Abstract
Subjective social status (SSS) reflects one’s perception of one’s standing within society. SSS has been linked with health outcomes, over and above socioeconomic status, and is thought to influence health in part by shaping stress responsivity. To test this, the present study examined the links between SSS and psychological, hypothalamic-pituitary-adrenal (HPA) axis, and cardiovascular responsivity in a sample of 87 ethnically diverse late adolescents (Mage = 18.39 years). Participants rated their family’s SSS while either in high school (n = 50) or one year afterward (n = 37). Participants completed the Trier Social Stress Task (TSST) and reported their fear during baseline and after task completion, provided six saliva samples throughout the task, and had their heart rate monitored continuously throughout the task. Multilevel models, with time points nested within participants, were conducted to assess reactivity and recovery for each outcome. Results indicated that lower SSS was associated with greater fear reactivity and faster rates of HPA axis reactivity and recovery to baseline. Regarding cardiovascular responses, no differences were observed regarding heart rate. Lower SSS predicted increased respiratory sinus arrhythmia during the stress task only among participants who rated their SSS while in high school; no association was observed for those who rated SSS after high school. Results suggest that perceptions of one’s family’s standing in society can shape responses to stress and potentially broader health.
Keywords: subjective social status, cortisol, respiratory sinus arrhythmia, stress, physiology, adolescents
Introduction
Subjective social status (SSS) refers to one’s perception of relative standing within a social context (Adler, Epel, Castellazzo, & Ickovics, 2000). Contrasting single measures of objective socioeconomic status (SES), such as income and education, SSS accounts for people’s feelings about their status in society and thereby incorporates multiple facets of life circumstances (e.g., relative financial security, standard of living; Singh-Manoux, Adler, & Marmot, 2003). As such, SSS tends to be only weakly to moderately correlated with markers of objective SES (e.g., Adler et al., 2000; Demakakos, Nazroo, Breeze, & Marmot, 2008; Elizabeth Goodman, Huang, Schafer-Kalkhoff, & Adler, 2007); Goodman, Huang, Schafer-Kalkhoff, & Adler, 2007), and lower SSS has been linked to poorer mental and self-rated health in adolescents and adults (Cundiff & Matthews, 2017; Quon & McGrath, 2014). Unique effects of SSS on health suggest that, in addition to one’s objective status, one’s perception of their status may negatively impact their health. The chronic toll of low SSS, or feeling of low status relative to others, may promote greater threat sensitivity and ultimately worse health outcomes, regardless of income or education (Brosschot, Verkuil, & Thayer, 2018).
Association of low SSS with poorer health may be partly mediated by alterations in negative affect, hypothalamic-pituitary-adrenal (HPA) axis, and cardiovascular responses to stress. People of lower SSS report more negative affect, with all results maintained over and above SES (Adler et al., 2000; Ghaed & Gallo, 2007; Kraus, Adler, & David Chen, 2013; Operario, Adler, & Williams, 2004). In the context of stress reactivity, people temporarily placed in positions of less social power show greater increases in negative affect following stress (Cundiff, Smith, Baron, & Uchino, 2016; Mendelson, Thurston, & Kubzansky, 2008). Young adults with lower SSS show blunted HPA axis responses to stress, and adults of lower SSS show lower resting heart rate even after controlling for socioeconomic status and lower salivary alpha-amylase activity throughout the day relative to adults with higher SSS (Adler et al., 2000; Gruenewald, Kemeny, & Aziz, 2006; Habersaat, Abdellaoui, Geiger, Urben, & Wolf, 2018; Hellhammer, Buchtal, Gutberlet, & Kirschbaum, 1997). These health indicators have been linked, in turn, with poorer health outcomes (e.g., Burke, Davis, Otte, & Mohr, 2005; Heim, Ehlert, & Hellhammer, 2000; Tang, Rashid, Godley, & Ghali, 2016; Thayer, Yamamoto, & Brosschot, 2010). In many of the above studies, SSS predicted health indicators over and above SES, and meta-analyses suggest that SSS predicts health outcomes even after controlling for SES (Cundiff & Matthews, 2017; Quon & McGrath, 2014). Given this unique effect of SSS on health, it is likely that SSS may uniquely impact the stress response as well. However, although it seems plausible that feeling of lower status can influence health by shaping the stress response, a paucity of research has rigorously assessed this mechanism using an experimental paradigm.
Thus, the current study aimed to examine links between SSS and psychological, HPA axis, and cardiovascular reactivity and recovery. We hypothesized that lower SSS would be linked to greater psychological reactivity, indexed by increased fear; slower rates of HPA axis activity, indexed by less cortisol secretion per minute; and greater cardiovascular responses, indexed by both increased heart rate and reduced respiratory sinus arrhythmia (RSA), a measure of PNS activity. Importantly, we assessed whether effects were maintained over and above SES in order to distinguish between whether the perception of low status has an effect unique from that of having low objective status. We addressed these aims in a sample of late adolescents, as adolescence may be an optimal time to examine these associations. Low SSS has been linked with differences in the stress responses previously in adults rather than adolescents (Akinola & Mendes, 2014). SSS is robustly related to health during adolescence (Quon & McGrath, 2014; Starfield, Riley, Witt, & Robertson, 2002), and heightened social consciousness may amplify the unique effect of low SSS (i.e., viewing oneself as low status relative to others) on health as well as predispose late adolescents to show enhanced physiological and psychological responses to social-evaluative stress (Rankin, Lane, Gibbons, & Gerrard, 2004; Sebastian, Viding, Williams, & Blakemore, 2010; Stroud et al., 2009). Many previous studies also did not control for aspects of objective socioeconomic status when assessing how status shapes the stress response. By controlling for income and education, analyses test not only the effect of SSS but the unique effect of perceiving oneself as low status. Moreover, consistent with previous studies of SES and SSS (Andersson, 2018; Brown, Richardson, Hargrove, & Thomas, 2016; Kahneman & Deaton, 2010), we examined non-linear associations between SSS and responsivity by including quadratic terms of SSS, in order to differentiate whether associations were driven by having a distinctly low SSS or lacking a high SSS. Finally, SSS generally decreases across the transition to adulthood as adolescents gain experience beyond their home community and develop a less optimistic view of their social status (Goodman et al., 2001). Because the transition to college can be especially influential in shaping adolescents’ views of their status (Loeb & Hurd, 2017), we also explored whether associations between SSS and responsivity differed between participants rating SSS while in high school and those rating SSS after graduating.
Methods
Participants
Participants (n = 91) were recruited from an ongoing three-wave longitudinal study examining the transition from adolescence into adulthood. Participants were recruited for the larger study via in-class presentations in four high schools in the Los Angeles area. After completing the second wave of data collection for the larger study, individuals who were at least 18 years of age and self-identified as either Latino or European-American were contacted via phone to participate in an additional experimental task for $150. Participants provided informed consent. Among these participants, 87 reported measures of SSS, income, and parental education and comprised the analytic sample. Of the analytic sample, 50 had recently completed or were currently seniors in high school and 37 had graduated approximately one year prior. Approximately two-thirds (64.4%) were from Latino backgrounds and one-third (35.6%) was from European-American ethnic backgrounds, and slightly over half (57.5%) identified as female. Participants in high school did not differ from those who had already graduated when reporting SSS with respect to ethnicity, gender, income, education, or SSS. Previously-published papers from the experimental sample focused on the role of stress, adiposity, depressive symptoms, and psychological resources in stress reactivity (Chiang et al., 2017, 2019).
Procedures
As part of the second wave of data collection, participants completed questionnaires and caregivers participated in an interview. Late adolescents rated their SSS, and caregivers reported income and highest levels of parental education. All study procedures of this and the larger study were approved by the UCLA Institutional Review Board.
Participants came to UCLA an average of 5 months (± 2.7) after completing the questionnaires to take part in the Trier Social Stress Test (TSST), a well-established social-evaluative stress task (Kirschbaum, Pirke, & Hellhammer, 1993). Participants completed the TSST in the Clinical and Translational Research Center (CTRC) at the University of California, Los Angeles between the hours of 12 pm and 6 pm, with most visits beginning at 12 or 1 pm. Heart rate was measured continuously throughout the session and six samples of salivary cortisol were collected throughout. A nurse assessed vital signs after participants entered, and participants then watched a neutral-content video for 20 minutes to facilitate acclimation to the environment. After this baseline period, participants provided one cortisol sample and completed the fear subscale of the Positive and Negative Affect Schedule-Short Form (PANAS-SF).
Participants then learned that they would be preparing and presenting a speech in front of an evaluative panel on why they were qualified for their ideal job. This marked the beginning of the TSST. Participants had five minutes to prepare for the task and subsequently presented for five minutes to two confederates who were trained to provide nonverbal negative feedback. After finishing the presentation, participants completed a mental arithmetic task. The confederates asked participants to subtract by 13’s from 2935 as quickly as possible. Confederates instructed participants to start from the beginning after each error and to go more quickly after any pauses or after three consecutive correct answers. After five minutes, the participant was asked to stop. The confederates left and the experimenter reentered the room and collected another cortisol sample from the participant. Participants completed the fear-subscale of the PANAS-X again and other psychosocial questionnaires. During this recovery period, participants provided four cortisol samples 15, 30, 45, and 60 minutes after recovery began. The experimenter removed the sensors and fully debriefed the participant.
Measures
Subjective social status
Participants completed the MacArthur Scale of Subjective Social Status–Youth Version (Adler et al., 2000; Goodman et al., 2001) during the second wave of the larger parent study. Participants were presented with a picture of a 10-rung ladder and the following prompt: “Imagine that this ladder pictures how American society is set up. At the top of the ladder are the people who are the best off – those who have the most money, the highest amount of schooling, and the jobs that bring the most respect. At the bottom are the people who are the worst off – they have the least money, little or no education, no job or jobs that no one wants or respects.” Using the ladder, participants then rated their family’s standing relative to the rest of society on a 10-point scale, with the bottom of the ladder (1) representing people who are worst off and the top of the ladder (10) representing people who were best off.
Family income and parental education
As part of the interview during the second wave of data collection, participants’ primary caregivers (94.5% of whom were mothers) reported the family’s total household income from all sources before taxes from all family members who contribute to household expenses. They also reported how far each parent went in school on an 11-point scale (1 = Some elementary school, 11 = Graduated from medical, law, or graduate school). Values were averaged when information was provided on two caregivers.
Fear
Participants completed the fear sub-scale of the PANAS X-Short form, a common measure of situational emotions and emotional reactivity (Watson & Clark, 1994), at the end of the baseline period and after completing the TSST. Items included “afraid”, “scared”, “nervous”, “frightened”, and “shaky” and participants rated each item on a 5-point scale (1 = Not at all, 5 = Extremely), and responses were averaged across items. The scale showed good reliability at baseline and post-task (αs = .83 and .85, respectively).
Cortisol
Salivary cortisol was collected using oral swabs (Salimetrics). Each participant provided six samples. These samples were collected after baseline, immediately after the TSST, and 15, 30, 45, and 60 minutes after recovery began. Samples were stored at −80°C and assayed using high-sensitivity chemiluminescence-immunoassays in the Laboratory of Biological Psychology at the Technical University of Dresden, Germany. Inter- and intra-assay coefficients of variation were below 10%, which is considered good and acceptable (Schultheiss & Stanton, 2009).
Landmark registration was used to identify individuals’ peaks because this method has been shown to be more sensitive than traditional methods (rANOVA, AUC, etc.) in the identification of subtle differences in distinct aspects of the response (i.e., reactivity, recovery) and better accounts for timing effects and individual variability in timing of peaks (Lopez-Duran, Mayer, & Abelson, 2014). Each participant’s individual peak was identified, defined as being the first point at least 10% greater than the baseline cortisol level and followed by a decline. These individual peaks were used to create a new time axis reflecting minutes before and after peak, with all peaks at the 0 point. For participants who did not display a peak that met these criteria, the mode time of peak among responders was used as their peak. A total of 67 participants (77.0% of the sample) had peaks that met criteria; all other participants’ cortisol curves were anchored at the mode time of peak (15 min after TSST completion).
Heart rate and respiratory sinus arrhythmia
Electrocardiogram (ECG) data were collected continuously throughout baseline, the stress task, and the first 10 min of recovery using a physiological recording system (BIOPAC Systems, Inc., Santa Barbara, CA). There were technical issues in recording ECG for one participant, and one participant had a heart rate of 106.40 bpm during baseline which rose to 120.95 bpm during task preparation, values 3.83 and 4.13 standard deviations above the mean, respectively. These two participants were excluded from heart rate and RSA analyses, leaving 85 participants for these analyses. Average heart rate values were calculated in beats per minute (bpm) across each section (i.e., baseline, task preparation, recovery), after sampling using a 500 msec sampling interval. ECG data were converted to inter-beat-intervals and artifacts were edited in CardioEdit by two research assistants certified CardioEdit Reliable (Brain-Body Center, 2007). RSA values were generated from the software CardioBatch based on 30-second epochs using the Porges-Bohrer Method (Brain-Body Center, 2007; Porges, 1985; Porges & Bohrer, 1990). Research assistants separately edited six participants’ data, and all RSA values derived were within 0.02 between research assistants.
Mean values were calculated for the nonverbal parts of the task (baseline, preparation for the task, and recovery). Baseline and recovery were each 10 min, twice as long as the preparation for the stress task. The RSA values from first 5 min and last 5 min were strongly correlated for both baseline and recovery, as were the reactivity and recovery RSA values derived for the first versus last 5 min of each section (rs (83) = .87 - .93, all ps < 0.001). No significant differences were found between the first versus last 5 min of both reactivity or recovery (ts (84) = 0.98 – 1.15, ps = .25 - .33). Therefore, the entire baseline and recovery periods were used for analysis.
Analysis plan
Piecewise multilevel modeling was used to assess fear reactivity and both reactivity and recovery for cortisol, heart rate, and RSA. Using gPower (Faul, Erdfelder, Buchner, & Lang, 2013), we found that we had adequate power to detect associations between SSS and reactivity and recovery changes scores for all analyses in regression (power ranging from .96-.99), including interactions; power should be maintained and potentially increased by using multilevel modeling with properly nested data (Lehman, Taylor, Kiefe, & Seeman, 2005). Time was nested within participants in all analyses. There were only two estimates of fear reactivity, so one time variable was computed for the contrast of baseline (−1) and post-task (1). Because each participant provided six cortisol samples, multilevel models nested time within individuals. Cortisol was not normally distributed, so values were natural log transformed to approximate a more normal distribution. Landmark registration was first used to account for individual variability in individuals’ peal cortisol responses; time variables were centered with 0 corresponding to the peak cortisol response. Two time variables were included as predictors—one to correspond to reactivity and one to correspond to recovery, such that reactivity and recovery could be assessed separately, while simultaneously controlling for the other. The reactivity time variable coded the peak and all times afterward as 0, and the recovery time variable coded the peak and all times before as 0 (Kahle, Miller, Lopez, & Hastings, 2016). Hence, the reactivity time variable included the times corresponding to reactivity and 0 for all other values, and likewise for the recovery time variable. For heart rate and RSA, aggregate values were taken across baseline, prep, and recovery. The time variables were dummy coded with preparation as the comparison group; reactivity time was coded to compare baseline with task preparation (baseline = 1, task preparation = 0, recovery = 0) and recovery time was coded to compare recovery with task preparation (baseline = 0, task preparation = 0, recovery = 1). Again, by including both time variables in the same model, estimates of reactivity and recovery control for the variability in one another. Gender, ethnicity, and high school status were included as level 2 predictors. SSS was included as a level 2 predictor, and its interaction with time was the predictor of interest. To confirm that associations were unique to SSS, controls for income and education, as well as their interactions with time, were included in Model 2 for each outcome.
In Model 1 of all analyses, stress responses were predicted from SSS, over and above demographic controls (i.e., gender, ethnicity, whether the participant was in high school when reporting SSS). Gender and ethnicity were effect coded (Male = −1, Female = 1; European American = −1, Latino =1). For ease of interpretation of interaction terms, whether the participant was in high school when reporting SSS was dummy coded (Graduated from High School = 0, Enrolled in High School = 1). In Model 2, family income and parental education were included to assess whether there were unique associations with SSS over and above SES. All continuous predictors—SSS, family income, and parental education—were grand mean-centered. To assess non-linear associations between SSS and outcome variables, SSS was mean-centered and quadratic terms were included in the model if significant.
These primary analyses were then followed by exploratory analyses of whether the association between SSS and the stress response differed according to high school status by including the interaction between SSS and high school status in Model 1. If an interaction term was significant, we assessed whether the association was maintained after including family income and parental education in Model 2. Interactions were probed by separately estimating the simple slopes of participants who had completed high school and participants who had not yet completed high school at the time that they reported SSS.
Results
Descriptive statistics and correlations
Youth reported levels of SSS that were generally above the midpoint of the scale (M = 7.30, SD = 1.60). Median family income was $79,000 and the average parental education (across both parents, when available) was 7.41 (SD = 2.00), which was around “a 4-year college degree.” Family income and parental education were significantly associated with SSS (rs = 0.41, 0.34, respectively; ps < 0.01). SSS did not differ by gender or ethnicity (ps > .1).
Psychological reactivity
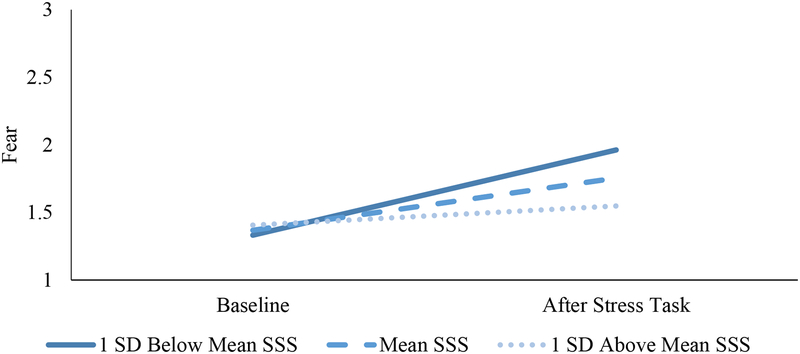
Self-reported fear increased from baseline (M = 1.38, SD = .49) to immediately after the task (M = 1.80, SD = 0.85; t (86) = 4.291, p < .001). As shown in Table 1 and Figure 1, lower SSS was associated with greater fear reactivity (b (SE) = −0.08 (0.03), p = .008), over and above demographic variables and SES. There was no quadratic effect of SSS on fear reactivity (p = .39). Although fear was the primary outcome of interest in this paper, the full negative affect subscale of the PANAS was administered at both time points. SSS was not associated with negative affect reactivity (b = −0.03, se = .02, p = .12)
Table 1.
Fear reactivity as a function of SSS.
| Fear Reactivity Summary | ||||
|---|---|---|---|---|
| Variable | B | SE | B | SE |
| Constant | 1.45*** | 0.11 | 1.47*** | 0.04 |
| SSS | −0.06 | 0.05 | −0.08 | 0.05 |
| Time | 0.20*** | 0.04 | 0.19*** | 0.04 |
| SSS × Time | −0.07** | 0.02 | −0.08** | 0.03 |
| Gender | 0.07 | 0.09 | 0.07 | 0.09 |
| Ethnicity | −0.01 | 0.09 | −0.09 | 0.10 |
| High School | 0.15 | 0.09 | 0.14 | 0.09 |
| Income | 2.24** | 0.86 | ||
| Income × Time | 1.20 | 0.72 | ||
| Education | −0.05 | 0.03 | ||
| Education × Time | −0.04 | 0.02 | ||
Note.
p < .05,
p < .01,
p < .001; SSS=subjective social status; Time was effect-coded (Baseline = −1; Post-task = 1); Gender was effect-coded (Male = −1, Female); Ethnicity was effect-coded (European American = −1, Latino =1); High School was dummy coded and refers to whether participants were not in high school when reporting SSS (High School = 0) or were in high school when reporting SSS (High School = 1); family income was divided by 106.
Figure 1.
Fear Reactivity as a Function of SSS.
Cortisol reactivity and recovery rates
Participants showed an average increase in cortisol concentration from baseline to peak of 9.55 nmol/L and an average decrease in cortisol concentration from peak to the final time point of 11.20 nmol/L. The natural log of cortisol significantly changed across the task (F (5, 81) = 27.49, p < .001), such that each of the six samples significantly differed in concentration from the previous and subsequent samples; ts (86) = 2.371 – 8.601, all ps < .05. The natural log of the concentration of cortisol in the first and last samples did not differ from one another (t (86) = 0.79, p = .43), suggesting that participants’ cortisol values generally returned close to their initial level by the end of the recovery period. Time of visit was not related to baseline cortisol; (r [89] = −.19, p = .071).
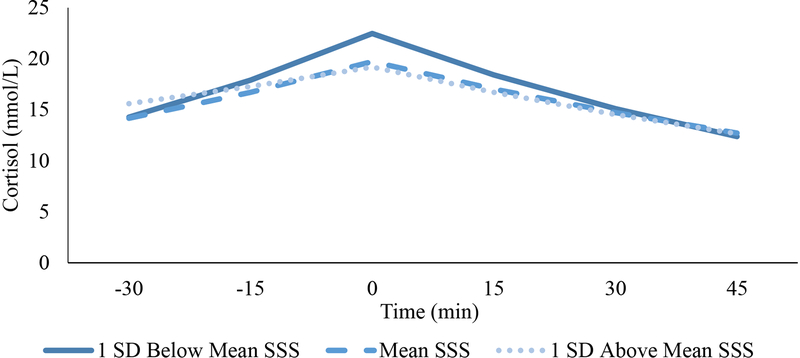
As shown in Table 2 and Figure 2, lower SSS was associated with greater cortisol reactivity rates (b (SE) = −0.003 (0.01), p = .027). There were no quadratic relationships between SSS and cortisol reactivity rate (p = .2). In contrast, although there was no significant linear relation between SSS and cortisol recovery rate, there was a significant quadratic relationship between SSS and cortisol recovery rate (b (SE) = −0.001 (0.003), p = .009; Table 2; Fig. 2), such that low SSS was associated with higher (i.e., faster) rates of cortisol recovery relative to mean or high SSS, over and above demographic variables and objective SES. After accounting for both the linear and quadratic effects of SSS on cortisol recovery, SSS was associated with quicker rates of cortisol recovery (−0.013 ln(nMol/L)/min) at the lower end of SSS (i.e., one SD below the mean) compared to at the mean and at the higher end of SSS (−0.009 ln(nMol/L)/min and −0.22 nMol/L/min, respectively). Both the linear association between low SSS and faster cortisol reactivity (b (SE) = −0.003 (0.001), p = .029) and the quadratic association between low SSS and faster cortisol recovery (b (SE) = −0.0008 (0.0003), p = .005) remained significant over and above baseline cortisol.
Table 2.
Cortisol reactivity and recovery slopes as a function of SSS.
| Cortisol Responsivity Summary | ||||
|---|---|---|---|---|
| Variable | B | SE | B | SE |
| Constant | 2.50*** | 0.08 | 2.48*** | 0.08 |
| SSS | −0.10* | 0.05 | −0.10 | 0.05 |
| SSS2 | 0.01 | 0.01 | 0.01 | 0.01 |
| Reactivity Time | 0.01*** | 0.002 | 0.01*** | 0.002 |
| SSS × Reactivity Time | −0.003** | 0.001 | 0.003* | 0.001 |
| Recovery Time | −0.01*** | 0.001 | −0.01*** | 0.001 |
| SSS × Recovery Time | 0.001 | 0.001 | 0.001 | 0.001 |
| SSS2 × Recovery Time | −0.001** | 0.0003 | −0.001** | 0.0002 |
| Gender | −0.04 | 0.03 | −0.04 | 0.03 |
| Ethnicity | 0.08 | 0.03 | 0.09** | 0.03 |
| High School | 0.003 | 0.06 | 0.01 | 0.06 |
| Income | 0.00 | 0.00 | ||
| Income × Reactivity Time | 0.00 | 0.00 | ||
| Income × Recovery Time | 0.00 | 0.00 | ||
| Education | −0.04 | 0.04 | ||
| Education × Reactivity Time | −0.002 | 0.001 | ||
| Education × Recovery Time | 0.00 | 0.001 | ||
Note.
p < .05,
p < .01,
p < .001; SSS=subjective social status; Reactivity Time was coded as 0 for all values after the time of peak cortisol level; Recovery Time was coded as 0 for all values after the time of peak cortisol level; Gender was effect-coded (Male = −1, Female); Ethnicity was effect-coded (European American = −1, Latino =1); High School was dummy coded and refers to whether participants were not in high school when reporting SSS (High School = 0) or were in high school when reporting SSS (High School = 1); family income was divided by 106.
Figure 2.
HPA Axis Reactivity Rates and Recovery Rates as a Function of SSS Using Landmark Registration.
Heart rate reactivity and recovery
Heart rate significantly increased from 68.51 bpm at baseline to 73.87 bpm during preparation and remained elevated at 73.90 bpm during recovery; F (2, 82) = 55.621, p < .001. SSS was not related to heart rate reactivity (b (SE) = −0.78 (0.71), p = .27) or recovery (b (SE) = 0.88 (0.70), p = .21). Results remained non-significant after accounting for income and parental education for both reactivity (p = .64) and recovery (p = .99). There was also not a quadratic effect for reactivity (p = .25) or recovery (p = .46).
Respiratory sinus arrhythmia reactivity and recovery
Participants’ mean RSA for baseline, preparation for the stress task, and task recovery were 7.14, 7.12, and 7.19, such that on average participants did not show a strong parasympathetic response to the task (F (2, 82) = 0.28, p = .74). SSS was not associated with reactivity (b (SE) = −0.06 (0.05), p = .30) but lower SSS was associated with greater recovery over and above demographic factors (b (SE) = 0.12 (0.06), p = .029). Quadratic associations between SSS and reactivity (p = .87) and recovery (p = .31) were non-significant.
Interactions with high school status
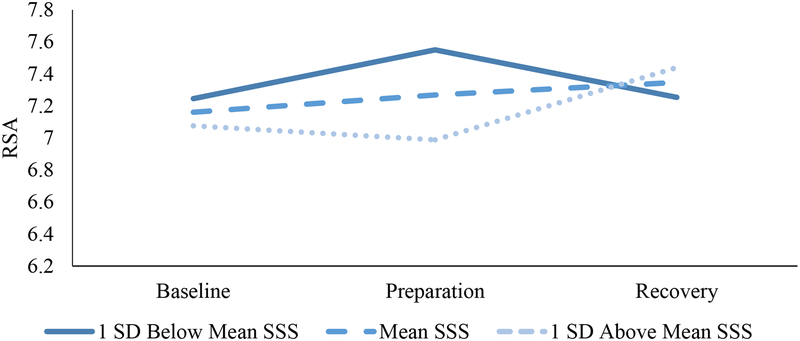
Given potential developmental changes in the significance of SSS, we explored interaction effects between high school status and SSS. No interactions emerged for fear, cortisol, or heart rate responsivity (ps = .28 - .73). However, as shown in Table 3 and Figure 3, an interaction emerged for RSA reactivity (b (SE) = −0.20 (0.10), p = .036) and a significant interaction emerged for RSA recovery (b (SE) = 0.29 (0.10), p = .008) after accounting for SES. Simple slopes analyses indicated that SSS predicted RSA reactivity (b (SE) = −0.18 (0.08), p = .023) and recovery (b (SE) = 0.26 (0.11), p = .015) among youth reporting SSS while in high school, over and above income and parental education. By contrast, SSS was not linked with cardiovascular changes in RSA reactivity (b (SE) = 0.03 (0.08), p = .75) or recovery (b (SE) = 0.0005 (0.06), p = .99) among those who had already graduated high school when SSS was assessed. Despite variability in time between the report of SSS and completion of the TSST, all results were maintained after controlling for the number of months elapsed, and observed associations did not vary by the number of months that had elapsed (ps > .1).
Table 3.
RSA reactivity and recovery as a function of SSS.
| RSA Responsivity Summary | ||||
|---|---|---|---|---|
| Variable | B | SE | B | SE |
| Constant | 7.09*** | 0.17 | 7.07*** | 0.16 |
| SSS | 0.01 | 0.12 | −0.01 | 0.13 |
| Reactivity Time | 0.20 | 0.11 | −0.19 | 0.11 |
| SSS × Reactivity Time | −0.05 | 0.07 | 0.04 | 0.08 |
| Recovery Time | 0.04 | 0.11 | 0.05 | 0.11 |
| SSS × Recovery Time | −0.01 | 0.07 | −0.02 | 0.08 |
| High School | 0.12 | 0.21 | 0.12 | 0.21 |
| SSS × High School | −0.16 | 0.17 | −0.14 | 0.16 |
| Reactivity Time × High School | −0.31* | 0.14 | 0.30* | 0.14 |
| Recovery Time × High School | −0.01 | 0.07 | 0.04 | 0.14 |
| SSS × Reactivity Time × High School | −0.20* | 0.10 | −0.20* | 0.10 |
| SSS × Recovery Time × High School | 0.28** | 0.10 | 0.29** | 0.10 |
| Gender | −0.06 | 0.09 | −0.07 | 0.09 |
| Ethnicity | −0.03 | 0.10 | −0.10 | 0.11 |
| Income | 3.52 | 1.91 | ||
| Income × Reactivity Time | 0.52 | 1.29 | ||
| Income × Recovery Time | 2.72 | 1.31 | ||
| Education | −0.04 | 0.06 | ||
| Education × Reactivity Time | 0.00 | 0.04 | ||
| Education × Recovery Time | 0.01 | 0.04 | ||
Note.
p < .05,
p < .01,
p < .001; SSS=subjective social status; Reactivity Time was coded as 0 for all values after the time of peak cortisol level; Recovery Time was coded as 0 for all values after the time of peak cortisol level; Gender was effect-coded (Male = −1, Female); Ethnicity was effect-coded (European American = −1, Latino =1); High School was dummy coded and refers to whether participants were not in high school when reporting SSS (High School = 0) or were in high school when reporting SSS (High School = 1); family income was divided by 106.
Figure 3.
RSA Reactivity and Recovery as a Function of SSS among Participants Reporting SSS while in High School.
Discussion
Low SSS has been robustly linked with poorer health outcomes, but the mechanisms by which SSS shapes health remain unclear. This study aimed to assess whether SSS could potentially shape health by influencing psychological and physiological responses to stress. Specifically, the study assessed the association of SSS with psychological, HPA axis, and cardiovascular responses to stress in a sample of late adolescents and whether these effects were maintained over and above income and parental education. By controlling for income and parental education, two major facets of socioeconomic status, we were able to assess the unique effect of SSS on these stress response systems. Our findings suggest that SSS predicts differences in psychological, HPA axis, and PNS responses, even after controlling for income and education. Lower SSS was associated with greater fear reactivity as well as faster HPA axis reactivity and recovery, over and above family income and parental education. Finally, low SSS was associated with reduced RSA reactivity and recovery (i.e., vagal augmentation while preparing for the task and vagal withdrawal following the task) among participants who evaluated their SSS while in high school. Taken together, the perception of being of low status appears to be linked with differences in the stress response across all systems and may thereby uniquely shape health outcomes among low-status adolescents.
SSS was related to fear reactivity, such that lower SSS was associated with greater increases in fear following the TSST. People of lower SSS may appraise ambiguous cues as more threatening, similar to people of low SES (Chen, Langer, Raphaelson, & Matthews, 2004). This may be because low SSS may signify more negative life events in general or that being lower in the social hierarchy is an insecure position that necessitates greater sensitivity to others and to potential threats. Greater sensitivity to threat can result in greater responses of negative affect, and previous work has suggested that lower SSS results in poorer mental health in part through chronic negative affect (Kraus, Tan, & Tannenbaum, 2013). Our results suggest this dynamic exists during late adolescence, potentially setting the stage for longer-term mental health problems during adulthood.
With respect to the HPA axis, low SSS was associated with faster reactivity and recovery. This finding suggests that adolescents of lower status show more dynamic cortisol levels, in that they are mounting a greater cortisol response but also able to recover to levels comparable to baseline. In general, rapid HPA axis reactivity is maladaptive because of the greater exposure to cortisol throughout the body, whereas recovery is considered beneficial and more commonly seen in younger populations (Miller, Chen, & Zhou, 2007; Seeman & Robbins, 1994). If this recovery becomes dysregulated later in development, adolescents of low SSS may be poised for poorer health outcomes from greater cortisol secretion. This finding differs from recent work suggesting lower SES relates to slower rates of HPA axis recovery in adults (Lê-Scherban et al., 2018). SSS may be associated with faster HPA axis reactivity and recovery over and above SES because it is especially relevant to developmental changes during adolescence. Further work will be needed in adults to identify whether low SSS is linked with blunted responses in adults, similar to how lower SES is associated with blunted responses in adults. For instance, the more dynamic response observed among low SSS adolescents may become further dysregulated and correspond to blunting later in development.
In terms of cardiovascular responsivity, SSS was not related to heart rate. This finding is at odds with previous literature; lower SSS has been linked with higher resting heart rate and greater risk and prevalence of cardiovascular disease among adults (Adler et al., 2000; Tang et al., 2016). Associations between SSS and heart rate responses may emerge during adulthood rather than during adolescence, or SSS may correspond to poorer cardiovascular health through another mechanism. For instance, lower SSS has been linked with poorer health behaviors, including substance use (Finkelstein, Kubzansky, & Goodman, 2006; Reitzel, Nguyen, Strong, Wetter, & McNeill, 2013; Russell & Odgers, 2019).
Lower SSS was associated with cardiovascular recovery with respect to RSA. Specifically, people of lower SSS had smaller increases in RSA following the task, suggesting greater recovery of the PNS specifically, although this effect was not unique from that of SES. Greater PNS recovery following stress is associated with better health (Fuller-Rowell et al., 2013), so these results may suggest that PNS recovery specifically may be a mechanism by which lower SSS is associated with poorer physiological health. This effect was primarily driven by participants who evaluated their SSS while in high school, as indicated by the significant interactions between high school status and SSS in predicting PNS reactivity and recovery. A significant association of SSS with PNS reactivity emerged for those who reported SSS in high school when probing this interaction. SSS was unrelated to changes in PNS activity among participants who reported SSS after graduating from high school. However, for adolescents who reported SSS in high school, lower SSS was associated with increases in RSA from baseline to task preparation and decreases in RSA during recovery, whereas higher SSS was linked with decreases in RSA during task preparation and increases in RSA during recovery. Among non-clinical populations, the response observed among adolescents with higher SSS has been linked with emotion regulation whereas that observed among adolescents with lower SSS has been linked with poorer mental health and emotion regulation (Graziano & Derefinko, 2013). This finding aligns with past work suggesting that SSS is more related to mental than physical health (Quon & McGrath, 2014).
Participants of low SSS may have shown such a pattern because they were less invested during the task or unable to effectively cope. These results correspond well to the fear reactivity results, as youth of lower SSS also reported greater fear immediately after completing the task. They may be unable to regulate their fear, which is reflected in their physiology, as lower SSS has been linked with maladaptive coping previously (Jackson, Richman, LaBelle, Lempereur, & Twenge, 2015; Schubert, Süssenbach, Schäfer, & Euteneuer, 2016). They may have feared the evaluation of their performance and consequently showed a sustained reduction in RSA during recovery rather than during the preparation for the task itself. Reduced PNS activity can result in difficulty attending to and maintaining normative function, as well as poorer health if sustained over time (Porges, 1995).
It is possible that SSS predicted PNS responses only among the younger cohort because SSS changes with the transition from high school. Studies suggest SSS generally decreases with age (Goodman et al., 2007). Additionally, the predictive importance of SSS for psychological and physiological responses to threat may vary with time. The transition out of high school may be especially influential for SSS as adolescents generally experience broader contexts (i.e., college, work environment) which enable them to better evaluate their status within society. By developing a broader understanding of their family’s status, their SSS could change, and this difference may explain the interaction observed between society SSS and cohort. Future studies should assess the trajectory of SSS and changes in its relation to health outcomes.
Interestingly, lower SSS is linked with faster HPA axis rates of reactivity and recovery, which could potentially be positive for health, and responses of fear and PNS activity linked with poorer health. These differences across systems may be due to the temporal differences in measuring HPA axis and PNS responses. Changes in PNS activity are apparent on the scale of seconds to minutes, whereas, changes in HPA axis activity have a 20–30 minute lag before being apparent in salivary cortisol. Adolescent of low SSS may fully recover—faster than adolescents of mean or high SSS—by this later timepoint.
Taken together with the fear and parasympathetic findings, it seems that adolescents of low SSS are reacting and responding to the stress differently from those of moderate or high SSS. Lower SSS has been linked with differences in coping with stress (i.e., more depressive thinking, rumination), which may influence psychological and physiological responses to stress (Jackson et al., 2015; Schubert et al., 2016). Adolescents of lower SSS may show greater increases in fear because these youth are either more sensitive to threat or are having more difficulty regulating their affective responses to the task. Differences in autonomic activity have been thought to index coping. Greater vagal withdrawal is often related to engagement with the stressor (e.g., Porges, 2007). It is possible that adolescent of low SSS, and not those of mean or high SSS, engage in a coping mechanism that is especially beneficial for HPA axis recovery but not PNS or fear reactivity. Indeed, discordance between physiological systems has been previously documented (e.g., Gordis, Granger, Susman, & Trickett, 2006; Laurent, Lucas, Pierce, Goetz, & Granger, 2016). Future work can interrogate whether SSS influences adolescents’ coping strategies and whether such strategies contribute to discordance across psychological and physiological responses to stress.
Because of the nature of this study, causal relations cannot be inferred from these data. Although we find it unlikely that participants rate their SSS based on their stress responsivity (and measures of SSS were taken prior to the stress session), further studies manipulating SSS will be needed to assess whether such changes can induce temporary changes in physiological responsivity. Given that such a manipulation induced changes in RSA in a previous study (Pieritz, Süssenbach, Rief, & Euteneuer, 2016), these effects may carry over to responsivity. More rigorous measures of SNS activity such as pre-ejection period can be used to more thoroughly assess relations between SSS and SNS activity. It should also be noted that participants’ SSS was reported 4–10 months prior to experience of the stressor. Again, although there is evidence that SSS is largely stable (Goodman et al., 2007), the transition from high school is a major, stressful turning point that can likely impact SSS. Although SSS undergoes normative changes, participants’ rating of SSS while in high school appear more predictive than their ratings one year afterward.
Conclusions
These findings suggest that people of low SSS have differences in their psychological and physiological stress responses. Differences in stress responses can contribute to poorer health outcomes and may be one mechanism by which lower SSS relates to consistently poorer health and well-being, and SSS may influence physiology distinctly from SES. Increased fear reactivity suggests that adolescents of lower SSS may feel overwhelmed facing a challenging or novel task, and differences in stress physiology may reflect differences in emotion regulation which can have consequences for health. Although SSS did not relate to heart rate, there were differences in HPA axis responses and cardiovascular responses with respect to RSA. These findings suggest that adolescents interpret stressful situations differently based on their SSS.
Adolescents who feel undervalued in society or their more proximal community are especially likely to have low SSS and be preoccupied with stressors. These stressors can activate the psychological and physiological stress response, thereby exacerbating differences between adolescents of low and high SSS. Repeated activation of the stress response can worsen health by redirecting physiological and psychological resources from daily processes, such as academic learning (Levy, Heissel, Richeson, & Adam, 2016). In light of these findings, it is especially important to consider adolescents’ perceived SSS rather than solely their objective standing and resources in order for youth to succeed.
Highlights.
Subjective social status (SSS) was linked with differences in stress responsivity. Specifically, lower SSS was associated with greater increases in fear following an acute stressor and faster rates of cortisol reactivity and recovery. Adolescents with lower SSS in high school showed less cardiovascular reactivity and recovery with respect to respiratory sinus arrhythmia, a marker of parasympathetic nervous system activity.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under Grant R01-HD062547, UCLA California Center for Population Research funded by the National Institute of Child Health and Human Development under Grant R24-HD041022, UCLA Older Americans Independence Center funded by the National Institute of Aging under Grant P30-AG028748, UCLA Cousins Center for Psychoneuroimmunology, University of California Institute for Mexico and the US, American Psychological Association, and Division 38 of the American Psychological Association.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
- Adler NE, Epel ES, Castellazzo G, & Ickovics JR (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women. Health Psychology, 19(6), 586–592. 10.1037//0278-6133.19.6.586 [DOI] [PubMed] [Google Scholar]
- Akinola M, & Mendes WB (2014). It’s good to be the king: Neurobiological benefits of higher social standing. Social Psychological and Personality Science. doi: 10.1107/s0108270113015370/sk3488sup1.cif [DOI] [Google Scholar]
- Andersson MA (2018). An odd ladder to climb: Socioeconomic differences across levels of subjective social status. Social Indicators Research, 136(2), 621–643. 10.1007/s11205-017-1559-7 [DOI] [Google Scholar]
- Brain-Body Center (2007). CardioEdit/CardioBatch [computer software]. Chicago: University of Illinois. [Google Scholar]
- Brosschot JF, Verkuil B, & Thayer JF (2018). Generalized unsafety theory of stress: Unsafe environments and conditions, and the default stress response. International Journal of Environmental Research and Public Health, 15(3), 464 10.3390/ijerph15030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Richardson LJ, Hargrove TW, & Thomas CS (2016). Using multiple-hierarchy stratification and life course approaches to understand health inequalities: The intersecting consequences of race, gender, SES, and age. Journal of Health and Social Behavior, 57(2), 200–222. 10.1177/0022146516645165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- CardioBatch software. Brain-Body Center, University of Illinois at Chicago; 2007. [Google Scholar]
- CardioEdit software. Brain-Body Center, University of Illinois at Chicago; 2007. [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, & Matthews KA (2004). Socioeconomic status and health in adolescents: The role of stress interpretations. Child Development, 75(4), 1039–1052. 10.1111/j.1467-8624.2004.00724.x [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Ko A, Bower JE, Taylor SE, Irwin MR & Fuligni AJ (2019). Stress, psychological resources, and HPA and inflammatory reactivity during late adolescence. Development and Psychopathology, 31, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, & Fuligni AJ (2017). Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain Behavior, and Immunity, 66, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff JM, & Matthews KA (2017). Is subjective social status a unique correlate of physical health? A meta-analysis. Health Psychology, 36(12), 1109–1125. 10.1037/hea0000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundiff JM, Smith TW, Baron CE, & Uchino BN (2016). Hierarchy and health: Physiological effects of interpersonal experiences associated with socioeconomic position. Health Psychology, 35(4), 356–365. 10.1037/hea0000227 [DOI] [PubMed] [Google Scholar]
- Demakakos P, Nazroo J, Breeze E, & Marmot M (2008). Socioeconomic status and health: The role of subjective social status. Social Science & Medicine (1982), 67(2), 330–340. 10.1016/j.socscimed.2008.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2013). G*Power (Version 3.1.2) [Computer software]. Kiel, Germany: Universität Kiel: Retrieved from http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3/download-and-register [Google Scholar]
- Finkelstein DM, Kubzansky LD, & Goodman E (2006). Social status, stress, and adolescent smoking. Journal of Adolescent Health, 39(5), 678–685. 10.1016/j.jadohealth.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Williams DR, Love GD, McKinley PS, Sloan RP, & Ryff CD (2013). Race differences in age-trends of autonomic nervous system functioning. Journal of Aging and Health, 25(5), 839–862. 10.1177/0898264313491427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaed SG, & Gallo LC (2007). Subjective social status, objective socioeconomic status, and cardiovascular risk in women. Health Psychology, 26(6), 668–674. 10.1037/0278-6133.26.6.668 [DOI] [PubMed] [Google Scholar]
- Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, & Colditz GA (2001). Adolescents’ perceptions of social status: Development and evaluation of a new indicator. Pediatrics, 108(2), e31–e31. 10.1542/peds.108.2.e31 [DOI] [PubMed] [Google Scholar]
- Goodman E, Huang B, Schafer-Kalkhoff T, & Adler NE (2007). Perceived socioeconomic status: A new type of identity which influences adolescents’ self rated health. Journal of Adolescent Health, 41(5), 479–487. 10.1016/j.jadohealth.2007.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis E, Granger D, Susman E, & Trickett P (2006). Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31(8), 976–987. 10.1016/j.psyneuen.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. 10.1016/j.biopsycho.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, & Aziz N (2006). Subjective social status moderates cortisol responses to social threat. Brain, Behavior, and Immunity, 20(4), 410–419. 10.1016/j.bbi.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Habersaat S, Abdellaoui S, Geiger AM, Urben S, & Wolf JM (2018). Low subjective social status in the police is linked to health-relevant changes in diurnal salivary alpha-amylase activity in Swiss police officers. Stress, 21(1), 11–18. 10.1080/10253890.2017.1389882 [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, & Hellhammer DH (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25(1), 1–35. 10.1016/S0306-4530(99)00035-9 [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Buchtal J, Gutberlet I, & Kirschbaum C (1997). Social hierarchy and adrenocortical stress reactivity in men. Psychoneuroendocrinology, 22(8), 643–650. 10.1016/S0306-4530(97)00063-2 [DOI] [PubMed] [Google Scholar]
- Jackson B, Richman LS, LaBelle O, Lempereur MS, & Twenge JM (2015). Experimental evidence that low social status is most toxic to well-being when internalized. Self & Identity, 14(2), 157–172. 10.1080/15298868.2014.965732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle S, Miller JG, Lopez M, & Hastings PD (2016). Sympathetic recovery from anger is associated with emotion regulation. Journal of Experimental Child Psychology, 142, 359–371. 10.1016/j.jecp.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Deaton A (2010). High income improves evaluation of life but not emotional well-being. Proceedings of the National Academy of Sciences, 107(38), 16489–16493. 10.1073/pnas.1011492107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘Trier Social Stress Test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kraus MW, Adler N, & Chen TWD (2013). Is the association of subjective SES and self-rated health confounded by negative mood? An experimental approach. Health Psychology, 32(2), 138–145. 10.1037/a0027343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MW, Tan JJX, & Tannenbaum MB (2013). The social ladder: A rank-based perspective on social class. Psychological Inquiry, 24(2), 81–96. 10.1080/1047840X.2013.778803 [DOI] [Google Scholar]
- Laurent HK, Lucas T, Pierce J, Goetz S, & Granger DA (2016). Coordination of cortisol response to social evaluative threat with autonomic and inflammatory responses is moderated by stress appraisals and affect. Biological Psychology, 118, 17–24. 10.1016/j.biopsycho.2016.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, & Seeman TE (2005). Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine, 67(6), 846–854. 10.1097/01.psy.0000188443.48405.eb [DOI] [PubMed] [Google Scholar]
- Lê-Scherban F, Brenner AB, Hicken MT, Needham BL, Seeman T, Sloan RP, … Diez Roux AV (2018). Child and adult socioeconomic status and the cortisol response to acute stress: Evidence from the multi-ethnic study of atherosclerosis. Psychosomatic Medicine, 80(2), 184 10.1097/PSY.0000000000000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ, Heissel JA, Richeson JA, & Adam EK (2016). Psychological and biological responses to race-based social stress as pathways to disparities in educational outcomes. The American Psychologist, 71(6), 455–473. 10.1037/a0040322 [DOI] [PubMed] [Google Scholar]
- Loeb E, & Hurd NM (2017). Subjective social status, perceived academic competence, and academic achievement among underrepresented students. Journal of College Student Retention: Research, Theory & Practice, 1521025117696821. 10.1177/1521025117696821 [DOI] [Google Scholar]
- Lopez-Duran NL, Mayer SE, & Abelson JL (2014). Modeling neuroendocrine stress reactivity in salivary cortisol: Adjusting for peak latency variability. Stress, 17(4), 285–295. 10.3109/10253890.2014.915517 [DOI] [PubMed] [Google Scholar]
- Mendelson T, Thurston RC, & Kubzansky LD (2008). Affective and cardiovascular effects of experimentally-induced social status. Health Psychology, 27(4), 482–489. 10.1037/0278-6133.27.4.482 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Operario D, Adler NE, & Williams DR (2004). Subjective social status: Reliability and predictive utility for global health. Psychology & Health, 19(2), 237–246. 10.1080/08870440310001638098 [DOI] [Google Scholar]
- Pieritz K, Süssenbach P, Rief W, & Euteneuer F (2016). Subjective social status and cardiovascular reactivity: An experimental examination. Frontiers in Psychology, 7, 1091 10.3389/fpsyg.2016.01091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1985). U.S. Patent No. 4,510,944 Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Porges SW (1995). Cardiac vagal tone: A physiological index of stress. Neuroscience & Biobehavioral Reviews, 19(2), 225–233. 10.1016/0149-7634(94)00066-A [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, & Bohrer RE (1990). The analysis of periodic processes in psychophysiological research In Principles of psychophysiology: Physical, social, and inferential elements (pp. 708–753). New York, NY, US: Cambridge University Press. [Google Scholar]
- Quon EC, & McGrath JJ (2014). Subjective socioeconomic status and adolescent health: A meta-analysis. Health Psychology, 33(5), 433–447. 10.1037/a0033716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin JL, Lane DJ, Gibbons FX, & Gerrard M (2004). Adolescent self-consciousness: Longitudinal age changes and gender differences in two cohorts. Journal of Research on Adolescence, 14(1), 1–21. 10.1111/j.1532-7795.2004.01401001.x [DOI] [Google Scholar]
- Reitzel LR, Nguyen N, Strong LL, Wetter DW, & McNeill LH (2013). Subjective social status and health behaviors among African Americans. American Journal of Health Behavior, 37(1), 104–111. 10.5993/AJHB.37.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, & Odgers CL (2019). Adolescents’ subjective social status predicts day-to-day mental health and future substance use. Journal of Research on Adolescence. 10.1111/jora.12496 [DOI] [PubMed] [Google Scholar]
- Schubert T, Süssenbach P, Schäfer SJ, & Euteneuer F (2016). The effect of subjective social status on depressive thinking: An experimental examination. Psychiatry Research, 241, 22–25. 10.1016/j.psychres.2016.04.081 [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, & Stanton SJ (2009). Assessment of salivary hormones. Methods in Social Neuroscience, 17–44. [Google Scholar]
- Sebastian C, Viding E, Williams KD, & Blakemore S-J (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition, 72(1), 134–145. 10.1016/j.bandc.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Seeman TE, & Robbins RJ (1994). Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocrine Reviews, 15(2), 233–260. 10.1210/edrv-15-2-233 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Adler NE, & Marmot MG (2003). Subjective social status: Its determinants and its association with measures of ill-health in the Whitehall II study. Social Science & Medicine, 56(6), 1321–1333. 10.1016/S0277-9536(02)00131-4 [DOI] [PubMed] [Google Scholar]
- Starfield B, Riley AW, Witt WP, & Robertson J (2002). Social class gradients in health during adolescence. Journal of Epidemiology & Community Health, 56(5), 354–361. 10.1136/jech.56.5.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21(01), 47 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KL, Rashid R, Godley J, & Ghali WA (2016). Association between subjective social status and cardiovascular disease and cardiovascular risk factors: A systematic review and meta-analysis. BMJ Open, 6(3), e010137 10.1136/bmjopen-2015-010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, & Brosschot JF (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (n.d.). The PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded Form. 28. [Google Scholar]