Abstract
Farnesoid X receptor (FXR) induces fibroblast growth factor 15 (FGF15, human ortholog FGF19) in the gut to potently inhibit bile acid (BA) synthesis in the liver. FXR activation in hepatic stellate cells (HSCs) reduces liver fibrosis. Fgf15−/− mice develop attenuated liver fibrosis but the underlying mechanisms for this protection are unclear. We hypothesized that FGF15/19 functions as a profibrotic mediator or mitogen to HSCs and increased BAs in Fgf15−/− mice leads to enhanced FXR activation in HSCs, subsequently reducing fibrogenesis. In this study, complimentary in-vivo and in-vitro approaches were used: 1) carbon tetrachloride (CCl4)-induced liver fibrosis model in wild type (WT), Fgf15−/−, and Fgf15 transgenic (TG) mice with BAs levels modulated by feeding cholestyramine- or cholic acid-containing diets, 2) analysis of primary HSCs isolated from WT and Fgf15−/− mice, and 3) treatment of a human HSC line, LX-2, with FXR activators and/or recombinant FGF19 protein. The results showed that Fgf15−/− mice had lower basal collagen expression, which was increased by BA sequestration. CCl4-induced fibrosis with similar severity in all genotypes, however, cholestyramine increased fibrosis severity only in Fgf15−/− mice. HSCs from Fgf15−/− mice showed increased FXR activity and reduced expression of profibrotic mediators. In LX-2 cells, FXR activation increased PPARγ activity and reduced proliferation. FGF19 activated both STAT3 and JNK pathways, and reduced NFκB signaling without increasing fibrogenic gene expression or cell proliferation. Conclusion: FGF15/19 does not act as a direct profibrotic mediator or mitogen to HSCs in our models, and the protection against fibrosis by FGF15 deficiency may be mediated through increased BA activation of FXR in HSCs.
Keywords: Hepatic fibrosis, Hepatic stellate cells, Farnesoid X receptor, bile acids, FGF15, FGF19
Hepatic fibrosis is the result of chronic injuries manifested as the accumulation of extracellular matrix primarily produced by the hepatic stellate cells (HSCs). Chronic injuries, caused by viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, or obstructive biliary diseases, trigger a persistent activation of HSCs leading to constant production of extracellular matrix and simultaneous inhibition of the matrix-degrading enzymes.1 Advanced liver fibrosis can develop to cirrhosis, liver failure and/or hepatocellular carcinoma. Despite the dire consequences of hepatic fibrosis, treatment options are limited as there are no therapeutic agents approved to prevent or reverse the disease. In the clinic, removing the causative factor(s) and supportive treatment are the first line of therapy, and when failed, the only option left is liver transplant. Therefore, identifying mechanisms of hepatic fibrogenesis and developing therapeutic agents to treat hepatic fibrosis are medically urgent.
Fibroblast growth factor 15 (FGF15) is the mouse ortholog of human FGF19, however, only shares 50% amino acid sequence homology.2, 3 FGF15 and FGF19 are members of the endocrine FGFs as they do not bind extracellular heparin sulfate, which allows them to escape the extracellular matrix and thus circulate systemically as endocrine hormones.4 In the intestine, Fgf15/FGF19 are highly induced by the bile acid (BA)-activated nuclear receptor farnesoid X receptor (FXR) and travel through portal circulation to the liver where it activates its predominate, obligate co-receptor FGFR4 /βKLOTHO (βKL) on hepatocytes. This suppresses BA synthesis through inhibition of classical BA synthesis genes, including cholesterol 7-α-hydroxylase (Cyp7a1/CYP7A1) and sterol 12-α-hydroxylase (Cyp8b1/ CYP8B1).5–8 Therefore, FGF15/19 signaling serves as a major negative feedback loop for BA synthesis. It is important to note that FGF15 and FGF19 can also activate the co-receptor FGFR1c/βKL.9 Though HSCs express lower levels of FGFR4 than hepatocytes, they express greater levels of FGFR1. Therefore, it is possible that FGF15 and FGF19 activate FGFRs on HSCs.10, 11 HSCs also express FXR with a level much lower than that in hepatocytes.12, 13 Activation of FXR in HSCs has been shown to reduce hepatic fibrogenesis by mitigating collagen expression, increasing matrix metalloprotease activity, inhibiting transforming growth factor beta (TGFβ) signaling, sensitizing HSCs to apoptotic signals, and reducing HSC contractility.13–20
FGF15 deficiency has been shown to reduce the development of hepatic fibrosis in both a high-fat-diet (HFD)-induced non-alcoholic steatohepatitis (NASH) model and a CCl4-induced fibrosis model.11, 21 In the CCl4 model, FGF15/19 increased fibrosis by inducing connective tissue growth factor (CTGF) in hepatocytes, which in turn activated HSCs.11 Interestingly, no difference in Ctgf expression was observed with HFD feeding.21 Therefore, mechanisms in addition to induction of CTGF may underlie the regulation of hepatic fibrogenesis by FGF15 and FGF19.
In this study, we hypothesized that FGF15 and FGF19 affect hepatic fibrogenesis by both FGF15/19- and BA-dependent mechanisms: 1) FGF19 may act as a profibrogenic factor that directly modulates HSC activation and proliferation and 2) alteration of FXR activity in HSCs via modulation of BA pool size. To test these hypotheses, we used complimentary in vivo and in vitro models that utilize gain- or loss-of-function of BAs or FGF15/19.
METHODS
Animals and treatments:
Male Fgf15 knockout (KO, Fgf15−/−) and transgenic (TG) mice were used. The Fgf15−/− mice were raised with a 75%/25% mixed C57BL/6J/A129 background respectively, because in a C57BL/6J background, knockout was embryonically lethal. TG mice were generated with a C57BL/6J background.22 At 8–10 weeks of age, the mice were fed either a control chow diet, 0.2% CA-containing diet (Sigma Aldrich; Cat. # C1129), or a 2% cholestyramine-containing diet (Sigma Aldrich; Cat. # C4650; PicoLab Rodent Diet 5053; LabDiet, New Brunswick, NJ). After 2 weeks on the assigned diets, the mice were i.p. injected with 1 mL/kg CCl4 dissolved in olive oil twice a week for 4 weeks to induce liver fibrosis. Control mice were injected with olive oil alone (Figure 1A). Assigned diets were continued until the end of the study. At the end of the treatment, serum, gall bladder, intestines, and livers were collected. All animal experiments were conducted with the approval of the Rutgers University Institutional Animal Care and Use Committee. Please refer to supplemental information for description of other study methodology.
Figure 1.
Effect of genotype, diets, and CCl4 on BA homeostasis and markers of liver injury. (A) The three genotypes of mice (WT-C57, Fgf15−/− and FABP1-Fgf15) were assigned to the indicated diets to create multiple combinations of TBAP and FGF15 activity and treated with vehicle or CCl4 according to the above timeline. (B) BA levels in the intestine, liver, and serum were measured and TBAP calculated. (C) Expression of FXR target genes was measured in the ileum. (D) Expression of genes regulating BA synthesis and BA transport was measured in the liver. Data presented as mean plus SD. Data analyzed by 3-Way ANOVA with posthoc Tukey’s HSD. * significant across diet; # significant across genotype; † significant across CCl4 treatment (p ≤ 0.05). Abbreviations: BW - Body weight, Bsep - Bile salt export pump, CA - Cholic acid, CCl4 - Carbon tetrachloride, Cyp - Cytochrome P450, FABP1-Fgf15 - overexpressing FGF15 transgenic mice, Fgf15 - Fibroblast growth factor 15, GI+GB - Gastrointestinal and gall bladder, Ibabp - Ileal bile acid binding protein, KO - Knockout mice, Ntcp - Sodium-taurocholate co-transporting polypeptide, Shp – Small heterodimer partner, TBAP - Total bile acid pool, TG - Transgenic mice, WT - Wild type mice.
Statistical tests & analysis
Levene’s test was performed to assess the equity of variance of each variable. Should the assumption of homogeneity of variance not be met for subsequent analyses, data were logarithmically transformed. Statistical analyses were selected based upon variable type, number of variables, and number of groups. Comparison between two groups was performed using Student’s T-test. Analysis of 3 or more groups within the same variable was performed using 1-Way ANOVA with post-hoc Duncan’s Multiple Range Test. Data from the in vivo CCl4 study were analyzed via 3-Way ANOVA and post-hoc Tukey’s test. Correlations between endpoints of interest and total bile acid pool (TBAP) size and hepatic small heterodimer partner (Shp) expression were analyzed using simple linear regression and F-test.
RESULTS
Effects on BA homeostasis
The dosages of cholestyramine (2%) and cholic acid (CA) (0.2%) were chosen because they alter the BA pool size in WT mice without apparent toxicity.23 In agreement, the TBAP in WT mice was lowered modestly by cholestyramine while increased by CA (Figure 1B). KO mice had an increased basal TBAP, which was brought back to levels comparable to those in WT mice by cholestyramine feeding, and was also decreased by CCl4. In TG mice, the TBAP was reduced compared to WT, and was greatly increased by CA feeding. Genotype and diet predominantly altered the BA concentrations in the intestine. Liver and serum BA concentrations were not affected by genotype, CCl4 or cholestyramine treatment alone. CA alone increased the concentrations of BAs in the liver and serum of TG mice. When co-administered with CCl4, CA increased liver and serum BAs in WT mice and further elevated serum BAs in TG mice.
Changes in BA concentration in the intestine led to corresponding alterations in FXR activity in the ileum (Figure 1C). In WT mice, cholestyramine feeding did not alter ileal FXR activity as expression of FXR target genes Fgf15, Shp, and Ibabp remained unchanged. However, CA diet increased FXR activity and led to a 43-fold induction of Fgf15 and trend for increased Ibabp expression. FXR activity in the intestines of KO mice was enhanced evident by increased expression of Shp. Cholestyramine reduced intestinal FXR activity and led to reduced Shp and Ibabp expression. In the TG mice, Fgf15 was overexpressed in the ileum by 578-fold and was not further increased by CA feeding. Despite the lowered levels of BAs in the intestine, TG mice had comparable expression of other FXR target genes.
Expression of genes involved in BA synthesis and transport in the liver was affected by genotype and diet (Figure 1D). KO and TG mice had increased and suppressed expression of Cyp7a1 compared to WT, respectively. In WT mice, cholestyramine led to inductions of genes involved in BA synthesis with a trend for increased expression of Cyp7a1 and increased expression of Cyp8b1. Conversely, exogenous CA feeding suppressed the expression of Cyp7a1. CCl4 treatment in KO mice reduced Cyp7a1 expression. Expression of major BA transporters, sodium taurocholate co-transporting polypeptide (Ntcp) and bile salt efflux pump (Bsep), was not affected by FGF15 deficiency or cholestyramine diet (Figure 1D). In WT mice, CA diet led to a trend for decreased expression of Ntcp. CA diet alone increased Bsep expression in TG mice, and when combined with CCl4, led to reductions in Ntcp expression.
We also investigated the effects of CCl4 on serum BA composition in WT, KO, and TG mice (Supplemental Table 1). In all genotypes, CCl4 did not change the percentage of conjugated/unconjugated or primary/secondary BAs. Vehicle dosed KO mice had a greater percentage of taurine conjugated BAs compared to WT. In WT mice, CCl4 increased the serum concentrations of all BAs species except taurine-conjugated deoxycholic acid (T-DCA) and taurine-conjugated ωMCA (T-ωMCA). Unlike in WT mice, CCl4 did not increase CA, T-CA, DCA, and T-DCA levels in KO mice or DCA and T-DCA in TG mice. However, CCl4 led to greater increases in T-CDCA, CDCA, and UDCA. Overall, the percentage of primary BAs in KO mice was increased by CCl4.
Effects on liver biomarkers and histology
Biomarkers for liver injury were not affected by genotype, cholestyramine diet, or CCl4 treatment, but were elevated by CA diet (Figure 2A). In WT and TG mice, the CA diet increased both the liver-to-body-weight ratio and activities of ALT. Co-treatment of TG mice with CA and CCl4 led to elevations in serum total bilirubin levels. ALP activities were similar across all groups.
Figure 2.
Liver injury markers and histology scores from H&E stained liver sections. (A) Serum biomarkers of liver injury were measured and liver-to-BW ratio calculated. Data analyzed by 3-Way ANOVA with posthoc Tukey’s HSD. * significant across diet; # significant across genotype; † significant across CCl4 treatment (p ≤ 0.05). (B) Sections were scored for severity of biliary hyperplasia, centrilobular necrosis, inflammation, and fibrosis. Data are presented as histology pies. Each pie is divided into slices equal to the number of mice comprising the group. Slices colored according to histology score; white (0), yellow (1), orange (2), red (3), black (4). Hyperplasia, centrilobular necrosis, and inflammation were scored from 0 to 3. Fibrosis was scored from 0 to 4. Abbreviations: ALP - Alkaline phosphatase, ALT – Alanine aminotransferase, BW - Body weight, CA - Cholic acid, CCl4 - Carbon tetrachloride, FABP1-Fgf15 - overexpressing FGF15 transgenic mice, KO - Knockout mice, TBAP - Total bile acid pool, TG – Transgenic mice, WT - Wild type mice.
Liver histology was examined and scored for severity of biliary hyperplasia, centrilobular necrosis, inflammation, and fibrosis (Figure 2B). In WT mice, CCl4 caused minimal biliary hyperplasia and inflammation in a third of mice and induced mild or marked fibrosis in every mouse. When given with CCl4, cholestyramine increased incidence of biliary hyperplasia and inflammation. Cholestyramine did not affect fibrosis scores in WT mice. CA diet alone led to the development of biliary hyperplasia in half of the treated WT mice. Co-treatment of WT mice with CA and CCl4 led to increased incidence and severity of biliary hyperplasia, centrilobular necrosis, and inflammation compared to CCl4 treatment alone. Fibrosis severity in these WT mice with CA/CCl4-cotreatment was reduced.
FGF15 deficiency was not protective against CCl4-induced fibrosis, revealed by similar incidence and severity of fibrosis between KO and WT mice (Figure 2B). Chronic treatment with CCl4 caused mild to moderate centrilobular necrosis in KO mice, which differs greatly from WT mice in which no centrilobular necrosis was observed. The incidence and severity of inflammation in KO mice appeared to be greater than in WT mice. Cholestyramine feeding led to mild fibrosis in half of the treated KO mice. When co-administered with CCl4, cholestyramine increased the severity of inflammation and fibrosis but not centrilobular necrosis.
Overexpression of Fgf15 did not alter the severity of CCl4-induced fibrosis (Figure 2B). CA diet led to biliary hyperplasia and inflammation in majority of TG mice. Treatment of TG mice with CA diet alone caused mild and moderate fibrosis in two out of 9 mice (22%). Lastly, TG mice treated with CA were found to have steatosis, a finding not observed in any other group (Supplemental Figures 1–3). Co-treatment of TG mice with CA and CCl4 led to marked biliary hyperplasia and worsened inflammation compared to CCl4 treatment alone, but fibrosis severity was comparable between these two groups.
Effects on expression of genes involved hepatic inflammation and fibrosis
Despite a previous report which suggests 0.2% CA-containing diet as non-toxic, the presence of cholestasis in the CA-treated WT and TG mice confounds the interpretation of the effects of BAs and FGF15 on CCl4-induced inflammatory and fibrotic endpoints.23 Therefore, CA-treated mice were excluded from subsequent analyses regarding these endpoints.
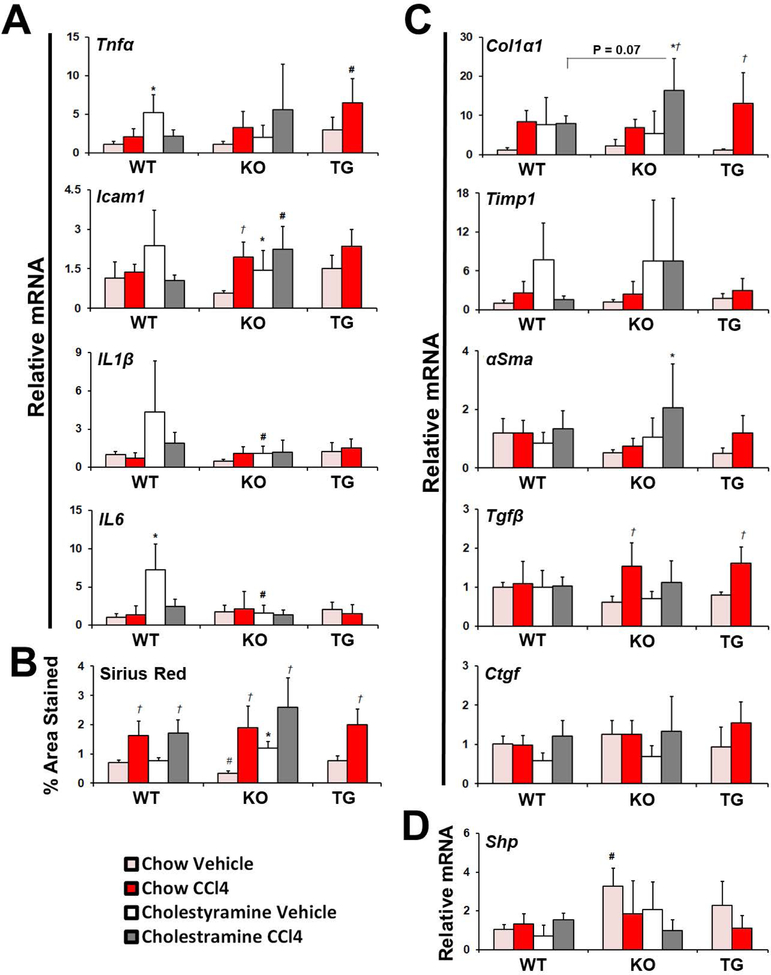
To identify effects on liver inflammation, the gene expression of inflammatory cytokines in the liver was measured (Figure 3A). In WT mice, expression of tumor necrosis factor alpha (Tnfα), intracellular adhesion molecule 1 (Icam1), interleukin-1β (Il1β), and Il6 was not increased by CCl4, but Tnfα and Il6 expression was increased by cholestyramine. In KO mice, Icam1 expression was increased by cholestyramine or CCl4 treatment alone, and was greater in KO than in WT mice when co-treated. Compared to WT mice, Tnfα expression was greater in TG mice when treated with CCl4.
Figure 3.
Effect of genotype, diet, and CCl4 treatment on hepatic inflammation and fibrosis. (A) Expression of inflammatory mediators was measured. (B) The area stained in liver sections by SR was determined and (C) expression of fibrotic mediators and (D) FXR target gene was measured. Data presented as mean plus SD. Data were analyzed by 3-Way ANOVA with post-hoc Tukey’s HSD. * significant across diet; # significant across genotype; † significant across CCl4 treatment (p ≤ 0.05). Abbreviations: αSma – Alpha smooth muscle actin, CA - Cholic acid, CCl4 - Carbon tetrachloride, Col1α1 - Collagen type 1 alpha 1 chain, Ctgf - Connective tissue growth factor, Icam1 - Intercelluar adhesion molecule 1, IL - Interleukin, KO - Knockout mice, Shp - Small heterodimer partner, Tgfβ - Transforming growth factor beta, Timp1 – Tissue inhibitor of metalloproteases, Tnfα - Tumor necrosis factor alpha, TG - Transgenic mice, WT - Wild type mice.
Shown in Figure 3B and Supplemental Figure 4, hepatic collagen levels were comparable between WT and TG mice, but lower in chow fed, vehicle-treated KO mice compared to WT, consistent to our previous finding.21 Cholestyramine feeding did not change collagen levels in WT mice but almost tripled in KO mice. Hepatic collagen levels were increased to similar extent by CCl4 in WT, KO, and TG mice. Additionally, CCl4 led to comparable induction of collagen 1α1 (Col1α1) and tissue inhibitor of metalloproteases 1 (Timp1) (Figure 3C). In WT and KO mice, cholestyramine treatment led to increased Col1α1 and Timp1 expression, but only in KO mice, co-treatment of cholestyramine and CCl4 tended to further increase the induction of Col1α1 (P = 0.07). The expression levels of αSma trended lower in KO and TG mice than WT mice. None of the treatments affected αSma expression in WT mice, but was increased in KO mice by co-treatment with cholestyramine and CCl4. Similarly, expression of Tgfβ was increased by CCl4 in KO and TG mice but was not altered in WT mice by any treatment. Consistent with the increased TBAP size, the expression of a classical FXR target gene, Shp, in the liver (Figure 3D), was increased in KO mice compared to WT mice.
To determine if TBAP size and hepatic FXR activity mediated the extent of hepatic inflammation and fibrosis, TBAP size and expression of hepatic Shp were compared to Tnfα, Icam1, and Col1α1 expression as well as to the extent of hepatic collagen levels, revealed by Sirius red staining, by linear regression (Figure 4). In WT mice, no correlation was observed between TBAP and hepatic Shp to collagen, Icam1, or Col1α1. Tnfα was inversely correlated to TBAP size, however, not to Shp. In KO mice, collagen, Col1α1, Icam1 and Tnfα were inversely correlated to TBAP size and hepatic Shp. In TG mice, Icam1, Tnfα, collagen and Col1α1 were not correlated to TBAP size. However, hepatic Shp expression was correlated to both collagen levels and Col1α1.
Figure 4.
Correlation of Tnfα, Icam1, % area stained by SR, and Col1α1 expression to TBAP size and hepatic Shp expression. Expression of hepatic Shp was logarithmically transformed. Data were analyzed using simple linear regression and F-test. Analyses were performed classified by genotype. Resulting R2 and Pvalues are present on each respective graph. Overlay graph contains the lines of best fit from each genotype and line of best fit (black regression line), R2, and P-value for the entire dataset when analyzed across all genotypes. Abbreviations: BW - Body weight, Col1α1 - Collagen type 1 alpha 1 chain, Icam1 - Intercellular adhesion molecule 1, KO - Knockout mice, Shp - Small heterodimer partner, TBAP - Total bile acid pool, TG - Transgenic mice, Tnfα - Tumor necrosis factor alpha, WT - Wild type mice.
The correlations of Icam1, Tnfα, Col1α1, and collagen to TBAP size and Shp expression were then repeated not classified by genotype. Each comparator was weakly and inversely correlated to TBAP size and Shp expression. For every comparison, the line-of-best-fit for the KO mice alone was nearly identical to that for all animal groups analyzed together. Additionally, the regression lines comparing collagen and Col1α1 expression to Shp expression were similar for KO and TG mice.
Analysis of primary HSCs isolated from WT and KO mice:
Hepatocytes and HSCs were isolated from WT and KO mice. To verify purity of isolated HSCs, expression of Cyp7a1 was measured with relative expression of Cyp7a1 in isolated WT HSCs 0.07x that of hepatocytes (data not shown). Primary HSCs isolated from untreated KO mice had increased FXR activity indicated by increased Ostb mRNA (Figure 5A) and OSTβ protein levels (Figure 5B) and, as well as by increased mRNA levels of Bsep (Figure 5A). Interestingly, the mRNA levels of Shp were unaltered. The levels of mRNA of Col1a1, Timp1, and αSma were decreased. FXR has been previously reported to reduce inflammatory mediator expression in HSCs by up-regulating Ppary.15 The isolated HSCs from KO mice had a trend for increased Pparγ mRNA expression and reduced mRNA levels of several inflammatory mediators. HSCs from KO mice showed increased protein levels of secondary messengers of FGFR signaling (Figure 5B), including extracellular signal-related kinase (ERK) and signal transducer and activator of transcription 3 (STAT3), and increased levels of phosphorylated ERK (pERK).
Figure 5.
Protein and gene expression in primary HSCs isolated from WT and KO mice. (A) Relative gene expression of fibrotic, inflammatory, and bile acid homeostatic genes was measured. (B) Protein levels of OSTβ and phosphorylated and total FGFR secondary messengers in HSCs isolated from WT and KO mice were detected by Western Blot and semi-quantified. Data are presented as mean + SD and analyzed by Student’s T-test; * significant across genotype (p ≤ 0.05). Abbreviations: Asbt – Apical sodium bile-acid transporter, αSma - Alpha smooth muscle actin, Bsep - Bile salt export pump, Col1α1 - Collagen 1 alpha 1, Ctgf - Connective tissue growth factor, ERK - Extracellular signal-related kinase, Icam1 – Intercellular adhesion molecule 1, IκBα - Inhibitor of kappa B alpha, Il- Interleukin, JNK - c-Jun N-terminal kinase, Ntcp - Sodium taurocholate co-transporting protein, OSTβ - Organic solute transporter beta, Pparγ – Peroxisome proliferator-activated receptor gamma , Shp - Small heterodimer partner, STAT3 - Signal transducer and activator of transcription 3, TGFβ - Transforming growth factor beta, Timp1 - Tissue inhibitor of metalloproteases, Tnfα - Tumor necrosis factor alpha.
Effects of FGF19 on HSCs in vitro
To determine if FGF19 can activate FGFRs on HSCs, LX-2 cells were treated with 50 ng/mL recombinant FGF19 for 15 and 30 minutes with changes in secondary messenger phosphorylation measured. The 50 ng/mL concentration increased the levels of pERK and altered CYP7A1 and CYP8B1 expression in HepG2 cells (Supplemental Figure 5). Levels of phosphorylated STAT3 (pSTAT3) and phosphorylated c-Jun N-terminal kinase (pJNK), but not pERK, were increased (Figure 6A). Additionally, levels of inhibitor kappa B alpha (IκBα) were increased and the ratio of pIκBα/IκBα was reduced. Gene expression was measured in LX-2 cells treated with TGFβ and/or FGF19 for 24 hours (Figure 6B). As expected, TGFβ increased the expression of COL1α1 while reducing that of PPARγ and its response genes, abhydrolase domain containing 5 (ABHD5), adipose triglyceride lipase (ATGL), and CCAAT enhancer binding protein alpha (C/EBPα). FXR and SHP were down-regulated by TGFβ. Treatment of LX-2 cells with FGF19 for 24 hours did not alter COL1α1 expression and led to a slight decrease in αSMA. Expression of FXR was also decreased. Co-treatment with FGF19 and TGFβ reduced SHP expression greater than either treatment when given alone, and FGF19 modestly decreased the induction of COL1α1 by TGFβ.
Figure 6.
Effect of FGF19 on FGFR activation and gene expression in LX-2 cells. (A) Relative levels of phosphorylated and total FGFR secondary messengers in LX-2 cells treated with 50 ng/mL FGF19 were detected by Western Blot and semi-quantified. (B) LX-2 cells were treated with FGF19 and/or TGFβ for 24 hours and changes in gene expression measured. (C) LX-2 cells were treated with FGF19 for 48 hours and changes in gene expression measured. (D) Relative levels of p-p65, pIκBα, and IκBα in LX-2 cells treated with FGF19 for 72 hours were detected by Western Blot and semi-quantified. Data are presented as mean + SD and analyzed by (A, B) one-way ANOVA with post-hoc Duncan’s Multiple Range Test or (C,D) Student’s T-test. Duncan groups indicated by letters (a, b, c) and by * means significant across treatment (p ≤ 0.05). Abbreviations: ABHD5 – Abhydrolase domain containing 5, αSMA - Alpha smooth muscle actin, ATGL - Adipose triglyceride lipase, βKL - Beta-Klotho, CEBPα - CCAAT-enhancer-binding protein alpha, COL1α1 - Collagen type 1 alpha 1 chain, ERK - Extracellular signal-related kinase, FGF19 - Fibroblast growth factor 19, FGFR - Fibroblast growth factor receptor, FXR - Farnesoid X receptor, IκBα - Inhibitor kappa B alpha, IL - Interleukin, PPARγ - Peroxisome proliferator-activated receptor gamma, SHP - Small heterodimer partner, STAT3 - Signal transducer and activator of transcription 3, TGFβ - Transforming growth factor beta.
Effects of FGF19 on gene expression in LX-2 cells were investigated. After 48 hours of treatment, FGF19 decreased expression of COL1α1 and genes encoding cytokines IL1β, IL6, and IL8 (Figure 6C). To determine if IκBα levels may contribute to the reduction in cytokine expression, levels were measured 72 hours after FGF19 treatment (Figure 6D). Indeed, levels of IκBα were increased 72 hours after treatment with FGF19. Moreover, levels of phosphorylated p65 were reduced.
LX-2 cells were treated with FGF19 for 48 hours and effects on proliferation were investigated. For cell count and Alamar Blue assays, cells were seeded at 5000 cells per well. After 48 hours, there was an average of 9,375 cells in wells treated with vehicle compared to FGF19 treated wells that contained an average of 4,700 cells (Figure 7A). FGF19 treatment was not toxic as viability of the treated cells was 97% (Figure 7B). Therefore, LX-2 cells treated with FGF19 did not proliferate. Corresponding to decreased proliferation, the gene expression of Cyclin D1 was reduced (Figure 7C). Interestingly, despite the decreased cell count observed after FGF19 treatment, no effect of FGF19 on proliferation was observed in the Alamar Blue assay (Figure 7D). Treatment of LX-2 cells for 48 hours with chenodeoxycholic acid (CDCA) at 100 μM led to decreased cell numbers, which may have been resultant of increased cell death as viability was reduced to 78%. Additionally, expression of Cyclin D1 was reduced by CDCA.
Figure 7.
Effect of FGF19 and CDCA treatment on LX-2 proliferation and receptor gene expression. 5,000 LX-2 cells were seeded into each well and treated with FGF19 and/or CDCA for 48 hours. (A) Cells were counted and (B) viability assessed via trypan blue. (C) Expression of Cyclins D1 and E1 was measured. (D) Alamar blue assay was performed and relative fluorescence measured. (E) LX-2 cells were treated with CDCA for 24 hours with changes in gene expression measured. (F) LX-2 cells were treated with FGF19 and/or CDCA for 48 hours and effects on the expression measured. Data are presented as mean + SD and analyzed by (A-D, F) 1-Way ANOVA with post-hoc Duncan’s Multiple Range Test and (E) by Student’s T-test or. Duncan groups indicated by letters (a, b, c) and * means significant across treatment (p ≤ 0.05). Abbreviations: ABHD5 - Abhydrolase domain containing 5, αSMA - Alpha smooth muscle actin, ATGL -Adipose triglyceride lipase, βKL - Beta-Klotho, CEBPα - CCAAT-enhancer-binding protein alpha, COL1α1 -Collagen type 1 alpha 1 chain, FGF19 - Fibroblast growth factor 19, FGFR - Fibroblast growth factor receptor, PPARγ - Peroxisome proliferator-activated receptor gamma, SHP - Small heterodimer partner, TGR5 - Takeda G-protein receptor 5.
LX-2 cells treated with CDCA for 24 hours and with CDCA and/or FGF19 for 48 hours were assessed for changes in gene expression. Treatment of LX-2 cells with CDCA for 24 hours induced the expression of FXR response gene SHP, as well as Takeda G-protein receptor 5 (TGR5), FGFR1, and βKL (Figure 7E). The expression of the FGF19 receptors, FGFR1 and FGFR4, underwent down-regulation in response to 48 hours of FGF19 treatment (Figure 7F). This down-regulation was prevented by co-treatment with CDCA, and SHP, TGR5, and βKL expression was induced significantly by co-treatment
DISCUSSION
FGF15 deficiency has been previously reported to protect mice from the development of fibrosis in both HFD-induced NASH model and CCl4-induced fibrosis model.11, 21 FGF15 is a strong regulator of BA homeostasis and BA-activated FXR in HSCs reduces liver fibrosis. Therefore, we hypothesized that the mechanism by which FGF15 increases hepatic fibrogenesis is dependent upon reduction of BAs and inactivation of FXR. Additionally, as other FGFs regulate HSC activation, we also hypothesized that FGF15 may function as a direct profibrotic stimulator to HSCs.24 These hypotheses were tested in both in vivo, and in vitro models: 1) cohorts of mice with various combinations of TBAP size and FGF15 expression were created via genetic and pharmacologic means and then treated with CCl4, 2) primary HSCs were isolated from WT and KO mice, and 3) the human HSC line, LX-2, was treated with recombinant FGF19 with or without FXR-activating BA.
The in vivo model showed that the TBAP size was modulated by FGF15 deficiency and overexpression corresponding to the known role of FGF15 as a negative feedback mediator of BA synthesis. The cholestyramine diet was well tolerated and did not increase serum liver injury biomarkers or liver-to-body weight ratio in WT and KO mice. Cholestyramine caused a slight decrease in TBAP size in the WT mice. However, it reduced the elevated TBAP size in KO mice back to levels comparable to WT mice. Corresponding with decreased intestinal BA levels, FXR activity in the ileum and liver of KO mice was reduced. The dose of 0.2% CA was selected due to reports suggesting that this concentration is tolerated well in WT mice.23 Unfortunately, this dosage led to the development of cholestasis when co-treated with CCl4 in our cohorts of mice, likely due to additive toxicity to hepatocytes. TG mice were more susceptible to this concentration of CA and developed more severe cholestasis than WT mice. We hypothesize the increased susceptibility in TG mice is due to low basal expression of Bsep (0.32x compared to WT). The presence of cholestasis in the CA-treated WT and TG mice confounds the interpretation of the effects of BAs and FGF15 on CCl4-induced inflammatory and fibrotic endpoints. Therefore, CA-treated mice were excluded from subsequent analyses regarding these endpoints.
The combination of the three genotypes and cholestyramine diet allowed for the dissociation of BA levels from expression of FGF15. This permitted the determination of the BA-dependent and -independent effects of FGF15 on CCl4-induced inflammation and fibrosis. Additionally, the identification of BA-dependent and -independent effects of FGF15/19 on HSCs was elucidated by isolation of primary HSCs from WT and KO mice as well as in vitro treatment of LX-2 cells with FGF19 and CDCA.
It has been previously reported that FXR activation mitigates hepatic inflammation.25–29 In agreement, in this study, TBAP size and expression of a FXR target gene, Shp, were correlated to Tnfα and Icam1 expression. One mechanism by which FXR has been shown to reduce inflammatory mediator expression in HSCs is via the induction of Pparγ.15 PPARγ expression was increased in LX-2 cells treated with CDCA and in HSCs isolated from KO mice, and correspondingly, inflammatory mediator expression in the LX-2 cells and isolated HSCs was reduced. FGF15 has also been shown to reduce nuclear factor kappa-light-chain-enhancer of activated B-cells (NFκB) signaling through the activation of FGFR4.30 NFκB signaling was reduced, evident as IκBα levels were increased following treatment of LX-2 cells with FGF19 protein, resulting in reduced inflammatory mediator gene expression. Therefore, in our CCl4 model, the data suggests increased inflammation in TG mice may be due to reduced BA levels and diminished FXR signaling whereas the increased inflammation in KO mice may have been due to reduced FGFR inhibition of NFκB signaling.
Contrary to findings in previous reports, KO mice were not protected from CCl4-induced fibrosis.11 This may be due in part to a higher dose of CCl4 used, or may also be resultant of differences in Ctgf expression. In the previous study, FGF15 deficiency was found to be protective against fibrosis by mitigating inductions of hepatocyte-derived CTGF. In the current study, induction of Ctgf was not observed in any genotype.
The current study supports that the effects of FGF15/19 deficiency on protecting from hepatic fibrosis are likely dependent on increasing BA levels, which subsequently enhances FXR signaling in HSCs. In agreement with our previous report using FGF15 deficient mice in a HFD-induced NASH model, untreated KO mice presented with lower amount of collagen in the liver.21 In this study, FXR activity was increased in primary HSCs from KO mice, which was associated with reduced expression of profibrotic mediators. Additionally, cholestyramine treatment resulted in raised the collagen levels in KO mice back to those comparable to WT mice, and cholestyramine treatment alone increased fibrosis histology scores in KO but not WT mice. Correlations further support the BA-dependent regulation of hepatic fibrosis by FGF15/19. Collagen levels and Col1α1 expression were inversely correlated with TBAP size and hepatic Shp expression in KO and TG mice. Though the analyses above focused on BA pool size and FXR and FGFR activation in HSCs, it is important to note that various BA species have differential effects on HSCs.31–34 Of importance in this study, after treatment with CCl4, serum concentrations of T-CDCA and CDCA increased to a greater extent in KO mice and WT mice. Therefore, the extent of FXR activation in HSCs after CCl4 may be affected by both BA pool size and composition. Our findings are aligned with the body of literature which states that FXR activation in HSCs reduces extracellular matrix production and mitigates HSC responsiveness to profibrotic signals such as TGFβ and thrombin.14, 16
To determine the direct effects of FGF19 on HSCs, the human HSC line LX-2 was treated with recombinant FGF19 protein. The relative expression of FGF19 receptors in LX-2 and HepG2 cells (Supplemental Table 2). FGF19 was found to increase both STAT3 and JNK phosphorylation indicating that FGF19 can activate FGFRs in LX-2 cells. However, FGF19 did not increase HSC activation or proliferation as initially hypothesized. The changes in expression of HSC phenotypic marker genes in response to FGF19 treatment were modest, indicating no change in HSC phenotype to either a more activated or quiescent state. Contrary to our hypothesis, our data indicate that FGF19 may slightly reduce Col1α1 expression and HSC proliferation.
In summary, FGF15 deficiency has been shown to be protective against hepatic fibrosis in HFD-induced NASH and CCl4 fibrosis models.11, 21 As an FGF19 analog protein and FXR agonists, which highly induce the expression of FGF19, are currently in human clinical trials for the treatment of cholestasis and NASH, these studies raised a concern that FXR and FGF19-based therapies may detrimentally affect fibrosis development. We therefore sought to determine the mechanisms by which FGF15 deficiency was protective against hepatic fibrosis in order to determine if this mechanism is clinically relevant. We demonstrated that the protective effect of FGF15 deficiency is mediated by increased BA activation of FXR in HSCs, in addition FGF19 does not directly act a profibrotic mediator or mitogen to HSCs (Figure 8). In agreement, clinical trials with the FGF19 analog protein and FXR agonists have demonstrated decreased severity of fibrosis in non-cirrhotic NASH patients after treatment.35, 36 BAs and FXR signaling are proposed key mediators in mitigating the development of hepatic fibrosis and our findings provide further evidence supporting the use of FXR agonists to mitigate the development of hepatic fibrosis. However, we acknowledge that further studies of FGF15/19 in fibrosis need to be designed as liver injury-induced fibrosis by NASH and chemicals may have differential underlying mechanisms.
Figure 8.
Summary of the direct and BA-mediated mechanisms by which FGF15/19 alters HSC function and development of liver fibrosis.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Fang Liu (Department of Chemical Biology, Rutgers University) for providing the TGFβ protein used in this study.
FUNDING
This study was supported by NIH grants R21ES028258, GM04030, T32ES019854 and VA-BX002741, as well as the Bristol Myers Squibb Graduate Fellowship in Toxicology awarded to Justin Schumacher.
ABBREVIATIONS
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- αSMA
Alpha-smooth muscle actin
- βKL
Beta-klotho
- BSEP
Bile salt export pump
- CA
Cholic acid
- CCl4
Carbon tetrachloride
- CDCA
Chenodeoxycholic acid
- C/EBPα
CCAAT/enhancer binding protein alpha
- COL1A1
Collagen type 1 alpha 1 chain
- CTGF
Connective tissue growth factor
- CYP7A1
Cholesterol 7-alpha-hydroxylase
- CYP8B1
Sterol 12-alpha-hydroxylase
- DCA
Deoxycholic acid
- ERK
Extracellular signal-related kinase
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptor
- FXR
Farnesoid X Receptor
- HFD
High fat diet
- HSC
Hepatic stellate cell
- IBABP
Ileal bile acid binding protein
- IκBα
Inhibitor kappa B alpha
- IL-1
Interleukin 1
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- Icam1
Intracellular adhesion molecule 1
- JNK
c-Jun N-terminal kinase
- KO
Whole body Fgf15 knockout mice/Fgf15−/− mice
- ωMCA
Omega muricholic acid
- NASH
Non-alcoholic steatohepatitis
- NFκB
Nuclear factor kappa-light-chain-enhancer of activated B-cells
- NTCP
Sodium-taurocholate co-transporting polypeptide
- PPARγ
Peroxisome proliferator-activated receptor gamma
- SHP
Small hetero-dimerization partner
- STAT3
Signal transducer and activator of transcription 3
- UDCA
Urdsodeoxycholic acid
- TBAP
Total bile acid pool
- TGFβ
Transforming growth factor beta
- TGR5
Takeda G-protein receptor 5
- TIMP1
Tissue inhibitor of metalloproteases 1
- TNFα
Tumor necrosis factor alpha
REFERENCES
- 1.Higashi T, Friedman SL & Hoshida Y Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 121, 27–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura T, Utsunomiya Y, Hoshikawa M, Ohuchi H & Itoh N Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim Biophys Acta 1444, 148–51 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Xie MH et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine 11, 729–35 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Ornitz DM & Itoh N The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4, 215–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagaki T et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2, 217–25 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Song KH, Li T, Owsley E, Strom S & Chiang JY Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49, 297–305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong B et al. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56, 1034–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S et al. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab 20, 320–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurosu H et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282, 26687–95 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoine M et al. Expression and function of fibroblast growth factor (FGF) 9 in hepatic stellate cells and its role in toxic liver injury. Biochem Biophys Res Commun 361, 335–41 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Uriarte I et al. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int J Cancer 136, 2469–75 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Fickert P et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol 175, 2392–405 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorucci S et al. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther 314, 584–95 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Fiorucci S et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127, 1497–512 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Fiorucci S et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther 315, 58–68 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Carino A et al. Disruption of TFGbeta-SMAD3 pathway by the nuclear receptor SHP mediates the antifibrotic activities of BAR704, a novel highly selective FXR ligand. Pharmacol Res 131, 17–31 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Verbeke L et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 59, 2286–98 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Li J et al. Roles of microRNA-29a in the antifibrotic effect of farnesoid X receptor in hepatic stellate cells. Mol Pharmacol 80, 191–200 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Lu C, Zhang F, Shao J & Zheng S Dihydroartemisinin restricts hepatic stellate cell contraction via an FXR-S1PR2-dependent mechanism. IUBMB Life 68, 376–87 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Li J et al. Inhibition of endothelin-1-mediated contraction of hepatic stellate cells by FXR ligand. PLoS One 5, e13955 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher JD et al. The effect of fibroblast growth factor 15 deficiency on the development of high fat diet induced non-alcoholic steatohepatitis. Toxicol Appl Pharmacol 330, 1–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong B et al. A Novel Fibroblast Growth Factor 15 Dependent- and Bile Acid Independent-Promotion of Liver Regeneration in Mice. Hepatology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P, Zhang Y & Klaassen CD Dose-response of five bile acids on serum and liver bile Acid concentrations and hepatotoxicty in mice. Toxicol Sci 123, 359–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher JD & Guo GL Regulation of Hepatic Stellate Cells and Fibrogenesis by Fibroblast Growth Factors. Biomed Res Int 2016, 8323747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Liu Q, Wang J & Harnish DC Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem Biophys Res Commun 379, 476–9 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Mencarelli A et al. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol 183, 6657–66 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Deshmane SL, Kremlev S, Amini S & Sawaya BE Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29, 313–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L et al. Activation of farnesoid X receptor downregulates monocyte chemoattractant protein-1 in murine macrophage. Biochem Biophys Res Commun 467, 841–6 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Kim DH et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. Embo j 34, 184–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drafahl KA, McAndrew CW, Meyer AN, Haas M & Donoghue DJ The receptor tyrosine kinase FGFR4 negatively regulates NF-kappaB signaling. PLoS One 5, e14412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawitza I, Kordes C, Gotze S, Herebian D & Haussinger D Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep 5, 13320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svegliati-Baroni G et al. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology 128, 1042–55 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Saga K et al. Secondary Unconjugated Bile Acids Induce Hepatic Stellate Cell Activation. Int J Mol Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z et al. Taurocholic acid is an active promoting factor, not just a biomarker of progression of liver cirrhosis: evidence from a human metabolomic study and in vitro experiments. BMC Gastroenterol 18, 112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrison SA et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Neuschwander-Tetri BA et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.