continuous), and education (3-level categorical). We further considered models that included the dichotomous variables of pig ownership, secondhand smoke exposure, having an enclosed kitchen, and self-report of having a cold in the past week by evaluating whether or not there was a meaningful change in the mean biomarker concentration difference as compared to the initial model. We repeated all analyses after removing 7 potential biomarker outliers (ng/mL), including those that were high for CRP (n=2; 297, 270) and IL-1β (n=1; 15), and low for CRP (n=1; 0.05) and SAA (n=3; 0.05, 0.05, 2.0) concentrations.
Sub-studies
For sub-study 1, dried blood spots were collected over four consecutive days in 2011 from 21 non-smoking participants, aged 9–55 years, from the same community in Nicaragua and using the same methods described above for collection, transportation, and analysis of the eight biomarkers via dried blood. We calculated the intraclass correlation coefficients (ICCs) for each biomarker using 80 observations from 21 participants. Study protocols were approved by the Colorado State University Institutional Review Board and the Nicaraguan Ministry of Health.
For sub-study 2 conducted in 2011 at CSU, we recruited a convenience sample of 21 healthy, non-pregnant volunteers, at least 18 years of age, weighing at least 110 pounds, and not currently using any anti-inflammatory medications. Among participants, we collected paired samples of finger-stick dried blood spots and 10 mL venous-drawn blood collected in serum separator tubes. Samples were shipped overnight to the U.S EPA and analyzed for the eight biomarkers of interest in dried blood spots as described above and in the serum (as described by Meso Scale Diagnostics). We calculated Spearman correlation coefficients between paired finger-stick dried blood spots and serum from venous-drawn blood samples for the eight biomarkers. Study protocols were approved by the CSU Institutional Review Board.
RESULTS
Participant characteristics among female cooks in Nicaragua (N=54) included a mean (standard deviation; SD) age of 31.7 years (15.1) and years of school ranged from none (n=17, 33%), 1–5 years (n=16, 31%), and 6 or more years (n=19, 37%). Nine participants (17%) owned a pig. Approximately one third of participants cooked in an enclosed kitchen (n=20), were obese (BMI of 30 or greater, n=18), and were exposed to secondhand smoke (n=19). Half of the sample (n=27) reported having a cold in the past week.
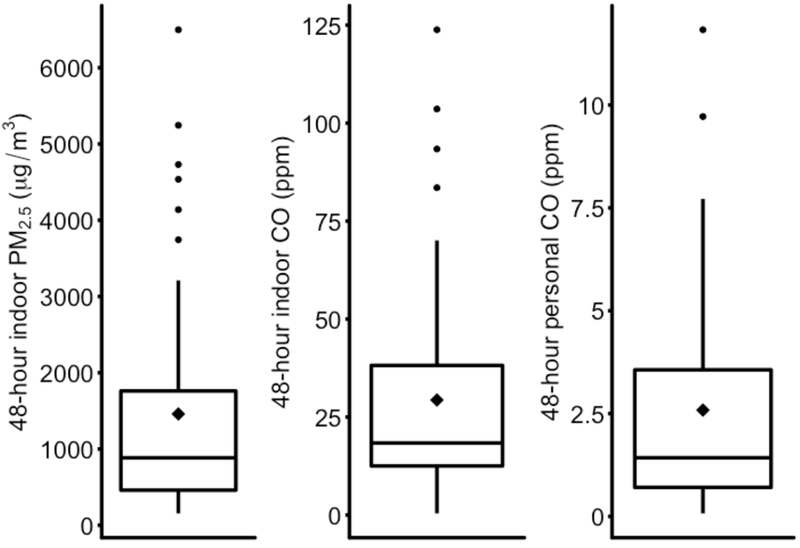
Distributions of the 48-hour HAP concentrations are shown in Figure 1. The mean (SD) and median (25th, 75th percentiles) concentrations were: indoor PM2.5, 1460 µg/m3 (1476), 884 (460, 1810); indoor carbon monoxide, 29.4 ppm (27.4), 18.4 (12.5, 38.2); and personal carbon monoxide, 2.6 ppm (2.6), 1.4 (0.7, 3.7).
Figure 1.
Distributions of 48-hour indoor PM2.5 (µg/m3) and indoor and personal CO (ppm) concentrations among female cooks in rural Nicaragua. The lower boundary of the box represents the 25th percentile; the upper boundary represents the 75th percentile; the “◊”symbol inside the box is the mean; the line within the box is the median. The upper whisker is the third quartile plus 1.5 times the interquartile range (IQR), and the lower whisker is the first quartile minus 1.5 times the IQR. The dots are outliers.
Abbreviations: CO: Carbon monoxide; PM2.5: Particulate matter with diameter <2.5 micrometers; ppm: parts per million; µg/m3: micrograms per cubic meter.
Descriptive statistics and Spearman correlation coefficients for the eight biomarkers (ng/mL) among the 54 participants are presented in Table 1. Spearman correlation coefficients for the biomarkers showed strong correlations between IL-6 and IL-8 (rho=0.87), IL-6 and TNF-α (rho=0.79), and IL-8 and TNF-α (rho=0.73) (Table 1).
Table 1.
Descriptive statistics and correlations of biomarkers measured from dried blood spots of capillary whole blood collected from female cooks in Nicaragua, 2008.
| Descriptive statistics | Spearman Correlation Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte (ng/mL) | N | Mean (SD); Median (25th, 75th percentiles) | CRP | SAA | sICAM | sVCAM | IL-1β | IL-6 | IL-8 | TNF-α |
| CRP | 54 | 41.2 (60.8); 20.0 (9.4, 37.1) | . | 0.54 | 0.35 | 0.29 | 0.04 | −0.03 | −0.27 | −0.10 |
| SAA | 54 | 36.2 (63.3); 16.2 (6.8, 33.3) | . | . | 0.57 | −0.03 | 0.25 | 0.20 | 0.05 | 0.03 |
| sICAM | 54 | 5.8 (2.6); 5.2 (4.0, 7.8) | . | . | . | 0.12 | −0.16 | −0.23 | −0.26 | −0.24 |
| sVCAM | 54 | 9.1 (8.3); 8.6 (0.9, 14.9) | . | . | . | . | −0.22 | −0.31 | −0.51 | −0.20 |
| IL-1β | 54 | 1.6 (2.1); 1.0 (0.8, 1.8) | . | . | . | . | . | 0.44 | 0.40 | 0.38 |
| IL-6 | 54 | 0.4 (0.5); 0.2 (0.08, 0.44) | . | . | . | . | . | . | 0.87 | 0.79 |
| IL-8 | 54 | 30.8 (41.1); 16.4 (6.7, 37.7) | . | . | . | . | . | . | . | 0.73 |
| TNF-α | 54 | 0.7 (0.8); 0.4 (0.2, 0.8) | . | . | . | . | . | . | . | . |
Abbreviations: CRP: C-reactive protein; IL-1β: interleukin 1-beta; IL-6: interleukin 6; IL-8; interleukin 8; SAA: serum amyloid A; sICAM: soluble intercellular adhesion molecule; sVCAM: soluble vascular cell adhesion molecule; TNF-α: tumor necrosis factor-alpha.
Several biomarkers were associated with age and BMI in the expected directions, as observed in other studies evaluating systemic injury and inflammation via venous-drawn sampling. For example, the mean natural-log transformed concentrations of CRP (natural-log transformation of ng/mL) increased across 3 levels of age: 11–25 years, mean=2.5 (95% confidence interval [CI]: 1.9, 3.1, n=22; reference category); 26–39 years, mean=2.9 (95% CI: 2.3, 3.6, n=17; p=0.33); and 40 or more years, mean=3.5 (95% CI: 2.8, 4.3, n=15; p=0.03). Similarly, higher sVCAM levels were observed among the oldest age group compared to the youngest (data not shown). Higher CRP levels were observed among obese women compared to non-obese women (obese group mean=3.9, 95% CI: 3.3, 4.5 vs. non-obese group mean=2.4, 95% CI: 2.0, 2.8; p=0.0001). Higher levels of mean analyte concentrations were also observed among obese compared to non-obese women for SAA and sVCAM (data not shown). We did not observe patterns of higher biomarker levels for older ages and obese participants for the other biomarkers; in fact, for several of the biomarkers, patterns in the direction opposite to that hypothesized were observed across age (IL-1B, IL-8) and obesity (IL-8, TNF-a) categories (data not shown).
Table 2 summarizes the adjusted associations between measures of HAP and biomarkers of systemic injury/inflammation. For example, a 25% increase in indoor PM2.5 was associated with a 7.4% increase (95% CI: 0.7, 14.5) in CRP. Similar effects were observed for elevated personal and indoor carbon monoxide on CRP (Table 2). Associations between carbon monoxide and sVCAM were also observed; however, higher HAP concentrations were not consistently associated with higher levels of the other biomarkers (Table 2). Results did not change after considering other potential confounders or when removing potential outliers.
Table 2.
Mean differences in concentrations of biomarkers of systemic injury and inflammation per 25% increase in the air pollutant concentrations measured over 48 hours among female cooks in rural Nicaragua, 2008.†
| Pollutant | N | Difference | 95% CI |
|---|---|---|---|
| CRP | |||
| Indoor PM2.5 | 48 | 7.4% | 0.7, 14.5 |
| Indoor CO | 51 | 4.8% | −0.5, 10.4 |
| Personal CO | 50 | 7.9% | 1.8, 14.3 |
| SAA | |||
| Indoor PM2.5 | 48 | 6.9% | −3.9, 18.9 |
| Indoor CO | 51 | 3.0% | −5.0, 11.6 |
| Personal CO | 50 | 1.9% | −7.1, 11.7 |
| sICAM | |||
| Indoor PM2.5 | 48 | 2.0% | −1.3, 5.4 |
| Indoor CO | 51 | 1.0% | −1.6, 3.6 |
| Personal CO | 50 | 2.4% | −0.5, 5.4 |
| sVCAM‡ | |||
| Indoor PM2.5 | 48 | 1.6 ng/ml | −1.1, 4.3 |
| Indoor CO | 51 | 1.8 ng/ml | −0.2, 3.8 |
| Personal CO | 50 | 2.7 ng/ml | 0.5, 5.1 |
| IL-1β | |||
| Indoor PM2.5 | 48 | 0.5% | −4.4, 5.7 |
| Indoor CO | 51 | 0.8% | −2.9, 4.8 |
| Personal CO | 50 | −1.6% | −5.6, 2.5 |
| IL-6 | |||
| Indoor PM2.5 | 48 | −6.7% | −41.8, 49.7 |
| Indoor CO | 51 | −10.5% | −37.2, 27.4 |
| Personal CO | 50 | 8.2% | −28.9, 64.6 |
| IL-8 | |||
| Indoor PM2.5 | 48 | −2.1% | −9.9, 6.4 |
| Indoor CO | 51 | −4.2% | −10.1, 2.0 |
| Personal CO | 50 | −3.1% | −9.6, 4.0 |
| TNF-α | |||
| Indoor PM2.5 | 48 | −6.5% | −15.1, 3.1 |
| Indoor CO | 51 | −4.0% | −11.0, 3.4 |
| Personal CO | 50 | −0.5% | −8.9, 8.7 |
All models were adjusted for age, body mass index, and education. Sample sizes less than 54 are due to missing pollutant or covariate data. With the exception of sVCAM, beta coefficients were entered into the formula ((1.25^β)-1) and multiplied by 100; therefore, we can interpret the estimate as a percent difference in inflammatory marker for each 25% increase in pollutant.
Values presented for sVCAM represent the mean change in units of ng/ml for each 1-unit increase in the natural-logarithmic transformed pollution variable.
Abbreviations: CI: confidence interval; CO: Carbon monoxide; CRP: C-reactive protein; IL-1β: interleukin 1-beta; IL-6: interleukin 6; IL-8; interleukin 8; PM2.5: Particulate matter with diameter <2.5 micrometers; SAA: serum amyloid A; sICAM: soluble intercellular adhesion molecule; sVCAM: soluble vascular cell adhesion molecule; TNF-α: tumor necrosis factor-alpha.
Sub-studies
For sub-study 1, the highest ICC was observed for CRP (ICC=0.88); 88% of the variance in CRP levels over four days was estimated to be from between-person differences. The ICCs for the other biomarkers were as follows: SAA, 0.70; TNF-α, 0.55; sICAM, 0.53; IL-6, 0.39; IL-1β, 0.24; sVCAM, 0.13; and IL-8, 0.02.
For sub-study 2, CRP concentrations were highly correlated between the paired dried blood spots and serum from venous-drawn blood samples (Spearman rho=0.96). Spearman correlation coefficients between dried blood and venous-drawn samples for the other biomarkers were as follows: SAA, 0.72; IL-8, 0.39; TNF-α, 0.36; sVCAM, 0.29; IL-6, 0.29; sICAM, 0.14; IL-1β, 0.08.
DISCUSSION
Although our study was limited by a small, cross-sectional design, limited measurements of potential confounders and air pollution exposures (including a lack of pollutant source identification)[16], and the possibility that results were observed by chance, CRP as measured via dried blood spots was positively associated with exposure to 48-hour PM2.5 and carbon monoxide among Nicaraguan women. Additionally, CRP was positively associated with age and BMI in our study population, and in our sub-study the majority of the estimated variability in concentrations over a 4-day period was between-person. In general, CRP is considered an important biomarker in clinical and public health practice; compared to other markers of inflammation, it is a stable and independent predictor of cardiovascular disease, with seemingly little diurnal or seasonal variability.[8] Furthermore, although not consistently, previous research reported associations between short- and long-term ambient air pollution exposures and CRP.[17–20] Two previous feasibility studies (our study among Honduran women[11] and the Shan et. al. study among rural Chinese women)[10], also cross-sectional and limited by small sample sizes, evaluated measures of household air pollution and CRP via dried blood spots; both reported results consistent with the null association.[10, 11] Finally, two cross-sectional studies reported differences in CRP concentrations assessed via venous-drawn blood samples among populations using different fuel or stove types. CRP was higher among women using biomass stoves as compared to liquified petroleum gas stoves in rural India[21] but lower among biomass-exposed compared to non-biomass-exposed men and women living in Puno, Peru;[22] the heterogenous study populations and residual confounding are limitations that may explain these discrepancies.
The paired concentrations from finger-stick dried blood spots and serum from venous-drawn blood samples were highly correlated for CRP, similar to results from another paired sample study.[5] Although correlations were not as strong, SAA demonstrated similar patterns. For other biomarkers, the weak correlations between dried blood spot and serum samples indicate that dried blood as measured via our protocol may not be an appropriate substitute for serum. It is possible that some biomarker concentrations are different in capillary whole blood as compared to venous-drawn serum; however, further research utilizing dried blood spots to assess these markers should investigate options for improving upon the field and laboratory methods employed here. For example, different techniques may improve upon potential analyte degradation and measurement error issues with dried blood samples (e.g., modification of filter paper collection media, proper assay development [including enhancing dynamic range and limit of detection issues for capillary whole blood], and blood volume standardization).[4] Therefore, with the exception of CRP, our results for the associations between measures of HAP exposure and these dried blood markers of systemic injury and inflammation should be interpreted with caution.
Another limitation of our study is that the dried blood spot validation study was not conducted within the constraints for sample collection, storage, and transportation typically experienced in rural areas of low-resource settings and it is possible that correlations between dried blood spots and serum samples would have been impacted by these conditions.
Overall, our results are the first to suggest that measures of HAP exposure among biomass cookstove users is associated with higher levels of CRP, a well-accepted indicator of cardiovascular disease risk.[8] In the sub-studies, we observed low within-person variability for CRP over a 4-day period, and CRP concentrations were highly correlated between paired dried blood spots and serum from venous-drawn blood. We conclude that assessments of CRP via dried blood spots provide a reliable and feasible alternative to venous-drawn samples in low-resource field settings.
Practical Implications:
Household air pollution from the use of biomass for cooking is estimated to be an important risk factor for cardiovascular disease, but direct evidence is limited. Obtaining measurements of clinical cardiovascular disease for epidemiologic studies in rural, low-resource settings is time- and cost-prohibitive and logistically challenging. Dried blood spots collected with a finger-stick by trained non-medical personnel are less invasive and eliminate the logistical difficulties associated with collecting, transporting, and storing venous-drawn blood. Our results are the first to suggest that measures of HAP exposure among biomass cookstove users are associated with higher levels of C-Reactive protein (CRP), a well-accepted indicator of cardiovascular disease risk. In sub-studies, we observed low within-person variability for CRP over a 4-day period, and CRP concentrations were highly correlated between paired dried blood spots and serum from venous-drawn blood.
Acknowledgements:
We are grateful to our community partner in Granada, Nicaragua, Casa de la Mujer, for their collaboration. We thank our local field workers, Maria Teresa Nurinda, Maria del Trancito Murillo, Maria Jose Narvaez Murillo, and Gloria Elena Rodriguez, the CSU student field teams, and William Marquardt. We acknowledge the helpful collaboration from Trees, Water and People in Fort Collins, Colorado, Allison Shaw, Jacqueline D. Carter, and the NIOSH Mountain and Plains Education and Research Center (5T42 OH009229). This work was funded by the National Institutes of Health (R03 ES019696-01). The funder did not have any role in study design, data collection, analysis, interpretation, or writing of this report.
Funding: This work was funded by the National Institutes of Health (R03 ES019696-01). The funder did not have any role in study design, data collection, analysis, interpretation, or writing of this report.
Footnotes
Declarations: The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency or the National Institutes of Health. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The authors declare no conflicts of interest.
Human Subjects Research: The study was conducted in accordance with the Declaration of Helsinki of the World Medical Association and reviewed and approved by the Colorado State University Internal Review Board and the Nicaraguan Ministry of Health prior to its conduct. All participants gave informed consent prior to conducting the study.
References
- 1.Bonjour S, et al. , Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect, 2013. 121(7): p. 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Risk Factors Collaborators, Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet., 2018. 392: p. 1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, et al. , Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation, 2010. 121(21): p. 2331–78. [DOI] [PubMed] [Google Scholar]
- 4.McDade TW, Williams S, and Snodgrass JJ, What a drop can do: Dried blood spots as a minimally-invasive method for integrating biomarkers into population-based research. Demography, 2007. 44(4): p. 899–925. [DOI] [PubMed] [Google Scholar]
- 5.McDade TW, Burhop J, and Dohnal J, High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clinical Chemistry, 2004. 50(3): p. 652–4. [DOI] [PubMed] [Google Scholar]
- 6.Freeman JD, et al. , State of the Science in Dried Blood Spots. Clinical Chemistry, 2018. 64(4): p. 656–679. [DOI] [PubMed] [Google Scholar]
- 7.Wellenius G and Kaufman J, Choosing Study Outcomes that Reflect Cardiovascular Disease: From “Biomarkers” to Burden of Disease, in Evaluating the health benefits of clean cooking adoption: Indicators and Biomarkers of Noncommunicable Disease (NCDs) Global Alliance for Clean Cookstoves, Editor. 2014, United Nations Foundation: Washington DC. [Google Scholar]
- 8.Pearson TA, et al. , Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 2003. 107(3): p. 499–511. [DOI] [PubMed] [Google Scholar]
- 9.Pepys MB and Hirschfield GM, C-reactive protein: a critical update. J Clin Invest, 2003. 111(12): p. 1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan M, et al. , A feasibility study of the association of exposure to biomass smoke with vascular function, inflammation, and cellular aging. Environ Res, 2014. 135: p. 165–72. [DOI] [PubMed] [Google Scholar]
- 11.Clark ML, et al. , Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. International Journal of Environmental Health Research, 2009. 19(5): p. 357–368. [DOI] [PubMed] [Google Scholar]
- 12.Clark ML, et al. , A baseline evaluation of traditional cook stove smoke exposures and indicators of cardiovascular and respiratory health among Nicaraguan women. International Journal of Occupational & Environmental Health, 2011. 17(2): p. 113–21. [DOI] [PubMed] [Google Scholar]
- 13.Mirowsky JE, et al. , Repeated measures of inflammation, blood pressure, and heart rate variability associated with traffic exposures in healthy adults. Environ Health, 2015. 14: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hejl AM, et al. , Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. J Occup Environ Hyg, 2013. 10(4): p. 173–80. [DOI] [PubMed] [Google Scholar]
- 15.Adetona AM, et al. , Impact of Work Task-Related Acute Occupational Smoke Exposures on Select Proinflammatory Immune Parameters in Wildland Firefighters. J Occup Environ Med, 2017. 59(7): p. 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark ML, et al. , Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect, 2013. 121(10): p. 1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, et al. , Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol, 2017. 37(9): p. 1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, et al. , Effect of particulate matter air pollution on C-reactive protein: a review of epidemiologic studies. Rev Environ Health, 2012. 27(2–3): p. 133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajat A, et al. , Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology, 2015. 26(3): p. 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilz V, et al. , C-reactive protein (CRP) and long-term air pollution with a focus on ultrafine particles. Int J Hyg Environ Health, 2018. 221(3): p. 510–518. [DOI] [PubMed] [Google Scholar]
- 21.Dutta A, Ray MR, and Banerjee A, Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicology and Applied Pharmacology, 2012. 261(3): p. 255–262. [DOI] [PubMed] [Google Scholar]
- 22.Caravedo MA, et al. , Chronic exposure to biomass fuel smoke and markers of endothelial inflammation. Indoor Air, 2016. 26(5): p. 768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]