SUMMARY
Background:
We previously showed that a food-based empirical dietary inflammatory pattern (EDIP) score is associated with circulating inflammatory biomarkers. Metabolomic profiling of inflammatory diets may therefore provide insights on mechanisms contributing to disease etiology and prognosis. We aimed to elucidate metabolites associated with inflammatory diets among postmenopausal women, utilizing a robust study design that incorporates independent discovery and validation datasets.
Methods:
This baseline cross-sectional investigation evaluated associations between continuous EDIP scores calculated from food frequency questionnaires and 448 log-transformed plasma metabolites as outcomes in multivariable-adjusted linear regression analyses. Metabolites were measured with liquid chromatography tandem mass spectroscopy. Metabolite discovery was conducted among 1,109 Women’s Health Initiative (WHI) Hormone Therapy trial participants and results were replicated in an independent dataset of 810 WHI Observational Study participants. Secondary analyses were stratified by standard body mass index (BMI, kg/m2) categories. In discovery and replication datasets statistical significance was based on false-discovery rate adjusted P<0.05.
Results:
After adjusting for energy intake, BMI, physical activity, and other confounding variables, 23 metabolites were significantly associated with EDIP score in the discovery dataset. Of these, the following ten were replicated: trigonelline, caffeine, acethylamino-6-amino-3-methyluracil, 7-methylxanthine, 1,7-dimethyluric acid, 3-methylxanthine, C18:3CE, glycine, associated with lower dietary inflammatory potential; whereas C52:3 triacylglycerol and linoleate associated with higher dietary inflammatory potential. Four of the ten were associated [glycine (inversely), caffeine, 1,7-dimethyluric acid, C52:3 triacylglycerol, (positively)], with C-reactive protein levels. In secondary analyses, associations showed differences by BMI category. Four metabolites, related to coffee/caffeine metabolism were inversely associated among normal weight women, and 83 metabolites associated with EDIP among overweight/obese women, including 40 (48%) that were also associated with C-reactive protein.
Conclusion:
Metabolites associated with coffee/caffeine and lipid metabolism may reflect the inflammatory potential of diet. Potential differences by BMI and the linkage to disease outcomes, require further study.
Keywords: dietary patterns, inflammatory diets, metabolomics, postmenopausal women
INTRODUCTION
Diet is known to influence development and progression of chronic diseases, such as cancer, cardiovascular disease and diabetes. However, to date the biological pathways through which diet influences chronic disease risk have not been fully elucidated. Findings in this area, show systemic inflammation as one potential pathway through which diet may influence chronic disease risk in men and women [1–4]. In place of single analyte biomarkers, metabolomics approaches allow for a holistic view of multiple pathways implicated in the pathophysiology of disease by profiling multiple metabolites in biofluids, cells, and tissues. High-throughput metabolomics assays may shed light on specific metabolites and metabolic pathways influenced by diet, which could provide insights into the underlying biological mechanisms that drive dietary pattern - disease associations; and may inform dietary intervention strategies and/or intervention monitoring. Metabolomic analyses of human samples have now extended to population-based analyses, where the aim is to identify new or novel biomarkers of disease or metabolite profiles that are associated with different human exposures such as diet and lifestyle [5, 6]. Given the influence of dietary patterns on chronic systemic inflammation [1, 2, 7], dietary patterns associated with inflammation may therefore influence chronic disease outcomes [3, 8]. Elucidating the metabolomic profiles of inflammatory diets may therefore provide insights into specific biological pathways.
We previously developed the empirical dietary inflammatory pattern (EDIP) score to identify the dietary components that explain maximal variation in inflammatory biomarkers, including c-reactive protein (CRP), interleukin-6 (IL6) and tumor necrosis factor (TNF) alpha receptor 2 [1]. The EDIP is a food-based index that characterizes the inflammatory potential of a diet based on circulating levels of inflammatory biomarkers. Its relative validity was evaluated in two independent cohorts of health professionals [1, 9]; and in the Women’s Health Initiative cohort [2]. Previous studies have shown differences by BMI in the association of the EDIP and several inflammatory markers and with weight change [1, 2, 7, 10]. For example, long-term changes in the EDIP were associated with greater weight changes among overweight/obese men and women than among normal weight participants: overweight/obese participants who changed their diets towards more anti-inflammatory dietary patterns experienced significantly less weight gain compared to those who made minimal changes to their diets [10]. In other previous studies, EDIP predicted higher concentrations of inflammatory biomarkers among overweight or obese participants than among normal weight participants [1, 2, 7]. These findings suggest that the EDIP may be sensitive to dietary patterns associated with obesity and therefore may be more strongly associated with obesity-related inflammation. In this cross-sectional study in a population of postmenopausal women, we aimed to identify metabolites associated with the inflammatory potential of diet and to examine the role of body weight in these associations.
METHODS
Study population
The Women’s Health Initiative (WHI) enrolled 161,808 postmenopausal women 50 to 79 years old with a predicted >3-year survival, in 40 sites in the United States between 1993 and 1998. Women of racial or ethnic minority groups represented 17.1% of the overall WHI sample [11]. Participants were enrolled into an observational study (OS) or one or more of four clinical trials, two of which were hormone therapy (HT) trials. One of the HT trials randomly assigned 16,608 women to estrogen plus progestin or placebo, whereas the other randomized 10,739 women with prior hysterectomy, to estrogen or placebo. The full WHI-OS consisted of 93,676 women not eligible or unwilling to participate in the clinical trials [11].
At the baseline clinic visit, certified staff drew blood samples and performed physical measurements including blood pressure, height and weight. For the current study, we used data from 2,306 participants from the Metabolomics of CHD in the WHI study [12], a matched case-control study that selected participants from the OS and HT. The cases (who developed coronary heart disease (CHD) after the baseline blood draw) were frequency matched to controls on 5-year age groups, race/ethnicity, hysterectomy status, and 2-year enrollment window. We excluded women with implausible total energy intake values (≤600 kcal/d or ≥5000 kcal/d, n=92), very low or very high body mass index (BMI) values (<15 kg/m2 or >50 kg/m2, n=15), or those who self-reported diabetes at baseline (n=280). After exclusions, the analytic dataset included 1,919 women: 1,109 in the WHI-HT (discovery dataset) and 810 in the WHI-OS (replication dataset) (Supplemental Figure 1). The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center in Seattle, WA, and at each of the 40 Clinical Centers [11]. The current study was approved by the institutional review board at the Brigham and Women’s Hospital.
Dietary assessment and calculation of the empirical dietary inflammatory pattern (EDIP) score
At baseline, all participants completed a 122-item semi-quantitative food frequency questionnaire (FFQ) by self-report, developed for the WHI to estimate average daily dietary intake over the previous 3-month period [11, 13]. The WHI FFQ has produced results reasonably comparable to those from four 24-hour dietary recall interviews and four days of food diaries recorded within the WHI [14].
The EDIP development and validation studies have been described [1, 9]. The EDIP score was developed in a sample of 5,230 women in the Nurses’ Health Study (NHS) and validated in NHS-II (n=1,002) and Health Professionals Follow-up Study (HPFS: n=2632) [1]. Also, the score was previously applied in a multi-racial sample of 31,472 WHI participants with diet and biomarker data, and was found to be significantly associated with several inflammatory biomarkers [2]. The goal for the EDIP was to empirically create a score for overall inflammatory potential of whole diets defined using food groups. Thirty-nine pre-defined food groups [15] were entered into reduced rank regression models [16] followed by stepwise linear regression analyses to identify a dietary pattern most predictive of three plasma inflammatory biomarkers: CRP, IL6 and TNF receptor 2 [1]. The EDIP score is the weighted sum of 18 food groups which assesses the inflammatory potential of diet on a continuum from maximally anti-inflammatory to maximally pro-inflammatory, with lower (more negative) scores indicating anti-inflammatory diets and higher (more positive) scores indicating pro-inflammatory diets. Food groups that were positively related to concentrations of inflammatory biomarkers are the following: processed meat, red meat, organ meat, non-dark (non-oily) fish, other vegetables (i.e., vegetables other than green leafy vegetables and dark-yellow vegetables), refined grains, high-energy beverages (cola and other carbonated beverages with sugar, fruit drinks), low-energy beverages (low-energy cola and other low-energy carbonated beverages - the WHI FFQ did not separately assess low-energy beverages), and tomatoes. The remaining food groups were inversely related to concentrations of the inflammatory biomarkers, and include: beer, wine, tea, coffee, dark-yellow vegetables, green leafy vegetables, snacks (popcorn, corn chips, potato chips, crackers), fruit juice, and pizza [1].
Metabolite assessment
Plasma samples were collected using EDTA tubes and processed immediately. Specimens were stored in a –70°C freezer within 2 hours of collect ion or stored at –20°C for up to 2 days and shipped on dry ice and stored at −70°C until proces sing. Metabolomics measurements were conducted at the Broad Institute (Boston, MA), using 4 complimentary liquid chromatography tandem mass spectroscopy (LC-MS) methods, described in detail elsewhere [12], resulting in 509 metabolites. For each method, pooled plasma reference samples were included every 20 samples, and results were standardized using the ratio of the value of the sample to the value of the nearest pooled reference multiplied by the median of all reference values for the metabolite [12]. MultiQuant 1.2 software (AB SCIEX) was used to identify and quantify the metabolites [17]. All signals were inspected to ensure quality and integration, and a signal-to-noise ratio <10 was considered unquantifiable [18–20]. For each method, metabolite identities were confirmed using authentic reference standards or reference samples. Coefficients of variation (CVs) were calculated using pooled plasma samples from the first 800 WHI-OS participants [12]. After excluding metabolites with a CV >20%, 448 known metabolites were retained for analyses. In the pilot testing of the metabolomics platform, 92% of metabolites had acceptable assay reproducibility (CV<20%) and almost 90% of metabolites were stable over 1 – 2 years (Spearman correlation or ICC ≥0.4) [21].
Covariates
Data on potential confounding variables were collected by self-administered questionnaires on demographics, medical history, and lifestyle factors at baseline, and has been previously described [11]. Covariates included in the models were the following: total energy intake (kcal/day); age at WHI baseline (years); body mass index [BMI=weight (kg)/(height (m) x height (m))]; racial/ethnic groups (American Indian or Alaskan Native, Asian or Pacific Islander, Hispanic/Latino, African American, European American, and other race groups); educational levels categorized into some high school or lower educational level, high school graduate or some college or associate degree, and ≥4 years of college; smoking status (current, former, and never); regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAID) (yes/no); where regular use of medications was defined as: ≥2 times in each of the two weeks preceding the interview; physical activity, calculated by summing the metabolic equivalent tasks for all reported activities for each individual (e.g., walking, aerobics, jogging, tennis, swimming, biking outdoors, exercise machine, calisthenics, popular or folk dancing) (MET-hours/week) [22]; category and duration of estrogen use and category and duration of combined estrogen and progestin use categorized into five groups (none, ≤4.9y, 5–10.0y, 10.1–14.9y, and ≥15.0y); CHD case-control status, dietary modification trial arm (intervention, control, not randomized to the trial); hormone therapy trial arm (estrogen-alone intervention, estrogen-alone control, combined estrogen and progestin intervention, estrogen and progestin control, not randomized to trial); and calcium and vitamin D arm (intervention, control, not randomized to trial).
Statistical analysis
All metabolites were log-transformed and scaled to render with mean of zero and unit variance. Missing values below the limit of detection were assigned to half the lowest observed value. We described participants’ characteristics using means (standard deviations) for continuous variables, and frequencies (%) for categorical variables across quintiles of the EDIP score. Analyses were adjusted for covariates listed above.
For the discovery analysis in the larger WHI-HT dataset (n=1109), each metabolite was evaluated individually in multivariable-adjusted linear regression models in relation to 5-SD increments in the EDIP score. All models included BMI and the other factors listed above. Statistical significance was based on a two-sided P<0.05 and a corresponding false discovery rate-adjusted P-value of 0.05. Metabolites discovered in WHI-HT were evaluated individually in the independent WHI-OS dataset (n=810) using the same models as in the discovery analysis. Metabolite associations were considered to have been replicated based on a two-sided P<0.05 and a corresponding false discovery rate-adjusted p value of 0.05.
In addition, the subset of metabolites that were significantly associated with the EDIP score in the discovery dataset were evaluated in the discovery and validation datasets for association with CRP as the main predictor of interest, adjusting for the same factors as in the EDIP models. Plasma CRP was measured using a high sensitivity immunoturbidimetric assay on the Hitachi 911 (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN).
In secondary analyses, we conducted subgroup analysis in BMI categories: normal weight (15 to <25 kg/m2), overweight/obese (25 to ≤50 kg/m2), while adjusting for continuous BMI within BMI categories. Due to power considerations, stratified analyses were conducted in the combined WHI-OS and WHI-HT datasets. Statistical significance was based on a two-sided P<0.05 and a corresponding false discovery rate-adjusted P-value of 0.05. Additionally, and even though statistical power was limited, we conducted exploratory analyses in the BMI subgroups in separate discovery and replication datasets, similar to the primary analyses but at an FDR-adjusted P<0.10 for statistical significance.
RESULTS
Table 1 presents characteristics of study participants by EDIP quintiles. In both the discovery and replication datasets, women consuming the most anti-inflammatory diets (EDIP quintile 1) showed lower CRP concentrations, lower BMI and reported higher physical activity, compared to the women consuming the most pro-inflammatory diets (quintile 5). The proportions of obese women, African Americans, Hispanic/Latinos, and those with lower educational levels increased across EDIP quintiles (Table 1). Table 2 shows the distribution of foods and nutrients across EDIP quintiles. In terms of average weekly food intake, women with anti-inflammatory diets consumed 1.3 servings less red meat, 1.7 servings less processed meat, 5.9 servings less sugar-sweetened beverages, 1.3 servings less refined grains than women classified with pro-inflammatory diets. Also, women with anti-inflammatory diets consumed 17 glasses more wine, 4.2 cups more tea or coffee, and about 2.2 servings more dark-yellow vegetables and green leafy vegetables than the women with pro-inflammatory dietary patterns. Servings are defined in Table 2, footnote #3.
Table 1.
| Characteristic | WHI-HT: discovery (n=1109) | WHI-OS: replication (n=810) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quintile 1 (−4.99, <- 0.76) n=221 | Quintile 2 (−0.76, <- 0.18) n=222 | Quintile 3 (−0.18, <0.28) n=222 | Quintile 4 (0.28, <0.77) n=222 | Quintile 5 (0.77, 5.14) n=222 | Quintile 1 (−4.23, <- 0.75) n=162 | Quintile 2 (−0.75, <- 0.21) n=162 | Quintile 3 (−0.21, <0.30) n=162 | Quintile 4 (0.30, 0.74) n=162 | Quintile 5 (0.74, 3.63) n=162 | |
| Race/ethnicity | ||||||||||
| Black | 3.6 | 7.2 | 10.4 | 10.4 | 17.6 | 6.8 | 8.6 | 11.1 | 12.3 | 24.7 |
| Hispanic | 1.4 | 0.9 | 0.9 | 4.5 | 5.4 | 3.1 | 1.2 | 1.8 | 1.9 | 6.2 |
| White | 94.6 | 90.5 | 86.9 | 82.0 | 73.9 | 85.2 | 84.6 | 80.9 | 74.7 | 54.9 |
| Other3 | 0.4 | 1.4 | 1.8 | 3.1 | 3.1 | 4.9 | 5.6 | 6.2 | 11.1 | 14.2 |
| CRP, mg/L | 3.9 ± 5.0 | 4.3 ± 6.0 | 4.6 ± 8.3 | 5.1 ± 6.1 | 5.4 ± 5.3 | 4.1 ± 7.3 | 4.1± 5.2 | 4.3 ± 5.4 | 5.3 ± 7.5 | 5.6 ± 10.7 |
| Age, years | 66.6 ± 7.4 | 67.4 ± 6.5 | 67.5 ± 6.8 | 67.1 ± 6.4 | 65.9 ± 7.6 | 67.2 ± 6.6 | 68.6 ± 6.1 | 68.2 ± 6.4 | 67.8 ± 6.4 | 66.0 ± 7.8 |
| BMI, kg/m2 | 27.5 ± 5.1 | 28.7 ± 5.5 | 28.3 ± 5.2 | 28.7 ± 5.5 | 30.3 ± 6.1 | 26.4 ± 5.4 | 26.7 ± 4.4 | 26.7 ± 4.9 | 27.2 ± 5.7 | 28.6 ± 5.9 |
| Body mass index categories, % | ||||||||||
| 15 - < 18.5 | 0.5 | 0.5 | 0.9 | 0.9 | 1.8 | 1.9 | 0.6 | 0 | 1.9 | 1.8 |
| 18.5 - < 25 | 34.4 | 28.8 | 27.5 | 27.5 | 20.3 | 43.8 | 35.8 | 41.4 | 35.8 | 30.3 |
| 25-<30 | 38.0 | 31.5 | 41.9 | 33.3 | 27.5 | 37.0 | 39.5 | 37.0 | 36.4 | 31.5 |
| 30–50 | 27.1 | 39.2 | 29.7 | 38.3 | 50.4 | 17.3 | 24.1 | 21.6 | 25.9 | 36.4 |
| Physical activity, MET- hours/week | 8.2 ± 11.5 | 7.4 ± 12.6 | 5.7 ± 8.5 | 5.1 ± 7.8 | 4.0 ± 8.0 | 11.3 ± 13.7 | 8.5 ± 11.5 | 7.8 ± 11.5 | 7.2 ± 9.8 | 6.1 ± 9.8 |
| Smoking, % | ||||||||||
| Never | 42.1 | 53.1 | 46.4 | 48.2 | 52.3 | 45.1 | 47.5 | 49.4 | 46.9 | 49.4 |
| Former | 40.3 | 32.9 | 40.1 | 41.0 | 32.4 | 46.9 | 46.3 | 45.1 | 44.5 | 39.5 |
| Current | 17.6 | 14.0 | 13.5 | 10.8 | 15.3 | 8.0 | 6.2 | 5.6 | 8.6 | 11.1 |
| Aspirin/NSAIDs use, % | 15.4 | 18.5 | 20.3 | 14.0 | 14.9 | 14.8 | 15.4 | 17.3 | 12.3 | 10.5 |
| Educational level, % | ||||||||||
| Less than high school | 5.4 | 4.5 | 6.3 | 9.4 | 15.3 | 3.1 | 4.3 | 3.7 | 6.2 | 13.6 |
| High school/GED | 57.9 | 63.1 | 68.0 | 68.5 | 65.3 | 56.8 | 57.4 | 63.6 | 64.8 | 54.9 |
| ≥4 years of college | 36.7 | 32.4 | 25.7 | 22.1 | 19.4 | 40.1 | 38.3 | 32.7 | 29.0 | 31.5 |
EDIP, empirical dietary inflammatory pattern score; MET-h, metabolic equivalent hours; NSAID, nonsteroidal anti-inflammatory drugs; CRP, C-reactive protein; WHI, Women’s Health Initiative; HT, Hormone Therapy trial; OS, Observational Study.
Values are percentages or means ± SDs.
EDIP scores were adjusted for total energy intake using the residual method. Lower EDIP scores indicate anti-inflammatory diets whereas higher scores indicate pro-inflammatory diets.
Other race category included American Indian or Alaskan Native, Asian or Pacific Islander
Table 2.
Distribution of dietary intakes across quintiles of the EDIP score (combined discovery and replication datasets)
| Quintile 1 (−4.99 to <−0.76) n=383 |
Quintile 2 (−0.76 to <−0.19) n=384 |
Quintile 3 (−0.19 to <0.29) n=384 |
Quintile 4 (0.20 to <0.76) n=384 |
Quintile 5 (0.76 to 5.14) n=384 |
|
|---|---|---|---|---|---|
| Food/food groups, servings/week | |||||
| Red meat | 3.4 ± 3.0 | 3.2 ± 2.7 | 3.2 ± 2.8 | 3.3 ± 2.9 | 4.5 ± 4.5 |
| Processed meat | 1.7 ± 2.0 | 1.6 ± 1.7 | 1.6 ± 1.7 | 1.9 ± 2.1 | 2.9 ± 3.5 |
| Sugar-sweetened beverages | 0.7 ± 2.4 | 0.6 ± 2.0 | 0.6 ± 1.7 | 0.9 ± 2.2 | 4.1 ± 8.9 |
| Tomatoes | 3.9 ± 3.5 | 3.6 ± 3.2 | 3.4 ± 3.6 | 3.6 ± 3.6 | 4.1 ± 4.5 |
| Refined grains | 21.4 ± 12.2 | 20.3 ± 11.4 | 20.0 ± 11.6 | 21.6 ± 12.5 | 27.7 ± 16.6 |
| Wholegrains | 8.7 ± 5.7 | 8.1 ± 5.1 | 7.5 ± 5.3 | 7.5 ± 5.1 | 8.1 ± 6.4 |
| Wine | 3.4 ± 5.8 | 1.5 ± 2.8 | 0.8 ± 1.6 | 0.4 ± 1.3 | 0.2 ± 0.7 |
| Fruit juice | 4.9 ± 5.0 | 4.5 ± 4.4 | 4.3 ± 4.7 | 3.8 ± 4.0 | 3.8 ± 4.0 |
| Dark-yellow vegetables | 7.5 ± 5.3 | 6.0 ± 3.7 | 4.9 ± 4.1 | 4.5 ± 3.3 | 3.6 ± 2.9 |
| Green-leafy vegetables | 8.6 ± 6.5 | 6.8 ± 4.7 | 5.7 ± 4.0 | 4.7 ± 3.7 | 3.7 ± 3.2 |
| Coffee or tea | 29.4 ± 16.6 | 18.1 ± 9.9 | 13.5 ± 8.0 | 9.4 ± 7.1 | 7.0 ± 8.4 |
| Pizza | 0.5 ± 1.2 | 0.4 ± 0.5 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.2 ± 0.3 |
| Nutrient intakes | |||||
| Total fiber, g/d | 17.6 ± 7.2 | 16.3 ± 6.5 | 15.0 ± 6.4 | 14.5 ± 6.2 | 14.3± 6.9 |
| Total carbohydrate, g/d | 213 ± 76 | 200 ± 71 | 185 ± 74 | 186 ± 72 | 211 ± 95 |
| Total protein, g/d | 72.7 ± 28.8 | 66.0± 25.8 | 61.3 ± 26.1 | 61.5 ± 26.5 | 70.0 ± 34.6 |
| Total fat, g/d | 61.9 ± 33.0 | 57.0 ± 26.8 | 53.8 ± 30.9 | 55.4 ± 29.4 | 66.6 ± 41.7 |
| Saturated fat, g/d | 20.7 ± 11.7 | 19.3± 9.9 | 18.2 ± 11.4 | 18.2 ± 10.3 | 22.2± 14.8 |
| Total cholesterol, g/d | 230 ± 149 | 209 ± 116 | 194 ± 110 | 204 ± 138 | 246 ± 177 |
| Dietary calcium, mg/d | 928 ± 484 | 828 ± 437 | 758 ± 428 | 739 ± 422 | 739 ± 449 |
| Lycopene, mcg/d | 5362 ± 3647 | 4832 ± 3070 | 4551 ± 3187 | 4564 ± 3223 | 4559 ± 3456 |
EDIP, empirical dietary inflammatory pattern score
Values are means ± standard deviations.
EDIP scores were adjusted for total energy intake using the residual method. Lower EDIP scores indicate anti-inflammatory diets whereas higher scores indicate pro-inflammatory diets.
The EDIP component foods (servings per day) in the WHI were the following: processed meat (hot dogs, chorizo, other sausage, bacon, breakfast sausage, scrapple; lunch meat such as ham, turkey; other lunch meat such as bologna); red meat (ground meat including hamburgers, beef, pork, and lamb as a main dish or as a sandwich; stew, pot pie, and casseroles with meat; gravies made with meat drippings); organ meat (liver, including chicken liver; other organ meats); fish other than dark-meat fish (fried fish, shrimp, lobster, crab and oysters, canned tuna, tuna salad, and tuna casserole, white fish such as sole, snapper, cod); other vegetables (i.e., vegetables other than green leafy vegetables and dark yellow vegetables: red peppers and red chilies, green peppers, green chilies, jalapenos, and green chili salsa, corn, and hominy); refined grains (total grain variable minus whole grain variable, both WHI-computed food groups); high-energy beverages [all regular (not diet) soft drinks]; low-energy beverages (the WHI FFQ did not assess low-energy beverages); tomatoes (fresh tomato, tomato juice, tomato sauce, cooked tomato, salsa and salsa picante); beer (all types); wine (red wine, white wine); coffee or tea (all types); dark-yellow vegetables (carrots, including mixed dishes with carrots; summer squash, zucchini, nopales, and okra; winter squash, such as acorn, butternut, and pumpkin; sweet potatoes and yams; other potatoes, cassava, and yucca—boiled, baked, or mashed); green leafy vegetables (cooked greens such as spinach, mustard greens, turnip greens, collards; lettuce and plain lettuce salad; mixed lettuce or spinach salad with vegetables); pizza (low-fat pizza; other pizza); fruit juice (orange juice and grapefruit juice; other fruit juices such as apple and grape); snacks (snacks such as potato chips, corn chips, tortilla chips, Ritz and cheese crackers; saltines, Snackwell’s, fat-free tortilla chips and fat-free potato chips; popcorn).
In the discovery dataset, the EDIP score was associated with 77 metabolites at an FDR adjusted P-value <0.05 in multivariable-adjusted models and after additionally adjusting for BMI, 23 metabolites remained significantly associated (Table 3). Of the 23 metabolites, ten were replicated in the WHI-OS after adjustment for all covariates. The eight metabolites with inverse associations included: trigonelline, caffeine, 5-acethylamino-6-amino-3-methyluracil, 7-methylxanthine, 1,7-dimethyluric acid, 3-methylxanthine, C18:3 cholesterol ester, and glycine. Of the ten metabolites, four were also associated with CRP levels: Caffeine, 1,7-dimethyluric acid and C52:3 triacylglycerol were positively associated with CRP, whereas glycine was inversely associated (Table 4). Caffeine and 1,7-dimethyluric acid showed positive associations with CRP, in contrast with their inverse associations with the EDIP score.
Table 3.
Associations of the EDIP score with metabolites in the discovery (WHI-HT) and replication (WHI-OS) datasets1,2,3,4
| Associations in WHI-HT (discovery, n=1109) | Associations in WHI-OS (replication, n=810) | ||||
|---|---|---|---|---|---|
| Metabolite | HMDB # | Beta estimate (95%CI) | FDR –adjusted P-value | Beta estimate (95%CI) | FDR-adjusted P-value |
| Trigonelline | HMDB0000875 | −1.32 (−1.59, −1.05) | 8.88 E-19 | −1.11 (−1.44, −0.78) | 2.75 E-10 |
| Caffeine | HMDB0001847 | −0.48 (−0.74, −0.22) | 0.007 | −0.86 (−1.23, −0.49) | 1.34 E-05 |
| 5-acetylamino-6-amino-3-methyluracil | HMDB0004400 | −0.74 (−1.00, −0.48) | 2.63 E-06 | −0.80 (−1.16, −0.43) | 2.81 E-05 |
| 7-methylxanthine | HMDB0001991 | −0.52 (−0.81, −0.23) | 0.007 | −0.70 (−1.05, −0.34) | 1.47 E-04 |
| 1,7-dimethyluric acid | HMDB0011103 | −0.58 (−0.85, −0.31) | 0.002 | −0.72 (−1.10, −0.33) | 2.73 E-04 |
| 3-methylxanthine | HMDB0001886 | −0.55 (−0.84, −0.26) | 0.006 | −0.66 (−1.03, −0.30) | 4.00 E-04 |
| C52:3 TAG | HMDB0005384 | 0.43 (0.13, 0.73) | 0.042 | 0.50 (0.17, 0.82) | 0.003 |
| Linoleate | HMDB0000673 | 0.61 (0.30, 0.92) | 0.005 | 0.48 (0.11, 0.84) | 0.011 |
| C18:3 CE | HMDB0010370 | −0.56 (−0.84, −0.28) | 0.005 | −0.38 (−0.72, −0.03) | 0.032 |
| Glycine | HMDB0000123 | −0.42 (−0.70, −0.15) | 0.032 | −0.34 (−0.68, 0.01) | 0.055 |
| Isoleucine | HMDB0000172 | 0.38 (0.12, 0.65) | 0.042 | 0.23 (−0.11, 0.58) | 0.190 |
| Uracil | HMDB0000300 | −0.41 (−0.70, −0.13) | 0.042 | −0.31 (−0.61, 0.02) | 0.067 |
| C18:3 LPC | HMDB0010387 | −0.50 (−0.80, −0.20) | 0.016 | −0.26 (−0.59, 0.08) | 0.131 |
| C24:0 LPC | HMDB0008038 | −0.40 (−0.68, −0.13) | 0.042 | −0.19 (−0.51, 0.14) | 0.261 |
| Sebacate | HMDB0000792 | −0.50 (−0.81, −0.18) | 0.027 | 0.17 (−0.17, 0.52) | 0.328 |
| C34:0 PS | HMDB0012356 | −0.39 (−0.67, −0.12) | 0.045 | −0.16 (−0.53, 0.20) | 0.374 |
| Cortisol | HMDB0000063 | −0.55 (−0.84, −0.26) | 0.006 | −0.20 (−0.55, 0.15) | 0.265 |
| C12 carnitine | HMDB0002250 | 0.44 (0.15, 0.73) | 0.032 | 0.20 (−0.16, 0.57) | 0.277 |
| C12:1 carnitine | HMDB0013326 | 0.53 (0.24, 0.81) | 0.007 | 0.16 (−0.21, 0.52) | 0.402 |
| C14:1 carnitine | HMDB0002014 | 0.44 (0.15, 0.72) | 0.032 | 0.22 (−0.15, 0.58) | 0.245 |
| C14:2 carnitine | HMDB0013331 | 0.42 (0.14, 0.71) | 0.040 | 0.22 (−0.15, 0.58) | 0.238 |
| C56:5 TAG | HMDB0005406 | 0.45 (0.13, 0.77) | 0.048 | −0.06 (−0.40, 0.28) | 0.910 |
| C56:8 TAG | HMDB0005392 | 0.50 (0.21, 0.79) | 0.012 | −0.02 (−0.29, 0.26) | 0.721 |
HMDB, Human Metabolome Database
All values are beta estimates obtained from multivariable-adjusted linear regression models with 5-SD increments of the EDIP score as the main predictor of interest and metabolite as the main response variable of interest.
Metabolites in bold font are the 10 replicated metabolites
Models were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status.
Statistical significance was defined as false-discovery rate adjusted p<0.05.
Table 4.
Associations of CRP with metabolites in the discovery (WHI-HT) and replication (WHI-OS) datasets1,2,3,4
| HMDB # | Associations in WHI-HT (discovery, n=1109) | Associations in WHI-OS (replication, n=810) | |||
|---|---|---|---|---|---|
| Metabolite | Beta estimate (95%CI) | FDR-adjusted P-value | Beta estimate (95%CI) | FDR –adjusted P-value | |
| Trigonelline | HMDB0000875 | −0.09 (−0.13, −0.04) | 2.96 E-04 | −0.004 (−0.05, 0.04) | 0.867 |
| Caffeine | HMDB0001847 | 0.01 (−0.04, 0.05) | 0.767 | 0.08 (0.04, 0.13) | 9.67 E-04 |
| 5-acetylamino-6-amino-3-methyluracil | HMDB0004400 | −0.02 (−0.06, 0.03) | 0.431 | −0.01 (−0.06, 0.03) | 0.603 |
| 7-methylxanthine | HMDB0001991 | −0.02 (−0.07, 0.02) | 0.341 | −0.02 (−0.06, 0.03) | 0.477 |
| 1,7-dimethyluric acid | HMDB0011103 | 0.01 (−0.04, 0.05) | 0.673 | 0.07 (0.02, 0.12) | 0.007 |
| 3-methylxanthine | HMDB0001886 | −0.01 (−0.06, 0.04) | 0.708 | 0.01 (−0.04, 0.06) | 0.642 |
| C52:3 TAG | HMDB0005384 | 0.07 (0.02, 0.12) | 0.012 | 0.06 (0.02, 0.11) | 0.005 |
| Linoleate | HMDB0000673 | 0.08 (0.03, 0.13) | 0.004 | 0.02 (−0.02, 0.07) | 0.307 |
| C18:3 CE | HMDB0010370 | −0.07 (−0.11, −0.02) | 0.007 | −0.02 (−0.07, 0.02) | 0.339 |
| Glycine | HMDB0000123 | −0.18 (−0.23, −0.14) | 1.58 E-13 | −0.14 (−0.18, −0.10) | 1.72 E-09 |
| Isoleucine | HMDB0000172 | 0.12 (0.08, 0.16)) | 8.32 E-07 | 0.04 (−0.002, 0.09) | 0.062 |
| Uracil | HMDB0000300 | −0.02 (−0.07, 0.02) | 0.314 | −0.06 (−0.10, −0.01) | 0.011 |
| C18:3 LPC | HMDB0010387 | −0.18 (−0.23, −0.13) | 2.65 E-11 | −0.10 (−0.14, −0.05) | 2.33 E-05 |
| C24:0 LPC | HMDB0008038 | −0.16 (−0.20, −0.11) | 3.82 E-10 | −0.12 (−0.16, −0.08) | 5.59 E-08 |
| Sebacate | HMDB0000792 | −0.05 (−0.10, −0.001) | 0.047 | 0.03 (−0.01, 0.08) | 0.158 |
| C34:0 PS | HMDB0012356 | −0.04 (−0.09, 0.003) | 0.069 | 0.03 (−0.02, 0.08) | 0.202 |
| C12 carnitine | HMDB0002250 | 0.03 (−0.01, 0.08) | 0.152 | 0.01 (−0.04, 0.06) | 0.665 |
| C12:1 carnitine | HMDB0013326 | 0.07 (0.02, 0.12) | 0.005 | 0.02 (−0.02, 0.07) | 0.300 |
| C14:1 carnitine | HMDB0002014 | 0.06 (0.01, 0.10) | 0.024 | 0.01 (−0.04, 0.06) | 0.703 |
| C14:2 carnitine | HMDB0013331 | 0.03 (−0.02, 0.08) | 0.187 | 0.01 (−0.04, 0.05) | 0.785 |
| Cortisol | HMDB0000063 | −0.02 (−0.07, 0.03) | 0.462 | 0.002 (−0.04, 0.05) | 0.932 |
| C56:5 TAG | HMDB0005406 | −0.05 (−0.10, 0.002) | 0.058 | −0.01 (−0.04, 0.05) | 0.699 |
| C56:8 TAG | HMDB0005392 | 0.02 (−0.03, 0.07) | 0.407 | −0.02 (−0.06, 0.01) | 0.212 |
All values are beta estimates obtained from multivariable-adjusted linear regression modeling 5-unit increments of CRP levels as the main predictor of interest and metabolite as the main response variable of interest.
Metabolites in bold font are the 10 replicated metabolites
Models were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status.
Statistical significance was defined as false-discovery rate adjusted p<0.05.
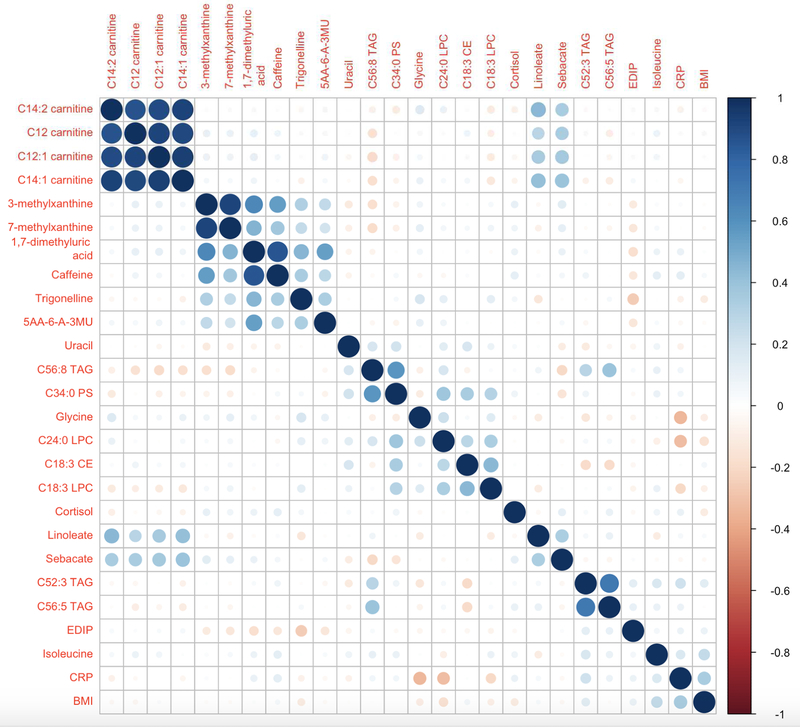
Figure 1 is a heat map in the replication dataset showing partial Spearman correlations between the 23 discovered metabolites with FDR P-value <0.05 and EDIP score, BMI and CRP, ordered by hierarchical clustering. The carnitines were highly clustered and showed no association with EDIP, CRP and BMI. The strongest inverse correlations with the EDIP were shown by trigonelline, −0.26, 1,7-dimethyluric acid, −0.17, 5-acetylamino-6-amino-3-methyluracil, −0.13, 7-methylxanthine, −0.13, and 3-methylxanthine, −0.12 (all P<0.001); whereas the strongest positive correlations with the EDIP were shown by C52:3 TAG, 0.12, C56:5 TAG, 0.10 (all P<0.004) (Figure 1).
Figure 1.
Heat map in the replication dataset showing partial Spearman correlations between the 23 discovered metabolites and EDIP score, BMI and CRP. Ordering is by hierarchical clustering. Correlations were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status. 5AA-6-A-3MU, 5-acetylamino-6-amino-3-methyluracil; BMI, body mass index; CE, cholesterol ester; CRP, C-reactive protein; EDIP, empirical dietary inflammatory pattern score; LPC, lysophosphatidylcholine; TAG, triacylglycerol
Secondary analyses suggested differences by BMI category. Among normal weight women (BMI: 15 to <25 kg/m2, n=630, including 20 thin women with BMI 15 - <18.5), after adjustment for covariates, including continuous BMI; four metabolites, related to coffee/caffeine metabolism (5-acetylamino-6-amino-3-methyluracil, trigonelline, 1,7-dimethyluric acid and caffeine) were inversely associated with the EDIP score (FDR adjusted P<0.05). None of the four metabolites was associated with CRP levels (Table 5). In contrast, among normal weight women, 63 metabolites showed significant associations with CRP after multivariable adjustment (FDR adjusted P<0.05). However, none of the four metabolites that associated with EDIP were among the 63 associated with CRP concentrations (data not shown). In the exploratory analyses, 26 metabolites were associated with EDIP at the raw P<0.05, and none at FDR-adjusted P<0.10, among normal weight women in the discovery dataset (Hormone Therapy trial: n=317) (Supplemental Table 1); therefore no replication was conducted among normal weight women.
Table 5.
Metabolite discovery among normal weight women (n=630), and an evaluation of association with C-reactive protein1,2,3
| Association with EDIP score | Association with CRP | ||||
|---|---|---|---|---|---|
| Metabolite | HMDB # | Beta estimate (95%CI) | FDR-adjusted P-value | Beta estimate (95%CI) | FDR-adjusted P-value |
| 5-acetylamino-6-amino-3-methyluracil | HMDB0004400 | −1.07 (−1.48, −0.67) | 9.72 E-05 | −0.003 (−0.07, 0.07) | 0.932 |
| Trigonelline | HMDB0001847 | −0.86 (−1.24, −0.48) | 0.002 | −0.01 (−0.08, 0.05) | 0.736 |
| 1,7-dimethyluric acid | HMDB0011103 | −0.86 (−1.28, −0.43) | 0.008 | 0.05 (−0.02, 0.12) | 0.392 |
| Caffeine | HMDB0001847 | −0.89 (−1.33, −0.45) | 0.008 | 0.05 (−0.02, 0.13) | 0.372 |
All values are beta estimates obtained from multivariable-adjusted linear regression modeling 5-SD increments in EDIP or 5-unit increments in CRP as the main predictor of interest and metabolite as the main response variable of interest.
Models were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status.
Statistical significance was defined as false-discovery rate adjusted p<0.05.
Among overweight or obese women (BMI: 25 to 50 kg/m2, n=1289), after adjustment for covariates, including continuous BMI, 110 metabolites were significantly associated with the EDIP score after multivariable adjustment (FDR adjusted P<0.05) (data not shown); and 83 remained significant after additionally adjusting for BMI (FDR adjusted P<0.05) (Table 6). In multivariable-adjusted models, 196 metabolites were significantly associated with CRP concentrations and 178 remained significant with additional adjustment for continuous BMI (FDR adjusted P<0.05) (data not shown). Of the 83 metabolites associated with EDIP, 40 (48%) were also associated with CRP (Table 6). The 83 metabolites included alkaloids and derivatives, cholesterol esters, lysophosphatidylcholines, sphingomyelins, amino acids, purines and pyrimidines and that were significantly higher with lower dietary inflammatory potential, whereas diacyleglycerols, triacylglycerols and bile acids increased with higher dietary inflammatory potential. In the exploratory analyses among overweight and obese women, we found 45 metabolites to be significantly associated with EDIP at FDR-adjusted P<0.10 in the discovery dataset (Hormone Therapy trial, n=792) (Supplemental Table 2). In the replication dataset (Observational Study: n=497), 16 of these metabolites associated with EDIP at FDR-adjusted P<0.05 (Supplemental Table 3).
Table 6.
Metabolite discovery among overweight or obese women (n=1289), and an evaluation of the associations with C-reactive protein1,2,3,4,5
| Association with EDIP score | Association with CRP | |||||
|---|---|---|---|---|---|---|
| Metabolite | Category and metabolic pathway | HMDB # | Beta estimate (95%CI) | FDR adjusted P-value | Beta estimate (95%CI) | FDR adjusted P-value |
| Trigonelline | Alkaloids and derivatives | HMDB0000875 | −1.38 (−1.63, −1.13) | 1.44 E-24 | −0.04 (−0.07, −00009) | 0.049 |
| 1,7−dimethyluric acid | Alkaloids and derivatives | HMDB0011103 | −0.68 (−0.94, −0.42) | 2.59 E-05 | 0.02 (−0.02, 0.06) | 0.346 |
| 7−methylxanthine | Alkaloids and derivatives | HMDB0001991 | −0.63 (−0.88, −0.37) | 1.08 E-04 | −0.02 (−0.06, 0.01) | 0.202 |
| 3−methylxanthine | Alkaloids and derivatives | HMDB0001886 | −0.62 (−0.87, −0.36) | 1.08 E-04 | −0.01 (−0.05, 0.03) | 0.612 |
| Caffeine | Alkaloids and derivatives | HMDB0001847 | −0.54 (−0.79, −0.29) | 7.33 E-04 | 0.02 (−0.01, 0.06) | 0.199 |
| Piperine | Alkaloids and derivatives | HMDB0029377 | 0.33 (0.08, 0.58) | 0.034 | 0.01 (−0.02, 0.05) | 0.482 |
| C18:1 CE | Cholesterol esters | HMDB0000918 | −0.43 (−0.70, 0.17) | 0.010 | −0.06 (−0.09, −0.02) | 0.006 |
| C18:2 CE | Cholesterol esters | HMDB0000610 | −0.45 (−0.75, −0.19) | 0.007 | −0.05 (−0.09, −0.01) | 0.009 |
| C18:3 CE | Cholesterol esters | HMDB0010370 | −0.52 (−0.78, −0.25) | 0.002 | −0.04 (−0.07, 0.002) | 0.064 |
| C20:2 CE | Cholesterol esters | HMDB0006734 | −0.39 (−0.64, −0.13) | 0.015 | −0.05 (−0.09, −0.02) | 0.006 |
| C20:3 CE | Cholesterol esters | HMDB0006736 | −0.34 (−0.61, −0.07) | 0.043 | 0.02 (−0.02, 0.05) | 0.394 |
| C20:4 CE | Cholesterol esters | HMDB0006726 | −0.33 (−0.59, −0.06) | 0.047 | −0.01 (−0.04, 0.03) | 0.741 |
| C22:4 CE | Cholesterol esters | HMDB0006729 | −0.40 (−0.67, −0.14) | 0.016 | −0.04 (−0.08, −0.002) | 0.040 |
| C15:0 LPC | Lysophosphatidylcholines | HMDB0010381 | −0.33 (−0.60, −0.06) | 0.047 | −0.12 (−0.15, −0.08) | 2.16 E-08 |
| C18:2 LPC | Lysophosphatidylcholines | HMDB0010386 | −0.40, −0.67, −0.14) | 0.014 | −0.15 (−0.19, −0.12) | 1.31 E-14 |
| C18:3 LPC | Lysophosphatidylcholines | HMDB0010387 | −0.44 (−0.72, −0.17) | 0.011 | −0.10 (−0.14, −0.06) | 1.75 E-06 |
| C20:1 LPC | Lysophosphatidylcholines | HMDB0010391 | −0.51 (−0.79, −0.24) | 0.003 | −0.09 (−0.13, −0.05) | 3.89 E-05 |
| C20:5 LPC | Lysophosphatidylcholines | HMDB0010397 | −0.40 (−0.66, −0.14) | 0.015 | −0.15 (−0.19, −0.11) | 6.29 E-14 |
| C24:0 LPC | Lysophosphatidylcholines | HMDB0008038 | −0.44 (−0.70, −0.18) | 0.007 | −0.12 (−0.16, −0.08) | 2.72 E-09 |
| C36:0 PC | Phosphatidylcholines | HMDB0008036 | −0.33 (−0.60, −0.06) | 0.048 | −0.03 (−0.07, 0.01) | 0.111 |
| C40:11 PC plasmalogen | Phosphatidylcholine plasmalogens | NA | −0.34 (−0.59, −0.08) | 0.038 | 0.01 (−0.03, 0.05) | 0.653 |
| C18:1 LPE | Lysophosphatidylethanolamines | HMDB0011506 | −0.33 (−0.60, −0.06) | 0.047 | −0.07 (−0.11, −0.04) | 5.37 E-04 |
| C14:0 SM | Sphingomyelins | HMDB0012097 | −0.45 (−0.72, −0.19) | 0.007 | 0.01 (−0.03, 0.05) | 0.633 |
| C20:0 SM | Sphingomyelins | HMDB0012102 | −0.34 (−0.61, −0.07) | 0.043 | 0.01 (−0.02, 0.05) | 0.471 |
| C22:0 SM | Sphingomyelins | HMDB0012103 | −0.33 (−0.61, −0.06) | 0.047 | 0.03 (−0.01, 0.07) | 0.109 |
| C22:1 SM | Sphingomyelins | HMDB0012104 | −0.32 (−0.59, −0.05) | 0.050 | −0.01 (−0.05, 0.03) | 0.634 |
| C24:0 SM | Sphingomyelins | HMDB0011697 | −0.41 (−0.68, −0.14) | 0.015 | −0.01 (−0.04, 0.03) | 0.764 |
| C24:1 SM | Sphingomyelins | HMDB0012107 | −0.34 (−0.60, −0.07) | 0.043 | 0.05 (0.01, 0.09) | 0.016 |
| 5−acetylamino−6−amino−3−methyluracil | Amino acids | HMDB0004400 | −0.84 (−1.09, −0.58) | 1.70 E-08 | −0.01 (−0.04, 0.03 | 0.723 |
| Phenylalanine−d8 | Amino acids | Internal Standard | −0.44 (−0.72, −0.17) | 0.012 | 0.003 (−0.04, 0.04) | 0.887 |
| Glycine | Amino acids | HMDB0000123 | −0.40 (−0.65, −0.15) | 0.013 | −0.14 (−0.18, −0.11) | 3.12 E-13 |
| Asparagine | Amino acids | HMDB0000168 | −0.36 (−0.61, −0.11) | 0.022 | −0.10 (−0.13, −0.06) | 1.27 E-06 |
| Taurine | Amino acids | HMDB0000251 | −0.36 (−0.63, −0.09) | 0.032 | 0.005 (−0.03, 0.04) | 0.817 |
| Proline betaine | Amino acids | HMDB0004827 | −0.35 (−0.60, −0.09) | 0.034 | −0.02 (−0.06, 0.02) | 0.311 |
| ADMA | Amino acids | HMDB0001539 | −0.34 (−0.61, −0.07) | 0.043 | −0.05 (−0.09, −0.01) | 0.016 |
| Serine | Amino acids | HMDB0000187 | −0.33 (−0.60, −0.06) | 0.048 | −0.12 (−0.16, −0.08) | 2.96 E-08 |
| N−methylproline | Amino acids | NA | −0.31 (−0.57, −0.05) | 0.050 | −0.03 (−0.06, 0.01) | 0.174 |
| Isoleucine | Amino acids | HMDB0000172 | 0.31 (0.07, 0.56) | 0.043 | 0.07 (0.03, 0.10) | 4.45 E-04 |
| Uracil | Purines and pyrimedines | NA | −0.47 (−0.74, −0.21) | 0.005 | −0.03 (−0.07, 0.01) | 0.130 |
| Uridine | Purines and pyrimedines | NA | −0.38 (−0.64, −0.13) | 0.016 | −0.04 (−0.07, 0.001) | 0.060 |
| AMP | Purines and pyrimedines | NA | −0.33 (−0.60, −0.06) | 0.046 | 0.03 (−0.01, 0.07) | 0.110 |
| GMP | Purines and pyrimedines | NA | −0.33 (−0.60, −0.06) | 0.047 | 0.01 (−0.03, 0.05) | 0.495 |
| Indole−3−propionate | Indoles and derivatives | NA | −0.31 (−0.57, −0.06) | 0.047 | −0.05 (−0.09, −0.01) | 0.010 |
| Cortisol | Steroids | HMDB0000063 | −0.46 (−0.73, −0.20) | 0.006 | −0.01 (−0.05, 0.03) | 0.605 |
| Pantothenate | Vitamins and minerals | HMDB0000210 | −0.37 (−0.63, −0.11) | 0.022 | 0.01 (−0.03, 0.05) | 0.555 |
| Hexose−monophosphate | NA | NA | −0.31 (−0.58, −0.05) | 0.050 | 0.02 (−0.02, 0.06) | 0.320 |
| Eicosapentaenoate | Fatty acids | HMDB0001999 | −0.46 (−0.71, −0.21) | 0.004 | −0.09 (−0.12, −0.05) | 1.19 E-05 |
| 2−hydroxyhexadecanoate | Fatty acids | HMDB0031057 | 0.33 (0.06, 0.59) | 0.047 | 0.02 (−0.02, 0.06) | 0.302 |
| C32:0 DAG | Diacylglycerols | HMDB0007098 | 0.49 (0.23, 0.75) | 0.003 | 0.07 (0.03, 0.11) | 6.73 E-04 |
| C32:1 DAG | Diacylglycerols | HMDB0007099 | 0.40 (0.14, 0.66) | 0.014 | 0.04 (0.01, 0.08) | 0.022 |
| C34:0 DAG | Diacylglycerols | HMDB0007100 | 0.49 (0.22, 0.76) | 0.004 | 0.04 (0.002, 0.08) | 0.037 |
| C34:1 DAG | Diacylglycerols | HMDB0007102 | 0.53 (0.27, 0.79) | 0.001 | 0.06 (0.03, 0.10) | 0.001 |
| C34:2 DAG | Diacylglycerols | HMDB0007103 | 0.51 (0.25, 0.77) | 0.002 | 0.05 (0.01, 0.09) | 0.008 |
| C34:3 DAG | Diacylglycerols | HMDB0007132 | 0.40 (0.14, 0.66) | 0.014 | 0.02 (−0.01, 0.06) | 0.193 |
| C36:1 DAG | Diacylglycerols | HMDB0007216 | 0.50 (0.24, 0.76) | 0.002 | 0.05 (0.01, 0.08) | 0.018 |
| C36:0 DAG | Diacylglycerols | HMDB0007158 | 0.36 (0.10, 0.61) | 0.028 | 0.002 (−0.03, 0.04) | 0.918 |
| C36:2 DAG | Diacylglycerols | HMDB0007218 | 0.47 (0.20, 0.73) | 0.005 | 0.03 (−0.01, 0.07 | 0.108 |
| C36:3 DAG | Diacylglycerols | HMDB0007219 | 0.44 (0.17, 0.71) | 0.010 | 0.01 (−0.03, 0.05) | 0.664 |
| C36:4 DAG−A | Diacylglycerols | HMDB0007248 | 0.34 (0.07, 0.60) | 0.042 | −0.01 (−0.03, 0.04) | 0.739 |
| C36:4 DAG−B | Diacylglycerols | HMDB0007248 | 0.36 (0.11, 0.61) | 0.022 | 0.04 (0.003, 0.07) | 0.034 |
| C38:3 DAG | Diacylglycerols | HMDB0007168 | 0.41 (0.14, 0.68) | 0.014 | 0.02 (−0.02, 0.06) | 0.291 |
| C48:0 TAG | Triacylglycerols | HMDB0005356 | 0.39 (0.09, 0.61) | 0.035 | 0.07 (0.03, 0.11) | 5.37 E-04 |
| C48:1 TAG | Triacylglycerols | HMDB0005359 | 0.38 (0.12, 0.64) | 0.021 | 0.05 (0.01, 0.09) | 0.012 |
| C50:0 TAG | Triacylglycerols | HMDB0005357 | 0.36 (0.10, 0.62) | 0.028 | 0.06 (0.02, 0.09)) | 0.005 |
| C50:1 TAG | Triacylglycerols | HMDB0044109 | 0.54 (0.29, 0.80) | 7.33 E-04 | 0.08 (0.04, 0.12) | 8.94 E-05 |
| C50:2 TAG | Triacylglycerols | HMDB0005377 | 0.50 (0.24, 0.75) | 0.002 | 0.07 (0.04, 0.11) | 4.02 E-04 |
| C50:3 TAG | Triacylglycerols | HMDB0005433 | 0.39 (0.13, 0.65) | 0.015 | 0.04 (0.002, 0.08) | 0.040 |
| C51:1 TAG | Triacylglycerols | HMDB0042104 | 0.36 (0.11, 0.62) | 0.024 | 0.04 (0.01, 0.08) | 0.024 |
| C51:2 TAG | Triacylglycerols | HMDB0049721 | 0.30 (0.05, 0.56) | 0.050 | 0.03 (−0.01, 0.07) | 0.106 |
| C51:3 TAG | Triacylglycerols | NA | 0.36 (0.10, 0.62) | 0.028 | 0 (−0.04, 0.04) | 0.990 |
| C52:0 TAG | Triacylglycerols | HMDB0005365 | 0.34 (0.08, 0.61) | 0.039 | 0.03 (−0.01, 0.07) | 0.103 |
| C52:1 TAG | Triacylglycerols | HMDB0005367 | 0.47 (0.22, 0.73) | 0.004 | 0.08 (0.04, 0.12) | 0.002 |
| C52:2 TAG | Triacylglycerols | HMDB0005369 | 0.56 (0.31, 0.82) | 5.77 E-04 | 0.06 (0.03, 0.10) | 0.002 |
| C52:3 TAG | Triacylglycerols | HMDB0005384 | 0.56 (0.30, 0.82) | 7.33 E-04 | 0.05 (0.01, 0.08) | 0.018 |
| C52:4 TAG | Triacylglycerols | HMDB0005363 | 0.41 (0.15, 0.68) | 0.014 | 0.01 (−0.03, 0.05) | 0.590 |
| C54:1 TAG | Triacylglycerols | HMDB0005395 | 0.36 (0.10, 0.62) | 0.028 | 0.04 (−0.001, 0.07) | 0.057 |
| C54:2 TAG | Triacylglycerols | HMDB0005403 | 0.47 (0.21, 0.73) | 0.004 | 0.04 (0.01, 0.08) | 0.020 |
| C54:3 TAG | Triacylglycerols | HMDB0005405 | 0.43 (0.17, 0.70) | 0.010 | 0.02 (−0.02, 0.05) | 0.397 |
| C56:8 TAG | Triacylglycerols | HMDB0005392 | 0.43 (0.16, 0.70) | 0.013 | 0.03 (−0.01, 0.06) | 0.197 |
| Glycolithocholate | Bile acids | HMDB0000698 | 0.32 (0.08, 0.56) | 0.036 | −0.03 (−0.06, 0.01) | 0.120 |
| Deoxycholate isomer−G | Bile acids | NA | 0.30 (0.05, 0.55) | 0.050 | −0.04 (−0.07, −0.001) | 0.044 |
| C6 carnitine | Acylcarnitines | HMDB0000705 | 0.32 (0.05, 0.59) | 0.047 | 0.04 (0.01, 0.08) | 0.022 |
All values are beta estimates obtained from multivariable-adjusted linear regression modeling 5-SD increments in EDIP score or 5-unit increments in CRP levels as the main predictor of interest and metabolite as the main response variable of interest.
Metabolites in bold font are 9 of the 10 replicated metabolites or metabolites (n=40 metabolites) also significantly associated with CRP.
Models were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status.
Statistical significance was defined as false-discovery rate adjusted p<0.05.
In the multivariable models, 110 metabolites were significantly associated with the EDIP score and 83 remained significant after additionally adjusting for BMI. In multivariable-adjusted models, 196 metabolites were significantly associated with CRP and 178 remained significant with additional adjustment for continuous BMI. Of the 83 metabolites associated with EDIP, 40 (48%) were also associated with CRP.
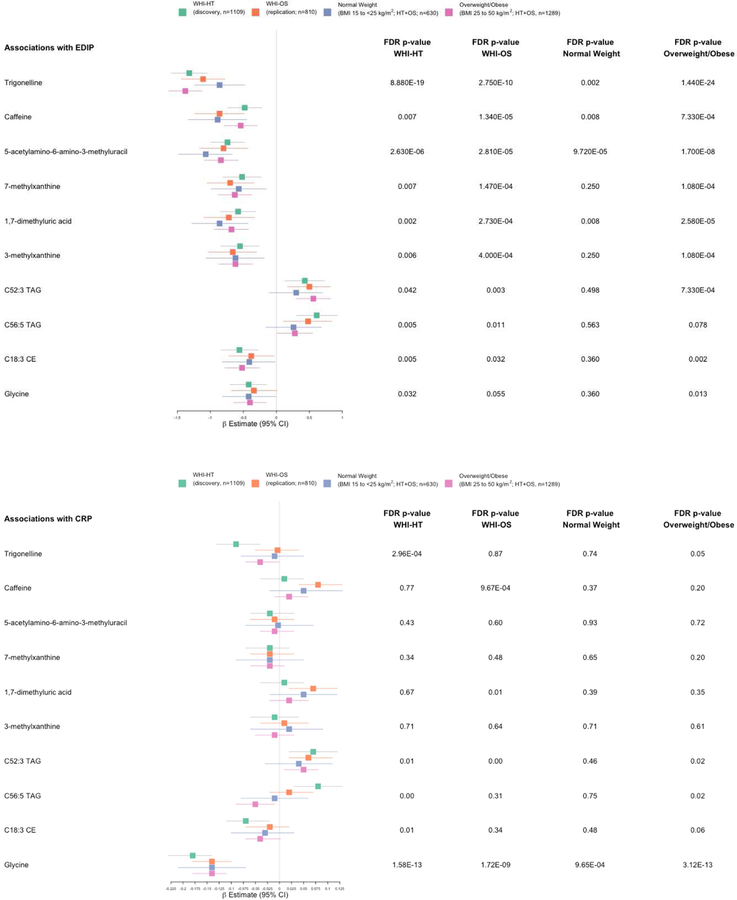
A summary of the associations of the ten replicated metabolites in the discovery and replication datasets and in BMI categories, is presented in Figure 2. In both the discovery and validation datasets, all ten metabolites were significant, both nominally at the 2-sided P<0.05 and at the FDR adjusted P<0.05. Among normal weight women, eight of the ten (except the two TAGs) were nominally significant and four of these were significant at FDR adjusted P<0.05; whereas among overweight or obese women, nine of the ten (except C56:5 TAG) were significant both nominally and at FDR adjusted P<0.05. For CRP associations, in the discovery dataset, three of the ten metabolites were nominally significant and two of these were also FDR significant, whereas in the replication dataset, four metabolites were both nominally and FDR significant. Among normal weight women, only one metabolite (glycine) was associated both nominally and at FDR adjusted P<0.05, whereas among overweight or obese women, four metabolites were associated both nominally and at FDR adjusted P<0.05. All associations (including in the subgroups) were adjusted for continuous BMI.
Figure 2.
Summary of EDIP and CRP associations with the 10 replicated metabolites in the discovery and replication datasets and in body mass index categories. Associations were adjusted for body mass index (continuous) age, physical activity, educational level, race/ethnicity, aspirin/NSAIDs use, smoking status, WHI Hormone Therapy trial arm, CHD case-control status.
DISCUSSION
In the current study, using a robust methodology, we identified ten metabolites correlated with the inflammatory potential of diet, independent of energy intake, race/ethnicity, BMI, physical activity and smoking; Adding biologic plausibility, four of the ten were also significantly associated with CRP levels.
Body weight appeared to play an important role in modifying these associations. For example, among normal weight women, four metabolites related to coffee/caffeine metabolism (5-acetylamino-6-amino-3-methyluracil, trigonelline, 1,7-dimethyluric acid and caffeine) were inversely associated with the EDIP score, but none of them were associated with CRP levels. In contrast, among overweight or obese women, up to 83 metabolites including lower levels of alkaloids and derivatives (n=6), cholesterol esters (n=7), lysophosphatidylcholines (n=6), sphingomyelins (n=6), amino acids (n=10) and purines & pyrimidines (n=4) decreased with higher dietary inflammatory potential; and higher levels of diacyleglycerols (n=13), triacylglycerols (n=18) and bile acids (n=2) were associated with higher inflammatory potential of the diet.
Six of the ten replicated metabolites were coffee/caffeine-related metabolites (trigonelline, caffeine, 7-methylxanthine, 5-acethylamino-6-amino-3-methyluracil, 3-methylxanthine, 1,7-dimethyluric acid) which were all associated with lower inflammatory potential of the diet. Two of the other four metabolites, a cholesterol ester (C18:3 CE) and a nonessential amino acid (glycine) were also inversely associated with dietary inflammatory potential whereas the remaining two replicated metabolites contributing to higher dietary inflammatory potential were a triacylglycerol (C52:3 TAG) and an omega-6 fatty acid (linoleate). These ten metabolites also reflect the food group composition of the EDIP score. For example, the six caffeine-related metabolites are indicative of coffee and tea, two inverse components of the EDIP score with a naturally high caffeine content [23], and C18:3 CE (a cholesterol ester) may be indicative of the vegetable components of the EDIP. Cholesterol esters generally contain high proportions of C18 fatty acids including alpha-linolenic acid found in plant-based oils [24]. However, excess omega-6 fatty acids (e.g., linoleate) from vegetable oils and meat-based foods may interfere with the health benefits of omega-3 fats, partly because they compete for the same rate-limiting enzymes [25]. In addition, omega-6 fatty acids are typically associated with pro-inflammatory responses [26, 27] in line with the current study findings for linoleate. The triacylglycerol is also indicative of the meat-based components which contributes to higher dietary inflammatory potential in the EDIP score [28]. Triacylglycerols are major components of very low density lipoprotein cholesterol, and play an important role in metabolism as energy sources and transporters of dietary fat [28].
In the biologic validation analysis, four of the ten replicated metabolites were also associated with CRP levels. The two caffeine-related metabolites (caffeine, and 1,7-dimethyluric acid), although inversely associated with the EDIP score, were positively associated with CRP levels. This is in line with previous studies of coffee and caffeine showing a predominant anti-inflammatory action of coffee but not of caffeine consumption [29]. A review of 15 clinical trials of coffee (8 studies) and caffeine (7 studies) found increased adiponectin levels in four of seven trials comparing filtered or caffeinated coffee with placebo; or comparing its levels at baseline and after consumption of medium or dark roasted coffee; but no change in adiponectin levels in the caffeine trials. Caffeine increased interleukin (IL)-6 levels in three of five studies and IL-10 levels in two of three trials [29]. Moreover, in a large previous study of 15,538 women and 7,393 men, we found that habitual coffee consumption (≥4 cups/day of total coffee compared to nondrinkers) was associated with lower concentrations of inflammatory biomarkers (CRP, IL6, TNF alpha receptor 2), and the associations were not different by caffeine content [30]. These findings suggest that caffeine may actually have a pro-inflammatory effect and that the anti-inflammatory effects of coffee may therefore be mediated through other metabolites. For example, in the current study, high trigonelline levels were associated with lower dietary inflammatory potential but not associated with systemic inflammation (CRP). Trigonelline is an alkaloid in coffee, and is degraded to an extent during coffee roasting to produce niacin (vitamin B3), which has been shown to suppress colonic inflammation [31] and colon cancer in mice [32]. In addition, a randomized crossover trial that enrolled 15 overweight men to evaluate the acute effects of decaffeinated coffee and major coffee compounds including chlorogenic acid and trigonelline, found that these two metabolites reduced early glucose and insulin secretion [33].
Glycine was inversely associated with both EDIP and CRP whereas C52:3 TAG was positively associated with both predictors. Several amino acids including glycine have been shown to have anti-inflammatory properties [34]. In a study that treated 74 diabetes patients with 5g/day glycine or 5 g/day placebo, patients treated with glycine had a significant decrease in HbA1C, pro-inflammatory cytokines and IFN-gamma [35]. C52:3 TAG (palmitic acid, C16:0) is obtained mainly from vegetable oil and animal fats, and though endogenous synthesis contributes a significant portion of this saturated fatty acid in the circulation [36], serum levels have been found to correlate well with dietary intake [37]. Recent findings suggest that dietary fats can influence gut microbiota composition and that this can affect inflammatory status in vivo (31) but whether dietary fats substantially influence disease outcomes by affecting the inflammatory status of people via alterations in their gut microbiome remains untested.
Associations differed by body weight. The metabolites identified among normal weight women were all inversely associated with the EDIP score, indicating that these women were generally consuming an anti-inflammatory (high quality) dietary pattern. Additionally, normal weight women had lower (more anti-inflammatory) EDIP scores. An anti-inflammatory dietary pattern would be expected to be associated with lower concentrations of circulating CRP concentrations. However, none of the four metabolites was associated with CRP. It is possible that among normal weight women, the beneficial effects of a high-quality dietary pattern are mediated via pathways other than those directly involving systemic inflammation. In contrast, among overweight or obese women, 83 metabolites were significantly associated with the EDIP score, and about half were also associated with CRP. In contrast to normal weight women, the 83 metabolites identified in overweight/obese women include several metabolite categories other than alkaloid and derivatives. In addition, the alkaloid trigonelline that was inversely associated with EDIP but not with CRP among normal weight women, was significantly inversely associated with both predictors among overweight/obese women (n=1289), suggesting that the lack of associations with CRP among normal weight women (n=630) could also be due to low statistical power. Among the overweight/obese group, most of the metabolite associations with EDIP were consistent with the CRP associations. Higher CRP concentrations were associated with higher levels of most triacylglycerols and diacylglycerols and with lower levels of most lysophosphatidylcholines, cholesterol esters and fatty acids, but were generally not associated with alkaloids and derivatives or sphingomyelins.
The differences by body weight in the metabolomic profiles observed in the current study, reflect differences by body weight in the association of the EDIP score with weight change and with several inflammatory biomarkers in previous studies [1, 2, 7, 10]. In these previous studies, EDIP predicted higher concentrations of inflammatory biomarkers and weight changes among overweight or obese participants than among normal weight participants. Interestingly, higher EDIP scores were strongly associated with lower concentrations of adiponectin in these previous studies, especially among overweight or obese participants. Adiponectin is a hormone secreted by adipose tissue [38] and lower adiponectin concentrations are associated with higher adiposity and inflammation [39]. Taken together, these findings suggest that the EDIP is sensitive to dietary patterns associated with obesity and therefore may be more strongly associated with obesity-related inflammation in line with the metabolomic profile identified among overweight/obese women.
Among overweight or obese women, results showed a positive association between higher EDIP scores and higher levels of diacylglycerols and triacylglycerols consistent with reduced lipid turnover rate and increased storage of triglycerides observed in obesity [40]. Specifically, lysophospholipids are signaling molecules involved in modulating processes such as inflammation, insulin secretion and insulin sensitivity through their interaction with G protein-coupled receptors [41, 42]. Lysophospholipids may therefore be important in obesity and related diseases such as cancer. A study assessing plasma lysophospholipids in obesity found that a multivariable-adjusted combination of 26 lysophospholipids could discriminate between normal weight and obese participants with an accuracy of 98% [43]. Among the obese participants, the authors found decreased concentrations of different lysophospholipid species including lysophosphatidylcholines, and lysophosphatidylethanolamines [43]. These results are consistent with those observed in the current study, in which nine lysophospholipids were inversely associated with higher dietary inflammatory potential among overweight/obese women, and seven of these were also inversely associated with CRP, a widely studied biomarker of inflammation.
Our study has several strengths, including a well-validated metabolomics platform, detailed covariate data, and a robust methodology. Notably, our methodology includes the integration of dietary data with metabolomics and biomarker data to characterize the metabolomics profiles of inflammatory diets – an analytic approach that to t he best of our knowledge, has not yet been conducted. Limitations of our study include known measurement error in using an FFQ for the assessment of diet, such as underreporting of energy and protein intake [44, 45]. Given that energy misreporting may depend on BMI, we adjusted all models for BMI including the adjustment of BMI subgroup analysis for continuous BMI. In addition, BMI as a measure of body fatness (adiposity) is limited in not being able to distinguish between excess fat, muscle, or bone mass, and does not measure body fat distribution. However, BMI is a reasonable measure of weight status in populations and as is a good screening tool to identify potential weight problems in individuals. Our findings may also have limited generalizability, therefore additional data are needed to identify whether these results are specific to postmenopausal women or whether there are similar profiles in younger premenopausal women or in men. In addition, we had limited sample sizes in BMI subgroup analyses and could not conduct statistical replication of the metabolites found, but we note that nine of the ten replicated metabolites were among the 83 significant metabolites in overweight/obese women at the FDR adjusted P<0.05. In addition, we also conducted similar subgroup analyses using CRP. Furthermore, we did not have data on other inflammatory biomarkers (e.g., IL6, TNF alpha receptor 2 etc), and CRP may not be comprehensively reflective of systemic inflammation.
In summary, in two separate samples of postmenopausal women, multiple metabolites associated with the inflammatory potential of diet were identified, statistically replicated and biologically validated using plasma CRP levels. We found that metabolites associated with coffee/caffeine and lipid metabolism may inform on the underlying biological pathways through which inflammatory diets may influence disease outcomes. Also, metabolites identified may provide insights on the role of inflammatory dietary patterns in obesity-related inflammation. The linkage of these findings to inflammation-related disease requires further study.
Supplementary Material
Highlights.
Diet may influence disease risk and progression through inflammation
The specific mechanisms involved are not fully understood
Metabolomic profiling of inflammatory diets may therefore provide insights
We identified and replicated 10 metabolites associated with diet-related inflammation
Metabolites associated with lipid metabolism may reflect diet-related inflammation
Acknowledgments
FUNDING
Dr. Fred K. Tabung was supported by National Cancer Institute grant numbers K99CA207736 and R00CA207736. Dr. Paulette D. Chandler received support from grant U01CA138962 from the National Cancer Institute and grant 127524-MRSG-15-012-01-CNE from the American Cancer Society. The National Institutes of Health funded the WHI program through contracts #: HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C and HHSN268201600004C. The funding agencies played no role in the project planning, data analysis, results interpretation and manuscript preparation.
Abbreviations:
- BMI
Body mass index
- EDIP
Empirical dietary inflammatory pattern score
- FDR
False Discovery Rate
- FFQ
Food frequency questionnaire
- HMDB
Human Metabolome Database
- MET-hours
Metabolic equivalent hours
- NHS
Nurses’ Health Study
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PA
Physical activity
- US
United States
- WHI-HT
Women’s Health Initiative – Hormone Therapy
- WHI-OS
Women’s Health Initiative – Observational Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
REFERENCES
- [1].Tabung FK, Smith-Warner Stephanie A, Chavarro Jorge E, Wu Kana, Fuchs Charles S, Hu Frank B, Chan Andrew T, Willett Walter C, Giovannucci Edward L. Development and validation of an empirical index of dietary inflammatory potential. The Journal of Nutrition 2016;146:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tabung FK, Giovannucci Edward L, Giulianini Franco, Liang Liming, Chandler Paulette D, Balasubramanian Raji, Manson JoAnn E, Cespedes Feliciano Elizabeth M, Hayden Kathleen M, Van Horn Linda, Rexrode Kathryn M An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. The Journal of Nutrition 2018;148:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tabung FK, Liu Li, Wang Weike, Fung Teresa T, Wu Kana, Smith-Warner Stephanie A, Cao Yin, Hu Frank B, Ogino Shuji, Fuchs Charles S, Giovannucci Edward L. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncology 2018;4:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang C-Y, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. The Journal of nutrition 2012;142:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Playdon MCN, Lampe JW, Tinker LF, Prentice R, Hayden KM, Van Horn L, Sampson J, Stolzenberg-Solomon R, Moore SC. Objective biomarkers of usual diet: a metabolomics analysis of weighed food intake 2018.
- [6].Playdon MC, Moore SC, Derkach A, Reedy J, Subar AF, Sampson JN, et al. Identifying biomarkers of dietary patterns by using metabolomics. The American journal of clinical nutrition 2017;105:450–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Willett WC, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. The Journal of Nutrition 2017;147:1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, Zhang X, et al. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology 2017;156:1517–30.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Chan AT, Willett WC, Giovannucci EL. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. The Journal of Nutrition 2017;147:1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tabung FK, Satija Ambika, Fung Teresa T, Clinton Steven K, Giovannucci Edward L. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. The Journal of Nutrition 2019;149:804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- [12].Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018;137:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. Journal of the American Dietetic Association 2003;103:323–8. [DOI] [PubMed] [Google Scholar]
- [14].Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Annals of Epidemiology 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- [15].Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. The American Journal of Clinical Nutrition 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- [16].Hoffmann K, Schulze MB, Schienkiewitz A, Nöthlings U, Boeing H. Application of a New Statistical Method to Derive Dietary Patterns in Nutritional Epidemiology. American Journal of Epidemiology 2004;159:935–44. [DOI] [PubMed] [Google Scholar]
- [17].Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013;59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A 2006;1125:76–88. [DOI] [PubMed] [Google Scholar]
- [19].Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011;121:1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Townsend M, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, Tworoger SS, Wolpin BM. Reproducibility of Metabolomic Profiles among Men and Women in 2 Large Cohort Studies. Clinical Chemistry 2013;59:1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical Activity and Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. Cancer prevention research (Philadelphia, Pa) 2011;4:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mejia EGd, Ramirez-Mares MV. Impact of caffeine and coffee on our health. Trends in Endocrinology & Metabolism 2014;25:489–92. [DOI] [PubMed] [Google Scholar]
- [24].Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. The American Journal of Clinical Nutrition 2007;85:385–91. [DOI] [PubMed] [Google Scholar]
- [25].Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009;91:791–5. [DOI] [PubMed] [Google Scholar]
- [26].Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids 2018;132:41–8. [DOI] [PubMed] [Google Scholar]
- [27].Julia C, Meunier N, Touvier M, Ahluwalia N, Sapin V, Papet I, et al. Dietary patterns and risk of elevated C-reactive protein concentrations 12 years later. British Journal of Nutrition 2013;110:747–54. [DOI] [PubMed] [Google Scholar]
- [28].Flock MR, Kris-Etherton PM. Diverse physiological effects of long-chain saturated fatty acids: implications for cardiovascular disease 2013;16:133–40. [DOI] [PubMed] [Google Scholar]
- [29].Paiva C, Beserra BTS, Reis CEG, Dorea JG, Da Costa THM, Amato AA. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Critical Reviews in Food Science and Nutrition 2017:1–12. [DOI] [PubMed]
- [30].Dong Hang ASK, Ma Wenjie, Hu Yang, Tabung Fred K., Hongmei, Hu Zhibin, Shen Hongbing, Mucci Lorelei A., Chan Andrew T., Giovannucci Edward, Song Mingyang. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. American Journal of Clinical Nutrition 2018. [DOI] [PMC free article] [PubMed]
- [31].Li J, Kong D, Wang Q, Wu W, Tang Y, Bai T, et al. Niacin ameliorates ulcerative colitis via prostaglandin D(2)-mediated D prostanoid receptor 1 activation. EMBO Molecular Medicine 2017;9:571–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of the receptor (Gpr109a) for niacin and the commensal metabolite butyrate suppresses colonic inflammation and carcinogenesis. Immunity 2014;40:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van Dijk AE, Olthof MR, Meeuse JC, Seebus E, Heine RJ, van Dam RM. Acute Effects of Decaffeinated Coffee and the Major Coffee Components Chlorogenic Acid and Trigonelline on Glucose Tolerance. Diabetes Care 2009;32:1023–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wheeler MD, Ikejema K, Enomoto N, Stacklewitz RF, Seabra V, Zhong Z, et al. Glycine: a new anti-inflammatory immunonutrient. Cellular and Molecular Life Sciences CMLS 1999;56:843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cruz M, Maldonado-Bernal C, Mondragón-Gonzalez R, Sanchez-Barrera R, Wacher NH, Carvajal-Sandoval G, et al. Glycine treatment decreases proinflammatory cytokines and increases interferon-γ in patients with Type 2 diabetes. Journal of Endocrinological Investigation 2008;31:694–9. [DOI] [PubMed] [Google Scholar]
- [36].Fuhrman BJ, Barba M, Krogh V, Micheli A, Pala V, Lauria R, et al. Erythrocyte Membrane Phospholipid Composition as a Biomarker of Dietary Fat. Annals of Nutrition and Metabolism 2006;50:95–102. [DOI] [PubMed] [Google Scholar]
- [37].Song X, Huang Y, Neuhouser ML, Tinker LF, Vitolins MZ, Prentice RL, et al. Dietary long-chain fatty acids and carbohydrate biomarker evaluation in a controlled feeding study in participants from the Women’s Health Initiative cohort. The American Journal of Clinical Nutrition 2017;105:1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dyck D, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiologica 2006;186:5–16. [DOI] [PubMed] [Google Scholar]
- [39].Díez J,J, Iglesias P The role of the novel adipocyte-derived hormone adiponectin in human disease. European Journal of Endocrinology 2003;148:293–300. [DOI] [PubMed] [Google Scholar]
- [40].Sam S, Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Translational Research 2014;164:284–92. [DOI] [PubMed] [Google Scholar]
- [41].Grzelczyk A, Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: New data – New insight into their function. Biochim ie 2013;95:667–79. [DOI] [PubMed] [Google Scholar]
- [42].Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2002;1582:81–8. [DOI] [PubMed] [Google Scholar]
- [43].del Bas JM, Caimari A, Rodriguez-Naranjo MI, Childs CE, Paras Chavez C, West AL, et al. Impairment of lysophospholipid metabolism in obesity: altered plasma profile and desensitization to the modulatory properties of n–3 polyunsaturated fatty acids in a randomized controlled trial. The American Journal of Clinical Nutrition 2016;104:266–79. [DOI] [PubMed] [Google Scholar]
- [44].Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, Beresford SA, Caan B, Thomson C, Satterfield S, Kuller L, Heiss G, Smit E, Sarto G, Ockene J, Stefanick ML, Assaf A, Runswick S, Prentice RL. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. American Journal of Epidemiology 2008;167:1247–59. [DOI] [PubMed] [Google Scholar]
- [45].Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, Tinker L, Schoeller D, Bingham S, Eaton CB, Thomson C, Johnson KC, Ockene J, Sarto G, Heiss G, Neuhouser ML. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. American Journal of Epidemiology 2011;174:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.