Abstract
Stretchable and tough hydrogels have drawn a lot of attention recently. Due to their unique properties, they have great potential in the application in areas such as mechanical sensing, wound healing, and drug delivery. In this review, we will summarize recent developments of stretchable and tough hydrogels in these areas.
Keywords: highly stretchable, tough, hydrogel, polyacrylamide, chemical crosslinking, physical crosslinking, sensor, drug delivery, wound healing
1. Introduction
Due to the high water content and excellent biocompatibilities, hydrogels have been used for wound healing, tissue culture, tissue/cartilage replacement [1], scaffolds for cell growth [2], etc. When combined with electronics, hydrogels have been used to develop a variety of biomedical devices, including sensors [3,4,5,6,7,8,9,10,11,12,13], switchable micro patterns [14], self-oscillators [15], etc. Most hydrogels have relatively low tensile strength and low elasticity, which limits their application capacity in the biomedical field. In order to improve the mechanical properties of hydrogels, highly stretchable and tough hydrogels have been invented recently [16]. These highly stretchable and tough hydrogels generally consist of one or more gel networks of two or more polymer chains crosslinked both chemically (typically via covalent bond) and physically (typically via intermolecular interactions) [17,18]. Recently, we wrote a review on the synthesis and fundamental properties of highly stretchable and tough hydrogels [19]. In this review, we will summarize the applications of the most up-to-date highly stretchable and tough hydrogels for mechanical sensing, drug delivery, and wound dressing. This review is a part II, with a focus on the applications.
2. Highly Stretchable and Tough Hydrogels for Mechanical Sensing
Mechanical sensors are a class of sensors used to measure the mechanical properties of an object. One major approach of measurement is through piezoresistivity, which is the effect exhibited when there is a change in resistance due to applied pressure. Many of these piezoresistive sensors, also known as strain gauges, were made from a polyester base with wire or metallic foil. These sensors generally have a low stress limit and cannot be readily repaired when damaged. Highly stretchable, tough hydrogels have high stress limits and can self-heal over time. Mechanical hydrogel sensors are wearable, due to the high stress limit that they possess, i.e., their reasonable stretchability. When appraising the use of hydrogels in sensors, one must consider the gauge factor of the gel, the healing time and efficiency, and the mechanical property of hydrogels.
2.1. Gauge Factor
Gauge factor is the ratio of the change in resistance of the gel to the strain applied, which is defined as , where ε is the strain, L is the change in length, L is the original length, ν is the Poisson’s ratio, ρ is the resistivity, R is the change in strain resistance, L is the unstrained resistance.
In 2015, Frutiger et al. [20] developed a soft strain sensor, by modifying a commercially available silicone, that exhibited a high stretchability (up to 700%), however, this elastomer tended to have low gauge factors (0.348 ± 0.11). In 2016, Cai et al. [21] developed a polyvinylalcohol (PVA)-based hydrogel with a single wall carbon nanotube (SWCNT) conductor in an attempt to create a highly stretchable gel with high gauge factor (Figure 1). The hydrogel exhibited a gauge factor of 0.24 at 100% strain and 1.51 at 1000% strain. The gauge factor was shown to have improved due to the addition of the SWCNT conductor, based on the gauge factors that were recorded of the gel without the SWCNT conductor (0.09 at 100% and 0.53 at 1000%).
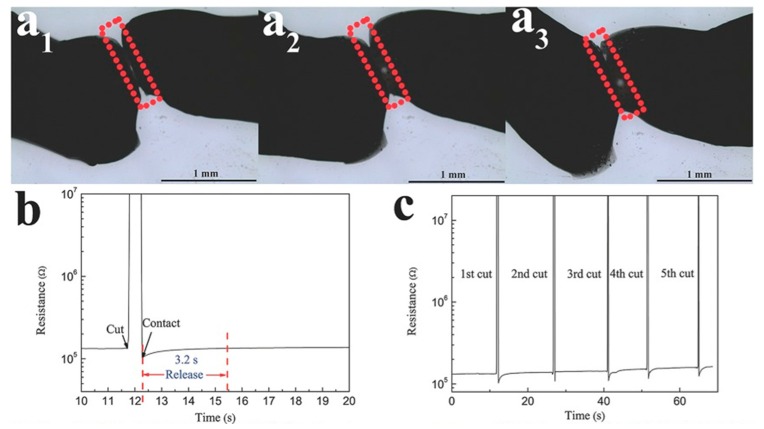
Figure 1.
(a1–a3) Images of polyvinylalcohol (PVA)/single wall carbon nanotube (SWCNT) hydrogel healing at times 0, 30, 60 s, respectively, at room temperature. (b) Electrical healing process by measuring resistance with time under ambient conditions. (c) Cycles of cutting and healing of hydrogel. (Adapted from [21] Figure 2, with permissions from Wiley).
Wang et al. [22] determined that the gauge factor can be improved with a synthesized hydrogel with polyaniline (PANI) and polyacrylic acid (PAA) in 2018 (Figure 2). After cutting and healing (a), the hydrogel can hold a ~500 g mass (b). Strain percentage vs. stress over multiple healing cycles showed the gel had a gauge factor of 11.6 for up to 100% strain and 4.7 for strains above 100% (c). The gel showed the combination of PANI and PAA attributes to the high stretchability and conductivity of the gel. Electrical conductivity test showed that the hydrogel healed with a green LED bulb could stretched more than 400% before breaking (d), and the gel can be healed multiple times without losing the conductivity (e). The hydrogel healing process was also discussed (f).
Figure 2.
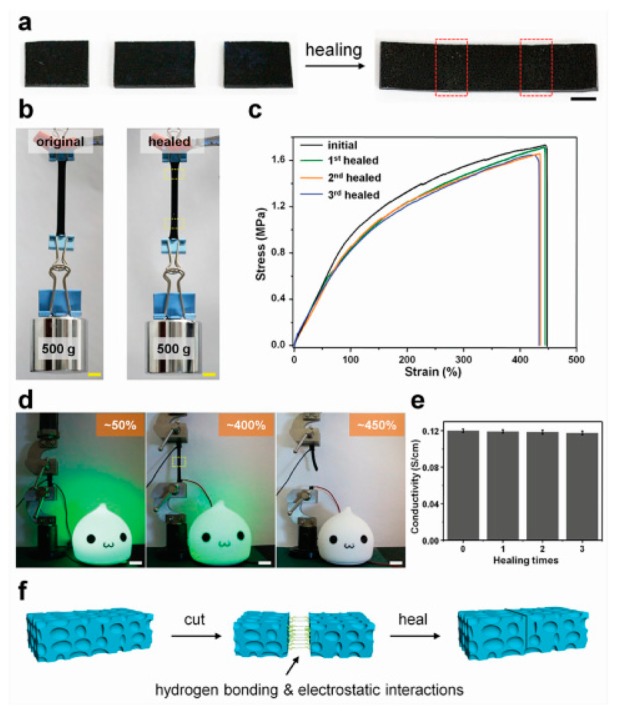
(a) Cutting and healing of polyacrylic acid (PAA)/polyaniline (PANI) hydrogel. (b) Hydrogel supporting ~500 g mass. (c) Strain percentage vs. stress over multiple healing cycles. (d) Electrical conductivity test of healed hydrogel with a green LED bulb. (e) Electrical conductivity graph over multiple healing cycles. (f) Illustration of hydrogel healing process. (Adapted from [22], Figure 3, with permissions from Wiley).
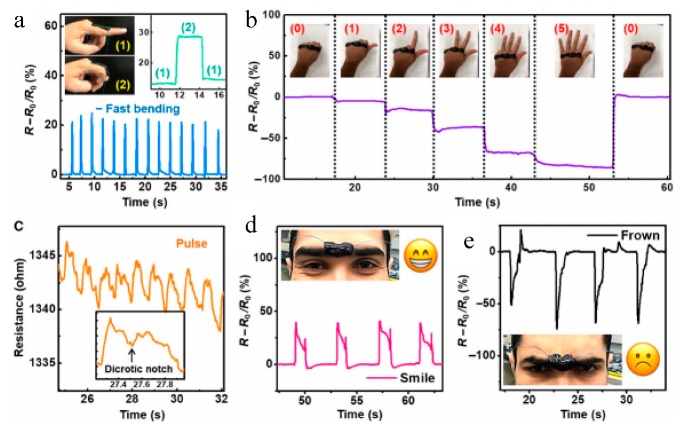
At a similar time in 2018, Zhang et al. [23] proposed the use of MXenes in hydrogels to improve the sensor performances (Figure 3). The PVA/MXene hydrogel demonstrated high stretchability and a high gauge factor. The recorded gauge factor was 25 at 40% strain. The conductive MXene filler increased strain sensitivity and mechanical properties of the PVA gel, due to MXene nanosheets having an abundance of surface functional groups. These functional groups can alter the surfaces to become negatively charged and hydrophilic.
Figure 3.
(a–e) Change in resistance of PVA/MXene hydrogel due to various gestures and facial expressions. (Adapted from [23], Figure 4, with permissions from AAAS).
2.2. Healing Time and Efficiency
Hydrogel sensors are able to heal from any deformations that occur. This includes the self-healing of the gel’s appearance, electrical recovery, and mechanical recovery. The inability to heal in any of those ways would negatively impact the use of the hydrogel as a sensor.
The PVA/SWCNT hydrogel was able to heal its appearance partially in 30 s and completely within 60 s at room temperature, with no scarring left on the gel. While the gel may have only partially healed, the PVA/SWCNT gel manages an electrical healing time of 3.2 s [21].
Liu et al. [24,25] prepared a PVA-based hydrogel that is self-healing and self-adhesive. They used polydopamine (PDA) to assist in self-healing and adhesiveness, and achieved complete self-healing in 250 milliseconds under ambient temperature. Similarly, Zhang et al. [26] utilized MXenes to increase the sensitivity of PVA-based hydrogel. The PVA/MXene hydrogel showed an ‘instantaneous’ healing time. MXenes, having numerous surface functional groups, contributes to the hydrogen bonding found in PVA, which explains the incredible healing time.
2.3. Stretchability
If a sensor is to be wearable, it should generally be highly stretchable to ensure comfort for the wearer. Shao et al. [25] formulated an ionic hydrogel based on polyacrylic acid (PAA) and aluminum ions. The gel achieved a fracture strain of 2952%, showing that the gel is ultra-stretchable. The above-mentioned PVA/MXenes hydrogels was able to achieve a stretchability of 3400%. Most recently, in 2019, Yang et al. developed a PVA/Borax gel. The biocompatible hydrogel has the ability to stretch up to a strain of 5000%.
Table 1 summarizes other hydrogel-based mechanical sensors that have been developed from a variety of groups.
Table 1.
The properties of highly stretchable and tough hydrogels.
| Gel | Problems of Traditional Gels that the New Gel Tried to Fix | Design Strategy of the New Gel in the Paper | Gauge Factor (Strain %) | Healing Time and Efficiency | Mechanical Properties | Year | Ref. |
|---|---|---|---|---|---|---|---|
| PVA/SWCNT | No sensing properties over 100% strain, Low Gauge Factor | Introduce SWCNT to increase stretchability, gauge factor, and recovery | 0.24 (100%) 1.51 (1000%) |
Electrical Healing: 3.2 s Appearance: 30–60 s Self-Healing Efficiency: ~98% |
No change in sensor properties after 1000 cycles at 700% strain Excellent Sensing Performance |
2016 | [21] |
| PVA/Graphene | No sensing properties over 100% strain, Low Gauge Factor | Introduce Graphene to increase stretchability, gauge factor, and recovery | 0.92 (1000%) | - | Excellent Sensing Performance | 2016 | [21] |
| PVA/Silver Nanowire | No sensing properties over 100% strain, Low Gauge Factor | Introduce Silver Nanowire to increase stretchability, gauge factor, and recovery | 2.25 (1000%) | Silver nanowire is easily oxidized by air and water | Excellent Sensing Performance | 2016 | [21] |
| Aromatic Polyamic Acid Salt (PAAS) Hydrogel | Poor Mechanical Properties, Preparation is toxic | Prepare in an environmentally friendly way, Adding p-PDA/s-BPDA enhance mechanical properties | - | Self-healed within 1 min at room temperature | Mechanical stress of 500 kPa at 1350% strain, Storage Modulus of 5 × 105 Pa | 2019 | [27] |
| DCh/PPy/PAA | Low Conductivity, Sensitivity, Mechanical Recovery | Create a mechanically/electrically self-healing hydrogel with pressure/extension sensitivity | - | Mechanical Recovery: 2 min 90% Electrical Recovery: 30 s |
Conductivity increases with strength of compression on Hydrogel | 2017 | [28] |
| PVA/PVP/Fe3+ | Low Mechanical Properties, Self-healability, sensitivity | Fabricate a conductive, elastic, self-healing, and strain-sensitive hydrogel | 0.478 (200%) | Self-healing within 5 min, and self-recovery within 30 min | Mechanical Strength of 2.1 MPa of tensile stress | 2017 | [29] |
| PVA/PDA | Low Detection Ranges and sensitivity | A low-modulus PVA hydrogel that is self-healing, PDA makes the hydrogel self-adhesive | - | Self-Healing in 250 ms at ambient temperature | High Sensing Performance in the ranges of Ultralow (0.1%) to High (500%) Strain | 2018 | [24] |
| PEDOT:SL/PAA | Not wearable, Insensitive to pressure/strain Can freeze at subzero temperatures |
PEDOT:SL improves softness and elasticity-promotes strain sensitivity | 7 (100%) | - | Stretched to 7 times original length, recovers with negligible residual strain | 2019 | [30] |
| PAAm/Graphene | Poor mechanical consistence and electrical conductivity | Hydrogel acts as potential scaffold for neuronal growth | 9 (30%) | - | Conductivity:5.4 × 10−5 S/cm | 2018 | [31] |
| PAA/PANI | Self-healing electronics have low durability and stretchability | PANI-based self-healing electronic composite with high stretchability and electrical conductivity | 11.6 (Within 100%) 4.7 (Over 100%) |
Electrical Conductivity Healing Efficiency: 88.2% in 5 min Mechanical Healing Efficiency: 24.3% in 5 min |
Stretchability up to 400% Electrical Conductivity of 0.12 S/cm |
2018 | [22] |
| PAAm/LiCl | Ionogels have lower conductivity than hydrogels | Soft, stretchable electrical devices integrating a conductive hydrogel | 0.84 (40%) | - | Conductivity: 10.39 ± 0.31 S/m | 2017 | [32] |
| PAA/Graphene/Fe3+ | Low stretchability, self-healing, mechanical properties | Covalent bonds -strong, stable network for the hydrogel, Reduced graphene oxide and ions are highly sensitive | 0.31 (100%) 1.32 (500%) |
Recovered nearly 100% initial conductivity | Resistance: 5.8 kΩ Strength: ~300 kPa at 45% strain Tensile Strength: ~400 kPa at 300% strain |
2018 | [33,34] |
| PAA/Al3+ | Poor mechanical properties, Require adhesives | Ions allow high sensitivity to large and subtle motions | 5.5 (100%) 7.8 (2000%) |
Healing efficiency of 88% at 20 min and 92% at 30 min | Ultra-stretchability with a 2952% fracture strain, Compression Performance: 95% strain without fracture Toughness: 5.60 MJ/m3 |
2018 | [25] |
| Dopamine/Talc/PAAm (DTPAM) | Low stretchability and recoverability | Polydopamine-modified talc particles uniformly disperse in PAAm—Enhance mechanical properties/adhesiveness | 0.125 (100%) 0.693 (1000%) |
Appearance healed after 30 min at room temperature | After healing, can still be stretched over 800% Strongly adhesive |
2018 | [34] |
| PAAm/Alginate | Low mechanical robustness and stretchability | PAAm and alginate form a ‘tough’ hydrogel that has a high stretchability and fracture toughness | - | - | Fracture Toughness of ~9000 J/m2 Fatigue Fracture of 53 J/m2 Cycle 1000: Constant resistance to high stretching |
2016 2017 |
[35,36] |
| PAAm/Alginate/CaCl2 | Desired properties lost below freezing point of water | Gel soaked in 30 wt % CaCl2 retains stretchability/toughness/conductivity at below 0 °C | - | - | Fracture Toughness of ~5000 J/m2 | 2018 | [37] |
| PAAm/Alginate Optical Fibers | Fragile against external strain, Low mechanical strength |
Make a tough hydrogel, which has high stretchability and mechanical strength | - | - | Fracture Energy of ~9000 J/m2 Can be elongated to 700% strain |
2016 | [38] |
| PAMPS/PAAm Double Network Gel |
Single network hydrogels showed poor mechanical properties, Fatigue Damage under low cyclic load |
Double Network hydrogels have outstanding mechanical properties | - | - | Average Toughness ~3358 J/m2, Fracture Energy 3779 J/m2, Fatigue Threshold 418 J/m2 | 2018 | [39] |
| PVA/PAAm | Low stretchability and sensitivity | Adhesive Wrinkled microarchitectures and interconnected ridges increase contact area |
- | - | Stretchability up to 500%, Response time of 150 ms, Sensitivity of 0.05 kPa−1 at 0 to 3.27 kPa | 2018 | [40] |
| AAm/2-hydroxyethylacrylate/Liquid Gallium | Low sensitivity, limited stretchability, and poor stability | Use liquid metals as soft fillers in hydrophilic polymer networks to make highly stretchable, force-sensitive hydrogels | - | - | Tensile Strain ~1500%, Compressive Sensitivity of 0.25 kPa | 2019 | [41] |
| PAA/PANI | Limited by fragile and weak properties, like low flexibility | Highly Stretchable PAA/PANI hydrogel | 0.60 (0–800%) 1.05 (800–1130%) |
- | Tensile Deformation: 1160% strain Sensing Range: 0 to 1130% |
2018 | [42] |
| PVA/MXene | Low sensitivity | MXenes have high conductivity and strain sensitivity. MXenes improve mechanical properties |
2, 0 wt % MXene (40%) 25, 4.1 wt % MXene (40%) |
Instantaneous Self-Healing | Stretchability of 3400% Conformability and adhesive to various surfaces, including human skin |
2018 | [23] |
| PAAm/Alginate/Eutectic Gallium | Low Conductivity, Stretchability, High Power Consumption | Eutectic Gallium is highly conductive and used in a known tough hydrogel | - | - | Sensitivity of 100 Pa, can be rehydrated to most of its initial weight (>85%) after 30 drying/soaking cycles | 2018 | [43] |
| PAAm/Agar/LiCl | Low stretchability, Opaque, Poor Mechanical Strength | Conductive, Excellent mechanical properties, stretchability, and sensitivity, Transparent | 1.8 (1100%) | - | Stretchability over 1600%, Tension Strength: 0.22 MPa, Compression Strength: 3.5 MPa | 2019 | [44] |
| PDMS/AAm/NaCl | Capacitance and resistance are affected by stretch, bend, and pressure | Low Cost Materials and methods | - | - | Ionic Resistivity of 0.06 Ω | 2017 | [45] |
| PAAm/LiCl | Low Sheet Resistances and transparency, Brittle | Used as an ionic conductor | - | - | Can operate with over 1000% areal strain Elastic Modulus of 12 kPa |
2016 | [46] |
| PAAm/LiCl/Silicone | LED-based systems are limited by low ultimate strain | Fabricate a hyperelastic light-emitting capacitor (HLEC), using a hydrogel | - | - | Stretches to >480% strain | 2016 | [47] |
| PAAm/Alginate/PDMS | Low mechanical robustness and compatibility | Hydrogel–Elastomer Hybrid that is stretchable, robust, and biocompatible | - | - | - | 2017 | [48] |
| PNAGA-PAMPS/PEDOT-PSSa | Conductive Hydrogels (CHs) are mechanically weak and brittle | PNAGA hydrogels demonstrate high strength, thermoplasticity, and self-healability | - | Self-healed after 3 h in a plastic syringe immersed in a 90 °C water bath | 0.22–0.58 MPa tensile strength, 1.02–7.62 MPa compressive strength, 817–1709% breaking strain | 2017 | [49] |
| PVA/CNF | Low sensitivity, stretchability, self-healability, and transparency | Highly sensitive, stretchable, and autonomously self-healing ionic skin—biocompatible | - | Spontaneously Self-Healed in 15 s | Highly Transparent—Transmittance as high as 90%, Modulus of 11.2 kPa, Elongation Rate of 1900% | 2019 | [50] |
| PVA/Borax | Low stretchability, self-healing, water retention, biocompatibility | PVA and Borax: biocompatible/highly stretchable/easily dissolvable in aqueous solution/have good mechanical performance | - | Self-healed 10 times without affecting electrical conduction of gel | Can be stretched to strains over 5000% | 2019 | [51] |
PVA—Polyvinyl Alcohol; SWCNT—Single-Wall Carbon Nanotube; p-PDA—p-Phenylenediamine; s-BPDA—Biphenyltetracarboxylic dianhydride; DCh—Double-bond Decorated Chitosan; PPy—Polypyrrole; PAA—Polyacrylic Acid; PVP—Polyvinylpyrrolidone; PDA—Polydopamine; PEDOT:SL—Poly (3,4-ethylenedioxythiophene): Sulfonated Lignin; PAAm—Polyacrylamide; PANI—Polyaniline; PAMPS—Poly (1-acrylanmido-2-methylpropanesulfonic acid); AAm—Acrylamide; PDMS—Polydimethylsiloxane; PNAGA-PAMPS—Poly (N-acryloyl glycinamide-co-2-acrylamide-2-methylpropanesulfonic); PEDOT-PSS—Poly (3,4-ethylenedioxythiophene)-poly (styrenesulfonate); CNF—Cellulose Nanofibril.
3. Highly Stretchable and Tough Hydrogels for Wound Healing
Although hydrogels are already being utilized for wound healing, many of these commercial hydrogels have flaws. For instance, commonly used in surgery to create a fibrin clot, fibrin glue has issues with weak adhesion strength and the risk of transfer of blood diseases [52,53]. Fibrin glue TISSEEL [54] is vulnerable to debonding. Polyethylene glycol–based adhesives like COSEAL, although it has good adhesion properties and effectively prevents leaking of blood from vessels, carries poor mechanical properties and induces swelling [55]. Similarly, cyanoacrylate-based adhesives, super glues, are hampered by their poor biomechanical compatibility [56]. Despite being the strongest class of tissue adhesives, these adhesives are cytotoxic and poor with wet surfaces, making it difficult to be applicable to wound healing [57]. In terms of self-healing, a commercial carboxymethyl hydrogel only healed 27% in a week and 70% in 10 days [58]. With these flaws, researchers have looked for alternative solutions and methods to create hydrogels better suited for wound healing with efficient healing time and effective adhesion strength.
3.1. Healing Properties
When hydrogels are examined for their uses in wound healing, the healing time is a critical factor. Healing time is the necessary time needed for a wound to heal. In 2013, Sakai et al. developed a simple yet effective method that allows a highly stretchable gel to in situ gelate when a solution is poured onto a wound [58]. This solution, containing a PVA derivative with phenolic hydroxyl properties (PVA-Ph), glucose oxidase (GOx), and horseradish peroxidase (HRP), allows the hydrogelation to occur as quickly as five seconds. This PVA-Ph hydrogel was able to heal 77% of the initial wounds in seven days and 96% within 10 days, which is more effective than the previously mentioned commercial carboxymethyl hydrogel. Le et al. combined dopamine-modified four-armed poly (ethylene glycol) (PEG) and poly (sulfamethazine ester urethane) (PSMEU) to better control physical and mechanical properties [59]. The PEG-PSMEU hydrogel was tested on longitudinal cuts to measure healing time. These wounds completely closed after a week. These developed hydrogels also had other healing characteristics. Consequently, Qu et al. reported that loading the quaternized chitosan/Pluronic® F127 (QCS/PF) gel with curcumin results a tunable antioxidant ability [60]. Ballance et al. reported that the addition of cyclodextrin in a polyacrylamide gel increases mechanical strength [61]. More importantly, this hydrogel demonstrated antibacterial properties when treated with quinine. The gel’s improved stretchability resulted in a greater release of quinine, leading to successfully inhibit the growth of E. Coli. Liu et al. reported the combination of PEG-D4 with Laponite forms an injectable gel that degrades nontoxically [53]. In fact, many of the developed hydrogels are applicable on tissue skin unlike the previously mentioned commercial adhesives.
3.2. Adhesion Strength
To be applicable to wound healing, gels must have great adhesion strength. Adhesion strength is how a polymer sticks and bonds to surfaces. When adhesion strength is measured, multiple tests are usually done on a variety of surfaces. To compare with human skin, porcine skin was commonly used. Qu et al. increased the adhesion strength of 4.4 kPa to 6.1 kPa on porcine skin with the increase of Pluronic® F127 (PF127-CHO) [60]. This strength was comparable to that of a fibrin glue adhesive. Li et al. developed tough adhesives, the toughest of which had an adhesion strength of 83 kPa on porcine skin [62]. The adhesion occurred in a few minutes, lending itself to more uses, unlike cyanoacrylate, which hardens upon contact with tissue skin. Han et al. added polydopamine (PDA)-intercalated clay nanosheets to a PAM hydrogel to make it more adhesive [63]. While the hydrogel only has an adhesion strength of 28.5 kPa on porcine skin, it showed 120 kPa when measured on glass. When adhered to the glass slides, this hydrogel could support a load of 500 g. Most recently, Wang et al. made a tyrosine hydrochloride gel with the adhesion strength of 453 kPa on pigskin [64]. As time progressed, the adhesion strength has rapidly improved in hydrogels.
3.3. Additional Mechanical Properties
He et al. reported that the introduction of a microgel to enhance adhesiveness and improve mechanical strength in its poly (N-isopropylacrylamide) microgel/polyacrylic acid-polyacrylamide-polydopamine (MR/PAAc-PAM-PDA) hydrogel [65]. Fukao et al. combined bioceramic hydroxyapatite (HAp) with double network hydrogels to increase mechanical strength and create a structure similar to bone tissue [66]. Guvendiren et al. reported the integration of 3,4-dihydroxy-l-phenylalanine (DOPA) to the gel increases cohesive and adhesive properties [67]. Unlike healing time, self-healing time refers to the time needed for the hydrogel to recover. In 2018, Chen et al. crosslinked oxidized sodium alginate–dopamine (OSA-DA) and polyacrylamide (PAM) to withstand large deformations and efficiently self-heal [68]. OSA-DA-PAM hydrogel was able to recover 80% within only six hours. Using the dynamic coupling reaction of tyrosine hydrochloride catalyzed by enzymes, Wang et al. developed a gel that completely self-healed within a day in 2019 [64]. The hydrogel was 25% healed in four hours and 68% in 12 h, showing much progress.
Table 2 summarizes other hydrogel-based wound dressings that have been developed from a variety of groups.
Table 2.
The properties of highly stretchable and tough hydrogels for wound healing.
| Gel | Problems the Gel Tried to Fix | Design Strategy | Healing Time | Adhesion Strength (kPa) | Conclusion | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Tyrosine Hydrochloride Gel | Poor mechanical properties and self-healing properties | Use dynamic coupling reaction to improve adhesion and self-healing properties | Self 4 h: 25% 12 h: 68% 24 h: 100% |
Pigskin: 453 Glass: 265 Stainless Steel: 265 PTFE:329 |
This gel exhibited great self-healing abilities. | 2019 | [64] |
| MR/PAAc-PAM-PDA Hydrogel | Limited adhesion strength | Introduce MR to enhance adhesion and improve mechanical strength | - | 40 after 60 s | Stretch up to 660% at a tensile strength of 110 kPa. Good self-healing properties | 2019 | [65] |
| PEG-D4 Laponite Gel | Weak adhesion, poor biomechanical compatibility. | Add Laponite to PEG-D4 to promote bioactivity increase adhesion and mechanical properties | - | Wt % Laponite 0%: 3.5 1%: 7 2%: 8 |
Injectable gel degrades nontoxically. Laponite increases adhesion strength. |
2014 | [53] |
| DOPA gel | Unknown effects of DOPA on cohesion and adhesion | Integrate DOPA to the gel to test cohesive and adhesive properties | - | - | Become worse in adhesion at higher pH levels. | 2008 | [67] |
| OSDA-DA-PAM Hydrogel | Poor mechanical property, lack of tissue adhesiveness | Crosslink OSA-DA and PAM chains to withstand large deformations | Self 6 h: 80% |
- | Improved mechanical properties, Useful self-healing ability. | 2018 | [68] |
| PEG-PSMEU Hydrogel | Nuclease degradation, lack of membrane permeability | Combine PEG and PSMEU to better control mechanical properties |
longitudinal cutaneous wounds: healed in 7 days | 90 | Higher copolymer concentration leads to higher adhesion strength. | 2018 | [59] |
| QCS/PF Gel | Questionable reliability of dressing materials on wound | Increasing content of PF127-CHO increases adhesive strength | - | 6.1 | Good blood-clotting ability | 2018 | [60] |
| Cur-QCS/PF Gel | Questionable reliability of dressing materials on wound | Loading the gel with Cur will result tunable antioxidant ability. Greater release rate | - | - | Better healing from greater release rate, Better Collagen levels after 15 days | 2018 | [60] |
| PAMPS/PAM DN Gel | Unable to be firmly fixed onto bones by glues. | Inducing bioceramic HAp on the gel surface for robust bonding to bone tissues. | - | - | Bonelike structure by controlling HAp crystal orientation. Bonded to bone tissue. |
2017 | [66] |
| PAM-cyclodextrin Gel | Nonstretchable PAM gel has intrinsic brittleness | Combine cyclodextrin acrylate to increase strain | - | - | Quinine inhibited growth of E. Coli. Stretched 16 times original | 2018 | [61] |
| PVA-Ph Hydrogel | Challenge in situ formation of hydrogel wound dressings | HRP-catalyzed reaction so the gel can form in situ on wound | 7 days: 77% 10 days: 96% |
- | Gelated as quickly as 5 s. Easily pour onto wound. Retained mechanical properties. | 2013 | [58] |
| PDA-clay-PAM Hydrogel | Weak adhesive materials and poor mechanical properties | Adding PDA-intercalated clay nanosheets will make it more adhesive | - | Glass: 120 Ti:80.8 PE:80.7 Porcine Skin:28.5 |
Strong adhesiveness, High stretchability, Good candidate for delicate surgical adhesive. | 2017 | [63] |
| Tough adhesives | Commercial adhesives have weak adhesion vulnerable to debonding |
Fabricate family of tough adhesives that can adhere to wet surfaces. | - | On beating porcine heart: 83 | Hemostatic dressing possible, High adhesion energy High matrix toughness |
2018 | [62] |
PTFE: Polytetrafluoroethylene; PNIPAM: Poly (N-isopropylacrylamide); MR: PNIPAM microgel; PAAc-PAM-PDA: Poly (acrylic acid)-poly (acrylamide)-poly (dopamine); PEG-D4: Dopamine-modified four-armed poly (ethylene glycol); DOPA: 3,4-Dihydroxy-l-phenylalanine; OSA-DA: Dopamine-grafted oxidized sodium alginate; PEG: Poly (ethylene glycol); PSMEU: Poly (sulfamethazine ester urethane); QCS: Quaternized chitosan; PF: Pluronic® F127; Cur: Curcumin; PAMPS: Poly (2-acrylamido-2-methylpropanesulfonic acid); DN: Double Network; HAp: Hydroxyapatite; SN: Single Network; PVA: Polyvinyl alcohol; Ph: Phenolic Hydroxyl; HRP: Horseradish peroxidase; GOx: Glucose oxidase; PDA: Polydopamine; PAM: Polyacrylamide; PAA: Polyallylamine.
4. Highly Stretchable and Tough Hydrogels for Drug Delivery
Highly stretchable, wearable hydrogels have a high potential in use for delivering drugs. In 2013, Zhang et al. formulated an enzyme-incorporated hydrogel made from PAAm and alginate [69]. This gel maintained homogenous throughout, even after being stretched 6.67 times its original size. The gel was put through a 48-h intensive straining and washing process that concluded with no leakage of proteins in the gel. Over a week of time, enzymes in the hydrogel maintained most of their initial activity when stored at room temperature. Any loose, free counterparts in solution, however, lost activity significantly. Park et al. created a hydrogel made from poly (methacrylic acid/ethylene glycol dimethacrylate) and Fe3O4 in 2015 [70]. The composite microcapsules in the gel are biocompatible, responsive to pH, and magnetic, which makes it appropriate for drug delivery. The amount of drug, in this case doxorubicin chloride (DOX), released was 43.8% at pH 2 compared to 9.5% at pH 7. In the same year, Di et al. [71] worked on a tensile strain-triggered drug delivery device. The gel was made of poly (lactic-co-glycolic acid) nanoparticles and Dragon Skin 30, which is a soft, strong, stretchy silicone rubber. The nanoparticles serve as drug delivery depots, while the Dragon Skin works for loading tensile strain. The amount of insulin release saturated after 10 testing cycles at 50% strain with two second intervals. With an interval of four hours, the same amount of insulin was released across several stretching events. Lin [35] demonstrated that when stretched, a polyacrylamide-based gel with alginate and titanium wire accommodates drug diffusion without any breakages or leaking, with a drug diffusion coefficient of 3 × 10−10 m2 s−1. A biocompatible, stretchable, and robust material was fabricated by Liu et al. [48]. The hydrogel is a polyacrylamide-based gel with alginate and polydimethylsiloxane. The design that Liu put forward could be programmed with desirable functionalities by designing the circuits in the cells and structures and patterns of the hydrogel.
5. Conclusions
Highly stretchable and tough hydrogels can be implemented in multiple different fields, such as mechanical sensors. Properties important to making a good mechanical sensor, such as gauge factor, self-healing, and having strong mechanical properties, can be seen within these hydrogels. Highly stretchable and tough hydrogels, with great adhesion strength and healing times, can be utilized for wound healing. The mechanical properties of tough, highly stretchable hydrogels can be used for drug delivery.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liao I.-C., Moutos F.T., Estes B.T., Zhao X., Guilak F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013;23:5833–5839. doi: 10.1002/adfm.201300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M., Smith A.M., Das A.K., Hodson N.W., Collins R.F., Ulijn R.V., Gough J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials. 2009;30:2523–2530. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Nicolson P.C., Vogt J. Soft contact lens polymers: An evolution. Biomaterials. 2001;22:3273–3283. doi: 10.1016/S0142-9612(01)00165-X. [DOI] [PubMed] [Google Scholar]
- 4.Ta H.T., Dass C.R., Larson I., Choong P.F., Dunstan D.E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials. 2009;30:4815–4823. doi: 10.1016/j.biomaterials.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 5.Jen A.C., Wake M.C., Mikos A.G. Review: Hydrogels for cell immobilization. Biotechnol. Bioeng. 1996;50:357–364. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida R., Uchida K., Kaneko Y., Sakai K., Kikuchi A., Sakurai Y., Okano T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 1995;374:240–242. doi: 10.1038/374240a0. [DOI] [Google Scholar]
- 7.Irie M., Misumi Y., Tanaka T. Stimuli-responsive polymers: Chemical induced reversible phase separation of an aqueous solution of poly(N-isopropylacrylamide) with pendent crown ether groups. Polymer. 1993;34:4531–4535. doi: 10.1016/0032-3861(93)90160-C. [DOI] [Google Scholar]
- 8.Lee K., Cussler E., Marchetti M., McHugh M. Pressure-dependent phase transitions in hydrogels. Chem. Eng. Sci. 1990;45:766–767. doi: 10.1016/0009-2509(90)87019-O. [DOI] [Google Scholar]
- 9.Bohon K., Krause S. An electrorheological fluid and siloxane gel based electromechanical actuator: Working toward an artificial muscle. J. Polym. Sci. Part B Polym. Phys. 1998;36:1091–1094. doi: 10.1002/(SICI)1099-0488(19980430)36:6<1091::AID-POLB16>3.0.CO;2-1. [DOI] [Google Scholar]
- 10.Horkay F., Tasaki I., Basser P.J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules. 2000;1:84–90. doi: 10.1021/bm9905031. [DOI] [PubMed] [Google Scholar]
- 11.Gupta K.M., Barnes S.R., Tangaro R.A., Roberts M.C., Owen D.H., Katz D.F., Kiser P.F. Temperature and pH Sensitive Hydrogels: An Approach Towards Smart Semen-Triggered Vaginal Microbicidal Vehicles. J. Pharm. Sci. 2007;96:670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- 12.Hong W., Hu X., Zhao B., Zhang F., Zhang D. Tunable Photonic Polyelectrolyte Colorimetric Sensing for Anions, Cations and Zwitterions. Adv. Mater. 2010;22:5043–5047. doi: 10.1002/adma.201002512. [DOI] [PubMed] [Google Scholar]
- 13.Caló E., Khutoryanskiy V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015;65:252–267. doi: 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- 14.Sidorenko A., Krupenkin T., Taylor A., Fratzl P., Aizenberg J. Reversible Switching of Hydrogel-Actuated Nanostructures into Complex Micropatterns. Science. 2007;315:487–490. doi: 10.1126/science.1135516. [DOI] [PubMed] [Google Scholar]
- 15.Maeda S., Hara Y., Sakai T., Yoshida R., Hashimoto S. Self-Walking Gel. Adv. Mater. 2007;19:3480–3484. doi: 10.1002/adma.200700625. [DOI] [Google Scholar]
- 16.Gong J.P., Katsuyama Y., Kurokawa T., Osada Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003;15:1155–1158. doi: 10.1002/adma.200304907. [DOI] [Google Scholar]
- 17.Sanabria-DeLong N., Crosby A.J., Tew G.N. Photo-Cross-Linked PLA-PEO-PLA Hydrogels from Self-Assembled Physical Networks: Mechanical Properties and Influence of Assumed Constitutive Relationships. Biomacromolecules. 2008;9:2784–2791. doi: 10.1021/bm800557r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branco M.C., Nettesheim F., Pochan D.J., Schneider J.P., Wagner N.J. Fast Dynamics of Semiflexible Chain Networks of Self-Assembled Peptides. Biomacromolecules. 2009;10:1374–1380. doi: 10.1021/bm801396e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao Z., Cao M., Michels K., Hoffman L., Ji H.-F. Design and Fabrication of Highly Stretchable and Tough Hydrogels. Polym. Rev. 2019 doi: 10.3390/polym11111773. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frutiger A., Muth J.T., Vogt D.M., Mengüç Y., Campo A., Valentine A.D., Walsh C.J., Lewis J.A. Capacitive Soft Strain Sensors via Multicore-Shell Fiber Printing. Adv. Mater. 2015;27:2440–2446. doi: 10.1002/adma.201500072. [DOI] [PubMed] [Google Scholar]
- 21.Cai G., Wang J., Qian K., Chen J., Li S., Lee P.S. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2016;4:1600190. doi: 10.1002/advs.201600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T., Zhang Y., Liu Q., Cheng W., Wang X., Pan L., Xu B., Xu H. A Self-Healable, Highly Stretchable, and Solution Processable Conductive Polymer Composite for Ultrasensitive Strain and Pressure Sensing. Adv. Funct. Mater. 2018;28:1705551. doi: 10.1002/adfm.201705551. [DOI] [Google Scholar]
- 23.Zhang Y.-Z., Lee K.H., Anjum D.H., Sougrat R., Jiang Q., Kim H., Alshareef H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018;4:eaat0098. doi: 10.1126/sciadv.aat0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Zheng R., Chen S., Wu Y., Liu H., Wang P., Deng Z., Liu L. A compliant, self-adhesive and self-healing wearable hydrogel as epidermal strain sensor. J. Mater. Chem. C. 2018;6:4183–4190. doi: 10.1039/C8TC00157J. [DOI] [Google Scholar]
- 25.Shao C., Wang M., Meng L., Chang H., Wang B., Xu F., Yang J., Wan P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018;30:3110–3121. doi: 10.1021/acs.chemmater.8b01172. [DOI] [Google Scholar]
- 26.Zhang J., Wan L., Gao Y., Fang X., Lu T., Pan L., Xuan F. Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin. Adv. Electron. Mater. 2019;5:1900285. doi: 10.1002/aelm.201900285. [DOI] [Google Scholar]
- 27.Zhou H., Zheng S., Qu C., Wang D., Liu C., Wang Y., Fan X., Xiao W., Li H., Zhao D., et al. Simple and environmentally friendly approach for preparing high-performance polyimide precursor hydrogel with fully aromatic structures for strain sensor. Eur. Polym. J. 2019;114:346–352. doi: 10.1016/j.eurpolymj.2019.01.043. [DOI] [Google Scholar]
- 28.Darabi M.A., Khosrozadeh A., Mbeleck R., Liu Y., Chang Q., Jiang J., Cai J., Wang Q., Luo G., Xing M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017;29:1700533. doi: 10.1002/adma.201700533. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y.-J., Cao W.-T., Ma M.-G., Wan P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces. 2017;9:25559–25570. doi: 10.1021/acsami.7b07639. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q., Pan X., Lin C., Lin D., Ni Y., Chen L., Huang L., Cao S., Ma X. Biocompatible, self-wrinkled, antifreezing and stretchable hydrogel-based wearable sensor with PEDOT:sulfonated lignin as conductive materials. Chem. Eng. J. 2019;370:1039–1047. doi: 10.1016/j.cej.2019.03.287. [DOI] [Google Scholar]
- 31.González-Domínguez J.M., Martín C., Durá Ó.J., Merino S., Vázquez E. Smart Hybrid Graphene Hydrogels: A Study of the Different Responses to Mechanical Stretching Stimulus. ACS Appl. Mater. Interfaces. 2018;10:1987–1995. doi: 10.1021/acsami.7b14404. [DOI] [PubMed] [Google Scholar]
- 32.Tian K., Bae J., Bakarich S.E., Yang C., Gately R.D., Spinks G.M., in het Panhuis M., Suo Z., Vlassak J.J. 3D Printing of Transparent and Conductive Heterogeneous Hydrogel—Elastomer Systems. Adv. Mater. 2017;29:1604827. doi: 10.1002/adma.201604827. [DOI] [PubMed] [Google Scholar]
- 33.Jing X., Mi H.-Y., Peng X.-F., Turng L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon. 2018;136:63–72. doi: 10.1016/j.carbon.2018.04.065. [DOI] [Google Scholar]
- 34.Jing X., Mi H.-Y., Lin Y.-J., Enriquez E., Peng X.-F., Turng L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces. 2018;10:20897–20909. doi: 10.1021/acsami.8b06475. [DOI] [PubMed] [Google Scholar]
- 35.Lin S., Yuk H., Zhang T., Parada G.A., Koo H., Yu C., Zhao X. Stretchable Hydrogel Electronics and Devices. Adv. Mater. 2016;28:4497–4505. doi: 10.1002/adma.201504152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai R., Yang Q., Tang J., Morelle X.P., Vlassak J., Suo Z. Fatigue fracture of tough hydrogels. Extrem. Mech. Lett. 2017;15:91–96. doi: 10.1016/j.eml.2017.07.002. [DOI] [Google Scholar]
- 37.Morelle X.P., Illeperuma W.R., Tian K., Bai R., Suo Z., Vlassak J.J. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018;30:1801541. doi: 10.1002/adma.201801541. [DOI] [PubMed] [Google Scholar]
- 38.Guo J., Liu X., Jiang N., Yetisen A.K., Yuk H., Yang C., Khademhosseini A., Zhao X., Yun S.-H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016;28:10244–10249. doi: 10.1002/adma.201603160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W., Liu X., Wang J., Tang J., Hu J., Lu T., Suo Z. Fatigue of double-network hydrogels. Eng. Fract. Mech. 2018;187:74–93. doi: 10.1016/j.engfracmech.2017.10.018. [DOI] [Google Scholar]
- 40.Ge G., Zhang Y., Shao J., Wang W., Si W., Huang W., Dong X. Stretchable, Transparent, and Self-Patterned Hydrogel-Based Pressure Sensor for Human Motions Detection. Adv. Funct. Mater. 2018;28:1802576. doi: 10.1002/adfm.201802576. [DOI] [Google Scholar]
- 41.Peng H., Xin Y., Xu J., Liu H., Zhang J. Ultra-stretchable hydrogels with reactive liquid metals as asymmetric force-sensors. Mater. Horiz. 2019;6:618–625. doi: 10.1039/C8MH01561A. [DOI] [Google Scholar]
- 42.Wang Z., Zhou H., Lai J., Yan B., Liu H., Jin X., Ma A., Zhang G., Zhao W., Chen W. Extremely stretchable and electrically conductive hydrogels with dually synergistic networks for wearable strain sensors. J. Mater. Chem. C. 2018;6:9200–9207. doi: 10.1039/C8TC02505C. [DOI] [Google Scholar]
- 43.Liu H., Li M., Ouyang C., Lu T.J., Li F., Xu F. Biofriendly, Stretchable, and Reusable Hydrogel Electronics as Wearable Force Sensors. Small. 2018;14:e1801711. doi: 10.1002/smll.201801711. [DOI] [PubMed] [Google Scholar]
- 44.Yang B., Yuan W. Highly Stretchable and Transparent Double-Network Hydrogel Ionic Conductors as Flexible Thermal–Mechanical Dual Sensors and Electroluminescent Devices. ACS Appl. Mater. Interfaces. 2019;11:16765–16775. doi: 10.1021/acsami.9b01989. [DOI] [PubMed] [Google Scholar]
- 45.Sarwar M.S., Dobashi Y., Preston C., Wyss J.K.M., Mirabbasi S., Madden J.D.W. Bend, stretch, and touch: Locating a finger on an actively deformed transparent sensor array. Sci. Adv. 2017;3:e1602200. doi: 10.1126/sciadv.1602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim C.-C., Lee H.-H., Oh K.H., Sun J.-Y. Highly stretchable, transparent ionic touch panel. Science. 2016;353:682–687. doi: 10.1126/science.aaf8810. [DOI] [PubMed] [Google Scholar]
- 47.Larson C., Peele B., Li S., Robinson S., Totaro M., Beccai L., Mazzolai B., Shepherd R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science. 2016;351:1071–1074. doi: 10.1126/science.aac5082. [DOI] [PubMed] [Google Scholar]
- 48.Liu X., Tang T.-C., Tham E., Yuk H., Lin S., Lu T.K., Zhao X. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc. Natl. Acad. Sci. USA. 2017;114:2200. doi: 10.1073/pnas.1618307114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Q., Wei J., Xu B., Liu X., Wang H., Wang W., Wang Q., Liu W. A robust, highly stretchable supramolecular polymer conductive hydrogel with self-healability and thermo-processability. Sci. Rep. 2017;7:41566. doi: 10.1038/srep41566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing X., Li H., Mi H.-Y., Liu Y.-J., Feng P.-Y., Tan Y.-M., Turng L.-S. Highly transparent, stretchable, and rapid self-healing polyvinyl alcohol/cellulose nanofibril hydrogel sensors for sensitive pressure sensing and human motion detection. Sens. Actuators B Chem. 2019;295:159–167. doi: 10.1016/j.snb.2019.05.082. [DOI] [Google Scholar]
- 51.Yang N., Qi P., Ren J., Yu H., Liu S.-X., Li J., Chen W., Kaplan D.L., Ling S. Polyvinyl Alcohol/Silk Fibroin/Borax Hydrogel Ionotronics: A Highly Stretchable, Self-Healable, and Biocompatible Sensing Platform. ACS Appl. Mater. Interfaces. 2019;11:23632–23638. doi: 10.1021/acsami.9b06920. [DOI] [PubMed] [Google Scholar]
- 52.Ten Hallers E.J.O., Jansen J.A., Marres H.A.M., Rakhorst G., Verkerke G.J. Histological assessment of titanium and polypropylene fiber mesh implantation with and without fibrin tissue glue. J. Biomed. Mater. Res. Part A. 2007;80:372–380. doi: 10.1002/jbm.a.30887. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y., Meng H., Konst S., Sarmiento R., Rajachar R., Lee B.P. Injectable Dopamine-Modified Poly(ethylene glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces. 2014;6:16982–16992. doi: 10.1021/am504566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sierra D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993;7:309–352. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 55.Wallace D.G., Cruise G.M., Rhee W.M., Schroeder J.A., Prior J.J., Ju J., Maroney M., Duronio J., Ngo M.H., Estridge T., et al. A tissue sealant based on reactive multifunctional polyethylene glycol. J. Biomed. Mater. Res. 2001;58:545–555. doi: 10.1002/jbm.1053. [DOI] [PubMed] [Google Scholar]
- 56.Fortelny R.H., Petter-Puchner A.H., Walder N., Mittermayr R., Öhlinger W., Heinze A., Redl H. Cyanoacrylate tissue sealant impairs tissue integration of macroporous mesh in experimental hernia repair. Surg. Endosc. 2007;21:1781–1785. doi: 10.1007/s00464-007-9243-7. [DOI] [PubMed] [Google Scholar]
- 57.Vakalopoulos K.A., Wu Z., Kroese L., Kleinrensink G.J., Jeekel J., Vendamme R., Dodou D., Lange J.F. Mechanical strength and rheological properties of tissue adhesives with regard to colorectal anastomosis: An ex vivo study. Ann. Surg. 2015;261:323–331. doi: 10.1097/SLA.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 58.Sakai S., Tsumura M., Inoue M., Koga Y., Fukano K., Taya M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J. Mater. Chem. B. 2013;1:5067. doi: 10.1039/c3tb20780c. [DOI] [PubMed] [Google Scholar]
- 59.Le T.M.D., Duong H.T.T., Thambi T., Phan V.G., Jeong J.H., Lee D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules. 2018;19:3536–3548. doi: 10.1021/acs.biomac.8b00819. [DOI] [PubMed] [Google Scholar]
- 60.Qu J., Zhao X., Liang Y., Zhang T., Ma P.X., Guo B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 61.Ballance W.C., Seo Y., Baek K., Chalifoux M., Kim D., Kong H. Stretchable, anti-bacterial hydrogel activated by large mechanical deformation. J. Control. Release. 2018;275:1–11. doi: 10.1016/j.jconrel.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Li J., Celiz A.D., Yang J., Yang Q., Wamala I., Whyte W., Seo B.R., Vasilyev N.V., Vlassak J.J., Suo Z., et al. Tough adhesives for diverse wet surfaces. Science. 2017;357:378–381. doi: 10.1126/science.aah6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han L., Lu X., Liu K., Wang K., Fang L., Weng L.-T., Zhang H., Tang Y., Ren F., Zhao C., et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano. 2017;11:2561–2574. doi: 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Xu Q., Chen T., Li M., Feng B., Weng J., Duan K., Peng W., Wang J. A dynamic-coupling-reaction-based autonomous self-healing hydrogel with ultra-high stretching and adhesion properties. J. Mater. Chem. B. 2019;7:3044–3052. doi: 10.1039/C9TB00244H. [DOI] [Google Scholar]
- 65.He X., Liu L., Han H., Shi W., Yang W., Lu X. Bioinspired and Microgel-Tackified Adhesive Hydrogel with Rapid Self-Healing and High Stretchability. Macromolecules. 2019;52:72–80. doi: 10.1021/acs.macromol.8b01678. [DOI] [Google Scholar]
- 66.Fukao K., Nonoyama T., Kiyama R., Furusawa K., Kurokawa T., Nakajima T., Gong J.P. Anisotropic Growth of Hydroxyapatite in Stretched Double Network Hydrogel. ACS Nano. 2017;11:12103–12110. doi: 10.1021/acsnano.7b04942. [DOI] [PubMed] [Google Scholar]
- 67.Guvendiren M., Messersmith P.B., Shull K.R. Self-Assembly and Adhesion of DOPA-Modified Methacrylic Triblock Hydrogels. Biomacromolecules. 2008;9:122–128. doi: 10.1021/bm700886b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen T., Chen Y., Rehman H.U., Chen Z., Yang Z., Wang M., Li H., Liu H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces. 2018;10:33523–33531. doi: 10.1021/acsami.8b10064. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Chen Q., Ge J., Liu Z. Controlled display of enzyme activity with a stretchable hydrogel. Chem. Commun. 2013;49:9815. doi: 10.1039/c3cc45837g. [DOI] [PubMed] [Google Scholar]
- 70.Park S.-J., Lim H.-S., Lee Y.M., Suh K.-D. Facile synthesis of monodisperse poly(MAA/EGDMA)/Fe3O4 hydrogel microspheres with hollow structures for drug delivery systems: The hollow structure formation mechanism and effects of various metal ions on structural changes. RSC Adv. 2015;5:10081–10088. doi: 10.1039/C4RA13904F. [DOI] [Google Scholar]
- 71.Di J., Yao S., Ye Y., Cui Z., Yu J., Ghosh T.K., Zhu Y., Gu Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano. 2015;9:9407–9415. doi: 10.1021/acsnano.5b03975. [DOI] [PubMed] [Google Scholar]