Abstract
The rostromedial tegmental nucleus (RMTg) receives inputs from the laterodorsal tegmental and pedunculopontine tegmental nuclei, the two principle brainstem cholinergic nuclei. We tested the effects of RMTg M3 and M4 muscarinic cholinergic receptor antagonism in a conditioned place preference (CPP) paradigm in mice. RMTg infusions of the M3 muscarinic cholinergic receptor antagonist 1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) do not result in the acquisition of CPP but increase locomotor activation. By contrast, RMTg infusions of the M4 muscarinic cholinergic receptor antagonist Tropicamide result in the acquisition of CPP but do not increase locomotor activation. The rewarding effects of RMTg Tropicamide infusions are dopamine-dependent as systemic pre-treatment with the broad-spectrum dopamine receptor antagonist flupenthixol prevents the acquisition of CPP induced by RMTg Tropicamide infusions. Under conditions of systemic dopamine receptor blockade, RMTg Tropicamide infusions significantly increase locomotor activation. These data provide further support for an important role of endogenous cholinergic input to the RMTg in reward function and suggest that the contributions of RMTg cholinergic input to rewarding and locomotor-activating effects involve differential contributions of RMTg M4 and M3 muscarinic receptors, respectively.
Keywords: laterodorsal tegmental nucleus, pedunculopontine tegmental nucleus, dopamine, acetylcholine, flupenthixol
The laterodorsal tegmental nucleus (LDTg) and the pedunculopontine tegmental nucleus (PPTg) – the two principle brainstem cholinergic nuclei – each send afferents to the ventral tegmental area (VTA) [1-4] and specifically to dopamine (DA) neurons in this site [3]. GABAergic neurons in the rostromedial tegmental nucleus (RMTg; [5,6]) or, alternatively called the tail of the VTA [7, 8], just caudal to the classically defined VTA, critically control the DA system. These GABAergic cells are continuous with those of the VTA, and project to [5, 6] and inhibit VTA DA neurons [9]. Similar to the VTA, the RMTg receives afferents from LDTg and PPTg [5, 6, 10]. Some of the LDTg/PPTg input to the RMTg input is cholinergic with the same LDT/PPTg cholinergic neurons sending collaterals to both RMTg and VTA [11]. While a role for cholinergic input to the VTA in reward function has been clearly established over several decades (see [12] for a review), the role of cholinergic input to the RMTg in reward function is less well understood. Previously we have shown that infusions of the M3 muscarinic receptor antagonist 1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), but not the M4 muscarinic receptor antagonist Tropicamide, increase drug-free and morphine-induced open-field locomotion in mice [13]. In the current studies we used conditioned place preference (CPP) – a commonly used paradigm that assesses the rewarding or aversive effects of drugs of abuse [14-16] – to test whether RMTg infusions of M3 or M4 muscarinic receptor antagonists induce rewarding effects.
To accomplish this 13 C57BL/6 mice (Charles River), maintained on a 12-hour light/dark cycle (lights on at 7:00 AM) with food and water available ad libitum throughout, were implanted with guide cannulae (26 ga; Plastics One Inc.) aimed at the RMTg (A-P -4.0, M-L ± 0.3, D-V – 4.3) as previously described [13, 17]. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were tested in a place conditioning apparatus consisting of two conditioning chambers (20 × 18 × 35 cm) differing in floor texture (smooth vs. wire grid floor) and color (black vs. black with vertical white stripes) connected by a third chamber (20 × 10 × 35 cm). The position of the mouse within the apparatus was monitored via a dedicated camera mounted above each apparatus and video tracking software (ANY-Maze; Stoelting Co.; Wood Dale, IL). ANY-Maze was used to measure time spent, and distance traveled in each of the chambers. All mice received an initial 30 min habituation session in which mice had free access to the entire apparatus. After 2-4 days baseline preferences for the two conditioning chambers were determined during a 20 min session in which mice had free access to the entire apparatus. We used a biased conditioning procedure in which the initially less preferred chamber was subsequently paired with RMTg infusions of either 10.32 μg Tropicamide or 2 μg 1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP; Tocris Bioscience), each dissolved in 0.05% DMSO (these doses were previously tested for their locomotor effects in RMTg [13]) and the alternate chamber was paired with RMTg vehicle infusion (4 alternating pairings of each). All intracranial infusions (injector cannulae 2 mm longer than guide cannulae) were made at a volume of 0.3 μl and at rate of 0.2 μl/min and mice were immediately confined to their respective conditioning chamber for a period of 30 min. During the subsequent CPP test, on the day following the 8th conditioning session, mice were again given free access to the entire apparatus. Following completion of behavioral testing mice were transcardially perfused and coronal cryosections throughout the extent of the RMTg were stained with cresyl violet to verify injection sites. Mice that did not have bilateral RMTg infusion sites were excluded from statistical analysis (2 for the Tropicamide and 1 for 4-DAMP CPP experiments). A difference score (time spent in the chamber during the CPP test – time spent in the chamber during the baseline test) was computed for each mouse. Total distance traveled while mice were confined to conditioning chambers was also measured. All data were analyzed using single-sample or paired-samples t-tests.
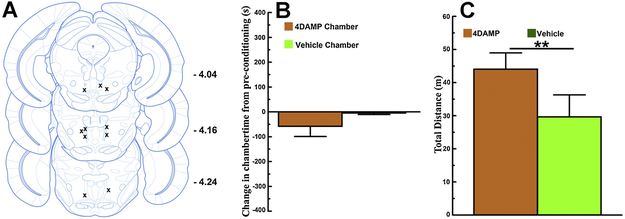
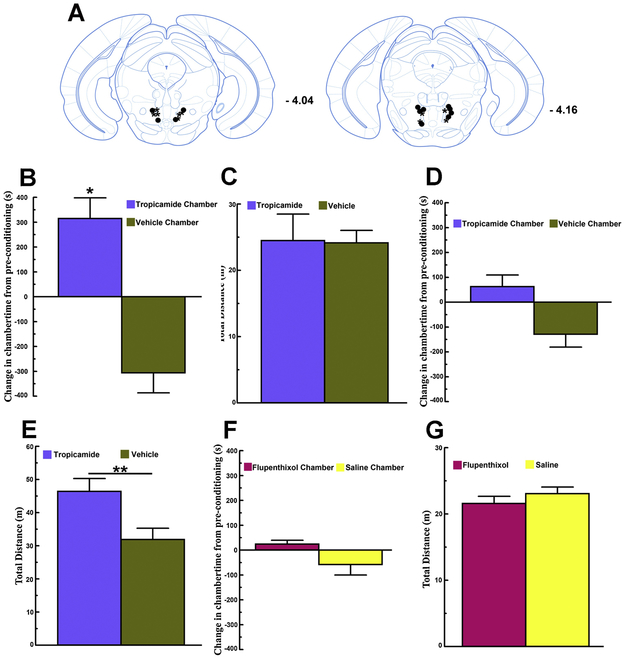
Infusions of the M3 muscarinic receptor antagonist 4-DAMP into RMTg sites (between Bregma -4.04 to – 4.28 [Fig 1A]) did not lead to the acquisition of conditioned place preference (paired-samples t-test comparing difference scores for 4-DAMP and vehicle chambers: t4 = 0.4, p > 0.1; single-sample t-test comparing the mean difference score for the 4-DAMP chamber to 0: t4 = - 1.45, p > 0.1 [Fig 1B]). By contrast, infusions of the M4 muscarinic receptor antagonist Tropicamide into RMTg sites (between Bregma -4.04 and -4.16 [Fig 2A, asterisk symbols]) did lead to the acquisition of conditioned place preference (paired-samples t-test comparing difference scores for Tropicamide and vehicle chambers: t4 = 3.19, p < 0.05; single-sample t-test comparing the mean difference score for the Tropicamide chamber to 0: t4 = 3.12, p < 0.05 [Fig 2B]). We also measured the total distance traveled while mice were confined to the conditioning chambers and computed the average total distance traveled across 4 vehicle and 4 antagonist (4-DAMP or Tropicamide) conditioning sessions. Consistent with our previous report [13] RMTg infusions of 4-DAMP [Fig 1C] but not RMTg infusions of Tropicamide [Fig 2C] increased locomotion relative to RMTg vehicle infusions (4-DAMP paired-samples t-test: t19 = 3.12, p < 0.01; Tropicamide paired-samples t-test: t19 = 0.24, p > 0.1). Thus RMTg antagonism of M3 or M4 muscarinic receptors, each of which are found in the RMTg [18], has opposite effects. RMTg 4-DAMP infusions do not result in the acquisition of CPP but increase locomotion, while RMTg Tropicamide infusions result in the acquisition of CPP but do not significantly affect locomotion. These data provide further support for an important role of endogenous cholinergic input to the RMTg, likely originating from the LDTg/PPTg, in reward function. More importantly, the contributions of cholinergic input to rewarding and locomotor-activating effects involve differential contributions of RMTg M4 and M3 muscarinic receptors, respectively.
Figure 1. RMTg infusions of the M3 muscarinic receptor antagonist 4-DAMP increase locomotor activation but do not lead to the acquisition of conditioned place preference.
A. Bilateral RMTg injection sites for mice used in 4-DAMP CPP studies (n = 5). B. RMTg 4-DAMP infusions do not lead to the acquisition of conditioned place preference. C. By contrast, in the same mice locomotor activity measured within the RMTg 4-DAMP infusion conditioning chamber is significantly greater than locomotor activity measured within the RMTg vehicle infusion conditioning chamber (** p < 0.01 [4-DAMP vs. vehicle conditioning chambers]). All error bars represent ±SEM.
Figure 2. RMTg infusions of the M4 muscarinic receptor antagonist Tropicamide lead to the acquisition of conditioned place preference but do not increase locomotor activation.
A. Bilateral RMTg injection sites for mice used in Tropicamide CPP studies (n = 5; asterisk symbols) and for mice used in Tropicamide CPP studies involving concurrent systemic pharmacological blockade of dopamine signaling (n = 6; solid circles). B. RMTg Tropicamide infusions result in the acquisition of conditioned place preference (* p < 0.05 [single-sample t-test: Tropicamide chamber difference score vs. 0]). C. By contrast, in the same mice locomotor activity measured within RMTg Tropicamide infusion and RMTg vehicle infusion conditioning chambers did not differ. D. Systemic pretreatment with the broad-spectrum dopamine receptor antagonist flupenthixol (0.08 mg/kg, i.p., given 1 hr prior to RMTg Tropicamide infusion conditioning sessions) prevents the acquisition of CPP induced by RMTg Tropicamide infusions (n = 6). E. By contrast, in the same mice locomotor activity measured within the RMTg Tropicamide infusion conditioning chamber following flupenthixol pre-treatment was significantly greater than locomotor activity measured within the RMTg vehicle infusions conditioning chamber following saline pre-treatment (** p < 0.01 [flupenthixol/Tropicamide vs. saline/vehicle chamber]). F. The dose of systemic flupenthixol used has no motivational effects on its own. Neither conditioned place preference nor conditioned place aversion are observed in mice (n = 7). G. Locomotor activity measured within the flupenthixol and saline conditioning chamber does not differ. All error bars represent ±SEM.
We next assessed whether the acquisition of CPP induced by RMTg Tropicamide infusions is dopamine-dependent. In an additional group of mice (n = 8, final statistical analysis based on n = 6) we tested whether RMTg Tropicamide infusions induce CPP under conditions of systemic dopamine receptor blockade. For these experiments mice were given a systemic injection of the broad-spectrum dopamine receptor antagonist flupenthixol (0.08 mg/kg, i.p.) 1 hour prior to each of the four RMTg Tropicamide infusion conditioning days. On RMTg vehicle infusion conditioning days mice received a saline injection (10 ml/kg i.p.) 1 hour prior to the intracranial injection. Under conditions of systemic dopamine receptor blockade, RMTg infusions of Tropicamide did not lead to the acquisition of CPP (paired-samples t-test comparing difference scores for Tropicamide and vehicle chambers: t5 = 2.24, p > 0.05; single-sample t-test comparing the mean difference score for the Tropicamide chamber to 0: t5 = 1.34, p > 0.1 [Fig 2D]). Importantly, we also tested whether the dose of flupenthixol used has any motivational effects on its own in a separate group of mice (n =8; final statistical analysis based on n = 7 as one mouse died during the experiment). For these CPP studies we also employed a biased conditioning procedure but paired systemic flupenthixol treatment with the initially more preferred chamber. Mice received a systemic injection of either flupenthixol (0.08 mg/kg, i.p.) or saline (10 ml/kg, i.p.) 1 hour prior to being confined to their respective conditioning chamber for a period of 30 min. Flupenthixol by itself did not result in the acquisition of either CPP or a conditioned place aversion (paired-samples t-test comparing difference scores for flupenthixol and saline chambers: t6 = 2.09, p > 0.05; single-sample t-test comparing the mean difference score for the Flupenthixol chamber to 0: t6 = 1.52, p > 0.1 [Fig 2F]). By contrast, following flupenthixol pretreatment, RMTg infusions of Tropicamide increased locomotion relative to RMTg vehicle infusions following saline pretreatment (paired-samples t-test: t23 = 11.86 p < 0.00001[Fig 2E]). The dose of flupenthixol used did not significantly affect locomotion on its own (paired-samples t-test: t27 = 1.31, p > 0.1 [Fig 2G]). Thus, the rewarding effects of RMTg M4 muscarinic receptor antagonism require intact dopamine signaling as flupenthixol pretreatment blocks the acquisition of CPP. At the same time, however, intact dopamine signaling appears to oppose the potential locomotor activating effects of RMTg M4 muscarinic receptor antagonism. The locomotor activating effects of RMTg Tropicamide infusions are only revealed under these conditions.
To summarize, first, we show that endogenous cholinergic input to the RMTg, mediated through M4 muscarinic, but not M3 muscarinic, receptors contributes to reward. Second, we provide additional support for the importance of cholinergic input to the RMTg in modulating locomotor activation [11, 13]. However, endogenous cholinergic input to the RMTg appears to differentially contribute to reward and locomotion through M4 and M3 muscarinic cholinergic receptors, respectively. Dissociations of rewarding and locomotor effects are not without precedent. In the rat VTA, for example, it has been shown that the locomotor-activating and rewarding effects of opioids can be dissociated, with AMPA glutamate receptor antagonists infused into the anterior or posterior portions of the VTA differentially affecting the rewarding and locomotor-activating effects of opiates, respectively [19]. Our data suggest that rewarding and locomotor-activating effects are dissociable in RMTg as well, but according to muscarinic receptor subtype. To our knowledge, this is the first such report in the RMTg. Whether this correlates with differential distribution of M3 and M4 muscarinic receptors within the RMTg remains to be determined.
In our experiments we measured locomotor activity during each of the conditioning sessions while mice were confined to a conditioning chamber. Previously we investigated the effects of RMTg 4-DAMP or Tropicamide infusions on open-field locomotion [13]. While the two testing conditions differ in obvious ways (i.e. chamber size, tactile and visual features of the chambers), the current data are consistent with and extend our previous report [13] that RMTg 4-DAMP, but not Tropicamide, infusions increase locomotor activation. Here we show that dopamine signaling contributes to RMTg Tropicamide locomotion as well, at least within the confines of the CPP apparatus conditioning chambers. The fact that RMTg Tropicamide only increased locomotion when dopamine receptor signaling was blocked suggests two thing. First, M4-mediated cholinergic signaling in RMTg modulates midbrain dopamine signaling and, second, its effects on dopamine signaling normally result in an inhibition of locomotion. Importantly, flupenthixol had no effects on locomotion by itself.
Whether M3 or M4 receptors found in the RMTg [18] are associated with GABA neurons or with the axon terminals of RMTg afferents is not clear. In the VTA, M3 receptors are associated with GABAergic neurons, while M4 receptors have been associated with LDTg/PPTg cholinergic terminals [20, 21]. If a similar arrangement exists in RMTg, and excitatory M3 receptors are associated with RMTg GABAergic cell bodies, then endogenous cholinergic input should increase RMTg GABAergic inhibition of VTA dopamine neurons. RMTg M3 receptor blockade then would increase locomotion by reducing GABAergic inhibition of VTA dopamine neurons. While we demonstrate this, the fact that this does not result in concurrent rewarding effects, as demonstrated by the lack of CPP acquisition following RMTg 4-DAMP infusions, is surprising.
The lack of a locomotor effect of RMTg Tropicamide infusions shown previously [13], and replicated here, has been interpreted [12] in light of the assumption that M4 receptors are associated with RMTg cholinergic afferents (from LDTg and/or PPTg) where they may act as inhibitory presynaptic autoreceptors, similar to what has been shown in the VTA [21]. RMTg Tropicamide infusions would then result in an accumulation of RMTg acetylcholine levels by blockade of the inhibitory presynaptic autoreceptor. In turn, this would result in increased cholinergic excitation of RMTg GABA neurons, possibly, via M3 receptors and consequently increased inhibition of midbrain dopamine neurons. Alternatively, if inhibitory M4 receptors are associated with RMTg GABA cell bodies then endogenous cholinergic input should inhibit RMTg GABA neurons and consequently decrease inhibition of midbrain dopamine neurons. RMTg Tropicamide infusions would then result in decreased cholinergic inhibition of RMTg GABA neurons and consequently increased inhibition of midbrain dopamine neurons. The dopamine-dependent rewarding effects of RMTg Tropicamide infusions, however, are not easily reconciled with either of these interpretations. Our data thus point to a need for a better understanding of where RMTg M3 and M4 muscarinic receptors are located. This will require detailed analysis at the level of electron microscopy. Furthermore, electrophysiological studies investigating the effects of RMTg 4-DAMP or Tropicamide application on RMTg GABA or VTA dopamine neuron activity are also lacking. Finally, while the VTA is a major projection target, the RMTg also projects to other brainstem targets, notably the LDTg/PPTg and the dorsal raphe [6]. The LDTg/PPTg (for a review see [12]) and the dorsal raphe [22, 23], in turn, each modulate the activity of midbrain dopamine neurons and reward related behavior. This raises the possibility that cholinergic modulation of RMTg GABA neurons may indirectly modulate dopamine signaling through these sites. Identification of the projection targets of RMTg GABA neurons receiving cholinergic input would be informative in this regard. The interpretation of the rewarding and locomotor activating effects of RMTg 4-DAMP or Tropicamide infusions will clearly be greatly facilitated by the aforementioned studies.
In conclusion, we show that locomotor and rewarding effects of endogenous cholinergic inputs to the RMTg are dissociable. M3 and M4 muscarinic receptor signaling in RMTg differentially contributes to locomotor activation and reward, respectively.
Highlights.
RMTg Tropicamide but not 4-DAMP induces CPP
RMTg 4-DAMP but not Tropicamide increases locomotion
RMTg Tropicamide CPP is dopamine-dependent
Flupenthixol pretreatment reveals RMTg Tropicamide locomotor effects
Acknowledgements
This work was funded by US National Institutes of Health grant R15 DA041694 to SS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Geisler S, Zahm DS, Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions, J. Comp. Neurol 490 (2005) 270–94. [DOI] [PubMed] [Google Scholar]
- [2].Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK, Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area, J. Neurosci 15 (1995) 5859–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N, Whole-brain mapping of direct inputs to midbrain dopamine neurons, Neuron 74 (2012) 858–73. [DOI] [PubMed] [Google Scholar]
- [4].Woolf NJ, Cholinergic systems in mammalian brain and spinal cord, Prog. Neurobiol 37 (1991) 475–524. [DOI] [PubMed] [Google Scholar]
- [5].Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC, The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses, Neuron 61 (2009) 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS, The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta, J. Comp. Neurol 513 (2009) 566–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC, Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions, J. Neurosci 32 (2012) 14094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perrotti L, Bolaños CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M, DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment, Eur. J. Neurosci 21 (2005) 2817–24. [DOI] [PubMed] [Google Scholar]
- [9].Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M, Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse, Neuropsychopharmacology 37 (2012) 1164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yetnikoff L, Cheng AY, Lavezzi HN, Parsley KP, Zahm DS, Sources of input to the rostromedial tegmental nucleus, ventral tegmental area, and lateral habenula compared: A study in rat. J. Comp. Neurol 523 (2015) 2426–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wasserman DI, Wang HG, Rashid AJ, Josselyn SA, Yeomans JS, Cholinergic control of morphine-induced locomotion in rostromedial tegmental nucleus versus ventral tegmental area sites, Eur. J. Neurosci 38 (2013) 2774–85. [DOI] [PubMed] [Google Scholar]
- [12].Steidl S, Wasserman DI, Blaha CD, Yeomans JS, Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons, Neurosci. Biobehav. Rev 83 (2017) 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Steidl S, Dhillon ES, Sharma N, Ludwig J, Muscarinic cholinergic receptor antagonists in the VTA and RMTg have opposite effects on morphine-induced locomotion in mice, Behav. Brain Res 323 (2017) 111–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tzschentke TM, Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade, Addict. Biol 12 (2007) 227–462. [DOI] [PubMed] [Google Scholar]
- [15].Huston JP, Silva MA, Topic B, Müller CP, What’s conditioned in conditioned place preference?, Trends Pharmacol. Sci 34 (2013) 162–66. [DOI] [PubMed] [Google Scholar]
- [16].Bardo MT, Bevins RA, Conditioned place preference: What does it add to our preclinical understanding of drug reward?, Psychopharmacology 153 (2000) 31–43. [DOI] [PubMed] [Google Scholar]
- [17].Steidl S, Yeomans JS, M5 muscarinic receptor knockout mice show reduced morphine-induced locomotion but increased locomotion after cholinergic antagonism in the ventral tegemental area, J. Pharmacol. Exp. Ther 329 (2009) 263–75. [DOI] [PubMed] [Google Scholar]
- [18].Wasserman DI, Tan JM, Kim JC, Yeomans JS, Muscarinic control of rostromedial tegmental nucleus GABA neurons and morphine-induced locomotion, Eur. J. Neurosci 44 (2016) 1761–70. [DOI] [PubMed] [Google Scholar]
- [19].Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A, Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area, J. Neurosci 28 (2008) 8406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Michel FJ, Fortin GD, Martel P, Yeomans J, Trudeau LE, M3-like muscarinic receptors mediate Ca2+ influx in rat mesencephalic GABAergic neurones through a protein kinase C-dependent mechanism, Neuropharmacology, 48 (2005) 796–809. [DOI] [PubMed] [Google Scholar]
- [21].Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG, M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies, FASEB J.18 (2004) 1410–12. [DOI] [PubMed] [Google Scholar]
- [22].Wang HL, Zhang S, Qi J, Wang H, Cachope R, Mejias-Aponte CA, Gomez JA, Mateo-Semidey GE, Beaudoin GMJ, Paladini CA, Cheer JF, Morales M, Dorsal raphe dual serotonin-glutamate neurons drive rewrd by establishing excitatory synapses on VTA mescoaccumbens dopamine neurons, Cell Rep. 26 (2019) 1128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qi J, Wang HL, Wang H, de Jesus Acevas Buendia J, Hoffman AF, Lupica CR, Seal RP, Morales M, A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons, Nat. Commun 5 (2014) 5390. [DOI] [PMC free article] [PubMed] [Google Scholar]