Research has increasingly recognized the role of the early caregiving environment in directing the development of executive function (EF), a set of higher-order cognitive abilities thought to be necessary for planning and executing goal-directed behavior. Because the specific skills that comprise EF (working memory, inhibitory control, and attention shifting) are rapidly developing between ages 3 and 5 (Garon, Bryson, & Smith, 2008), many of these studies have focused on caregiving’s contribution to EF development in the preschool years. For example, high quality home and childcare environments, marked by the presence of responsive caregivers and provision of learning materials, seem to make a positive contribution to the development of EF in this time period (Clark et al., 2013; Sarsour et al., 2011). Some evidence suggests that these factors may also serve as a buffer against the negative impacts of poverty and other more distal influences (Berry et al., 2016; Nelson et al., 2015).
In the home environment, children’s experiences of sensitive and supportive caregiving in infancy and toddlerhood have specifically been shown to aid in the development of EF (for a review, see Fay-Stammbach, Hawes, & Meredith, 2014). Specific parenting practices such as scaffolding (Bernier, Carlson, Deschênes, & Matte-Gagné, 2012; Bernier, Carlson, & Whipple, 2010; Hammond, Müller, Carpendale, Bibok, & Liebermann-Finestone, 2012; Hughes & Ensor, 2009) and attention directing/redirecting (Conway & Stifter, 2012) have also been investigated. This body of literature has concluded that interactions with sensitive caregivers, who provide opportunities and guidance to children in harnessing their emerging executive abilities, are indispensable for supporting optimal EF development.
However, relatively little research has considered whether there are earlier experiences, such as those afforded by the fetal environment, that make an independent contribution to EF development, controlling for postnatal interactions with sensitive caregivers in enriched learning environments. Recent literature has begun to elucidate the role of the prenatal environment in influencing child neurocognitive development more generally (Bale et al., 2010; O’Donnell, O’Connor, & Glover, 2009), suggesting that a similar framework could prove useful in the prediction of children’s EF trajectories. Theoretically, these extant findings provide support for the prenatal programming hypothesis, whereby prenatal experiences are thought to have long-lasting implications for infant health and development (O’Connor, Heron, Golding, Glover, & the ALSPAC Study Team, 2003). Similar to the prenatal programming hypothesis, the developmental origins of health and disease (DOHaD; Gillman, 2005) and fetal origins (Barker, 1995) hypotheses emphasize the importance of studying the earliest origins of health and developmental outcomes, by investigating adverse exposures during critical windows of development (including the prenatal period) that may confer increased risk. All three of these hypotheses are similar in their view that prenatal experiences may affect fetal development in ways that predispose individuals to disease and adverse developmental outcomes later in life. Depending on the ontogeny of the impacted systems, these predispositions may be further mediated or moderated by postnatal experiences, but nevertheless provide a starting point from which to understand subsequent child outcomes. The current study draws upon this framework to test the precursors and pathways that predict EF in young children.
Prenatal Programming of Executive Function
There is accumulating evidence supporting the prenatal programming of cognitive functioning, and specifically EF. Meta-analyses on outcomes for children born premature or low birth weight, two purported proxy variables for adverse prenatal exposure, have shown long-term deficits in IQ (Aylward, Pfeiffer, Wright, & Verhulst, 1989) and EF (Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever, & Oosterlaan, 2009) for these children, compared to full term, normal birth weight peers. Prospective, longitudinal studies beginning in pregnancy have begun to elucidate more specific prenatal risk factors that predict impaired EF development.
A set of studies have found that maternal emotional problems, such as depression, state anxiety and pregnancy-specific anxiety predict worse EF in school-aged children (Buss, Davis, Hobel, & Sandman, 2011; O’Donnell et al., 2017). These findings are often explained in terms of the deleterious effects of high maternal cortisol during pregnancy on the development of the hypothalamus-pituitary-adrenal (HPA) axis (O’Connor, Bergman, Sarkar, & Glover, 2013), with these effects possibly operating through epigenetic modification (Oberlander et al., 2008; Sandman, Davis, Buss, & Glynn, 2011). Elevated levels of maternal stress and cortisol have also been shown to alter child neurodevelopment (Sarkar et al., 2014), which would similarly have implications for behavior and cognitive development. A parallel line of work investigating maternal medical problems, such as obesity and obstetric complications, has reached similar conclusions regarding prenatal programming of EF. Two studies found that maternal pre-pregnancy obesity is associated with poorer child EF, compared to children of normal weight or overweight mothers (Buss et al., 2012; Theresia et al., 2017). One explanation for these findings is that maternal pre-pregnancy obesity contributes to a state of systemic inflammation, which exerts adverse prenatal programming effects on child neurodevelopment (Van Der Burg et al., 2016). Similarly, maternal obstetric complications, (e.g., gestational diabetes, pre-eclampsia) have been shown to be related to infant neurodevelopmental outcomes, including risk of ADHD (Seidman et al., 2000; Sprich-Buckminster, Biederman, Milberger, Faraone, & Lehman, 1993). However, these findings have not yet been extended to include EF as a cognitive outcome.
As illustrated by the studies described above, much of the research testing the prenatal programming hypothesis has thus far taken a piecemeal approach to identifying risk factors that independently predict EF. That is, single categories of risk factors (e.g., maternal emotional problems) have been studied in isolation, controlling for other possible confounds (e.g., maternal medical problems). While these studies have been successful in pinpointing a group of possible prenatal contributors, correlations among individual risk factors may obscure meaningful prediction of outcomes (Mosteller & Tukey, 1977), thus necessitating the use of a different approach. Cumulative risk approaches have benefits over single indicator models, including greater stability and increased power (Burchinal, Roberts, Hooper, & Zeisel, 2000; Evans, Li, & Whipple, 2013), and have been used extensively to study postnatal risk, including in the current sample (e.g., Burchinal & Willoughby, 2013).
One cumulative approach involves modeling prenatal conditions as a latent variable. Two studies have used this technique, modeling favorable fetal growth conditions as a latent variable with reflective indicators of infant birth weight, birth length, and gestational age (Bollen, Noble, & Adair, 2013; Camerota & Bollen, 2016). This model has been shown to provide better fit when compared to a model that includes each of the three indicators as independent, observed variables, although how this latent variable predicts child outcomes has not yet been tested.
A second cumulative approach utilizes data reduction techniques (e.g., principal components analysis, summation of dichotomous risk factors) to create a single indicator of prenatal risk, using multiple risk factors that have previously been studied in isolation (e.g., low birthweight, prematurity, maternal emotional problems, maternal pre-pregnancy obesity, and obstetric complications). Historically, this approach has been used to categorize LBW or preterm infants into groups of higher or lower medical risk, to better explain heterogeneity in developmental outcomes (e.g., Laucht, Esser, & Schmidt, 1997). More recently, cumulative prenatal risk (i.e., sum of maternal pregnancy complications, infant LBW, and prematurity) has been found to predict poorer social cognition in toddlers (Mark Wade, Madigan, Akbari, & Jenkins, 2015). Similarly, a composite of perinatal complications (e.g., infant Apgar scores less than six, respiratory distress syndrome) was found to predict lower IQ and less mature brain activation in adolescents born preterm (Carmody et al., 2006). Finally, Silveira and colleagues (2017) recently found that a cumulative prenatal adversity index (e.g., maternal chronic disease, smoking, and mental health during pregnancy, infant prematurity) significantly predicted child neurodevelopmental outcomes in two community birth cohorts, with the cumulative index acting as a stronger predictor than any isolated risk factor. Thus, there is precedence for using cumulative measures of prenatal risk, and some evidence to suggest that these indices are more predictive than individual risk factors. Therefore, applying a cumulative approach has the potential to enhance our understanding of the contributions of prenatal experience to child EF.
A second limitation of previous studies examining prenatal influences on EF is that they have not included comprehensive, objective measurement of the postnatal caregiving environment, beyond proxy variables such as maternal education or socioeconomic status. This omission is problematic for several reasons. For one, the prenatal and postnatal environment are likely correlated, leading to inflated estimates of the effect of the prenatal environment if the postnatal caregiving environment is not also considered. Further, the prenatal and postnatal environments may interact to predict developmental outcomes. For example, a number of studies have found that sensitive caregiving interacts with various indicators of prenatal risk (e.g., birth weight, prematurity, medical risk) to predict child cognitive outcomes (Camerota, Willoughby, Cox, & Greenberg, 2015; Madigan, Wade, Plamondon, Browne, & Jenkins, 2014; Wade et al., 2015). These findings suggest that the developmental trajectories associated with high prenatal risk are potentially modifiable by high-quality postnatal environments. However, it is currently unknown what factors in the postnatal environment, beyond maternal sensitivity, may have this protective effect, although this knowledge could inform prevention and intervention efforts.
Therefore, there is a need for additional studies that consider both prenatal and postnatal environmental influences on development, in order to elucidate what is specific to the prenatal period, as well as what aspects of the postnatal environment may exacerbate or ameliorate prenatal programming effects.
Role of Foundational Cognitive Abilities
Beyond uncovering the specific contributions of prenatal and postnatal experience to child development, it is also important to understand the earliest cognitive manifestations associated with adverse prenatal conditions, as these inquiries have clear implications for early detection and intervention efforts. It is well-established that child cognition shows at least some stability from infancy through early childhood (Bornstein & Sigman, 1986), with one study showing an indirect pathway from infant habituation time to child full-scale IQ, through the intermediary processes of general cognitive and language abilities (Bornstein et al., 2006). This indirect pathway may continue even further downstream to explain individual differences in adolescent academic achievement (Bornstein, Hahn, & Wolke, 2013). These studies illustrate the concept of a developmental cascade (e.g., Cox, Mills-Koonce, Propper, & Gariépy, 2010), the process by which functioning at one level of behavior (e.g., general cognitive abilities) affects functioning in later-developing, higher-level domains (e.g., IQ, achievement).
When considering EF specifically, there are a host of cognitive precursors emerging within the first three years of life that may exert cascading influences on EF development in the preschool years and beyond (for a review, see Hendry, Jones, & Charman, 2016). These precursors may include general cognitive abilities such as information processing and attention. Individual differences in these early-developing cognitive domains may be partially responsible for the long-term effects of prenatal conditions on children’s higher-order cognitive functioning. Indeed, many studies document deficits in general cognitive capacities (e.g., mental development scores, attention) for children born preterm and/or low birthweight (Murray, Scratch, Thompson, Inder, & Doy, 2014), and among children exposed to high levels of prenatal maternal stress (Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2003) and obstetric complications (Brinksma et al., 2017).
Several studies have found support for a cascade model linking foundational cognitive abilities to later differences in EF. Two studies have specifically examined infant information processing as an early cognitive precursor to preschool and child EF. Cuevas and Bell (2014) found that infants who were more efficient at processing a novel stimulus at 5 months (i.e., exhibited shorter median looking time) exhibited better EF performance on a battery of tasks when they were 24, 36, and 48 months. Similarly, Rose, Feldman, and Jankowski (2012) demonstrated that infant processing speed and memory at 7 and 12 months predicted better EF when children were 11 years old. A corollary question pertinent to the current investigation is the extent to which prenatal conditions are indirectly related to later EF through their effect on foundational cognitive abilities.
Only two studies have tested a full cascade model linking the prenatal environment to EF via foundational cognitive ability. Using a small sample of preterm and matched full-term 11-year-olds, researchers found that the relationship between prematurity and poorer EF was partially mediated through children’s slower processing speeds (Rose, Feldman, & Jankowski, 2011). Further, the negative effects of prematurity on academic achievement were entirely mediated through processing speed and EF. However, as all cognitive measures were administered concurrently in this study, it is unclear how the development of these processes unfolds longitudinally in children who experience different prenatal conditions. A second, longitudinal study found that the relationship between birth weight and EF at age 4 was indirect, operating through language ability at age 3 (Wade, Browne, Madigan, Plamondon, & Jenkins, 2014). However, it remains unclear whether these same relationships would be observed using a more comprehensive measure of prenatal risk. This issue constitutes the main impetus for the current study.
The Current Study
The overarching goal of the current investigation is to test a cascade model by which variations in prenatal conditions are linked to preschool cognitive outcomes (i.e., EF and IQ) through the mediating mechanism of infant general cognitive ability. In testing this model, we pose three research questions. First, to what extent do prenatal conditions predict child EF and IQ at age 3, controlling for quality of the postnatal environment? Although previous literature has explored the role of individual prenatal risk factors, we adopt a cumulative risk approach that tests whether variations in a wide range of variables indicative of prenatal risk (i.e., low birthweight, prematurity, maternal emotional problems, maternal pre-pregnancy obesity, and obstetric complications) predict preschoolers’ cognition, in a large, socioeconomically and ethnically diverse sample. Further, we include outcome measures of both EF and IQ in the current investigation, in order to determine whether prenatal programming effects are specific to EF, or whether they impact child cognitive development more broadly. Given that previous research on EF has not tended to include additional cognitive measures, such as IQ scores, it is unknown whether the magnitude of prenatal programming effects will be similar for both cognitive outcomes.
Our second question is whether the relationship between prenatal risk and preschool cognitive outcomes operates indirectly through infant general cognitive ability. Consistent with previous studies finding support for cascading effects of early cognitive deficits (e.g., Rose et al., 2011, 2008), we predict that the relationship will be largely indirect, with more risky prenatal environments predicting worse general cognition in infancy, which will negatively predict downstream EF. Because our mediator of infant cognitive abilities includes general cognitive processes, rather than specific precursors of EF, we predict that this cascade model will similarly predict deficits in IQ.
Finally, we test whether the relationship between prenatal risk and preschool cognitive outcomes is moderated by the postnatal environment, specifically observed caregiver responsivity and ratings of childcare quality. Previous research in this sample has demonstrated that mothers’ (Blair et al., 2011; Blair, Raver, & Berry, 2014) and fathers’ (Towe-Goodman et al., 2014) sensitive parenting from 6 to 36 months predicts child EF from ages 3 through 5. There is also evidence in this sample that sensitive parenting (Camerota et al., 2015) and high-quality childcare (Berry et al., 2016) buffer children from delays in EF given adverse prenatal or postnatal experiences. We expand these extant findings by simultaneously considering the postnatal caregiving environment in two settings (i.e., home and childcare). We predict that higher quality home and childcare environments will buffer children from the negative effects of risky prenatal environments, by moderating the links from prenatal risk to infant general cognitive ability, and from infant general cognitive ability to EF and IQ.
Methods
Participants
These data come from the Family Life Project (FLP), a longitudinal study of children living in primarily rural, low-income areas of the United States. Specifically, three counties in North Carolina and three counties in Pennsylvania were targeted. Participants were recruited during a one year period from September 2002 through September 2003, at the time that they gave birth to a child. Low-income and African-American families were oversampled in order to address study goals. Detailed sampling and recruitment information exist elsewhere (Burchinal, Vernon-Feagans, Cox, & Key Family Life Project Investigators, 2008). Out of the 5,471 families who gave birth during the recruitment period, 72% met eligibility criteria. Women were excluded if they did not live in a selected county, spoke a primary language other than English, or intended to move away from the target area within the next 3 years. The final sample consisted of 1,292 families who completed the 2 month home visit, at which point they were considered officially enrolled in the study. In the final sample, 51% of children were male and 41% were African-American.
Procedures
These analyses use data collected from home visits when children were approximately 2, 6, 15, 24, and 36 months of age. Home visits lasted approximately 2 to 3 hours and consisted of parent (e.g., semi-structured interviews), child (e.g., cognitive assessments), and dyadic (e.g., parent-child interaction tasks) activities. Pertinent to the current investigation, mothers retrospectively reported on pregnancy experiences during the 2 month home visit. At 6, 15, 24, and 36 months, infant and their caregivers engaged in a semi-structured free play activity which was recorded for later coding of parental warm sensitivity and harsh intrusiveness. At each of these time points, research assistants also rated the quality of the childcare environments, for children who attended childcare outside of the home. At 15 months, children’s general cognitive ability was assessed. At 36 months, children completed additional cognitive assessments including tasks to assess EF and IQ. All study protocols were approved by the institutional review boards at [blinded], and appropriate consent and assent were obtained from all participants.
Measures
Prenatal risk factors.
At 2 months, mothers responded to the pregnancy and delivery module of the Missouri Assessment of Genetics Interview for Children (MAGIC; Todd, Joyner, Heath, Neuman, & Reich, 2003), an interview surveying maternal experiences during pregnancy, including emotional problems and medical complications during pregnancy and delivery. The MAGIC module has been previously validated, with one study finding that mothers demonstrate good reliability and stability in reporting pre/perinatal events (Reich, Todd, Joyner, Neuman, & Heath, 2003).
Three yes/no questions were used to determine mothers’ emotional problems during pregnancy. These included, “Were you happy when you found out you were pregnant with [target child]?”, “Did you experience emotional problems, such as depression or anxiety, which upset you a lot, or for which you sought treatment or counseling?” and “Did you experience serious family problems which were upsetting to you?” The first question was reversed scored and added to the latter two questions to yield a sum score of emotional problems (range = 0 – 3). Nine medical problems during pregnancy were assessed, including heavy bleeding, excessive nausea or vomiting, weight loss over 10 pounds, water retention, convulsions, accidents requiring medical care, hypertension, infection, and other illnesses requiring medical care. The sum of all pregnancy complications was used as a composite of medical risk (range = 0 – 9).
Mothers self-reported their pre-pregnancy weight in pounds, as well as their current height in feet and inches. Using this information, we calculated mothers’ pre-pregnancy body mass index (BMI) and used this to determine whether she was obese (BMI ≥ 30) before her pregnancy with the target child. Mothers also reported her infant’s birth weight in pounds and ounces, which was converted into grams and used to categorize infants as low birthweight (< 2500 grams). Finally, mothers reported infant due date and birth date, which was used to calculate infant gestational age and subsequently, prematurity (< 37 weeks).
Descriptive statistics on all prenatal risk indicators are presented in Table 1. We utilized data reduction techniques to decrease the number of independent variables, and facilitate planned mediation and moderation analyses. Because of our interest in capturing variability among our entire set of prenatal risk factors, we conducted a principal components analysis (PCA) and retained the first principal component as our measure of prenatal risk. This approach allowed us to retain information about individual predictors while limiting the number of correlated independent variables included in analysis. We preferred this approach to a risk index (i.e., a count score) because it did not require arbitrary dichotomization of continuous variables, thus retaining the most information among its underlying set of indicators (Burchinal et al., 2000).
Table 1.
Descriptive statistics for prenatal risk variables
| Variable Name | N | M (SD) / % |
|---|---|---|
| Maternal Emotional Problems | 1287 | 0.47 (0.72) |
| Were you happy when found out you were pregnant? | 1282 | 82% |
| Did you experience emotional problems, such as depression or anxiety, for which you sought treatment or counseling? | 1287 | 13% |
| Did you experience serious family problems which were upsetting to you? | 1287 | 16% |
| Pregnancy Complications | 1287 | 1.45 (1.31) |
| Heavy bleeding | 1287 | 4.5% |
| Excessive nausea or vomiting | 1287 | 28% |
| Weight loss > 10 pounds | 1286 | 18% |
| Infection | 1287 | 27% |
| Hypertension | 1287 | 17% |
| Water retention | 1287 | 27% |
| Convulsions | 1287 | 0.4% |
| Accident requiring medical care | 1287 | 8.6% |
| Other illness requiring medical care | 1287 | 13% |
| Maternal Pre-Pregnancy Obesity | 1238 | 24% |
| Pre-pregnancy BMI | 1238 | 26.4 (7.16) |
| Infant Low Birthweight | 1287 | 8.0% |
| Birth weight (grams) | 1287 | 3276 (585.9) |
| Infant Premature | 1274 | 8.0% |
| Gestational age (weeks) | 1274 | 39.12 (1.91) |
Principal components analysis indicated that all five prenatal variables (i.e., maternal emotional problems, pregnancy complications, maternal pre-pregnancy obesity, low birthweight, prematurity) loaded positively onto a single factor (loadings = .28 – .76). This factor explained 31% of the variance in the underlying items. A single factor score was output for each individual and is subsequently referred to as prenatal risk, with higher values indicating more risk. Additional information about this factor score, including its distribution and correlation with individual indicators, is presented in Appendix A.
Postnatal caregiving quality.
Mother–child interactions were coded from video recordings of semi-structured play activities that lasted approximately 10 minutes. At 6 and 15 months, the dyad engaged in a free play activity. Parents were given a standardized set of toys (e.g., stacking rings, shape sorter) and instructed to play with their child as they normally would if they had some free time during the day. At 24 and 36 months, the dyads engaged in a puzzle task, in which experimenters provided a set of developmentally-appropriate puzzles of increasing difficulty for children to complete. Mothers were instructed to provide any assistance that they thought was necessary. In line with extant coding schemes (Cox & Crnic, 2002), these interactions were coded for the constructs of Sensitivity, Intrusiveness, Detachment, Stimulation of Cognitive Development, Positive Regard, Negative Regard and Animation. Global ratings of parents’ behaviors were made on a 1–5 scale at 6 and 15 months, and a 1–7 scale at 24 and 36 months, with values ranging from not at all characteristic (1) to highly characteristic (7) of the parent, based on the following definitions.
Sensitivity measured the degree to which the parent was aware of and responsive to the child’s signals and cues and engaged in synchronous interaction with the infant. Intrusiveness was defined by the parent controlling the interaction in a parent-centered way, such as inappropriately fast pacing or overstimulation. Detachment describes the degree to which a parent was emotionally distant or unaware of the child’s signals. Stimulation is defined by the parent engaging in age-appropriate behaviors that foster the child’s cognitive development, such as labelling objects. Positive Regard rates the quantity and intensity of the parent’s positive feelings towards the child, while Negative Regard rates the quantity and intensity of negative feelings towards the child. Finally, Animation captures the degree of parental energy and animation during the interaction, which could be vocal, physical, or affective.
Based on exploratory factor analysis conducted with oblique rotation (i.e., Promax), which suggested two underlying factors, we created composite scores of warm sensitivity and harsh intrusiveness by taking the mean across multiple parenting scales (Mills-Koonce et al., 2011; Vernon-Feagans, Cox, & FLP Key Investigators, 2013). Warm sensitivity was comprised of scores from the Sensitivity, Detachment (reverse coded), Stimulation of Cognitive Development, Positive Regard, and Animation scales, while harsh intrusiveness was comprised of scores from the Intrusiveness and Negative Regard scales. We used the composite scores of warm sensitivity and harsh intrusiveness in our analyses, as these scores capture both positive and negative dimensions of parenting. This composite score approach has been widely used in previous analyses with FLP data (Blair et al., 2014). High scores on the sensitivity composite indexed parental behavior that was warm, responsive, stimulating, and child-centered, whereas high scores on the intrusiveness composite indexed parental behavior that was harsh, negative, and parent-focused.
All coders were trained and certified by one master coder. In addition, reliability checks were completed on approximately 30% of tapes to ensure intraclass correlations (ICC) between all pairs of coders exceeded 0.80 (ICC=0.80–0.98 for all subscales and composite scores). To test the impact of timing of parental input, and to facilitate planned moderation analyses, we averaged warm sensitivity and harsh intrusiveness scores across 6–15 and 24–36 months to represent caregiver quality during infancy and toddlerhood, respectively.
Postnatal childcare quality.
Traditional childcare quality measures (e.g., Early Childhood Environment Rating Scale; Harms & Clifford, 1980) were unavailable in these data. Therefore, consistent with previous studies (e.g., Berry et al., 2016), we included independent ratings of the quality of the childcare environment using the Home Observation Measure of the Environment scale (HOME; Caldwell & Bradley, 1984). Quality of the childcare environment was only rated for children who attended childcare outside of the home (N = 454, 505, 427, 455 at 6, 15, 24, and 35 months, respectively). Childcare arrangements could include formal (e.g., center-based) or informal settings (e.g., family day care). At each time point, trained research assistants observed childcare settings for approximately 20 minutes for the purpose of completing the HOME scale.
The infant/toddler version of the measure was used at 6, 15, and 24 months, whereas the preschool version was used at 36 months. All three subscales (Responsivity, Acceptance, and Learning Materials) were used at all time points, but the number of items per subscale varied based on the version. The infant/toddler version contained 11 items for Responsivity, 8 items for Acceptance, and 9 items for Learning Materials. The preschool version contained 7 items for Responsivity, 4 items for Acceptance, and 11 items for Learning Materials. We summed items from all subscales to create a single index of childcare quality at each timepoint (range = 0 – 28 at 6, 15, and 24 months; range = 0 – 22 at 35 months). Internal consistency of all items was adequate at each of the four time points (α = .58 – .69). We averaged together ratings of childcare quality for 6 and 15 months (infancy), and 24 and 35 months (toddlerhood) to create two summary indicators of the postnatal childcare environment.
Child cognitive outcomes.
General cognitive ability.
Child developmental status was assessed at 15 months using the Bayley Scales of Infant Development (BSID-II; Bayley, 1993). The BSID-II is a widely used measure of cognitive development for children in the first two years of life, and measures abilities such as memory, problem solving, and language. The Mental Development Index (MDI), a norm-referenced standard score (M = 100, SD = 15), was used as our measure of general cognitive ability.
Executive function (EF).
Child executive function was assessed at 36 months using a battery of tasks presented in an open spiral bound flip-book format, with pages measuring 8×14 in. Each page presented stimuli to the child on one page and scripted instructions for the research assistant on the other. For each task, children first had to pass a set of training trials, assessing their comprehension of task constructs and procedures, before continuing on to the test trials. The battery of EF tasks included two measures of working memory (Working Memory Span; Pick the Picture Game), three measures of inhibitory control (Silly Sounds Stroop, Spatial Conflict, Animal Go/No-Go), and one measure of attention shifting (Something’s the Same). Details of these individual tasks, as well as psychometric properties of the battery, are explained elsewhere (Willoughby, Blair, Wirth, & Greenberg, 2010). We therefore omit full task descriptions here. Consistent with the analytic approach previously used in this sample (Willoughby, Wirth, & Blair, 2012), item response theory (IRT) models were used to create expected a-posteriori (EAP) scores for each task at each assessment. Individual EAP scores were averaged and used as an index of child EF ability.
Full-Scale IQ.
At 36 months, the Block Design and Receptive Vocabulary subscales of the Wechsler Preschool and Primary Scales of Intelligence (WPPSI-III; Wechsler, 2002) were used to assess child full-scale IQ. This two subscale short-form demonstrates the highest correlation (r = .88) with full scale IQ scores (Silverstein, 1975).
Demographic covariates.
At the 2 month home visit, primary caregivers reported on their years of education and their child’s gender. Household income-to-needs ratio (INR) was calculated at the 6, 15, 24, and 35 month data collection visits. Aggregated household income from all sources (e.g., earnings, child support) was divided by the U.S. poverty threshold for the year (adjusted for family size and household composition) to create an income-to-needs ratio for each family at each time point. Mirroring the approach described above, we averaged together INR at 6 and 15 month and 24 and 35 month to represent household income in infancy and toddlerhood, respectively.
Missing Data
Of the 1,292 primary caregivers enrolled in this study, 1,287 were biological mothers who could report on their pregnancies at the 2 month home visit. Of these 1,287 biological mothers, 49 (3.8%) were missing pre-pregnancy obesity data and 9 (0.7%) were missing infant due date data, meaning that prematurity could not be determined. Concerning postnatal data, 200 (16%) children were missing MDI data, 319 (25%) were missing EF data, and 238 (18%) were missing IQ data. There were 87 (6.8%) and 162 (13%) dyads missing ratings of caregiving quality in infancy and toddlerhood, respectively.
Because quality of the childcare environment was only collected for children attending childcare outside the home, there is a larger amount of missing data for this variable in infancy (N = 665; 52%) and toddlerhood (N = 701; 54%). Children who did not have childcare data in infancy were more likely to be white (χ2 (1) = 46.03, p < .001), but did not differ based on child gender or poverty status at recruitment (p > .05). Further, children with childcare data in infancy tended to have higher household INR [t(1174) = −5.30, p < .001], higher maternal education [t(1285) = −2.83, p = .005], and higher levels of maternal intrusiveness [t(1203) = −3.04, p = .002], but did not differ on prenatal risk, maternal sensitivity, general cognitive ability, EF, or IQ (p > .05). Similar results were found for children with and without childcare data in toddlerhood. Since differences in missing data were accounted for by variables included as covariates in the model, the assumption that these data were conditionally random, or missing at random (MAR), was supported. There was no reason to believe that childcare data were missing because of the level of childcare quality itself, which would render the data not missing at random (NMAR). Thus, we used full-information maximum likelihood (FIML) for all analyses (Schafer & Graham, 2002). A total of 343 (27%) of dyads had complete data.
Analytic Strategy
To address our first substantive aim, we used the prenatal risk factor score derived above in a total-effects model, to test whether prenatal risk predicted child EF and IQ above and beyond covariates and the postnatal caregiving and childcare environments. Because we hypothesized a cascading relationship between prenatal risk and preschool cognitive outcomes, we next tested a mediation model where we added an indirect path from prenatal risk to preschool EF and IQ through infant general cognitive ability (i.e., MDI score). Using this cascade model, we compared the significance and magnitude of the direct and indirect effects of prenatal risk on EF and IQ. Finally, we tested whether the cascade model described in step 2 was moderated by the postnatal caregiving environment. We accomplished this by adding six interaction terms to the indirect effects model (prenatal risk × infant caregiver sensitivity, prenatal risk × infant caregiver intrusiveness, prenatal risk × infant childcare quality, MDI × toddler caregiver sensitivity, MDI × toddler caregiver intrusiveness, MDI × toddler childcare quality), where the first three interaction terms predicted MDI and the latter three predicted EF and IQ. Non-significant interaction terms were trimmed and the final model was interpreted.
Descriptive statistics and path models addressing all substantive aims were estimated in Mplus version 8 (Muthén & Muthén, 2017). Path models allowed us to estimate a single model predicting multiple dependent variables (i.e., EF and IQ). All results accounted for the complex sampling design by adjusting for population weight and stratification variables, and all dependent variables (i.e., MDI, EF, IQ) were regressed on all covariates (e.g., INR). Additionally, all models accounted for possible relationships among exogenous variables (e.g., stability in parenting from infancy to toddlerhood, relationship between poverty and childcare quality) by allowing these variables to covary. Indirect effects were calculated as the products of coefficients linking the focal predictor to the outcome via the mediating variable(s) and the significance of these effects were determined using the delta method (MacKinnon, 2008).
Results
Preliminary Analyses
Weighted descriptive statistics, including means, standard deviations, and correlations for all study variables are displayed in Table 2. From this table, it is clear that prenatal risk was significantly, though modestly, correlated with child MDI (r = −.17, p < .001), EF (r = −.10, p < .01), and IQ (r = −.14, p < .001). Infant MDI was positively correlated with preschool EF (r = .23, p < .001) and IQ (r = .39, p < .001). Child male gender was negatively correlated with all cognitive outcomes of interest (r = −.10 - −.16, p < .01). Household INR was positively related to all cognitive outcomes in infancy and toddlerhood (r = .17 – .36, p < .001). Infant MDI was positively correlated with caregiver sensitivity (r = .22, p < .001) and childcare quality (r = .18, p < .001) and negatively correlated with caregiver intrusiveness (r = −.16, p < .001) in infancy. Similarly, preschoolers’ IQ was positively correlated with caregiver sensitivity (r = .34 – .41, p < .001) and childcare quality (r = .21 – .24, p < .001) and negatively correlated with caregiver intrusiveness (r = −.26 - −.45, p < .001) in both infancy and toddlerhood. Preschoolers’ EF was significantly correlated with caregiver sensitivity (r = .27 – .31, p < .001) and intrusiveness (r = −.22 - −.30, p < .001) in infancy and toddlerhood, but was not correlated with the quality of the childcare environment at either time point (r = .10, p > .07).
Table 2.
Weighted correlations and descriptive statistics for study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Prenatal Risk | - | |||||||||||||
| 2. Bayley MDI (15 months) | −.17 | - | ||||||||||||
| 3. EF (36 months) | −.10 | .23 | - | |||||||||||
| 4. IQ (36 months) | −.14 | .39 | .40 | - | ||||||||||
| 5. Male | −.00± | −.12 | −.10 | −.16 | - | |||||||||
| 6. Maternal education (years) | −.16 | .20 | .25 | .36 | .00± | - | ||||||||
| 7. Caregiver sensitivity(infant) | −.11 | .22 | .27 | .34 | −.02± | .49 | - | |||||||
| 8. Caregiver intrusiveness(infant) | .07 | −.16 | −.22 | −.26 | .05± | −.35 | −.29 | - | ||||||
| 9. Childcare quality (infant) | .01± | .18 | .10± | .21 | .02± | .14 | .19 | −.23 | - | |||||
| 10. Caregiver sensitivity (toddler) | −.16 | .30 | .31 | .41 | −.01± | .52 | .69 | −.32 | .20 | - | ||||
| 11. Caregiver intrusiveness (toddler) | .19 | −.31 | −.30 | −.45 | .10 | −.39 | −.37 | .45 | −.21 | −.57 | - | |||
| 12. Childcare quality (toddler) | .06± | .13 | .10± | .24 | .02± | .17 | .16 | −.06± | .25 | .13 | −.10 | - | ||
| 13. Income-to-needs ratio (infant) | −.12 | .17 | .22 | .35 | .05± | .58 | .39 | −.30 | .22 | .41 | −.30 | .22 | - | |
| 14. Income-to-needs ratio (toddler) | −.13 | .20 | .22 | .36 | .04± | .59 | .39 | −.27 | .21 | .42 | −.31 | .23 | .87 | - |
| n | 1227 | 1087 | 968 | 1049 | 1287 | 1287 | 1200 | 1200 | 622 | 1125 | 1125 | 586 | 1172 | 1147 |
| M | −.02 | 97.80 | −.45 | 96.90 | .52 | 13.02 | 2.98 | 2.23 | 25.32 | 3.02 | 2.21 | 20.99 | 2.23 | 2.18 |
| SD | 1.00 | 10.36 | .54 | 16.72 | 2.21 | .71 | .59 | 2.30 | .69 | .71 | 3.83 | 1.82 | 1.66 |
Note. MDI = Mental Development Index; EF = executive function. Sample sizes for correlations range from 448 to 1287, given variation among participants’ study completion. “Infant” measures are averages taken from 6- and 15-month observations. “Toddler” measures are averages taken from 24- and 35-month observations. All correlations are significant at p < .05, except where marked
(indicates p > .05).
Total Effects Model
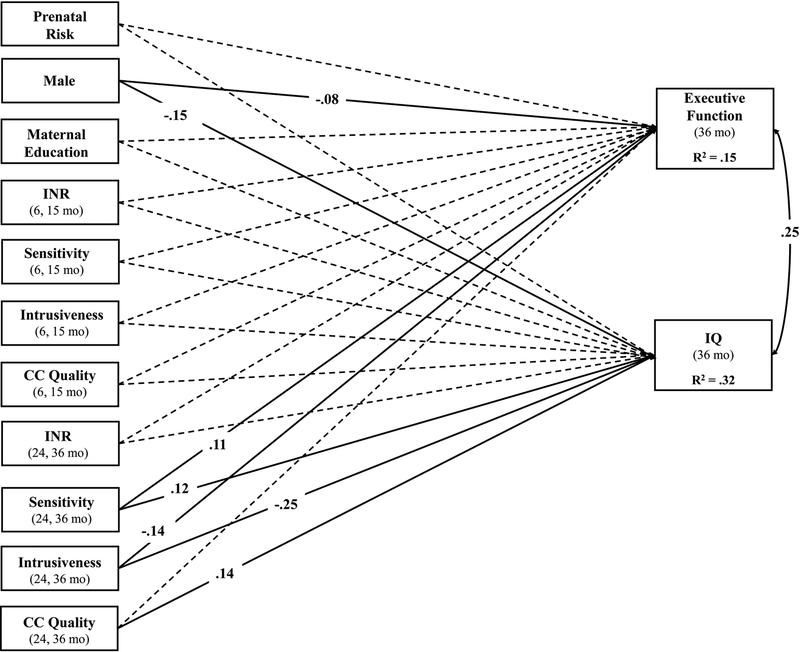
Our first path model included a direct effect of prenatal risk on EF and IQ, controlling for demographic covariates and caregiver and childcare quality in infancy and toddlerhood (see Figure 1). The model was fully saturated. There were no direct effects of prenatal risk on either EF (β = −.04, p = .24) or IQ (β = −.06, p = .13). Caregiver sensitivity (β = .12, p = .02), intrusiveness (β = −.25, p < .001), and childcare quality (β = .14, p < .001) in toddlerhood significantly predicted child IQ, whereas only caregiver sensitivity (β = .11, p = .04) and intrusiveness (β = −.14, p < .01) in toddlerhood significantly predicted child EF (Figure 1).
Figure 1.
Path model illustrating total effects of prenatal and postnatal factors on EF and IQ at age 3 years. Single-headed arrows represent regression paths, while curved, double-headed arrows represent correlations. Standardized parameter estimates are presented for all significant paths (p < .05) using solid lines, whereas non-significant paths are presented using dotted lines. All exogenous variables were allowed to covary. INR = income-to-needs ratio; CC = childcare.
Mediated (Cascade) Model
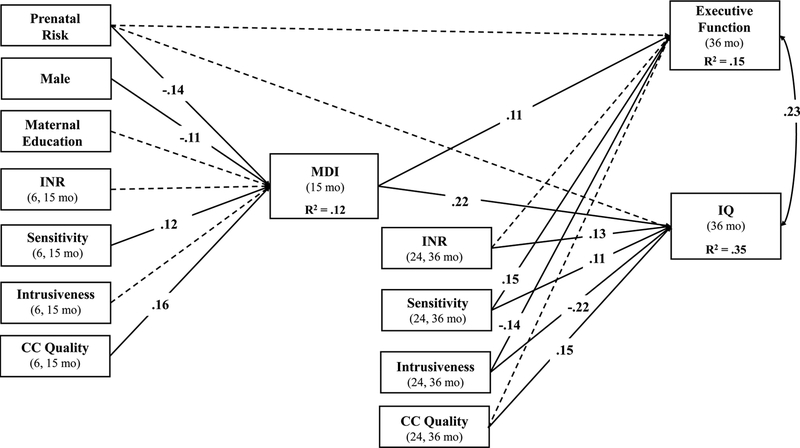
We next added an indirect path from prenatal risk to EF and IQ through child MDI. An important change in this model is that parenting and childcare quality in infancy were included as predictors of MDI, while parenting and childcare quality in toddlerhood were included as predictors of EF and IQ (see Figure 2). Model fit was adequate; χ2 (12) = 51.65, p < .001, CFI = .93, RMSEA = .05 (90% RMSEA confidence interval [.037, .065]). In this model, there was not a significant direct effect of prenatal risk on EF (β = −.03, p = .43) or IQ (β = −.03, p = .41). Prenatal risk did significantly predict child MDI (β = −.14, p < .001), which in turn predicted both EF (β = .11, p < .01) and IQ (β = .22, p < .001). The indirect path from prenatal risk to EF (β = −.02, p = .02; 95% CI [−.028, −.003]) and IQ (β = −.03, p < .001; 95% CI [−.045, −.014]) through MDI was significant. Caregiver sensitivity (β = .12, p = .001) and childcare quality (β = .16, p < .001) in infancy significantly predicted MDI. Caregiver sensitivity (β = .11, p < .01), intrusiveness (β = −.22, p < .001), and childcare quality (β = .15, p < .001) in toddlerhood significantly predicted IQ, whereas only caregiver sensitivity (β = .15, p = .001) and intrusiveness (β = −.14, p < .01) in toddlerhood significantly predicted child EF.
Figure 2.
Path model illustrating a cascade of effects from prenatal risk to EF and IQ at age 3 years via general cognitive ability (Bayley Mental Developmental Index [MDI]) at 15 months. Single-headed arrows represent regression paths, while curved, double-headed arrows represent correlations. Standardized parameter estimates are presented for all significant paths (p – .05) using solid lines. Non-significant paths are presented using dotted lines. For simplicity, paths from covariates (male, maternal education) to EF and IQ are not pictured. Additionally, all exogenous variables were allowed to covary. INR = income-to-needs ratio; CC = childcare.
Interactive Effects Model
In a final model, we added six interaction terms to test whether the indirect effects of prenatal risk on EF and IQ through MDI were moderated by the quality of the postnatal caregiving and childcare environments. This model fit the data well; χ2 (21) = 52.78, p < .001, CFI = .95, RMSEA = .03 (90% RMSEA confidence interval [.023, .046]). However, none of the added interaction terms (i.e., prenatal risk × infant caregiver sensitivity, prenatal risk × infant caregiver intrusiveness, prenatal risk × infant childcare quality, MDI × toddler caregiver sensitivity, MDI × toddler caregiver intrusiveness, MDI × toddler childcare quality) were significant predictors.
In light of possible concerns regarding multicollinearity among multiple interaction terms, we also tested each moderator individually. That is, we re-estimated six cascade models that each included a single interaction term (e.g., prenatal risk × infant childcare quality). None of these models yielded significant interaction terms, supporting our conclusions from the model that included all six interaction terms simultaneously.
Post-Hoc Analyses
Given the absence of interactive effects, we tested the alternative hypothesis that parenting and childcare quality might mediate the cascade from prenatal risk to general cognitive ability to EF and IQ. That is, we added paths from prenatal risk to parental sensitivity, intrusiveness, and childcare quality in infancy to general cognitive ability, and from general cognitive ability to parental sensitivity, intrusiveness, and childcare quality in toddlerhood to both EF and IQ. This model fit the data well, χ2 (9) = 12.55, p = .18, CFI = .99, RMSEA = .02 (90% RMSEA confidence interval [.000, .038]). In this model, the indirect paths from prenatal risk to MDI through maternal sensitivity (β = −.004, p = .32), intrusiveness (β = .000, p = .76), and childcare quality (β = .006, p = .24) in infancy were all non-significant. However, there were significant indirect paths from infant general cognitive ability to parental sensitivity in toddlerhood to EF (β = .02, p = .009) and IQ (β = .01, p = .02). Similarly, the indirect paths from general cognitive ability to parental intrusiveness in toddlerhood to EF (β = .02, p = .008) and IQ (β = .03, p < .001) were significant. The full indirect paths from prenatal risk to infant general cognitive ability to parental sensitivity (β = −.002, p = .02) and intrusiveness (β = −.003, p = .02) to child EF were significant. Similarly, there was a significant indirect path from prenatal risk to infant general cognitive ability to parental sensitivity (β = −.002, p = .03) and intrusiveness (β = −.005, p = .003) predicting child IQ.
Finally, given that infant MDI scores were not adjusted for prematurity, we conducted a sensitivity analysis excluding MDI data from all children born prematurely (< 37 weeks; n = 81). That magnitude of all effects remained largely unchanged, although coefficient standard errors increased with the exclusion of these scores, likely due to the decreased sample size. This change resulted in the indirect effect from prenatal risk to EF through general cognitive ability becoming marginally significant (β = −.014, SE =.008, p = .07; 95% CI [−.030, .001]), compared to the original model (β = −.015, SE = .006, p = .02; 95% CI [−.028, −.003]). In light of these relatively minor changes to the size of model coefficients, we concluded that unadjusted MDI scores were unlikely to be driving the effects reported in this manuscript.
Discussion
The current study tested the independent and interactive effects of prenatal and postnatal experience on preschoolers’ cognitive outcomes. We found that a composite of prenatal risk factors (i.e., low birthweight, prematurity, maternal emotional problems, maternal pre-pregnancy obesity, and obstetric complications) indirectly predicted child EF and IQ at age 3, above and beyond quality of home and childcare environments in infancy and toddlerhood. This is the first study of which we are aware that has found an independent effect of the prenatal environment on child EF while statistically controlling for the quality of the postnatal environment across several settings. Moreover, we found evidence for a cascade model linking prenatal risk to preschoolers’ cognitive outcomes via general cognitive abilities in infancy. Contrary to our hypotheses, we did not find evidence that the quality of the postnatal home and caregiving environments moderated the relationships among prenatal risk, general cognitive ability, and EF/IQ. Our findings demonstrate support for the Developmental Origins of Health and Disease (DOHaD) and prenatal programming hypotheses and suggest the importance of including measures of prenatal conditions in studies of child development.
Environmental contributions to children’s cognitive development, and in particular EF, have long been acknowledged in the literature. Thus far, the factors considered have primarily fallen under the domain of postnatal environmental characteristics, including poverty (Blair & Raver, 2012), parenting (Fay-Stammbach et al., 2014), and early education (Yoshikawa et al., 2013). These studies have not simultaneously considered the role of the prenatal environment. Likewise, the small but growing set of studies examining prenatal risk and childhood EF have tended to test only single indicators of prenatal risk and have not included comprehensive, objective assessments of the quality of the postnatal environment (Buss et al., 2011; Phua, Rifkin-Graboi, Saw, Meaney, & Qiu, 2012; Theresia et al., 2017; Wade & Jenkins, 2016). Therefore, we currently know very little about the independent contributions of prenatal and postnatal experience to the development of EF. The present study, indicating a small but significant effect of prenatal risk above and beyond the quality of the postnatal home and caregiving environments, corroborate and expand upon these previous findings. To the extent that prenatal and postnatal environmental quality are correlated with one another as well as with child EF, our inclusion of both indicators in the same model serves as a more stringent test of the hypothesis that prenatal experience shapes EF development.
Results of our mediation analyses provide support for a cascade model where the relationship between prenatal experience and preschool EF operates indirectly through infant’s fundamental cognitive abilities. In other words, exposure to adverse prenatal environments may initiate a cascade of developmental difficulties, due to their more proximal influence on foundational cognitive abilities (Rose et al., 2008). Our findings are consistent with developmental theories that suggest continuity in cognitive development starting in infancy (Bornstein et al., 2006) and more specifically, with studies that trace the origins of individual differences in EF to earlier differences in fundamental cognitive processes such as attention, language, and information processing (Cuevas & Bell, 2014; Rose et al., 2012; M. Wade et al., 2014). Because these general cognitive processes are measureable beginning in infancy, they provide an early identification and intervention point for children who may be at risk for later EF difficulties (Hendry et al., 2016), whether due to adverse prenatal experience or other factors.
However, one implication of these findings is that prenatal risk is not likely to influence EF alone. Rather, as observed in the current study, our cascade model explained the relationship between prenatal risk and both EF and IQ. It therefore remains to be seen whether there are developmental processes that link prenatal risk to EF specifically, or whether EF is just one of many cognitive abilities that are impacted by exposure to adverse prenatal environments. The inclusion of more discrete cognitive assessments in infancy may have improved our ability to test specific mechanisms by which prenatal risk relates to EF and IQ.
Recent research has elucidated several mechanisms that may be responsible for the long-lasting impact of prenatal experience on child cognitive development. Multiple studies have demonstrated alterations in neural structure and function for children born low birth weight (for a review, see Jobe, 2010) or exposed to elevated levels of maternal stress (Sarkar et al., 2014). In a separate set of studies, degree of neural abnormality was found to predict the extent of cognitive deficits in LBW and preterm infants (Lowe et al., 2011; Woodward, Clark, Bora, & Inder, 2012; Woodward, Clark, Pritchard, Anderson, & Inder, 2011). Therefore, exposure to risk factors in the fetal environment may alter trajectories of cognitive development through their impact on neural development, a process that is rapidly occurring during the prenatal period (Tau & Peterson, 2010).
A second compelling hypothesis concerns the role of maternal-placental-fetal neuroendocrine and immune systems as mediators of prenatal environmental influences on child development. Several reviews have postulated the ways in which the developing fetus acquires and integrates information about its environment using these distinct biological systems, the same systems that are involved in adaptation to stress and neurocognitive development later in the postnatal period (Wadhwa, Buss, Entringer, & Swanson, 2010; Wadhwa, 2005). Specifically, elevated fetal exposure to cortisol (O’Connor et al., 2013; Sandman et al., 2011) and corticotrophin-releasing hormone (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999) may be both the result of stressful pregnancy experiences (e.g., obstetric complications, emotional problems), and the common mechanism by which these diverse experiences exert programming effects, possibly by initiating epigenetic changes in the fetus (Oberlander et al., 2008). Relatedly, shared genetics between mothers and offspring may contribute to both higher-risk pregnancies and delays or deficits in child cognitive development (Leve et al., 2012).
Finally, behavioral differences in neonates exposed to higher levels of prenatal risk may interfere with the ability of caregivers to provide the type of sensitive responsiveness in the postnatal period that we know is important for cognitive development (e.g., Mills-Koonce et al., 2015). Studies using within-family designs have found evidence for this mechanism, reporting that siblings exposed to higher prenatal risk (e.g., lower birth weight) receive less sensitive caregiving, on average, compared to lower-risk (e.g., higher birth weight) siblings (Browne et al., 2018). The current study found some support for this mechanism in our post-hoc analyses, which indicated that deficits in infant general cognitive ability, which are partially due to variations in exposure to prenatal risk, predicted decreased maternal sensitivity and increased maternal intrusiveness, which in turn predicted poorer EF and IQ at preschool. As our study was not well placed to test all possible competing mechanisms, it is unclear how biological or genetic mechanisms contribute in addition to parenting.
Contrary to our hypotheses, we did not find evidence that prenatal and postnatal experience interacted to predict child cognitive outcomes at 15 months or 3 years. These findings suggest that high-quality caregiving and childcare experiences are equally beneficial for children regardless of their prenatal experiences. On the one hand, these findings are promising, as they suggest that all children have the potential to reap the benefits of supportive postnatal environments, regardless of level of prenatal risk (Austin et al., 2017). However, in an alternative model, we found that parenting quality partially mediated the cascade from prenatal risk to general cognitive ability to EF and IQ. Therefore, while supportive postnatal environments do not seem to buffer children from cognitive deficits resulting from increased prenatal risk, they may represent one pathway by which prenatal risk exerts enduring consequences for child development. Indeed, these intriguing findings suggest that it is not exposure to prenatal risk per se that results in suboptimal parenting. Rather, it is the subtle deficits in general cognitive ability that may result from such exposures that evoke decreased sensitivity and increased sensitivity in parents. If replicated, these findings have clear implications for prevention and intervention efforts, as promoting early cognitive development in children exposed to elevated levels of prenatal risk may disrupt the cascade leading to poor cognitive development in preschool and beyond.
Our findings are bolstered by the use of a large, longitudinal sample that contained repeated and objective measurement of key constructs, such as postnatal environmental quality and child cognition. Further, we overcome limitations of convenience sampling through our complex sampling design, which allows us to generalize our findings back to the counties from which our data were drawn. In spite of these strengths, an obvious limitation of the current study is our reliance on retrospective measurement of the prenatal environment. While previous studies have found maternal report of pre-pregnancy weight, obstetric complications, birthweight, and gestational age to be consistent with clinical records (Lederman & Paxton, 1998; Sou, Chen, Hsieh, & Jeng, 2006), there is also the possibility that mothers’ retrospective report of specific emotional or medical problems may be incomplete or subject to recall bias. Thus, future studies should attempt to replicate the current findings using prospectively obtained data on salient aspects of the prenatal environment. Further, although we attempted to capture a broad range of normative prenatal experiences, rather than specific, known toxic exposures (e.g., tobacco or alcohol use), there are other experiences that we were not able to capture given the current data. For example, maternal diet (e.g., Monk, Georgieff, & Osterholm, 2013), psychosocial stress (e.g., Laplante et al., 2004), and inflammation (e.g., Bastek, Weber, McShea, Ryan, & Elovitz, 2014) during pregnancy have all been linked to subsequent child outcomes. Among the variables we did include, low birthweight, prematurity, and pregnancy complications loaded most strongly on our factor score, while maternal emotional problems and pre-pregnancy obesity loaded less strongly. This observation may influence the interpretation of our study findings, and also suggests the need for further evaluation of single- and multi-dimensional models of prenatal risk. Similarly, although a cumulative risk approach provides important preliminary evidence of the relationship between prenatal experience and child cognitive development, this approach precludes us from identifying specific mechanistic pathways or specific periods of influence (e.g., influences from pre-conception vs. prenatal vs. perinatal periods). Clearly, additional research is needed in order to identify quantifiable aspects of the prenatal environment, as well as uncover mechanisms linking prenatal experience to postnatal development.
In sum, our findings demonstrate that increased prenatal risk is indirectly related to poorer EF and IQ during the preschool years via infant general cognitive ability. These findings provide support for DOHaD, prenatal programming, and fetal origins hypotheses first proposed in the medical literature. While some efforts have been made to apply these theories to developmental psychology (e.g., Pluess & Belsky, 2011), we hope that the current findings provide an impetus for others to consider the contributions of prenatal experience as an early driver of individual differences in cognitive processes.
Supplementary Material
Acknowledgements
This study is part of the Family Life Project [https://flp.fpg.unc.edu/]. The Family Life Project Phase I Key Investigators include Lynne Vernon-Feagans, University of North Carolina; Martha Cox, University of North Carolina; Clancy Blair, Pennsylvania State University; Peg Burchinal, University of North Carolina; Linda Burton, Duke University; Keith Crnic, Arizona State University; Ann Crouter, Pennsylvania State University; Patricia Garrett-Peters, University of North Carolina; Mark Greenberg, Pennsylvania State University; Stephanie Lanza, Pennsylvania State University; Roger Mills-Koonce, University of North Carolina; Emily Werner, Pennsylvania State University; and Michael Willoughby, University of North Carolina.
Data collection for this study was supported by NICHD P01 HD039667, with co-funding from the National Institute of Drug Abuse. Data analysis for this study was supported by the National Institutes of Health, Office of The Director under Award Number UG3OD023332. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, & Oosterlaan J (2009). Meta-Analysis of Neurobehavioral Outcomes in Very Preterm and/or Very Low Birth Weight Children. Pediatrics, 124(2), 717–728. 10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- Austin MP, Christl B, McMahon C, Kildea S, Reilly N, Yin C, … King S (2017). Moderating effects of maternal emotional availability on language and cognitive development in toddlers of mothers exposed to a natural disaster in pregnancy: The QF2011 Queensland Flood Study. Infant Behavior and Development, 49, 296–309. 10.1016/j.infbeh.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Aylward GP, Pfeiffer SI, Wright a, & Verhulst SJ (1989). Outcome studies of low birth weight infants published in the last decade: a metaanalysis. The Journal of Pediatrics, 115(4), 515–520. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2795341 [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, … Nestler EJ (2010). Early Life Programming and Neurodevelopmental Disorders. Biological Psychiatry, 68(4), 314–319. 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (1995). Fetal origins of coronary heart disease. BMJ (Clinical Research Ed.), 311(6998), 171–174. 10.1136/bmj.311.6998.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastek JA, Weber AL, McShea MA, Ryan ME, & Elovitz MA (2014). Prenatal inflammation is associated with adverse neonatal outcomes. American Journal of Obstetrics and Gynecology, 210(5), 450.e1–450.e10. 10.1016/j.ajog.2013.12.024 [DOI] [PubMed] [Google Scholar]
- Bayley N (1993). Bayley Scales of Infant Development (2nd ed). San Antonio, TX: Psychological Corp. [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, & Matte-Gagné C (2012). Social factors in the development of early executive functioning: A closer look at the caregiving environment. Developmental Science, 15(1), 12–24. 10.1111/j.1467-7687.2011.01093.x [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81(1), 326–339. 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Berry D, Blair C, Willoughby M, Garrett-Peters P, Vernon-Feagans L, Mills-Koonce WR, … Werner E (2016). Household chaos and children’s cognitive and socio-emotional development in early childhood: Does childcare play a buffering role? Early Childhood Research Quarterly, 34, 115–127. 10.1016/j.ecresq.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D. a., Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, … Fortunato CK (2011). Salivary Cortisol Mediates Effects of Poverty and Parenting on Executive Functions in Early Childhood. Child Development, 82(6), 1970–1984. 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2012). Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist, 67(4), 309–318. 10.1037/a0027493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, & Berry DJ (2014). Two approaches to estimating the effect of parenting on the development of executive function in early childhood. Developmental Psychology, 50(2), 554–565. 10.1037/a0033647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, Noble MD, & Adair LS (2013). Are gestational age, birth weight, and birth length indicators of favorable fetal growth conditions? A structural equation analysis of Filipino infants. Statistics in Medicine, 32(17), 2950–2961. 10.1002/sim.5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn C-S, & Wolke D (2013). Systems and cascades in cognitive development and academic achievement. Child Development, 84(1), 154–162. 10.1111/j.1467-8624.2012.01849.x.Systems [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn C, Bell C, Haynes OM, Slater A, Bornstein MH, … Slater A (2006). Stability in Cognition Across Early Childhood: A Developmental Cascade. Psychological Science, 17(2), 151–158. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, & Sigman MD (1986). Continuity in Mental Development from Infancy. Child Development, 57(2), 251–274. [DOI] [PubMed] [Google Scholar]
- Brinksma DM, Hoekstra PJ, van den Hoofdakker B, de Bildt A, Buitelaar JK, Hartman CA, & Dietrich A (2017). Age-dependent role of pre- and perinatal factors in interaction with genes on ADHD symptoms across adolescence. Journal of Psychiatric Research, 90, 110–117. 10.1016/j.jpsychires.2017.02.014 [DOI] [PubMed] [Google Scholar]
- Browne DT, Wade M, Plamondon A, Leckie G, Perlman M, Madigan S, & Jenkins JM (2018, April 16). Child and Contextual Effects in the Emergence of Differential Maternal Sensitivity Across Siblings. Developmental Psychology. 10.1037/dev0000506 [DOI] [PubMed] [Google Scholar]
- Burchinal MR, Roberts JE, Hooper S, & Zeisel SA (2000). Cumulative risk and early cognitive development: a comparison of statistical risk models. Developmental Psychology, 36(6), 793–807. 10.1037/0012-1649.36.6.793 [DOI] [PubMed] [Google Scholar]
- Burchinal M, Vernon-Feagans L, Cox M, & Investigators KFLP (2008). Cumulative social risk, parenting, and infant development in rural low-income communities. Parenting: Science and Practice, 8(1), 41–69. 10.1080/15295190701830672.Cumulative [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchinal M, & Willoughby M (2013). IV. Poverty and associated social risks: Toward a cumulative risk framework. Monographs of the Society for Research in Child Development, 78(5), 53–65. 10.1111/mono.12050 [DOI] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, & Sandman CA (2011). Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress, 14(6), 665–676. 10.3109/10253890.2011.623250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, & Sandman CA (2012). Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE, 7(6). 10.1371/journal.pone.0037758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, & Bradley RH (1984). Home observation for measurement of the environment. Little Rock, AR: University of Arkansas Press. [Google Scholar]
- Camerota M, & Bollen KA (2016). Birth Weight, Birth Length, and Gestational Age as Indicators of Favorable Fetal Growth Conditions in a US Sample. PLoS ONE, 11(4), e0153800 10.1371/journal.pone.0153800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerota M, Willoughby MT, Cox M, & Greenberg MT (2015). Executive function in low birth weight preschoolers: The moderating effect of parenting. Journal of Abnormal Child Psychology, 43(8), 1551–1562. 10.1007/s10802-015-0032-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody DP, Bendersky M, Demarco JK, Hiatt M, Dunn SM, Hegyi T, & Lewis M (2006). Early risk, attention, and brain activation in adolescents born preterm. Child Development, 77(2), 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Sheffield TD, Chevalier N, Nelson JM, Wiebe SA, & Espy KA (2013). Charting early trajectories of executive control with the shape school. Developmental Psychology, 49(8), 1481–1493. 10.1037/a0030578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway A, & Stifter C. a. (2012). Longitudinal Antecedents of Executive Function in Preschoolers. Child Development, 83(3), 1022–1036. 10.1111/j.1467-8624.2012.01756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M, & Crnic K (2002). Qualitative ratings for parent-child interaction at 3–12 months of age. Unpublished manuscript, The University of North Carolina at Chapel Hill. [Google Scholar]
- Cox MJ, Mills-Koonce R, Propper C, & Garié Py J-L (2010). Systems theory and cascades in developmental psychopathology. Development and Psychopathology, 22, 497–506. 10.1017/S0954579410000234 [DOI] [PubMed] [Google Scholar]
- Cuevas K, & Bell MA (2014). Infant attention and early childhood executive function. Child Development, 85(2), 397–404. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative Risk and Child Development. Cumulative Risk and Child Development. Psychological Bulletin. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Fay-Stammbach T, Hawes DJ, & Meredith P (2014). Parenting Influences on Executive Function in Early Childhood: A Review. Child Development Perspectives, 8(4), n/a–n/a. 10.1111/cdep.12095 [DOI] [Google Scholar]
- Garon N, Bryson SE, & Smith IM (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134(1), 31–60. 10.1037/0033-2909.134.1.31 [DOI] [PubMed] [Google Scholar]
- Gillman MW (2005). Developmental origins of health and disease. The New England Journal of Medicine, 353(17), 1848–1850. 10.1056/NEJMe058187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SI, Müller U, Carpendale JIM, Bibok MB, & Liebermann-Finestone DP (2012). The effects of parental scaffolding on preschoolers’ executive function. Developmental Psychology, 48(1), 271–281. 10.1037/a0025519 [DOI] [PubMed] [Google Scholar]
- Harms T, & Clifford RM (1980). Early Childhood Environment Rating Scale. New York, NY: Teachers College Press. [Google Scholar]
- Hendry A, Jones EJH, & Charman T (2016). Executive function in the first three years of life: Precursors, predictors and patterns. Developmental Review. 10.1016/j.dr.2016.06.005 [DOI] [Google Scholar]
- Hughes C, & Ensor R (2009). How do families help or hinder the emergence of early executive function? New Directions in Child and Adolescent Development, 123, 35–50. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, & Buitelaar JK (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry, 44(6), 810–818. 10.1111/1469-7610.00166 [DOI] [PubMed] [Google Scholar]
- Jobe AH (2010, November). Miracle extremely low birth weight neonates: Examples of developmental plasticity. Obstetrics and Gynecology. 10.1097/AOG.0b013e3181f60b1d [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Du Fort GG, Meaney ML, Saucier J-F, … King S (2004). Stress During Pregnancy Affects General Intellectual and Language Functioning in Human Toddlers. Pediatric Research, 56(3), 400–410. 10.1203/01.PDR.0000136281.34035.44 [DOI] [PubMed] [Google Scholar]
- Laucht M, Esser G, & Schmidt MH (1997). Developmental outcome of infants born with biological and psychosocial risks. Child Psychology & Psychiatry & Allied Disciplines, 38(7), 843–853. [DOI] [PubMed] [Google Scholar]
- Lederman SA, & Paxton A (1998). Maternal reporting of prepregnancy weight and birth outcome: Consistency and completeness compared with the clinical record. Maternal and Child Health Journal, 2(2), 123–126. 10.1023/a:1022996924094 [DOI] [PubMed] [Google Scholar]
- Leve LD, DeGarmo DS, Bridgett DJ, Neiderhiser JM, Shaw DS, Harold GT, … Reiss D (2012). Using an adoption design to separate genetic, prenatal, and temperament influences on toddler executive function. Developmental Psychology, 49(6), 1045–1057. 10.1037/a0029390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Duvall SW, MacLean PC, Caprihan A, Ohls R, Qualls C, & Phillips J (2011). Comparison of structural magnetic resonance imaging and development in toddlers born very low birth weight and full-term. Journal of Child Neurology, 26(5), 586–592. 10.1177/0883073810388418 [DOI] [PubMed] [Google Scholar]
- Mackinnon DP (2008). Introduction to statistical mediation analysis. Mahwah, NJ: Erlbaum. [Google Scholar]
- Madigan S, Wade M, Plamondon A, Browne D, & Jenkins JM (2014). Birth weight variability and language development: Risk, resilience, and responsive parenting. Journal of Pediatric Psychology, 40(9), 869–877. 10.1093/jpepsy/jsv056 [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger D.a., Blair C, & Cox MJ (2011). Father contributions to cortisol responses in infancy and toddlerhood. Developmental Psychology, 47(2), 388–395. 10.1037/a0021066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce WR, Willoughby MT, Zvara B, Barnett M, Gustafsson H, Cox MJ, … Cox MJ (2015). Mothers’ and fathers’ sensitivity and children’s cognitive development in low-income, rural families. Journal of Applied Developmental Psychology, 38, 1–10. 10.1016/j.appdev.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Georgieff MK, & Osterholm EA (2013). Maternal prenatal distress and poor nutrition - Mutually influencing risk factors affecting infant neurocognitive development. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(2), 115–130. 10.1111/jcpp.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller F, & Tukey JW (1977). Data analysis and regression: A second course in statistics. Reading, MA: Addison-Wesley Publishing Co. [Google Scholar]
- Murray AL, Scratch SFI, Thompson DK, Inder TE, & Doy (2014). Neonatal Brain Pathology Predicts Adverse Attention and Processing Speed Outcomes in Very Preterm and/or Very Low Birth Weight Children. Neuropsychology, 28(4), 552–562. 10.1002/nbm.3066.Non-invasive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus User’s Guide (Eighth Edi). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nelson JM, Choi HJ, Clark CAC, James TD, Fang H, Wiebe SA, & Espy KA (2015). Sociodemographic risk and early environmental factors that contribute to resilience in executive control: A factor mixture model of 3-year-olds. Child Neuropsychology, 21(3), 354–378. 10.1080/09297049.2014.910300 [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Bergman K, Sarkar P, & Glover V (2013). Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiology, 55(2), 145–155. 10.1002/dev.21007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V, & the ALSPAC Study Team. (2003). Maternal antenatal anxiety and behavioural / emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology and Psychiatry, 44(7), 1025–1036. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Lahti J, Lahti M, Edgar RD, Räikkönen K, & O’Connor TG (2017). Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS ONE, 12(6), 1–16. 10.1371/journal.pone.0177506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, & Glover V (2009). Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Developmental Neuroscience, 31(4), 285–292. 10.1159/000216539 [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, & Devlin AM (2008). Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics, 3(2), 97–106. 10.4161/epi.3.2.6034 [DOI] [PubMed] [Google Scholar]
- Phua DYL, Rifkin-Graboi A, Saw SM, Meaney MJ, & Qiu A (2012). Executive functions of six-year-old boys with normal birth weight and gestational age. PLoS ONE, 7(4), 2–6. 10.1371/journal.pone.0036502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, & Belsky J (2011). Prenatal programming of postnatal plasticity? Development and Psychopathology, 23(1), 29–38. 10.1017/S0954579410000623 [DOI] [PubMed] [Google Scholar]
- Reich W, Todd RD, Joyner CA, Neuman RJ, & Heath AC (2003). Reliability and stability of mothers’ reports about their pregnancies with twins. Twin Research, 6(2), 85–88. 10.1375/136905203321536209 [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2011). Modeling a cascade of effects: The role of speed and executive functioning in preterm/full-term differences in academic achievement. Developmental Science, 14(5), 1161–1175. 10.1111/j.1467-7687.2011.01068.x [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2012). Implications of Infant Cognition for Executive Functions at Age 11. Psychological Science, 23(11), 1345–1355. 10.1177/0956797612444902 [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, & Rossem R Van. (2008). A cognitive cascade in infancy: Pathways from prematurity to later mental development. Intelligence, 36(4), 367–378. 10.1016/j.intell.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, & Glynn LM (2011). Prenatal programming of human neurological function. International Journal of Peptides, 2011. 10.1155/2011/837596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite TJ (1999). Maternal Corticotropin- Releasing Hormone and Human Fetus. Developmental Psychobiology, 34(3), 163–173. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Dell’Acqua F, O’Connor TG, Catani M, Deeley Q, … Murphy DGM (2014). Prenatal stress and limbic-prefrontal white matter microstructure in children aged 6–9 years: a preliminary diffusion tensor imaging study. The World Journal of Biological PsychiatryOnline) Journal, 15(4), 346–352. 10.3109/15622975.2014.903336 [DOI] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, & Boyce AWT (2011). Family Socioeconomic Status and Child Executive Functions: The Roles of Language, Home Environment, and Single Parenthood. Journal of the International Neuropsychological Society, 17, 120–132. [DOI] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. 10.1037//1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Horton NJ, Rieder RO, & Tsuang MT (2000). The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal Project. Schizophrenia Bulletin, 26(2), 309–321. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10885633 [DOI] [PubMed] [Google Scholar]
- Silveira PP, Pokhvisneva I, Parent C, Cai S, Rema ASS, Broekman BFP, … Meaney MJ (2017). Cumulative prenatal exposure to adversity reveals associations with a broad range of neurodevelopmental outcomes that are moderated by a novel, biologically informed polygenetic score based on the serotonin transporter solute carrier family C6, member 4 (. Development and Psychopathology, 29(05), 1601–1617. 10.1017/S0954579417001262 [DOI] [PubMed] [Google Scholar]
- Silverstein AB (1975). Validity of WISC-R short forms. Journal of Clinical Psychology, 31(4), 696–697. [Google Scholar]
- Sou SC, Chen WJ, Hsieh WS, & Jeng SF (2006). Severe obstetric complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. Journal of Clinical Epidemiology, 59(4), 429–435. 10.1016/j.jclinepi.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Sprich-Buckminster S, Biederman J, Milberger S, Faraone SV, & Lehman BK (1993, September). Are perinatal complications relevant to the manifestation of ADD? Issues of comorbidity and familiality. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1097/00004583-199309000-00023 [DOI] [PubMed] [Google Scholar]
- Tau GZ, & Peterson BS (2010, January 30). Normal development of brain circuits Neuropsychopharmacology. Nature Publishing Group; 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theresia MH, Lahti M, Drake AJ, Denison FC, Raikkonen K, Norman JE, & Reynolds RM (2017). Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatric Research, 82, 47–54. 10.16192/j.cnki.1003-2053.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Todd RD, Joyner CA, Heath AC, Neuman RJ, & Reich W (2003). Reliability and stability of a semistructured DSM-IV interview designed for family studies. Journal of the American Academy of Child and Adolescent Psychiatry, 42(12), 1460–1468. 10.1097/00004583-200312000-00013 [DOI] [PubMed] [Google Scholar]
- Towe-Goodman NR, Willoughby M, Blair C, Gustafsson HC, Mills-Koonce WR, & Cox MJ (2014). Fathers’ sensitive parenting and the development of early executive functioning. Journal of Family Psychology, 28(6), 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Burg JW, Sen S, Chomitz VR, Seidell JC, Leviton A, & Dammann O (2016). The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatric Research, 79(1), 3–12. 10.1038/pr.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox MJ, & FLP Key Investigators. (2013). The Family Life Project: an epidemiological and developmental study of young children living in poor rural communities. Monographs Of The Society For Research In Child Development, 78(5), 1 10.1111/mono.12046 [DOI] [PubMed] [Google Scholar]
- Wade M, Browne DT, Madigan S, Plamondon A, & Jenkins JM (2014). Normal birth weight variation and children’s neuropsychological functioning: Links between language, executive functioning, and theory of mind. Journal of the International Neuropsychological Society, 20(9), 909–919. 10.1017/S1355617714000745 [DOI] [PubMed] [Google Scholar]
- Wade M, & Jenkins JM (2016). Pregnancy hypertension and the risk for neuropsychological difficulties across early development: A brief report. Child Neuropsychology, 22(2), 247–254. 10.1080/09297049.2014.958070 [DOI] [PubMed] [Google Scholar]
- Wade M, Madigan S, Akbari E, & Jenkins JM (2015). Cumulative biomedical risk and social cognition in the second year of life: Prediction and moderation by responsive parenting. Frontiers in Psychology, 6(APR), 1–11. 10.3389/fpsyg.2015.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD (2005). Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology, 30(8), 724–743. 10.1016/j.psyneuen.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, & Swanson JM (2010). Developmental origins of health and disease:Brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine, 27(5), 358–368. 10.1055/s-0029-1237424.Developmental [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2002). Wechsler Primary and Preschool Scale of Intelligence (3rd editio). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, & Greenberg M (2010). The measurement of executive function at age 3 years: Psychometric properties and criterion validity of a new battery of tasks. Psychological Assessment, 22(2), 306–317. 10.1037/a0018708 [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, & Blair CB (2012). Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment, 24(2), 418–431. 10.1037/a0025779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Clark CAC, Bora S, & Inder TE (2012). Neonatal White Matter Abnormalities an Important Predictor of Neurocognitive Outcome for Very Preterm Children. PLoS ONE, 7(12), e51879 10.1371/journal.pone.0051879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Clark CAC, Pritchard VE, Anderson PJ, & Inder TE (2011). Neonatal White Matter Abnormalities Predict Global Executive Function Impairment in Children Born Very Preterm. Developmental Neuropsychology, 36(1), 22–41. 10.1080/87565641.2011.540530 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Weiland C, Brooks-gunn J, Burchinal MR, Espinosa LM, Gormley WT, Zaslow MJ (2013). Investing in our future: The evidence base on preschool education. Ann Arbor, MI: Society for Research in Child Development. Retrieved from https://www.fcd-us.org/assets/2013/10/Evidence20Base20on20Preschool20Education20FINAL.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.