Abstract
Background:
We aimed to systematically assess a possible association of tofacitinib therapy with cardiovascular events (CVEs) and all-cause mortality.
Methods:
Systematic searches of PubMed, Embase, and Cochrane Library were conducted from inception through March 2019. Randomized controlled trials in patients with immune-mediated inflammatory diseases (IMIDs) reporting safety data were included. Included studies compared tofacitinib with placebo or 5 mg tofacitinib with 10 mg tofacitinib. The primary and secondary outcome measures were all CVEs [major adverse cardiovascular events (MACEs)/venous thromboembolism events (VTEs)] and all-cause mortality.
Results:
29 studies randomizing 13,611 patients were included. Compared with placebo, there was no significant increased risk of all CVEs (OR = 1.07, 95% CI 0.49–2.34), MACEs (OR 1.54, 95% CI 0.42–5.59), or all-cause mortality (OR = 1.13, 95% CI 0.26–4.95), but a decreased rate of VTEs (OR 0.03, 95% CI 0.00–0.21) in patients with IMIDs initiating tofacitinib. Meanwhile, paired comparison showed 10 mg tofacitinib twice daily was associated with a significantly lower incidence of all CVEs (OR = 0.56, 95% CI 0.33–0.96), MACEs (OR = 0.48, 95% CI 0.22–1.05), or all-cause mortality (OR = 0.47, 95% CI 0.19–1.17), but a trend toward an increase in VTEs risk (OR = 1.47, 95% CI 0.25–8.50), compared with the 5 mg regimen.
Conclusion:
Compared with placebo, there was no augmented risk of CVEs and all-cause mortality in patients with IMIDs following tofacitinib treatment in a short-term perspective, whereas 10 mg twice daily tofacitinib appeared to be associated with reduction in cardiovascular and all-cause mortality risks, except VTEs, relative to the 5 mg twice daily dose. Long-term studies and postmarketing risk monitoring are increasingly needed to develop a better understanding.
Keywords: all-cause mortality, cardiovascular risk, immune-mediated inflammatory diseases, meta-analysis, tofacitinib
Introduction
Patients with immune-mediated inflammatory diseases (IMIDs), including rheumatoid arthritis (RA), chronic plaque psoriasis (CPP), psoriatic arthritis (PsA), ulcerative colitis (UC), Crohn’s disease (CD), and ankylosing spondylitis (AS), have shown augmented risks of cardiovascular disease and cardiovascular-related mortality, largely fueled by systemic inflammatory burden, and subsequently accelerating atherosclerosis.1–6 Currently, the relationship between anti-inflammatory therapeutic approaches and a reduction in cardiovascular risk has been confirmed in experimental and clinical investigations, including recently published results from the CANTOS Trial.7–9 Likewise, in patients with IMIDs, cardiovascular benefits have also been suggested with systemic anti-inflammatory agents such as methotrexate and tumor necrosis factor inhibitor (TNFi).10–13 However, irrespective of anti-inflammation perspective, drug-specific mechanisms certainly have a role in the modulation of cardiovascular risk. For instance, in spite of limited control of inflammation, hydroxychloroquine could have a protective effect on cardiovascular outcomes in patients with RA, possibly through interference with lysosomal activity.14 Conversely, rofecoxib, which possesses potent anti-inflammatory properties, is found to be cardiotoxic.15
Tofacitinib, an orally administered small molecule Janus kinase (JAK) inhibitor, is being investigated for treatment of a range of IMIDs.16 After first securing US Food and Drug Administration (FDA) approval for the treatment of RA in 2012, the agent’s label has expanded to the areas of UC, CPP, and PsA. However, the impact of the agent on the risk of cardiovascular events (CVEs) remains undetermined. Given the abnormities in serum lipid profile and creatine phosphokinase, a safety trial enrolling older (⩾50) RA patients with at least one cardiovascular risk factor was required by the FDA.17 In the preliminary analysis of the ongoing phase IV study, a 10 mg twice daily dose of tofacitinib has been warned to increase occurrence of blood clots in the lungs and death, compared to the tofacitinib 5 mg twice daily or TNFi regimens.18 Additionally, growing cardiovascular concerns have been raised from other JAK inhibitor classes, including the FDA restricting approval to 2 mg baricitinib only, due to venous thromboembolism event (VTE) risk and a numerical excess of major adverse cardiovascular events (MACEs) in upadacitinib premarketing trials, although both low and high doses of baricitinib have been approved in European countries, Japan, Russia, and others.19–21
In light of the uncertain role of tofacitinib in cardiovascular outcomes and all-cause mortality indicated from previous studies, the purpose of this meta-analysis of randomized controlled trials (RCTs) was to assess the association of tofacitinib usage with cardiovascular events and mortality in adult patients with IMIDs.
Methods
This article has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).22
Literature search and inclusion criteria
We conducted separate PubMed, EMBASE, and Cochrane Library database searches of all relevant articles without language restrictions (from inception to March 3, 2019) and manually searched reference lists of potentially relevant studies. Search terms were comprised of tofacitinib (Xeljanz, CP-690 550), IMIDs, and combining the terms rheumatoid arthritis, Crohn’s disease, ulcerative colitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, systemic lupus erythematosus, or ankylosing spondylitis, and randomized controlled trial. An example of the search strategy is available in Supplementary Appendix S1. Abstracts of scientific meetings from the American College of Rheumatology, the European League Against Rheumatism, the American Gastroenterological Association, the American College of Gastroenterology, the United European Gastroenterology Week, the European Crohn’s and Colitis Organization, and the Inflammatory Skin Disease Summit were searched up to March 2019. To avoid publication bias, the results of all suitable unpublished but completed studies registered with the US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov) and FDA documents were procured.
We included all RCTs in adult patients with IMIDs that compared tofacitinib with placebo or simultaneously two dose regimens of tofacitinib (5 mg versus 10 mg, twice daily). Exclusion criteria included a noncomparative comparator, topical medicaments, pediatric patients, animal studies, and phase I and open-label extension studies. The eligibility of studies was independently evaluated by two reviewers (WX and SX). A third experienced reviewer (ZZ) selected the articles when the first two reviewers were in disagreement.
Data extraction and outcome measures
Data were extracted using piloted forms independently by both investigators (WX and SX), which included authors, publication year, location, study design, patients’ characteristics, treatment exposure, and the occurrence of CVEs. If ambiguity existed regarding the definition and number of CVEs, the clinical trial registration or relevant FDA documents were searched. If necessary, personal contact was made with the authors or sponsored pharmaceutical company. The overall number of CVEs during the randomized controlled phase was extracted for patients who received at least one dose of the agent or placebo. For extension RCTs in which treatment assignments were switched, the occurrence of CVEs was documented at the switching point. The longest randomized period was chosen to compare two dosage regimens for tofacitinib (5 mg versus 10 mg, twice daily), and total number of CVEs over the eligible period for each dosage regimen was extracted to identify dose-related cardiovascular effect.
A meta-analysis was performed for the primary outcomes of all CVEs, MACEs, and VTEs. The former was defined as a composite endpoint of angina pectoris, myocardial infarction, congestive heart failure, carotid artery disease, aortic aneurysm, cerebral vascular diseases (stroke and transient ischemic attack), VTEs (deep vein thrombosis, pulmonary embolism), and cardiovascular death. MACE was a composite of myocardial infarction, cerebrovascular accident (including ischemic and hemorrhagic strokes) or cardiovascular death. The secondary outcome of interest was comparative risk of all-cause mortality. In addition, an exploratory analysis of the possible association between anti-inflammatory efficacy (ACR 20 response rate for RA and PsA; PASI 75 response rate for CPP; remission rate for UA, CD; ASDAS 20 response rate for AS) and the incidence of all CVEs (MACEs/VTEs) or all-cause mortality was performed as an exploratory outcome. Two kinds of comparisons were made: (1) tofacitinib versus placebo; (2) pairwise comparisons of 5 mg and 10 mg twice daily tofacitinib. In the first comparison, all dosages of the agent in eligible RCTs were combined.
Data synthesis and analysis
Extracted data were combined for the meta-analysis using Review Manager 5.3 (RevMan 5.3) software (Cochrane Collaboration). For all outcomes, odd ratios (ORs) and 95% confidence intervals (CIs) were calculated as an effect measure to quantify the risk of MACEs in patients receiving tofacitinib compared with placebo or tofacitinib with different dosing using the Peto method, which has been generally considered better for rare events. Sensitivity analyses were conducted with Mantel–Haenszel fixed or random effects and restricted to multinational RCTs or the studies with a panel of independent cardiovascular experts to explore whether analytical methods or specific studies influenced the results of the comparisons. Risk of bias assessment was done using the Cochrane Collaboration risk of bias tool.23 Forest plots were constructed to summarize the OR estimates and their 95% CI. Heterogeneity across studies was tested as proposed by the χ2 test (p < 0.1 was regarded as statistically significant) and I2 statistics (significant heterogeneity, I2 > 50%). The effect was plotted as the inverse of its standard error to identify the risk of publication bias by visually assessing the symmetry of the funnel plots. To explore the association between anti-inflammatory effect and the cardiovascular or all-cause mortality risk, Spearman correlation coefficients were applied to determine the relationship between OR of cardiovascular events (all CVEs/MACEs/VTEs) or all-cause mortality and corresponding risk ratio (RR) of the above-mentioned reported efficacy for individual diseases.
Results
Study search and study characteristics
Of the 619 citations screened, 27 studies randomizing 13,611 patients met the predefined inclusion criteria as summarized in Figure 1. Among these studies, 13 and 6 items were in RA24–35 and CPP,36–42 respectively. Other reports, in the order of descending frequency, were in PsA,43,44 UC,45,46 CD,47,48 and AS.49 The remaining one combined patients with both CPP and PsA.50 Most commonly, one article specifically reported the results of a single trial, except two reports with two integrated trials39,48 and one report with three trials.46 Supplementary Table S1 shows the trial-level characteristics of the included studies. Most RCTs were multinational, except four which came only from Japan25,26,50 or the USA.33 From the 27 included studies, baseline characteristics of patients were generally comparable with regard to age, sex composition, disease duration, and disease activity across most arms for each class of diseases (Supplementary Table S1).
Figure 1.

Study flowchart of included randomized controlled studies for systematic review and meta-analysis.
RCT, randomized controlled trial.
Among the included studies, 24 were eligible for the comparison of tofacitinib and placebo and 20 were for paired comparisons of 5 mg and 10 mg twice daily tofacitinib. The available duration of the randomized controlled phase for tofacitinib compared to placebo and dose-comparison ranged from 4 to 52 (median 12) weeks and 8 to 104 (median 24) weeks respectively (Supplementary Table S2).
Cardiovascular outcomes
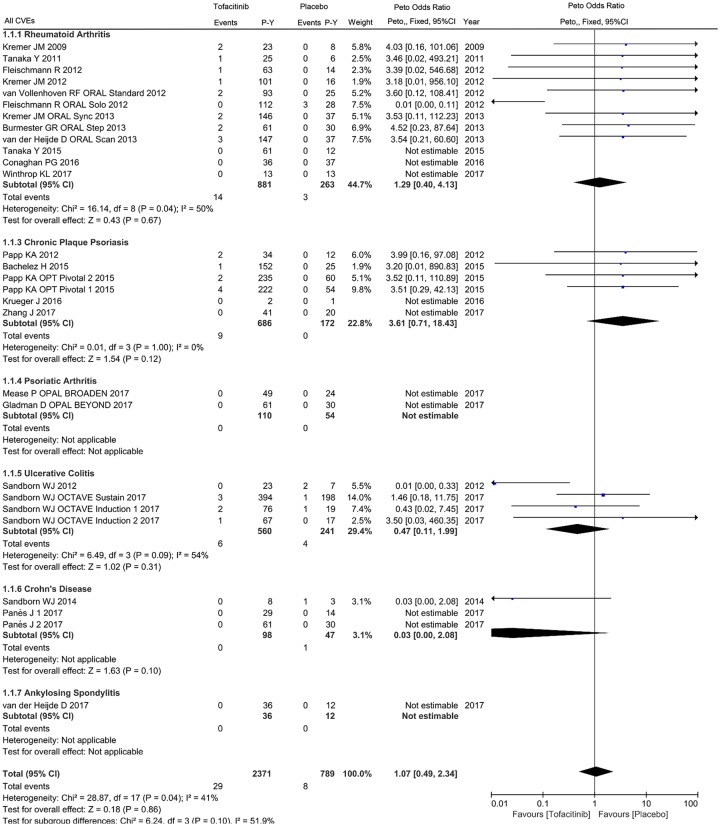
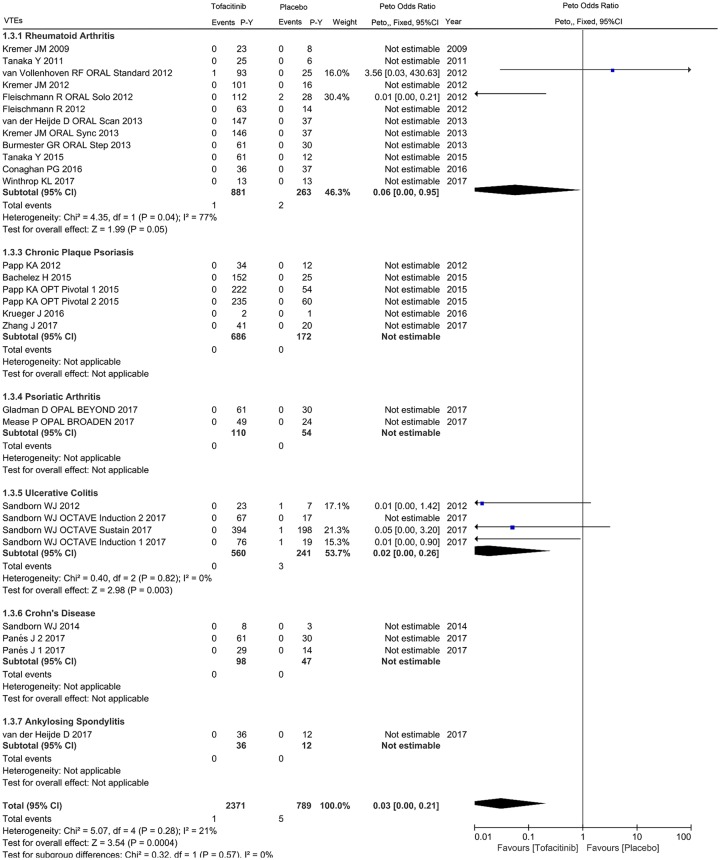
For the primary cardiovascular outcome, a total of 2371 and 789 patient-years of follow up in 24 eligible studies were included for tofacitinib and placebo arms respectively, with a crude incidence rate of all CVEs (MACEs/VTEs) of 1.223 (0.506/0.042) and 1.014 (0.253/0.634) per 100 patient-years, respectively (Supplementary Table 2). The pooled analysis of all CVEs found an odds ratio of 1.07 (95% CI 0.49–2.34) in patients initiating tofacitinib therapy, with 30 events in tofacitinib and 6 in placebo groups. Viewed separately, no statistically significant difference regarding the risk of all CVEs was observed in tofacitinib-treated patients with RA (OR 1.29, 95% CI 0.40–4.13), CPP (OR 3.61, 95% CI 0.71–18.43), UC (OR 0.47, 95% CI 0.11–1.99) or CD (OR 0.03, 95% CI 0.00–2.08) compared with placebo (Figure 2). There was no statistically significant correlation between tofacitinib treatment and the occurrence of MACEs in patients with IMIDs (OR 1.54, 95% CI 0.42–5.59) or individual diseases (Figure 3). Subanalysis of VTEs showed a deceased rate of VTEs in patients with IMIDs initiating tofacitinib, relative to placebo (OR 0.03, 95% CI 0.00–0.21), with one event in tofacitinib and five events in placebo arms (Figure 4). Little evidence of heterogeneity allowed for combination of trial results using the Peto method (Figures 2–4). Additionally, separate comparisons of different doses of tofacitinib (5 mg or 10 mg twice daily) with placebo were conducted. There was a trend toward increased cardiovascular risk in 5 mg tofacitinib twice daily (all CVEs: OR 1.96, 95% CI 0.80–4.79; MACEs: OR 3.38, 95% CI 0.92–12.41) and a trend toward decreased cardiovascular risk (all CVEs: OR 0.48, 95% CI 0.16–1.44; MACEs: OR 0.97, 95% CI 0.15–6.27) in 10 mg tofacitinib twice daily in comparison to placebo in a short-term perspective, but the differences did not reach statistical significance (Supplementary Figures S1–S4). A trend in decreased VTEs risk was observed in both the 5 mg (OR 0.07, 95% CI 0.01–0.74) or 10 mg (OR 0.13, 95% CI 0.02–0.72) groups in comparison to placebo (Supplementary Figures S5 and S6).
Figure 2.
Odds ratio of all cardiovascular events in patients treated with tofacitinib as compared with placebo.
Figure 3.
Odds ratio of major adverse cardiovascular events in patients with IMIDs treated with tofacitinib compared to placebo.
Figure 4.
Odds ratio of venous thromboembolism events in patients with IMIDs treated with tofacitinib compared to placebo.
There were 20 studies included comprising 2809 and 2862 patient-years of follow up in 5 mg and 10 mg dosages of tofacitinib respectively, which reported a total of 55 CVEs [35 with 5 mg (1.246 per 100 patient-years) and 20 with 10 mg (0.699 per 100 patient-years)], 25 MACEs [17 with 5 mg (0.605 per 100 patient-years) and 2 with 10 mg (0.253 per 100 patient-years)], and 5 VTEs [2 with 5 mg (0.071 per 100 patient-years) and 3 with 10 mg (0.105 per 100 patient-years)] (Supplementary Table S2). In comparison with 5 mg tofacitinib, decreased incidence of all CVEs favored 10 mg tofacitinib in individuals with IMIDs (OR 0.56, 95% CI 0.33–0.96), RA (OR 0.86, 95% CI 0.44–1.70), CPP (OR 0.30, 95% CI 0.11–0.82), PsA (OR 0.50, 95% CI 0.05–4.89), UC (OR 0.14, 95% CI 0.01–1.31), as shown in Figure 5. Further subanalyses revealed that the reduction was observed in MACEs (OR 0.48, 95% CI 0.22–1.05) rather than VTEs (OR 1.47, 95% CI 0.25–8.50) (Figures 6 and 7).
Figure 5.
Odds ratio of all cardiovascular events in patients with IMIDs treated with 10 mg tofacitinib twice daily as compared with 5 mg.
Figure 6.
Odds ratio of major adverse cardiovascular events in patients with IMIDs treated with 10 mg tofacitinib twice daily as compared with 5 mg.
Figure 7.
Odds ratio of venous thromboembolism events in patients with IMIDs treated with 10 mg tofacitinib twice daily as compared with 5 mg.
All-cause mortality outcome
For the all-cause mortality outcome, a total of nine events with tofacitinib (0.380 per 100 patient-years) and two with placebo (0.253 per 100 patient-years) were reported in eligible studies (Supplementary Table S2). Compared to placebo, there was no significant difference in the risk of all-cause mortality in patients receiving tofacitinib with IMIDs (OR 1.13, 95% CI 0.26–4.95), or any individual disease [RA (OR 3.46, 95% CI 0.45–26.84), CPP (OR 0.44, 95% CI 0.03–7.39), UC (OR 3.49, 95% CI 0.03–468.68), CD (OR 0.03, 95% CI 0.00–2.08)], with low heterogeneity (Figure 8). Separately, a trend to increased and decreased risk of all-cause mortality was noted in 5 mg (OR 1.92, 95% CI 0.49–7.52) and 10 mg tofacitinib (OR 0.22, 95% CI 0.02–2.61) twice daily, but fell short of statistical significance (Supplementary Figures S7 and S8).
Figure 8.
Odds ratio of all-cause mortality in patients with IMIDs treated with tofacitinib as compared with placebo.
For dose comparison, 20 studies reported 13 events in 5 mg tofacitinib (0.463 per 100 patient-years) and 6 events in 10 mg twice daily tofacitinib (0.210 per 100 patient-years) (Supplementary Table S2). Patients who were on the 10 mg dose tofacitinib had a trend toward a lower incidence of all-cause mortality than those on the lower dose (OR 0.47, 95% CI 0.19–1.17 for IMIDs; OR 0.66, 95% CI 0.24–1.83 for RA; OR 0.13, 95% CI 0.02–0.96 for CPP) (Figure 9).
Figure 9.
Odds ratio of all-cause mortality in patients with IMIDs treated with 10 mg tofacitinib twice daily as compared with 5 mg.
Exploratory association of efficacy with cardiovascular or mortality risk
For exploring the potential association between anti-inflammatory effect and the cardiovascular or all-cause mortality risk, efficacy data in matched weeks were available in 22 and 20 studies eligible for tofacitinib versus placebo and 10 mg versus 5 mg regimens. For the comparison of tofacitinib with placebo, the correlation coefficients between response rates and the risk of all CVEs, MACEs, VTEs, or mortality were 0.349, 0.130, −0.212, or −0.134. For RA alone, an inverse correlation was observed between all CVEs (MACEs/VTEs) or all-cause mortality and response rates with correlation coefficients of −0.176 (–0.042/–0.030), −0.515, which suggested the possibility of inverse correlation, although the correlation was weak (p > 0.1). For the dose comparison, there was a significant inverse correlation observed between all CVEs, MACEs, or all-cause mortality and therapeutic efficacy, with correlation coefficients of −0.440, −0.551, and −0.347 respectively (p values of 0.036, 0.006, and 0.105). For VTEs, the correlation was −0.071 (p = 0.747) (data available on request).
Sensitivity analyses
The sensitivity analyses using random or fixed effects and excluding the four USA- and Japan-based studies showed similar results for the main comparisons (Supplementary Figures S9–S14). After we specifically included the studies with an independent committee for cardiovascular safety adjudication, a numerically higher risk of all CVEs in patients with IMIDs initiating tofacitinib (OR 2.19, 95% CI 0.79–6.04) was observed in comparison to placebo. Moreover, the significant reduction in patients receiving 10 mg twice daily in all CVEs risk remained detectable (OR 0.40, 95% CI 0.19–0.84) (Supplementary Figures S15 and S16).
Risk of bias and publication bias
All trials were described as randomized; however, 25 out of 31 RCTs reported adequate sequence generation. Allocation concealment was assessed as low risk in 26 (84%) trials. Blinding of participants, personnel, and outcome assessor were performed in nearly all RCTs. Most of the trials (30 out of 31; 97%) were judged as low risk of attrition bias. We assessed all trials at low risk of selective reporting because all events are listed according to the system organ classes and ‘preferred terms’ in the Medical Dictionary for Regulatory Activities. Patient baseline characteristics in all intervention groups were well-balanced (Supplementary Table S3). For the Peto method, there was no evidence of publication bias across all the trials in a funnel plot analysis (Supplementary Figure S17).
Discussion
To our knowledge, this is the first systematic review with meta-analysis of the cardiovascular and all-cause mortality risks in patients with IMIDs receiving tofacitinib therapy based on pooled data from all currently controlled datasets. According to our results, short-term use of tofacitinib does not increase risk of CVEs and all-cause mortality in patients with IMIDs. Furthermore, 10 mg twice daily tofacitinib seems to be associated with a reduction in frequency of all-cause mortality and CVEs, except VTEs, when compared with 5 mg twice daily tofacitinib.
Despite lower total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) in RA patients, the risk of developing CVD in RA patients is 1.5–2-fold higher than for the general population.51 Tofacitinib has demonstrated significant efficacy in the treatment of a range of IMIDs, along with an increase in TC, LDL-c, and HDL-c in premarketing trials. The lipid abnormalities are worrisome and consequently prompted cardiovascular concern. In fact, RA patients generally experience >30% increase in LDL-c following treatment with a biological disease modifying anti-rheumatic drug (bDMARD), but recent basic investigations indicated the increases of LDL-c may not be linked to higher CV risk.51 For example, interleukin-6 (IL-6) receptor blockade with tocilizumab normalizes the hypercatabolism in active RA and the normalization of pathological LDL hypercatabolism seems unlikely to contribute to atherosclerosis development.52 Mechanistically, JAK inhibition with tofacitinib could partly influence lipid metabolism through the IL-6 pathway. A recent basic study found tofacitinib induced lipid release through increasing the levels of cellular liver X receptor α and reverse cholesterol transport activation, reflecting a potentially different mechanism.53 Simultaneously, tofacitinib treatment could increase HDL-c particle number and improve markers of HDL-c function.54 Clinically, a recent study showed tofacitinib therapy did not drive atherosclerosis development, but actually reduce carotid intima-media thickness in patients with an atherosclerosis event.55 This might be related to increase of TC together with HDL-c levels, while the TC/HDL cholesterol ratio was unchanged. TC/HDL-c ratio is a more important predictor of CVD than TC, fasting LDL, or LDL-c/HDL-c ratio. Besides, changing cholesterol esterification, increasing the size of the lipid molecules (from low density to high density), and improving antiatherogenic capacity of the lipid particle of tofacitinib therapy may account for this potential cardiovascular benefit. The present meta-analysis of RCTs showed no significant difference for tofacitinib (5 mg, 10 mg twice daily, or all dosage) therapy in patients with IMIDs as relative to placebo, and this is in line with the results of a most recent meta-analysis exploring the cardiovascular safety of JAK inhibitors (including tofacitinib) specifically in RA patients.56 However, previous noncomparative pooled analyses showed treatment with tofacitinib at both 5 mg and 10 mg twice daily is associated with a low occurrence of CVEs in patients with RA and UC (no statistical analysis performed).56,57,58 A possible explanation for these observations would be the length of available phase for direct comparison. The available duration of the randomized controlled phase for tofacitinib versus placebo ranged from 4 to 52 (median 12) weeks. Therefore, the direct comparison of tofacitinib with placebo in a relative short controlled phase had limited statistical power because of the relatively limited number of events. In our later dose comparison based on a median period of 24 weeks, a statistical difference is detected. Of note, in the subgroup of patients with CPP, all nine CVEs (including five MACEs) occurred in tofacitinib-treated groups, resulting in a numerically higher, but statistically nonsignificant, cardiovascular risk for tofacitinib use. In fact, different from the cardioprotective findings in patients with RA or UC following biologic therapies, no significant difference was reported in patients with CPP receiving biological agents.59–61 Thus, association between tofacitinib use and cardiovascular risk is noteworthy and needs to be addressed based on the ongoing long-term and real-world data.
Dosage analysis found lower incidences of all CVEs and MACEs in patients with IMIDs receiving higher doses of 10 mg twice daily compared to the lower dose arm. The possible interpretation is generally more potent anti-inflammatory effect of higher doses of tofacitinib, which was partially supported by the exploratory analysis with an inverse correlation between the anti-inflammatory effect and the cardiovascular or all-cause mortality risk, although the correlation was not strong enough based on the publicly available datasets. For dose-related impact on all-cause mortality, the last results from the ongoing postmarketing safety study required by the FDA, which enrolled RA patients at least 50 years old and having at least one cardiovascular risk factor, found an increased mortality in patients treated with tofacitinib 10 mg, as compared to those with tofacitinib 5 mg twice daily or a TNFi.18 The present study, based on a population closer to the real-world setting, found OR of 0.69 for patients with RA initiating higher dosage, relative to 5 mg regimen. Heterogeneity of the inclusion populations under study may underlie variation in mortality, and future real-world studies are warranted to confirm the safety profile of two regimens. Concerning the risk of VTEs, a recent published observational cohort study of RA patients using claims data found a numerically higher risk of VTEs than TNFi, but this finding did not reach significance (OR 1.33, 95% CI 0.78–2.24).62 Our findings from RCTs suggest a decrease in VTEs risk with this agent in patients with IMIDs when compared with placebo, with one and four events respectively in tofacitinib. The low occurrence of VTEs in placebo-controlled period obviously weakens the reliability of the results. For dose-related impact, the last results from an ongoing postmarketing safety trial required by the FDA, which enrolled RA patients at least 50 years old and having at least one cardiovascular risk factor, found an increased risk of blood clots in the lungs and mortality in patients treated with tofacitinib 10 mg twice daily compared to those with tofacitinib 5 mg twice daily or a TNFi.18 The present meta-analysis found a tendency toward an increase in VTE risk in the 10 mg twice daily dose regimen during the randomized controlled duration, which is similar to the last results of an increased risk of blood clots in the lungs in 10 mg twice daily tofacitinib group from the above-mentioned ongoing postmarketing study. To date, no clear indication of plausible biological mechanisms was established for the interpretation of the unfavorable increased risk of PE and mortality in 10 mg twice daily tofacitinib. The VTE concern was initially raised from the baricitnib clinical program. Although there is no plausible mechanism of action for baricitinib-induced thrombosis or for JAK inhibition to contribute to this risk, the FDA review team considered that the potential underlying pathogenic mechanisms based on the purported mechanism of action of baricitinib might be related to platelet count increase with the same dose-dependent trend.63 But no significant influence on platelet count was observed in the tofacitinib clinical program and the exploration of underlying mechanisms from basic research is sorely needed in the future.
In light of these findings of the present study and the FDA safety warnings, cardiovascular risk assessment including age, hypertension, prior cardiovascular disease, and medication patterns should be considered at the time of initiation of higher-dose tofacitinib therapy. Also, an independent cardiovascular safety endpoint adjudication committee reviewing all potential cardiovascular and thromboembolic events should be routinely established in subsequent JAK inhibitor clinical trials. Moreover, to develop a deep understanding of the association between tofacitinib therapy and CVEs, continued surveillance of emerging data from long-term studies and the exploration of underlying mechanisms from basic research is required in the future. On the other hand, the number needed to treat (NNT) and/or number needed to harm (NNH) is a powerful estimate of the effect of a treatment, which clearly tells healthcare professionals the effort needed to achieve a particular outcome.64 In the present study, the number NNH for all CVEs, MACEs, and all-cause mortality were 478,396 and 793 respectively, indicating the cardiovascular safety of tofacitinib. As a statistically valid and clinically useful indicator of treatment effect magnitude, NNT or NNH should become a part of the standard summary estimates in long-term and real-world studies in the future.
Several important limitations should be recognized when interpreting the findings of our meta-analysis. First, the limited duration of the randomized controlled phases, to some extent, would reduce the power of this meta-analysis to detect a change in risk of CVEs. Although the present study indicated the cardiovascular safety of tofacitinib treatment in a short-term perspective, which therapeutic dose of tofacitinib is safer for the general population in the treatment of RA and whether the cardiovascular risk increases or decreases over the time course need to be definitively demonstrated based on real-world data. Second, the relatively small number of CVEs (MACEs/VTEs) limited the scope of the study and the possibilities to perform more detailed subanalyses, for example, looking into myocardial infarction or congestive heart failure. Third, the ability to determine the possibility of racial disparity in CVE risk was hampered due to no ethnicity- or individual-level data provided by the study sponsor. Lastly, the background cardiovascular risk for patients in exposed and nonexposed groups in RCTs is likely to be lower than those seen in the context of real-world clinical practice. This may consequently limit the generalizability of the results.
Conclusion
This systematic review and meta-analysis of RCTs suggests that tofacitinib-based therapy did not increase the likelihood of CVEs or all-cause mortality in patients with IMIDs in a short-term perspective. Furthermore, 10 mg twice daily tofacitinib appeared to reduce the cardiovascular and all-cause mortality risk, except VTEs, relative to the 5 mg regimen. To develop a better understanding, both continuous postmarketing surveillance of emerging trial data and long-term prospective studies are required.
Supplemental Material
Supplemental material, Supplementary_file for Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials by Wenhui Xie, Shiyu Xiao, Yanrong Huang, Xiaoying Sun and Zhuoli Zhang in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We thank the pharmaceutical companies for kindly feedback on research data.
Footnotes
Authors’ contributions: ZZ conceived of the study, participated in its design and coordination, and critically revised the manuscript. WX and SX had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. WX drafted the manuscript. YH and XS contributed to the process of interpretation. All authors read and approved the final manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (grant number: 81971524, 81771740).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: All data for this study were obtained from existing publications and ethical approval was not required for this research.
ORCID iD: Wenhui Xie  https://orcid.org/0000-0002-3881-0266
https://orcid.org/0000-0002-3881-0266
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wenhui Xie, Department of Rheumatology and Clinical Immunology, Peking University First Hospital, West District, Beijing, China.
Shiyu Xiao, Department of Gastroenterology, Peking University Third Hospital, Haidian District, Beijing, China.
Yanrong Huang, Department of Rheumatology and Clinical Immunology, Peking University First Hospital, West District, Beijing, China.
Xiaoying Sun, Department of Rheumatology and Clinical Immunology, Peking University First Hospital, West District, Beijing, China.
Zhuoli Zhang, Director of Department of Rheumatology and Clinical Immunology, Peking University First Hospital, No.8, Xishiku Street, West District, Beijing 100034, China.
References
- 1. Baena-Díez JM, Garcia-Gil M, Comas-Cufí M, et al. Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart 2018; 104: 119–126. [DOI] [PubMed] [Google Scholar]
- 2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012; 71: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 3. Eder L, Wu Y, Chandran V, et al. Incidence and predictors for cardiovascular events in patients with psoriatic arthritis. Ann Rheum Dis 2016; 75: 1680–1686. [DOI] [PubMed] [Google Scholar]
- 4. Singh S, Kullo IJ, Pardi DS, et al. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol 2015; 12: 26–35. [DOI] [PubMed] [Google Scholar]
- 5. Eriksson JK, Jacobsson L, Bengtsson K, et al. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis 2017; 76: 364–370. [DOI] [PubMed] [Google Scholar]
- 6. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J 2015; 36: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1β decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2003; 23: 656–660. [DOI] [PubMed] [Google Scholar]
- 8. Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 10. Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011; 108: 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de La Forest Divonne M, Gottenberg JE, Salliot C. Safety of biologic DMARDs in RA patients in real life: a systematic literature review and meta-analyses of biologic registers. Joint Bone Spine 2017; 84: 133–140. [DOI] [PubMed] [Google Scholar]
- 13. Sarlos P, Szemes K, Hegyi P, et al. Steroid but not biological therapy elevates the risk of venous thromboembolic events in inflammatory bowel disease: a meta-analysis. J Crohns Colitis 2018; 12: 489–498. [DOI] [PubMed] [Google Scholar]
- 14. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2018; 77: 98–103. [DOI] [PubMed] [Google Scholar]
- 15. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005; 352: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 16. Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis?. Ann Rheum Dis 2018; 77: 175–187. [DOI] [PubMed] [Google Scholar]
- 17. Souto A, Salgado E, Maneiro JR, et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol 2015; 67: 117–127. [DOI] [PubMed] [Google Scholar]
- 18. European Medicines Agency. Increased risk of blood clots in lungs and death with higher dose of xeljanz (tofacitinib) for rheumatoid arthritis, https://www.ema.europa.eu/en/news/increased-risk-blood-clots-lungs-death-higher-dose-xeljanz-tofacitinib-rheumatoid-arthritis (2019).
- 19. Mullard A. FDA approves Eli Lilly’s baricitinib. Nat Rev Drug Discov 2018; 17: 460. [DOI] [PubMed] [Google Scholar]
- 20. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 2503–2512. [DOI] [PubMed] [Google Scholar]
- 21. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018; 391: 2513–2524. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JPT, Green S. (eds) Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons Ltd, 2008, pp.187–242. [Google Scholar]
- 24. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009; 60: 1895–1905. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka Y, Suzuki M, Nakamura H, et al. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthrit Care Res 2011; 63: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 26. Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012; 64: 617–629. [DOI] [PubMed] [Google Scholar]
- 27. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl Med 2012; 367: 495–507. [DOI] [PubMed] [Google Scholar]
- 28. Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012; 64: 970–981. [DOI] [PubMed] [Google Scholar]
- 29. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012; 367: 508–519. [DOI] [PubMed] [Google Scholar]
- 30. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013; 381: 451–460. [DOI] [PubMed] [Google Scholar]
- 31. Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic DMARDs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013; 159: 253–261. [DOI] [PubMed] [Google Scholar]
- 32. van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013; 65: 559–570. [DOI] [PubMed] [Google Scholar]
- 33. Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017; 69: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conaghan PG, Østergaard M, Bowes MA, et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis 2016; 75: 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 370: 2377–2386. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka Y, Takeuchi T, Yamanaka H, et al. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol 2015; 25: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol 2012; 167: 668–677. [DOI] [PubMed] [Google Scholar]
- 38. Bachelez H, van de Kerkhof PC, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015; 8; 386: 552–561. [DOI] [PubMed] [Google Scholar]
- 39. Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol 2015; 173: 949–961. [DOI] [PubMed] [Google Scholar]
- 40. Krueger J, Clark JD, Suárez-Fariñas M, et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J Allergy Clin Immunol 2016; 137: 1079–1090. [DOI] [PubMed] [Google Scholar]
- 41. Zhang J, Tsai TF, Lee MG, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci 2017; 88: 36–45. [DOI] [PubMed] [Google Scholar]
- 42. Bissonnette R, Iversen L, Sofen H, et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 43. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017; 377: 1525–1536. [DOI] [PubMed] [Google Scholar]
- 44. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017; 377: 1537–1550. [DOI] [PubMed] [Google Scholar]
- 45. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012; 367: 616–624. [DOI] [PubMed] [Google Scholar]
- 46. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 47. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 1485–1493.e2. [DOI] [PubMed] [Google Scholar]
- 48. Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017; 66: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017; 76: 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Asahina A, Etoh T, Igarashi A, et al. Oral tofacitinib efficacy, safety and tolerability in Japanese patients with moderate to severe plaque psoriasis and psoriatic arthritis: a randomized, double-blind, phase 3 study. J Dermatol 2016; 43: 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plutzky J, Liao KP. Lipids in RA: is less not necessarily more? Curr Rheumatol Rep 2018; 20: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Robertson J, Porter D, Sattar N, et al. Interleukin-6 blockade raises LDL via reduced catabolism rather than via increased synthesis: a cytokine-specific mechanism for cholesterol changes in rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1949–1952. [DOI] [PubMed] [Google Scholar]
- 53. Pérez-Baos S, Barrasa JI, Gratal P, et al. Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br J Pharmacol 2017; 174: 3018–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015; 67: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kume K, Amano K, Yamada S, et al. Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol Int 2017; 37: 2079–2085. [DOI] [PubMed] [Google Scholar]
- 56. Xie W, Huang Y, Xiao S, et al. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 2019; 78: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 57. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum 2016; 46: 261–271. [DOI] [PubMed] [Google Scholar]
- 58. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019; 17: 1541–1550. [DOI] [PubMed] [Google Scholar]
- 59. Ryan C, Leonardi CL, Krueger JG, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 2011; 306: 864–871. [DOI] [PubMed] [Google Scholar]
- 60. Rungapiromnan W, Yiu ZZN, Warren RB, et al. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2017; 176: 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Champs B, Degboé Y, Barnetche T, et al. Short-term risk of major adverse cardiovascular events or congestive heart failure in patients with psoriatic arthritis or psoriasis initiating a biological therapy: a meta-analysis of randomised controlled trials. RMD Open 2019; 5: e000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Desai RJ, Pawar A, Weinblatt ME, et al. Comparative risk of venous thromboembolism with tofacitinib versus tumor necrosis factor inhibitors: a cohort study of rheumatoid arthritis patients. Arthritis Rheumatol 2019; 71: 892–900. [DOI] [PubMed] [Google Scholar]
- 63. Food and Drug Administration. FDA briefing document, https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm605061.pdf (2018).
- 64. Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract 2013; 67: 407–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_file for Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials by Wenhui Xie, Shiyu Xiao, Yanrong Huang, Xiaoying Sun and Zhuoli Zhang in Therapeutic Advances in Musculoskeletal Disease