Abstract
Cellulose acetate is one of the most important cellulose derivatives. The use of ionic liquids in cellulose processing was recently found to act both as a solvent and also as a reagent. A recent study showed that cellulose dissolution in the ionic liquid 1-ethyl-3-methylimidazoliumacetate (EMIMAc) mixed with dichloromethane (DCM) resulted in controlled homogenous cellulose acetylation; yielding water-soluble cellulose acetate (WSCA). This research investigated the properties of cellulose acetate prepared in this manner, in an aqueous solution. The results revealed that WSCA fully dissolves in water, with no significant sign of molecular aggregation. Its conformation in aqueous solution exhibited a very large persistence length, estimated as over 10 nm. The WSCA exhibited surface activity, significantly reducing the surface tension of water. Because of the molecular dissolution of WSCA in water, augmented by its amphiphilicity, aqueous solutions of WSCA exhibited an overwhelmingly high rate of enzymatic hydrolysis.
Keywords: water soluble, cellulose acetate, polymer solution properties, enzymatic hydrolysis
1. Introduction
Cellulose acetate (CA), formed by partial or full acetylation of the three hydroxyl groups of anhydroglucose (AGU), the repeat unit of cellulose, is one of the oldest synthetic materials, studied since the end of the nineteenth century. Cellulose triacetate (degree of substitution DS~3) is usually manufactured by a direct irreversible cellulose acetylation reaction using acetic anhydride. The triacetate product can be subjected to partial hydrolysis with dilute sulfuric acid, to yield secondary acetates with a lower degree of substitution (DS), e.g., monoacetate (DS~1) and diacetate (DS~2). It is well-known that cellulose acetates with DS in the range of 0.4–0.9 are water-soluble [1,2,3,4]. Unfortunately, undesired degradation of the cellulose chain occurs during the deacetylation process [2]. A number of additional drawbacks are associated with this procedure rendering it commercially unattractive. These include long reaction times (8–48 h) at relatively high temperatures (140–160 °C) and the need for continuous or sequential addition of water to maintain reaction rates yielding dilute reaction mixtures (often <5% w/w), which require significant use of energy for subsequent solvent recycling [4,5].
The physical properties of CAs are determined by the conformational behavior of the chains and their intermolecular interactions. Chemically homogeneous sequences of un-derivatized or fully derivatized glucopyranose units can locally organize into a crystalline structure; whereas the heterogeneous ones comprised of differently substituted monomers randomly distributed along the chain, are amorphous [6,7]. X-ray diffraction observations revealed that the water-soluble CA is essentially noncrystalline [3]. CA samples merely presented a broad peak around the scattering angle 2θ = 20.0 degree, confirming that the original crystalline structure of cellulose was largely destroyed [8,9]. At the same time, it has been reported that the water solubility of CA depends on both the overall DS, as well as on the distribution of acetyl substituents along the chain. Substitution of secondary hydroxyls at C-2 and C-3 of AGU by acetyl groups plays a key role in the water solubility of CA [3,10]. Although the acetyl groups are less hydrophilic than the hydroxyl groups, their presence decreases the intra and inter molecular H-bonds along the cellulose chain, thus rendering it water soluble within a certain range of acetyl group substitution (DS 0.4–0.9). However, a further increase in acetyl substitution (DS > 0.9) increases the hydrophobicity of the cellulose chain, rendering it insoluble in water [11].
Innovations in cellulose solvents motivated the quest for new acetylation procedures that can enable homogenous acetylation in ionic liquids (IL), in particular 1-ethyl-3-methylimidazolium acetate (EMIMAc) [12,13,14], which promote unique cellulose solution behavior. Not only was EMIMAc found to dissolve cellulose to individual chains, it also proved less toxic and is biodegradable [13,15], which renders it an excellent solvent for downstream processing [16,17,18] and cellulose derivatization. On the other hand, it was reported that cellulose dissolution in 1-ethyl-3-methylimidazolium chloride-(EMIMCl) resulted in significant cellulose depolymerization [19]. In some cases, dissolving cellulose in IL with a co-solvent results in acetylation, such as in the case of a mixture of EMIMAc with bulky chlorides (p-toluenesulfonyl chloride, triphenylmethyl chloride, or 2-furoyl chloride) [20]. Water-soluble cellulose acetate (WSCA) was synthesized by adding dichloroacetyl chloride to a cellulose solution in a carboxylate ionic liquid such as EMIMAc [8]. Our previous work demonstrated the ability of dichloromethane (DCM), a simple and relatively small chloro-organic co-solvent, mixed with the ionic liquid EMIMAc, to facilitate direct homogenous acetylation. The DCM content controlled the degree of substitution, such that direct formation of WSCA was achieved. The work described details the reaction mechanism, which involves a methylene diacetate or chloromethyl acetate intermediate, formed by nucleophilic substitution of DCM, as the acetylation reagent [9].
ILs can serve as efficient solvents in biomass valorization, in particular processes that include enzymatic cellulose hydrolysis [21,22,23]. Pretreatment by IL disrupts the strong inter-chain interactions in the native cellulose crystal structure, and subsequent regeneration by a non-solvent such as water yields a larger and more accessible surface area for hydrolyzing enzymes [24,25,26,27]. It is thus of interest to evaluate the ultimate hydrolysis of cellulose fully dissolved in aqueous solution.
The objectives of this work were to evaluate some of the physical properties of WSCA prepared using EMIMAc and DCM, in particular the chain conformation and extent of aggregation in water, and to assess the amphiphilicity of the dissolved WSCA by its reduction of water surface tension. Moreover, we pose the hypothesis that molecular dissolution coupled with amphiphilicity lead to an overwhelmingly high rate of enzymatic cellulose hydrolysis.
2. Materials and Methods
2.1. Materials
Microcrystalline cellulose (MCC) powder (Avicel®), with particle size in the range of 70–250 µm, was purchased from Sigma-Aldrich Co., (Rehovot, Israel). EMIMAc of 96% purity, was supplied by Iolitec (Heilbronn, Germany). DCM was purchased from Bio-Lab Ltd (Jerusalem, Israel). EMIMAc and cellulose were dried in a vacuum oven (MRC, Holon, Israel) at 60 °C, 0.26 KPa, for at least 24 h.
2.2. Sample Preparation
Dissolution and acetylation of cellulose were performed in two stages. First, 0.8 g of cellulose powder was soaked in 0.6 g ice-cooled DCM and then 19.2 g EMIMAc was immediately added to the mixture, and was placed in a shaker incubator (MRC, Holon, Israel), at 70 °C and 150 rpm, for at least 12 h, until no cellulose crystals were visually observed [9]. This was carried out in 20-mL closed vials to avoid DCM evaporation. The remaining DCM, if any, was subsequently evaporated in a vacuum oven at 60 °C, 0.26 KPa, for at least 24 h [9]. The resultant solution was dialyzed against deionized water for 24 h, using dialysis tubing (Spectrum Laboratories, Inc., Spectra/Por 4 Regenerated Cellulose, MWCO 12–14 kDa, Rancho Dominguez, CA, USA filled by 20 mL solution), until electrical conductivity of the dialyzing fluid was below 1 mS/cm. The concentration of the WSCA extracted from the dialysis was determined gravimetrically. Different concentrations of WSCA were prepared by adding deionized water to the WSCA extracted for the dialysis.
Enzymatic hydrolysis was performed using Accellerase 1500 (DuPont, Rochester, NY, USA), at 30 FPU/g and a shaker incubator at 50 °C, 120 rpm as described earlier [28]. The amount of cellulose converted to fermentable sugars was determined using dinitrosalicylic acid (DNS, Sigma-Aldrich Co., Rehovot, Israe) in a redox reaction [29].
2.3. Equipment and Characterization Methods
Small-angle x-ray scattering (SAXS) measurements were performed using a molecular metrology SAXS/WAXS system (Rigaku Innovative Technologies, Auburn Hills, MI. USA) equipped with a sealed microfocus tube emitting CuKα radiation (MicroMax-002+S), two Gӧbel mirrors, three pinhole slits, and a generator powered at 45 kV and 0.9 mA. The scattering patterns were recorded by a two-dimensional position sensitive wire detector (Gabriel) positioned 150 cm behind the sample. The solutions were sealed in thin-walled glass capillaries about 2 mm in diameter and 0.01 mm wall thickness, and measured under vacuum at 25 °C. The scattered intensity I(q) was recorded as a function of the scattering vector q defined as: q = (4π/λ) sin(θ), where 2θ is the scattering angle, and λ is the radiation wavelength, at the interval 0.07 < q < 2.6 nm−1. The scattering intensity was normalized with respect to time, solid-angle, primary beam intensity, capillary diameter, and transmission, to yield the absolute specific scattering cross-section. The rheological properties of the solutions under steady-state shear flow were characterized using a Discovery DHR-2 rotational rheometer (TA Instruments, New Castle, DE. USA) equipped with cone-plate geometry (40 mm diameter, 0.58 cone angle) at 25 °C. Characterization of CA dissolved in water was performed using a size-exclusion chromatography instrument (SEC-MALLS-UV-RI) equipped with a multi-angle laser light scattering detector (MALLS, PN3609), refractive index detector (RI, PN3150) and UV detector, all from Postnova Analytics (Landsberg, Germany). Size exclusion separation was carried out at 35 °C, in a series of three waters columns (Waters, Milford, MA), namely, Ultra-hydrogel 250, 1000, and 2000, with exclusion limits of 8 × 104, 4 × 106, and 1 × 107 g/mol, respectively. The water-soluble samples were prepared with 0.1 M NaNO3 and filtered (0.22 μm) before injection. The specific refractive index increment (dn/dc) that was used to calculate the concentration from the RI detector was determined as 0.141 mL/g, as measured by a differential refractometer using four concentrations, which is in the range of values for many polysaccharides in aqueous solutions [30]. The injection volume and flow rate were set on 100 μL and 0.5 mL/min respectively. The molecular weight was calculated using random coil fitting method (which was chosen as the appropriate model by Auto fitting method) by the MALLS detector software (Nova Malls, version 1.5.0.7, Postnova analytics, Landsberg, Germany). The surface tension of aqueous WSCA solutions was measured through a pendant drop method using a surface tension apparatus (OCA 15 PLUS, Data-Basics, Inc., Filderstadt, Germany) with integrated software (SCA 22, Filderstadt, Germany)). The instrument records the geometry of drops formed by solutions of various concentrations, and the software calculates the surface tension using the Young-Laplace equation. The reference liquid was deionized water and the surrounding of the drop was air.
3. Results and Discussion
The unique reaction of DCM with EMIMAc yields an intermediate that can derivatize cellulose to different DSs, as described in a previous publication [9]. Mixing 2—4 wt% DCM with EMIMAc, to serve as a solvent for cellulose, resulted in cellulose acetylation upon dissolution and enabled the formation of cellulose acetate with 15—23 % acetyl content, that is equivalent to DS of 0.4—0.9. At such low DS, cellulose is considered water-soluble. Replacement by dialysis of the DCM/EMIMAc solvent with water yields a clear aqueous solution. Evaporation of water from this solution resulted in the formation of a transparent soluble film, as shown in Figure 1. This film can be readily re-dissolved in water.
Figure 1.

Water soluble cellulose acetate film.
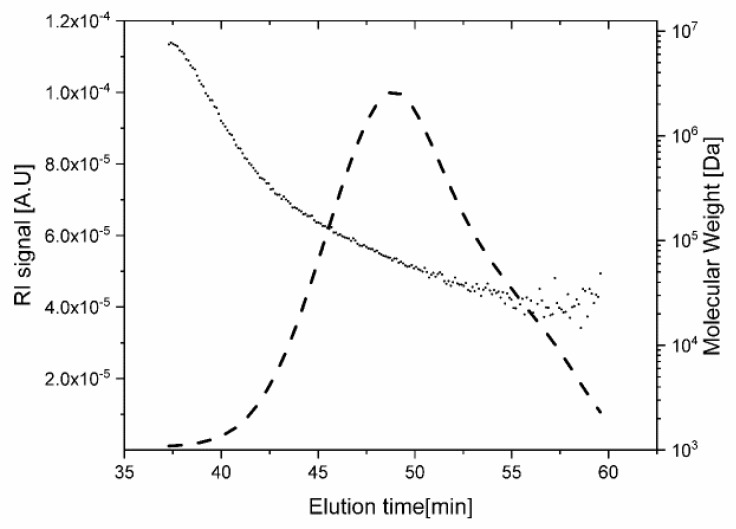
Previous works that studied molecular weight distribution of CA usually did not use aqueous solutions [31,32,33,34]. The SEC chromatogram, shown in Figure 2, demonstrates that the cellulose molecules were not degraded by the dissolution and derivation processes. The molecular weights determined by chromatography (MW average 50 kDa by number and 80 kDa by weight) were compatible with those of the cellulose precursor measured by light scattering from solutions in EMIMAC mixed with dimethylformamide [13], as well as those indicated by the manufacturer. The SEC chromatogram also shows that the derivatized cellulose is readily dissolved in water, with negligible sign of aggregates.
Figure 2.
SEC elution profile of cellulose acetate dissolved in water (0.1 M NaNO3). Dashed line—RI signal (axis at left); dotted line molecular weight (axis at right).
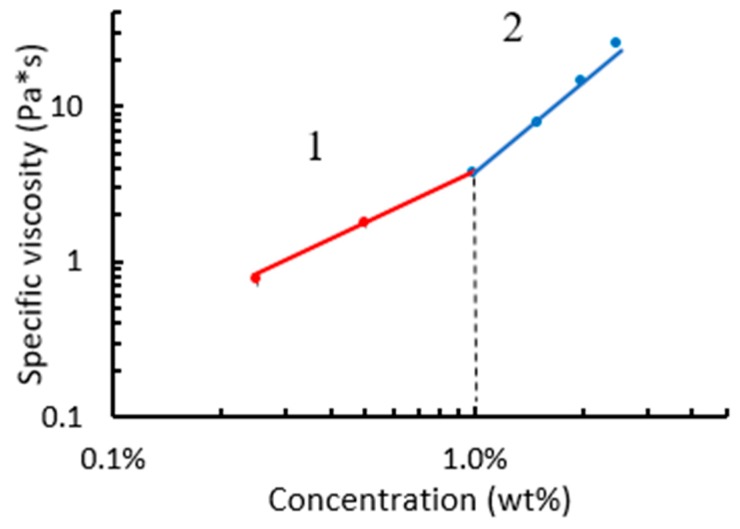
The viscosity of aqueous WSCA solutions was measured in steady shear experiments. The specific viscosity, defined as ηsp = (η − ηs)/ηs where η and ηs are the zero-shear viscosities of the solution and solvent (water), respectively, is presented in Figure 3 as a function of the solution concentration. Two different power-law relation regimes of specific viscosity to concentration can be noted. A linear regime (power exponent k~1) is observed at concentrations from 0.25 wt% to about 1.0 wt%, and a nonlinear regime is seen at concentrations of about 1.0–2.5 wt%, for which k~2.0. The former regime is typical of dilute solutions of neutral polymers, whereas the latter is characteristic of a semi-dilute un-entangled polymer solution at so-called “theta” solvent conditions. These measurements indicate that the water acts as a theta solvent for these cellulose acetate polymers, where the overlap concentration c* is about 1.0 wt%.
Figure 3.
Dependence of the specific viscosity on cellulose acetate solutions concentration in water at 25 °C, with power-law fits. The dashed line represents the overlap concentrations c*.
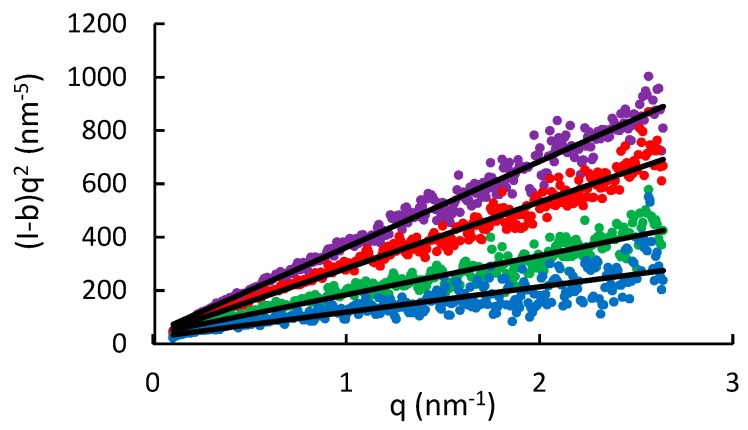
Figure 4 shows the SAXS patterns of aqueous WSCA solutions at four concentrations (1, 1.5, 2 and 2.5 wt%), at 25 °C. The information from the SAXS curves was extracted by fitting the data in the experimentally significant q range (0.01–0.25 Å−1). The patterns were fitted by the power law relation of Equation (1). Background subtraction allows a linear fit to Equation (2) as shown in Figure 5:
| (1) |
| (2) |
where I0 is the pre-factor of the power-law relation and b the background scattering. The q−1 intensity to scattering vector relation is characteristic of rod-like entities, and is typically observed in the range of scattering vectors (1/L) < q < (1/Rc), where L and Rc represent the rod length and the gyration radius of its cross-section, respectively.
Figure 4.
SAXS patterns of WSCA solutions in water, at concentrations, from bottom to top, of 1, 1.5, 2, 2.5 wt% (the plots have been spaced for clarity: 1.5% by 2, 2% by 3 and 2.5% by 5).
Figure 5.
Linear fitting of SAXS patterns of aqueous WSCA solutions (Equation 2), at concentrations, from bottom to top, of 1, 1.5, 2, 2.5 wt%.
In this case, the pre-factor, I0 is given by [14]:
| (3) |
where (N/V) is the number of rod-like particles per unit volume, A and φ are the rod cross-section area and volume fraction, respectively, and Δρ is the difference in scattering length density (SLD) between the rod and its surrounding medium. The values of I0 were obtained by fitting Equation (2) to the data in Figure 5, and the volume fractions of cellulose (φ), were calculated by Equation (3). For this calculation, the SLD of acetylated cellulose was calculated from the original cellulose parameters considering that at low DS, the values are similar to those of cellulose. Thus, SLD of 13.52 × 10−4 nm−2 was used, based on the cellulose formula (C6H10O5) with density of 1.5 g/cm3. The SLD of H2O is 9.47 × 10−4 nm−2, and a constant cross-section area A of 0.32 nm2 was used, as given for a chain in the cellulose I crystal structure [14]. The “experimental” values of the cellulose concentration (wt%), are compared in Table 1 with the “calculated” values determined from the volume fractions (φ) assuming a cellulose density of 1.5 g/cm3.
Table 1.
Comparison of the experiment cellulose concentrations (wt%) vs. those calculated from fitting the SAXS measurements.
| wt% Experimental | wt% Calculated |
|---|---|
| 2.5 | 2.3 |
| 2.0 | 1.8 |
| 1.5 | 1 |
| 1 | 0.7 |
The correspondence of the calculated and experimental φ values verified the validity of Equation (3) and confirmed the extended rod-like chain conformation of WSCA in water. A lower limit estimate of the persistence length (a) can be evaluated by the lowest q value of the linear fit (qmin), as , yielding a value of 11 nm. It also validated the SEC results, by showing no aggregation (no excess scattering) and indicated full dissolution of the cellulose molecules in water. This result also showed that no significant degradation occurred during dissolution and acetylation.
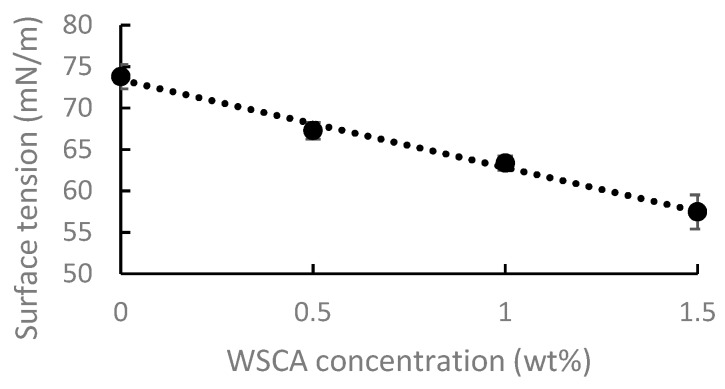
The structure of the cellulose chain is amphiphilic [35]. WSCA is expected to be even more amphiphilic because of the partial acetylation. It is thus of interest to evaluate its activity in reducing the surface tension of water. The effect of WSCA concentration on the surface tension of water was measured as shown in Figure 6. The results show a decrease in surface tension with increasing WSCA concentration. It is noted that the extent to which WSCA reduces the surface tension of water is similar to that of poly(vinyl pyrrolidone), a well-known water soluble amphiphilic polymer [36].
Figure 6.
The effect of WSCA concentration on surface tension of water.
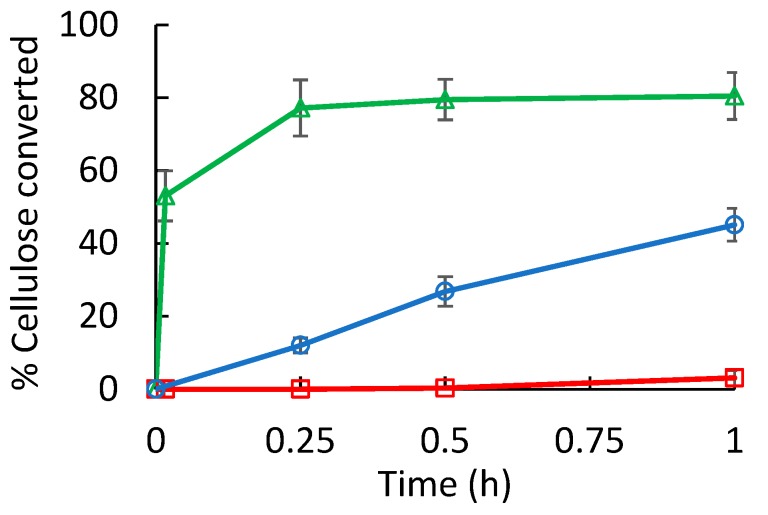
The foregoing results showed that WSCA dissolves in water, presenting in solution persistent chain segments (~10 nm) with an amphiphilic character. Enhanced enzymatic cellulose hydrolysis relies on disrupted crystalline packing of the cellulose chains presenting a high surface area exposed to water with some hydrophobic character for adsorption of the cellulose binding domains [37,38]. The hydrolysis rate of WSCA overwhelmingly increased as compared with microcrystalline cellulose and cellulose hydrogel particle dispersion (the fabrication of which has been reported previously [21]). In less than 5 min., more than 50 wt% of the cellulose was converted to reducing sugars. Hydrolysis rate was more than five times higher than the rate of samples prepared by the same procedure lacking the DCM [28,39], and hence lacking acetylation, as seen in Figure 7. This enhanced rate indicates that optimal conditions for enzymatic hydrolysis are achieved with water-soluble slightly acetylated cellulose.
Figure 7.
Hydrolysis of WSCA (∆) compared with that of microcrystalline cellulose (□) and cellulose hydrogel (○).
4. Conclusions
Direct cellulose acetylation by its dissolution in EMIMAc/DCM mixture can be used to fabricate water-soluble cellulose acetate. The reaction product dissolves completely in water, with no significant signs of chain aggregation, and exhibits a large persistence length (>10nm), having an amphiphilic character. As a result, it is highly accessible to enzymatic hydrolysis, exhibiting an extremely rapid hydrolysis rate indicating its high availability for enzymatic absorption and reactivity.
Author Contributions
Writing—review & editing, G.A., D.M.R., A.S. and Y.C.
Funding
This project was funded by the Israel Science Foundation Grant no. 123/18. The work of D. M. Rein was supported by a joint grant from the Center for Adsorption in Science of the Ministry of Immigrant Absorption and the Committee for Planning and Budgeting of the Council for Higher Education, under the framework of the KAMEA program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Malm C.J., Barkey K.T., Salo M. Far-Hydrolyzed Cellulose Acetates. Ind. Eng. Chem. 1957;49:79–83. [Google Scholar]
- 2.Kamide K., Okajima K., Kowsaka K., Matsui T. Solubility of cellulose acetate prepared by different methods and its correlationships with average acetyl group distribution on glucopyranose units. Polym. J. 1987;19:1405–1412. doi: 10.1295/polymj.19.1405. [DOI] [Google Scholar]
- 3.Miyamoto T., Sato Y., Shibata T. 13C-NMR Spectral Studies on the Distribution of Substituents in Water-Soluble Cellulose Acetate. J. Polym. Sci. 1985;23:1373–1381. doi: 10.1002/pol.1985.170230511. [DOI] [Google Scholar]
- 4.Buchanan C.M., Edgar K.J., Wilson A.K. Preparation and Characterization of Cellulose Monoacetates: The Relationship between Structure and Water Solubility. Macromolecules. 1991;24:3060–3064. doi: 10.1021/ma00011a005. [DOI] [Google Scholar]
- 5.Cao Y., Zhang J., He J., Li H., Zhang Y. Homogeneous acetylation of cellulose at relatively high concentrations in an ionic liquid. Chin. J. Chem. Eng. 2010;18:515–522. doi: 10.1016/S1004-9541(10)60252-2. [DOI] [Google Scholar]
- 6.Bocahut A., Delannoy J.Y., Vergelati C., Mazeau K. Conformational analysis of cellulose acetate in the dense amorphous state. Cellulose. 2014;21:3897–3912. doi: 10.1007/s10570-014-0399-8. [DOI] [Google Scholar]
- 7.Braun J.L., Kadla J.F. CTA III: A third polymorph of cellulose triacetate. J. Carbohydr. Chem. 2013;32:120–138. doi: 10.1080/07328303.2012.752493. [DOI] [Google Scholar]
- 8.Pang J., Liu X., Yang J., Lu F., Wang B., Xu F., Ma M., Zhang X. Synthesis of Highly Polymerized Water-soluble Cellulose Acetate by the Side Reaction in Carboxylate Ionic Liquid 1-ethyl-3-methylimidazolium Acetate. Sci. Rep. 2016;6:33725. doi: 10.1038/srep33725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfassi G., Rein D.M., Cohen Y. Partial cellulose acetylation in 1-ethyl-3-methylimidazolium acetate induced by dichloromethane. J. Polym. Sci. Part A Polym. Chem. 2018;56:2458–2462. doi: 10.1002/pola.29220. [DOI] [Google Scholar]
- 10.Miyamoto T., Takahashi S.I., Ito H., Inagaki H., Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989;23:125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 11.Bochek A.M., Kalyuzhnaya L.M. Interaction of water with cellulose and cellulose acetates as influenced by the hydrogen bond system and hydrophilic-hydrophobic balance of the macromolecules. Russ. J. Appl. Chem. 2002;75:989–993. doi: 10.1023/A:1020309418203. [DOI] [Google Scholar]
- 12.Swatloski R.P., Spear S.K., Holbrey J.D., Rogers R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002;124:4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- 13.Rein D.M., Khalfin R., Szekely N., Cohen Y. True molecular solutions of natural cellulose in the binary ionic liquid-containing solvent mixtures. Carbohydr. Polym. 2014;112:125–133. doi: 10.1016/j.carbpol.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Raghuwanshi V.S., Cohen Y., Garnier G., Garvey C.J., Russell R.A., Darwish T., Garnier G. Cellulose Dissolution in Ionic Liquid: Ion Binding Revealed by Neutron Scattering. Macromolecules. 2018;51:7649–7655. doi: 10.1021/acs.macromol.8b01425. [DOI] [Google Scholar]
- 15.Napso S., Rein D.M., Khalfin R., Cohen Y. Semidilute Solution Structure of Cellulose in an Ionic Liquid and Its Mixture with a Polar Organic Co-solvent Studied by Small-Angle X-Ray Scattering. J. Polym. Sci. PART B Polym. Phys. 2017:888–894. doi: 10.1002/polb.24337. [DOI] [Google Scholar]
- 16.Liu X., Pang J., Zhang X., Wu Y., Sun R. Regenerated cellulose film with enhanced tensile strength prepared with ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIMAc) Cellulose. 2013;20:1391–1399. doi: 10.1007/s10570-013-9925-3. [DOI] [Google Scholar]
- 17.Song H., Niu Y., Wang Z., Zhang J. Liquid crystalline phase and gel-sol transitions for concentrated microcrystalline cellulose (MCC)/1-ethyl-3-methylimidazolium acetate (EMIMAc) solutions. Biomacromolecules. 2011;12:1087–1096. doi: 10.1021/bm101426p. [DOI] [PubMed] [Google Scholar]
- 18.Lei L., Lindbråthen A., Sandru M., Gutierrez M.T.G., Zhang X., Hillestad M., He X. Spinning cellulose hollow fibers using 1-ethyl-3-methylimidazolium acetate-dimethylsulfoxide co-solvent. Polymers. 2018;10:972. doi: 10.3390/polym10090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno E., Miyafuji H. Reaction behavior of cellulose in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J. Wood Sci. 2013;59:221–228. doi: 10.1007/s10086-013-1322-x. [DOI] [Google Scholar]
- 20.Köhler S., Liebert T., Schöbitz M., Schaller J., Meister F., Günther W., Heinze T. Interactions of ionic liquids with polysaccharides 1. Unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate. Macromol. Rapid Commun. 2007;28:2311–2317. doi: 10.1002/marc.200700529. [DOI] [Google Scholar]
- 21.Dadi A.P., Schall C.A., Varanasi S. Mitigation of cellulose recalcitrance to enzymatic hydrolysis by ionic liquid pretreatment. Appl. Biochem. Biotechnol. 2007;137–140:407–421. doi: 10.1007/s12010-007-9068-9. [DOI] [PubMed] [Google Scholar]
- 22.Dadi A.P., Varanasi S., Schall C.A. Enhancement of Cellulose Saccharification Kinetics Using an Ionic Liquid Pretreatment Step. Biotechnol. Bioeng. 2006;95:904–910. doi: 10.1002/bit.21047. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H., Jones C.L., Baker G.A., Xia S., Olubajo O., Person V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009;139:47–54. doi: 10.1016/j.jbiotec.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Bernardo J.R., Gírio F.M., Łukasik R.M. The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules. 2019;24:808. doi: 10.3390/molecules24040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun S., Sun S., Cao X., Sun R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016;199:49–58. doi: 10.1016/j.biortech.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 26.Samayam I.P., Hanson B.L., Langan P., Schall C.A. Ionic-liquid induced changes in cellulose structure associated with enhanced biomass hydrolysis. Biomacromolecules. 2011;12:3091–3098. doi: 10.1021/bm200736a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling Z., Chen S., Zhang X., Takabe K., Xu F. Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-09885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfassi G., Rein D.M., Cohen Y. Cellulose emulsions and their hydrolysis. J. Chem. Technol. Biotechnol. 2019;94:178–184. doi: 10.1002/jctb.5760. [DOI] [Google Scholar]
- 29.Miller G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 30.Greenwood C.T., Hourston D.J. Specific refractive index increments of certain polysaccharide systems. Polymer. 1975;16:474–476. doi: 10.1016/0032-3861(75)90001-4. [DOI] [Google Scholar]
- 31.Wu C.-S. In: Handbook Of Size Exclusion Chromatography And Related Techniques. Wu C., editor. CRC Press; Boca Raton, FL, USA: 2003. [Google Scholar]
- 32.Ghareeb H.O., Radke W. Separation of cellulose acetates by degree of substitution. Polymer. 2013;54:2632–2638. doi: 10.1016/j.polymer.2013.03.041. [DOI] [Google Scholar]
- 33.Ghareeb H.O., Malz F., Kilz P., Radke W. Molar mass characterization of cellulose acetates over a wide range of high DS by size exclusion chromatography with multi-angle laser light scattering detection. Carbohydr. Polym. 2012;88:96–102. doi: 10.1016/j.carbpol.2011.11.071. [DOI] [Google Scholar]
- 34.Ghareeb H.O., Radke W. Characterization of cellulose acetates according to DS and molar mass using two-dimensional chromatography. Carbohydr. Polym. 2013;98:1430–1437. doi: 10.1016/j.carbpol.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 35.Rein D.M., Khalfin R., Cohen Y. Cellulose as a novel amphiphilic coating for oil-in-water and water-in-oil dispersions. J. Colloid Interface Sci. 2012;386:456–463. doi: 10.1016/j.jcis.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Águila-Hernández J., Trejo A., García-Flores B.E. Volumetric and surface tension behavior of aqueous solutions of polyvinylpyrrolidone in the range (288 to 303) K. J. Chem. Eng. Data. 2011;56:2371–2378. doi: 10.1021/je101330b. [DOI] [Google Scholar]
- 37.Boraston A.B., Bolam D.N., Gilbert H.J., Davies G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004;382:769–781. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimlos M.R., Beckham G.T., Matthews J.F., Bu L., Himmel M.E., Crowley M.F. Binding preferences, surface attachment, diffusivity, and orientation of a family 1 carbohydrate-binding module on cellulose. J. Biol. Chem. 2012;287:20603–20612. doi: 10.1074/jbc.M112.358184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfassi G., Rein D.M., Cohen Y. Enhanced hydrolysis of cellulose hydrogels by morphological modification. Bioprocess Biosyst. Eng. 2017;40:1635–1641. doi: 10.1007/s00449-017-1819-6. [DOI] [PubMed] [Google Scholar]