Flavopiridol (alvocidib) is a semi-synthetic flavon which inhibits cyclin-dependent kinases (CDKs) 1, 2, and 4 by outcompeting adenosine triphosphate (ATP), and CDK7 and CDK9 by noncompetitive binding.[1] Promising preclinical data in cell lines derived from mantle cell lymphoma (MCL) and activated B-cell subtype diffuse large B-cell lymphoma (DLBCL) led to a series of clinical trials of flavopiridol in various hematological malignancies; however, phase I and II trials in which the drug was given as either an intravenous bolus (IVB) or a continuous infusion (CIVI) resulted in modest responses in patients with newly diagnosed and relapsed MCL (overall response rate [ORR] 4–11%)[2, 3] and relapsed DLBCL (ORR 2%)[4]. This was attributed to the propensity of flavopiridol to bind to circulating albumin, resulting in a narrow therapeutic index. Herein, we report the results of a hybrid dosing schedule of flavopiridol in patients with relapsed and refractory (R/R) MCL and DLBCL, accompanied by an analysis of the in vivo effects of the drug on CDK targets seen in paired biopsy samples obtained before and after the first dose.
This was a single-center phase I/II trial of flavopiridol given as a 30-minute bolus, followed by a 4-hour infusion. In addition to the standard 3+3 inter-patient dose escalation, intra-patient escalation was also planned between the first and subsequent cycles to minimize the risk of tumor lysis syndrome (TLS). Primary objectives were to assess the response rate of flavopiridol hybrid dosing schedule in patients with relapsed MCL and DLBCL, and to assess the toxicity profile, maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of the regimen. The trial was approved by the National Cancer Institute Institutional Review Board and registered on ClinicalTrials.gov ().
28 patients with R/R MCL, DLBCL, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma were enrolled between February 2007 and May 2010 and received at least one dose of flavopiridol (Table 1). Median age was 60.75 (range 24.5–80.7). Most had advanced disease at diagnosis (Stage IV 82%), and were heavily pretreated, with median 3 (1–10) prior regimens. Dose levels on cycle 1 week 1 were 25 mg/m2 IVB + 25 mg/m2 4-hour CIVI (DL1, 4 patients), 30 mg/m2 IVB + 30 mg/m2 CIVI (DL2, 16 patients), and 30 mg/m2 IVB + 50 mg/m2 CIVI (DL3, 8 patients). Thereafter, all patients received 30 mg/m2 IVB + 50 mg/m2 4-hour CIVI weekly for four weeks in a six-week cycle, which was determined to be the MTD. Because of the occurrences of TLS seen in prior studies, including two deaths associated with uncontrolled hyperkalemia, all patients received allopurinol 300 mg orally once daily for the first five days of the first cycle along with intravenous hydration six hour prior to the first dose. The median number of cycles was 1 (1–6), with reasons for treatment discontinuation being disease progression (n=20), completion of all six planned cycles (6), adverse event (3), patient preference (1), and investigator discretion (1).
Table 1:
Patient demographics and disease data. MCL=mantle cell lymphoma, DLBCL=diffuse large B-cell lymphoma, FL-T=transformed follicular lymphoma, PMBCL=primary mediastinal B-cell lymphoma.
| Characteristic | All patients (N=28) | MCL (N=15) | Other (N=13) |
|---|---|---|---|
| No. of males (%) | 20 (71) | 12 (80) | 8 (62) |
| Median age, years (range) | 60.75 (24.5–80.7) | 60.7 (43.7–69.7) | 61.9 (24.5–80.7) |
| Performance status, no. (%) | |||
| 0 | 6 (21) | 2 (13) | 4 (30.5) |
| 1 | 21 (75) | 13 (87) | 8 (61.5) |
| 2 | 1 (4) | 0 | 1 (8) |
| Diagnosis, no. (%) | |||
| MCL | 15 (53) | 15 (100) | |
| DLBCL | 10 (36) | 0 | 10 (77) |
| FL-T | 2 (7) | 0 | 2 (15) |
| PMBCL | 1 (4) | 0 | 1 (8) |
| Stage | |||
| II | 2 (7) | 0 | 2 (15) |
| III | 3 (11) | 2 (13) | 1 (8) |
| IV | 23 (82) | 13 (87) | 10 (77) |
| Median LDH (range) | 199 (121–1177) | 178 (121–264) | 290 (121–1177) |
| Median no. of prior treatments (range) | 3 (1–10) | 1 (1–6) | 4 (1–10) |
Eight DLTs were observed in three patients: grade 4 metabolic acidosis and gastrointestinal perforation; and grade 3 TLS, non-neutropenic infection, hyperkalemia, hypoalbuminemia, and elevated AST and ALT. No single DLT occurred more than once. The most commonly reported treatment-related adverse events (AEs) were hematologic, including thrombocytopenia and anemia (9 patients each), neutropenia requiring prophylactic granulocyte colony-stimulating factor (G-CSF), leukocytosis, and lymphopenia (Table 2). The most common non-hematologic AEs were hypoalbuminemia and LFT abnormalities (9 patients each), and the most commonly reported symptom was fatigue (7 patients). Seven patients developed infections, most of them without concomitant neutropenia.
Table 2.
Adverse events deemed possibly, probably, and definitely related to the study drug. Grade 1 and 2 events with fever that 3 occurrences not shown. Dose-limiting toxicities denoted with an asterisk.
| Grade | Grade | |||||
|---|---|---|---|---|---|---|
| CTCAE term | 1/2 | 3/4 | CTCAE term | 1/2 | 3/4 | |
| Metabolic/laboratory | Hematologic | |||||
| Hypoalbuminemia* | 8 | 1 | Thrombocytopenia | 7 | 2 | |
| AST elevation* | 8 | 1 | Neutropenia | 6 | 2 | |
| Hyperkalemia* | 7 | 1 | Leukocytosis | 6 | 2 | |
| ALT elevation* | 6 | 1 | Lymphopenia | 5 | 2 | |
| Hyperbilirubinemia | 5 | 1 | Anemia | 8 | 1 | |
| Hypophosphatemia | 4 | 1 | ||||
| Hyponatremia | 3 | 1 | Other | |||
| Hyperuricemia | 2 | 1 | Infection without neutropenia* | 4 | 1 | |
| Hypokalemia | 2 | 1 | Hypotension | 4 | 1 | |
| Acidosis* | 0 | 1 | Diarrhea | 4 | 1 | |
| Cardiac troponin I elevation | 0 | 1 | Vomiting | 3 | 1 | |
| Renal failure | 0 | 1 | Infection with neutropenia | 1 | 1 | |
| Creatinine elevation | 8 | 0 | GI perforation (jejunum)* | 0 | 1 | |
| Hypocalcemia | 5 | 0 | Febrile neutropenia | 0 | 1 | |
| Hypernatremia | 5 | 0 | GI hemorrhage (rectum) | 0 | 1 | |
| Hypercalcemia | 4 | 0 | Syncope | 0 | 1 | |
| CPK elevation | 4 | 0 | Tumor lysis syndrome* | 0 | 1 | |
| Alkaline phosphatase elevation | 4 | 0 | Fatigue | 7 | 0 | |
| Low serum bicarbonate | 4 | 0 | Anorexia | 5 | 0 | |
| Hypomagnesemia | 3 | 0 | Fever (non-neutropenic) | 5 | 0 | |
| Hyperglycemia | 3 | 0 | Nausea | 5 | 0 | |
| Sinus tachycardia | 3 | 0 | ||||
| Bone pain | 3 | 0 | ||||
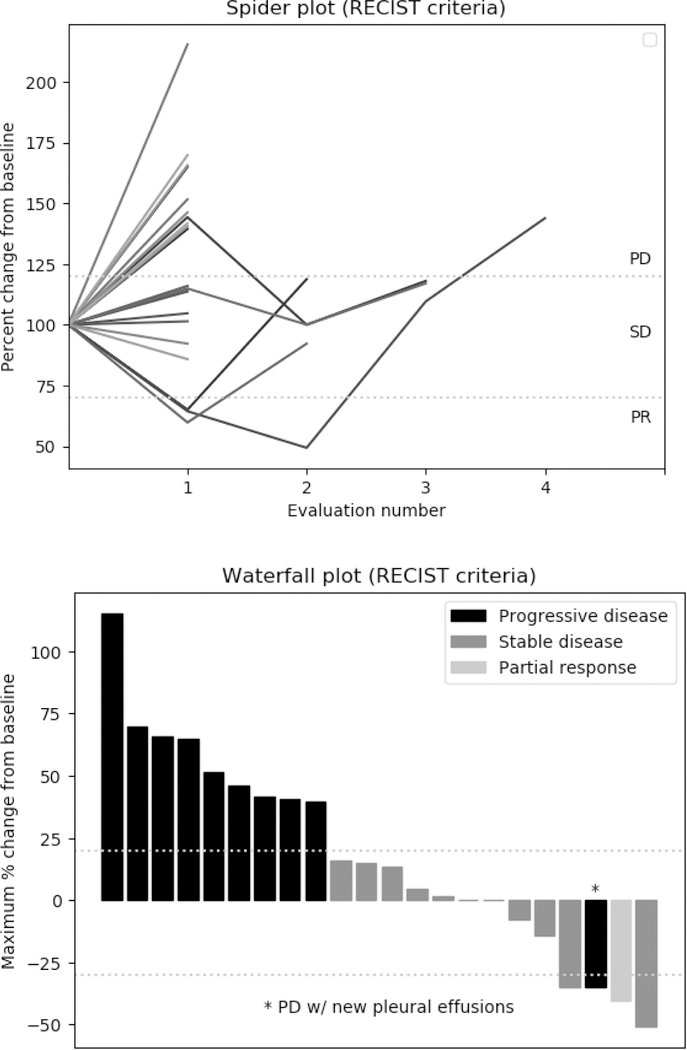
Of the 28 patients, 26 were evaluable at the time of study completion (Figure 1). One patient with DLBCL had a sustained partial response (PR) lasting 84 days, for an overall response rate of 3.8%. One patient with MCL had a 50% decrease in the size of target lesions at two months, which was not sustained at 4-month follow-up; another patient with DLBCL had >30% decrease but had developed new malignant pleural effusions. Nine of 26 patients (34.6%) had stable disease (SD), for a disease control rate (SD+PR) of 38.4%. There was decreased Rb staining at the S807/811 phospho-site in 8 of 9 paired samples analyzed, and at the S870 site in 7 of 8 paired samples, suggestive of the G1-related CDK2 and CDK4 inhibition. In one sample in which p53 was detected, there was an increase post-treatment, suggestive of CDK9 inhibition.
Figure 1.
Patient responses per protocol-defined RECIST 1.1 criteria. Evaluations were performed every two months.
Our findings are similar to those seen in a phase II trial conducted at the Ohio State University, which used a hybrid schedule of flavopiridol in 46 patients with various R/R non-Hodgkin lymphomas (of which 7 MCL and 17 DLBCL or other intermediate/aggressive B-cell lymphomas).[5] MTD for the two cohorts that completed accrual was not reach, with the maximum planned dose being 50 mg/m2 IVB + 50 mg/m2 CIVI. Eleven patients did not complete the first cycle, most for disease progression (7). Six patients (14%) achieved a PR, two with MCL, three with indolent B-cell lymphomas, and one with DLBCL. Median duration of response was 6 months. Five patients (12%) had SD, making the disease control rate 26%.
The hybrid dosing schedule of flavopiridol did seem to improve tolerability, and paired biopsies showed the appropriate pharmacodynamic effect of the drug. However, this did not translate into clinical responses, suggesting that broad CDK inhibition alone is insufficient to induce durable responses in MCL and the other included B-cell lymphomas. Other clinical studies have made similar observations. Phase I trial of a pan-CDK inhibitor roniciclib included 7 patients with lymphoid malignancies, and a phase II trial of the CDK1/4/9 inhibitor P276–00 had 13 patients with R/R MCL; no objective responses were seen in either trial.[6, 7] The small molecule AT7519M, which in addition to CDK1/2/4/9 also inhibits CDK5 but not CDK7, had ORR 17% (2 of 12) for R/R MCL in a phase II trial.[8]
Experience with other kinase inhibitors, most notably those of the phosphatidylinositol 3-kinase (PI3K), has shown that pan-inhibitors which target many isoforms have narrow therapeutic windows and limited efficacy in clinical trials. More specific CDK inhibitors have been investigated, including two of the three CDK4/6 inhibitors now approved by the US Food and Drug Administration for the treatment of certain breast cancer: palbociclib and ribociclib. In a phase Ib study of palbociclib, one of 17 patients had a complete response and two had PRs (ORR 18%);[9] and in the first-in human study of ribociclib, none of the seven patients with R/R MCL responded.[10] At least two selective CDK9 inhibitors, voruciclib and AZD4573, are in phase I studies in patients with R/R hematological malignancies with results yet to be reported.
CDK inhibitors may be more effective when used in combination with other drugs. One approach is to identify agents which could further sensitize lymphoma cells to CDK inhibition: metformin reduced the IC-50 value of the CDK9 inhibitor BAY1143572 3-fold and 10-fold in a Burkitt lymphoma and an MCL cell line respectively.[11] Alternatively, CDK inhibitors themselves may be used as sensitizing agents for drugs with known clinical activity in MCL. For example, flavopiridol caused downregulation of MCL-1 expression in BCL2high lymphoma cell lines and had marked synergy with venetoclax.[12] The combination is being tested in AML using CIVI dosing of flavopiridol in an ongoing multicenter Phase I trial (). Clinical trials of this combination in MCL may be limited by the risk of tumor lysis syndrome, but are a potentially rational doublet. The CDK9 inhibitor voruciclib, structurally similar to flavopiridol, has recently been shown to inhibit Mcl-1 in DLBCL cell lines, and sensitize them to the Bcl-2 inhibitor venetoclax both in vitro and in vivo.[13] If safety of voruciclib is demonstrated in the ongoing phase I trial (), its efficacy when combined with venetoclax in MCL and DLBCL should be explored. Similarly, palbociclib sensitized BTK-unmutated (but not BTK-mutated) ibrutinib-resistant lymphoma cell lines to ibrutinib by prolonging early G1 arrest.[14] In a recently reported phase I trial, palbociclib and ibrutinib were given together to 27 patients with R/R MCL with CR and ORR of 37% (10 patients) and 67% (18 patients) respectively.[15] However, the contribution of CDK4/6 inhibition to this activity will be challenging to establish even with the ongoing Phase II trial of the combination (), as single-agent ibrutinib has activity in MCL and is already approved for its treatment by the United Stated Food and Drug Administration. Notably, the trial excludes patients who have received prior ibrutinib even in cases of primary resistance, which are not usually associated with BTK mutations.
In summary, hybrid bolus/continuous dosing of the pan-CDK inhibitor flavopiridol was marginally better tolerated than prior dosing regimens and showed appropriate on-target effects detected in paired tumor biopsies. Despite this, it had minimal efficacy in patients with relapsed/refractory non-Hodgkin B-cell lymphoma, casting doubt on the utility of CDK inhibition in this disease. Ongoing trials of more specific CDK inhibitors in combination with other agents will help elucidate their role in lymphoma treatment.
Footnotes
Disclosure of interest: The authors report no conflict of interest.
Contributor Information
Milos D. Miljkovic, Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda MD, USA
Mark Roschewski, Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda MD, USA.
Kieron Dunleavy, Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda MD, USA; Department of Medicine, The George Washington University, Washington DC, USA.
Wyndham H. Wilson, Lymphoid Malignancies Branch, Center for Cancer Research, National Cancer Institute, Bethesda MD, USA.
References:
- 1.Shapiro GI. Preclinical and Clinical Development of the Cyclin-Dependent Kinase Inhibitor Flavopiridol. Clinical Cancer Research 2004;10(12):4270s–4275s. doi: 10.1158/1078-0432.Ccr-040020. [DOI] [PubMed] [Google Scholar]
- 2.Lin TS, Howard OM, Neuberg DS, et al. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma 2002. 2002/04//;43(4):793–797. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 3.Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2003. May 1;21(9):1740–5. [DOI] [PubMed] [Google Scholar]
- 4.Tan AR, Headlee D, Messmann R, et al. Phase I Clinical and Pharmacokinetic Study of Flavopiridol Administered as a Daily 1-Hour Infusion in Patients With Advanced Neoplasms. Journal of Clinical Oncology 2002;20(19):4074–4082. doi: 10.1200/jco.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Jones JA, Rupert AS, Poi M, et al. Flavopiridol Can Be Safely Administered Using a Pharmacologically Derived Schedule and Demonstrates Activity in Relapsed and Refractory Non-Hodgkin’s Lymphoma. American journal of hematology 2014. 09/30;89(1):19–24. doi: 10.1002/ajh.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahleda R, Grilley-Olson JE, Govindan R, et al. Phase I dose-escalation studies of roniciclib, a pan-cyclin-dependent kinase inhibitor, in advanced malignancies. Br J Cancer 2017. 2017/06/06/;116(12):1505–1512. doi: 10.1038/bjc.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassaday RD, Goy A, Advani S, et al. A phase II, single-arm, open-label, multicenter study to evaluate the efficacy and safety of P276–00, a cyclin-dependent kinase inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 2015. 2015/07//;15(7):392–397. doi: 10.1016/j.clml.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seftel MD, Kuruvilla J, Kouroukis T, et al. The CDK inhibitor AT7519M in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. A Phase II study of the Canadian Cancer Trials Group. Leuk Lymphoma 2017. 2017/06//;58(6):1358–1365. doi: 10.1080/10428194.2016.1239259. [DOI] [PubMed] [Google Scholar]
- 9.Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012. 2012/05/17/;119(20):4597–4607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 10.Infante JR, Cassier PA, Gerecitano JF, et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin Cancer Res 2016. 2016/12/01/;22(23):5696–5705. doi: 10.1158/1078-0432.Ccr-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chukkapalli V, Gordon LI, Venugopal P, et al. Metabolic changes associated with metformin potentiates Bcl-2 inhibitor, Venetoclax, and CDK9 inhibitor, BAY1143572 and reduces viability of lymphoma cells. Oncotarget 2018. 2018/04/20/;9(30):21166–21181. doi: 10.18632/oncotarget.24989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips DC, Xiao Y, Lam LT, et al. Loss in MCL-1 function sensitizes non-Hodgkin’s lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199). Blood Cancer J 2015. 2015/11/13/;5:e368. doi: 10.1038/bcj.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey J, Deckwerth TL, Kerwin WS, et al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci Rep 2017. 2017/12/21/;7(1):18007. doi: 10.1038/s41598-017-18368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014. 2014/09//;4(9):1022–1035. doi: 10.1158/2159-8290.Cd-14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P, Bartlett NL, Blum KA, et al. A phase I trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood 2019. 2019/01/28/. doi: 10.1182/blood-2018-11-886457. [DOI] [PMC free article] [PubMed] [Google Scholar]