Abstract
Of all the novel glucoregulatory molecules discovered in the past 20 years, bile acids (BAs) are notable for the fact that they were hiding in plain sight. BAs were well known for their requirement in dietary lipid absorption and biliary cholesterol secretion, due to their micelle-forming properties. However, it was not until 1999 that BAs were discovered to be endogenous ligands for the nuclear receptor FXR. Since that time, BAs have been shown to act through multiple receptors (PXR, VDR, TGR5 and S1PR2), as well as to have receptor-independent mechanisms (membrane dynamics, allosteric modulation of N-acyl phosphatidylethanolamine phospholipase D). We now also have an appreciation of the range of physiological, pathophysiological and therapeutic conditions in which endogenous BAs are altered, raising the possibility that BAs contribute to the effects of these conditions on glycaemia. In this Review, we highlight the mechanisms by which BAs regulate glucose homeostasis and the settings in which endogenous BAs are altered, and provide suggestions for future research.
Over the last 15 years, bile acids (BAs) have emerged as unexpected players in glucose homeostasis. In addition to their well-established role in promoting lipid absorption, BAs are also implicated in glucose metabolism and the secretion of glucoregulatory hormones1. In this Review, we highlight the mechanisms by which BAs influence glucose metabolism and suggest directions for future research.
BAs are cholesterol catabolites that are generated in hepatocytes (FIG. 1a). Following synthesis, BAs are conjugated to an amino acid and secreted into the bile. BAs are actively reabsorbed by enterocytes in the terminal ileum and travel via the portal vein to hepatocytes, where they are taken up and recycled. A proportion of BAs, however, escape ileal uptake, become modified by intestinal microorganisms, and are subsequently absorbed via passive diffusion in the colon2. Thus, BAs are found at high levels in the liver, bile and intestine (TABLE 1). Due to incomplete reuptake by hepatocytes, BAs are detected at low levels in plasma. The presence of BAs in the systemic circulation raises the possibility that BAs directly affect tissues throughout the body. High-affinity BA uptake transporters, however, are thought to be expressed predominantly in the liver and ileum2–5. Thus, it is unclear what concentrations of BAs could penetrate parenchymal cells or interstitial fluid in most tissues. The enterohepatic circulation of BAs has been reviewed extensively in REF.3.
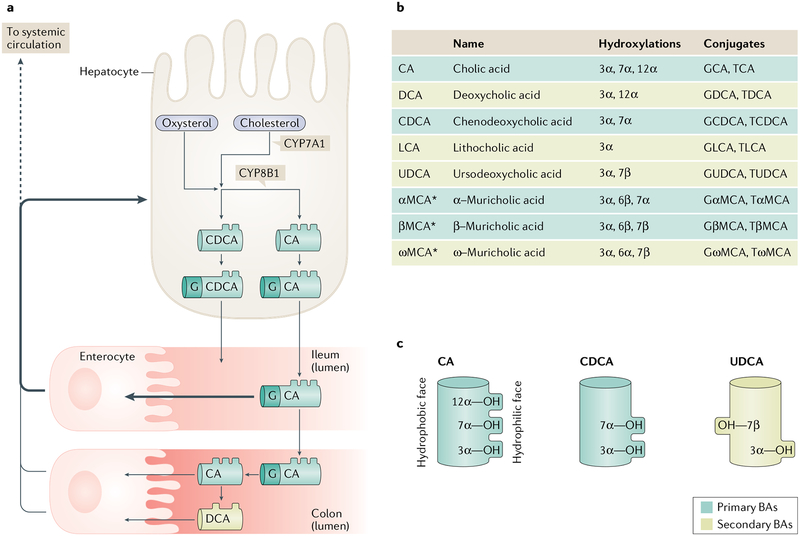
Fig. 1 |. Bile acid synthesis, modification and physicochemical properties.
a | Bile acid (BA) synthesis occurs only in the liver. In the classic pathway of BA synthesis, cholesterol is hydroxylated in the 7α position by the enzyme CYP7A1. Alternatively, cholesterol is first converted to an oxysterol prior to being 7α-hydroxylated by the enzymes CYP7B1 or CYP39A1. These oxysterols can arise in the liver, through the enzyme CYP27A1, or they can arise in other cells — such as macrophages via CYP27A1 or brain via CYP46A1 — then travel to the liver. After the initial step, which is considered rate-limiting, over a dozen enzymatic reactions proceed to generate the primary BA molecule chenodeoxycholic acid (CDCA). An intermediate in BA synthesis, 7α-hydroxy-4-cholesten-3-one, can undergo 12α-hydroxylation by the enzyme CYP8B1 and subsequently proceed through the additional steps. This process results in the generation of the second primary BA found in humans, cholic acid (CA). BAs are conjugated to an amino acid such as glycine (G) and secreted into the bile. BAs enter the duodenum directly or are stored in the gallbladder until postprandial gallbladder contraction. Most BAs are reabsorbed from the terminal ileum by the active transporter apical sodium-dependent bile acid transporter (ASBT). A minor fraction travel into the colon where they can be deconjugated and dehydroxylated by gut microorganisms, producing BAs that can be passively absorbed. From the portal vein, BAs are efficiently taken up into hepatocytes and recycled. A small fraction enter the systemic circulation. b | The major BA species found in humans and mice. c | Schematic demonstrating the amphipathic nature of BAs. *αMCA, βMCA and ωMCA are abundant in mice and rats but not humans.
Table 1 |.
Concentrations of bile acids found in human tissues and compartments
| Tissue | Concentration | Refsa |
|---|---|---|
| Systemic plasma and/or serum | 0.2–22.0 μM | 104,200,201 |
| Portal venous plasma and/or serum | 9–43 μM | 200,202,203 |
| Gallbladder bile | 31–234 mM | 143,144 |
| Common bile duct | 42–204 mM | 142,204 |
| Duodenum contents — fasting | 0.3–9.6 mM | 205,206 |
| Duodenum contents — postprandial | 8.3–11.9 mM | 206 |
| Jejunum contents — fasting | 0.8–5.5 mM | 205 |
| Jejunum contents — postprandial | 5–8 mM | 207 |
| Upper ileum contents — postprandial | 10 mM | 207 |
| Lower ileum contents — postprandial | 2 mM | 207 |
| Caecum contents | 0.2–1 mM | 208 |
| Faeces | ~4.5 μmol/g | 209 |
| Liver (liver biopsies contain bile canaliculi and ducts in addition to hepatocytes) | ~60 nmol/g | 130,210 |
| Subcutaneous white adipose tissue | ~0.2 nmol/g | 201 |
Different studies quantified BA levels using different extraction methods, different blood specimen processing methods (plasma versus serum), and different chromatography and mass spectrometry techniques. The individual references should be consulted for details.
Numerous BA species are detectable in humans (FIG. 1b). They differ primarily in their hydroxylation sites and the presence or absence of a conjugated amino acid, predominantly glycine in humans. Of note, in rodents BAs are predominantly conjugated with taurine. In both humans and mice, a minor proportion of BAs also undergo sulfation6,7. BA modifications alter their physicochemical properties, including the so-called ‘hydrophobicity’ of a BA molecule8,9. It is worth noting that this descriptor is derived from the chromatographic separation method, whereby BAs are designated more hydrophobic if they are retained longer on a nonpolar chromatography column during elution with a polar solvent9,10. BAs are more accurately described as amphipathic, meaning they have a hydrophobic surface and a hydrophilic surface, and the number and position of hydroxyl groups on a BA molecule determine its amphipathic nature10 (FIG. 1c). In addition to these physical descriptors, BAs can also be categorized as primary (synthesized in the liver) or secondary (generated by microbial modification of primary BAs in the gut11; FIG. 1). The composition of the BA pool is remodelled under numerous pathophysiological and experimental conditions12–16 and this change in composition could influence BA function.
A unique feature of BAs is that they can act via multiple completely distinct molecular mechanisms, for example, by emulsifying lipids, by affecting cellular membranes, through allosteric effects and via receptor-mediated pathways1. Some of the mechanisms by which BAs act are known to have effects on glycaemia, and a number of other mechanisms have the potential to effect glycaemia17,18. In the first part of this Review, we describe these mechanisms, many of which were revealed by studies in preclinical models. In the second section, we discuss conditions that affect BAs and which might, in turn, affect glycaemia. Such conditions include insulin sensitivity, the microbiome and liver diseases. We also examine interventions and therapeutic agents that alter BA-dependent pathways, deliberately or unexpectedly. Finally, we highlight gaps in our knowledge and questions for future consideration.
Non-receptor-mediated mechanisms
The canonical physicochemical effect of BAs is to support the emulsification of water-insoluble lipids. It is possible that this process and other non-receptor-mediated BA effects could affect glycaemia, directly or indirectly.
Lipid emulsification.
Because of their amphipathic nature, BAs, in combination with polar phospholipids, are able to incorporate dietary lipids into mixed micellar solutions in the intestinal lumen. This micellization process increases the surface area of luminal lipids and improves the accessibility of intestinal lipases and the efficiency of fat hydrolysis19. This property of BAs is essential to lipid absorption and total-body energy balance. Different BA species are differentially able to promote lipid absorption20,21. This ability to promote lipid absorption could be influenced by a BA’s micelle-forming properties8 and its permeability in the unstirred water layer lining the intestinal epithelium22. Evidence also suggests that enterocyte intracellular cholesterol esterification is regulated by BAs, although the mechanism of this is unknown23.
Effects on cell membranes.
BAs can insert into cell membranes, including the plasma membrane, and impact membrane dynamics24,25. A 2014 study showed that this is the mechanism by which BAs activate the BA-sensitive ion channel whose physiological function remains elusive26. At supraphysiological doses, BAs can disrupt cell membranes and cause cell lysis27–29. For example, deoxycholic acid (DCA), a secondary BA produced by dehydroxylation of cholic acid (CA), is particularly potent, and an injectable synthetic form of DCA has been developed that takes advantage of this attribute, and was approved by the FDA in 2016 for reduction of fat under the chin30.
BAs might affect intracellular membranes as well. DCA reportedly colocalizes with the mitochondrial outer membrane and perturbs its structure31. Tauroursodeoxycholic acid (TUDCA) has been reported to protect against endoplasmic reticulum (ER) stress, and treatment with TUDCA improves glycaemia in leptin-deficient ob/ob mice32,33. The molecular mechanisms by which TUDCA functions are not clear but could involve effects on the ER membrane itself.
Allosteric functions.
BAs can directly bind and modulate the activities of certain proteins. One notable example is N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), which is an enzyme found in the brain and intestine that converts membrane lipids into specialized bioactive lipids34. Products of NAPE-PLD include arachidonoylethanolamide (anandamide) and oleoylethanolamide, the latter of which promotes GLP1 secretion and both of which are involved in food intake regulation35. While solving the crystal structure of NAPE-PLD, researchers unexpectedly found DCA within the hydrophobic substrate binding pocket36. Moreover, DCA was found to bind and stabilize the enzyme and enhance its enzymatic activity. Further studies have shown that lithocholic acid (LCA), chenodeoxycholic acid (CDCA) and DCA bind NAPE-PLD (KD ~20, 25 and 43 μM, respectively)37. At sufficiently high concentrations, LCA inhibits NAPE-PLD, whereas CDCA and DCA activate it, but the physiological relevance of these effects, and any downstream effects on metabolism have not yet been investigated.
Receptor-mediated mechanisms
BAs activate several nuclear receptors and G protein-coupled receptors, with differing potencies (TABLE 2). Much of our understanding of the roles of BA receptors in glycaemia comes from experiments using genetically manipulated mouse models, as well as small-molecule agonists and antagonists. An important consideration for interpreting such studies is that BAs regulate their own synthesis through a series of negative feedback loops that converge on the key enzymes CYP7A1 and CYP8B1 (REFS3,38,39). Therefore, experimentally manipulating BA receptors frequently alters BA levels and composition (FIG. 2), which in turn influences other BA-sensitive pathways. In this section we review the reported involvement of BA receptors in glucose homeostasis; published mechanisms are summarized in FIG. 3. Of note, the reliance on preclinical models for these studies is a limitation. Mechanistic data in humans might be facilitated by identifying and studying individuals carrying genetic variants and by additional studies using receptor agonists.
Table 2 |.
Individual effects of bile acids on bile acid receptors
| Bile acid | FXR EC50 | FXR IC50 | TGR5 EC50 | VDR EC50 | PXR EC50 |
|---|---|---|---|---|---|
| Cholic acid | 100–200 μM197 | NA | 7.72 μM85, >10 μM84, 13.6 μM213 | No effect211 | No effect211 |
| Deoxycholic acid | 50 μM42, 50–75 μM212 | NA | 1.01–1.25 μM85,213 | No effect211 | 50.2 μM211 |
| Chenodeoxycholic acid | 1–2 μM212, 4.5 μM40, 5.2 μM185, 7 μM75, 10 μM41,212, (T, G) 10 μM40, 10–30 μM214, 20 μM41,212, 25–50 μM212, 50 μM42 | NA | 4–4.43 μM84,85, (T) 1.92 μM213, (G) 3.88 μM199 | No effect211 | (T) 104 μM211 |
| Lithocholic acid | 50 μM42 | NA | 35 nM84, (T) 0.33 μM85, 0.53 μM85, 3 μM84 | 8 μM41, 12.1 μM215, 21.6 μM211 | 10.2 μM211 |
| 3-Keto-lithocholic acid | NA | NA | NA | 3 μM75, 6.8 μM215 | 8.3 μM211 |
| Ursodeoxycholic acid | No effect40 | NA | 36.4 μM213, No effect85 | No effect75 | NA |
| α-Muricholic acid | NA | (T) 28 μM191 | NA | 101.7 μM211 | 56 μM211 |
| β-Murichoiic acid | NA | (T) 40 μM202 | NA | No effect211 | No effect211 |
| Hyodeoxycholic acid | NA | NA | 31.6 μM213 | NA | NA |
Note that different studies used different systems (cell lysates and different cell lines) and methods (such as, competitive binding assays, cAMP levels, cAMP-responsive luciferase reporter) to determine EC50 and IC50 values. The individual references should be consulted for details. EC50, the effective concentration for a half maximal response; FXR, farnesoid X receptor; G, values specifically for glycine conjugates; IC50, the concentration that reduces the response by half; NA, not applicable; PXR, pregnane X receptor; T, values specifically for taurine conjugates; TGR5, Takeda G protein-coupled receptor 5; VDR, vitamin D receptor.
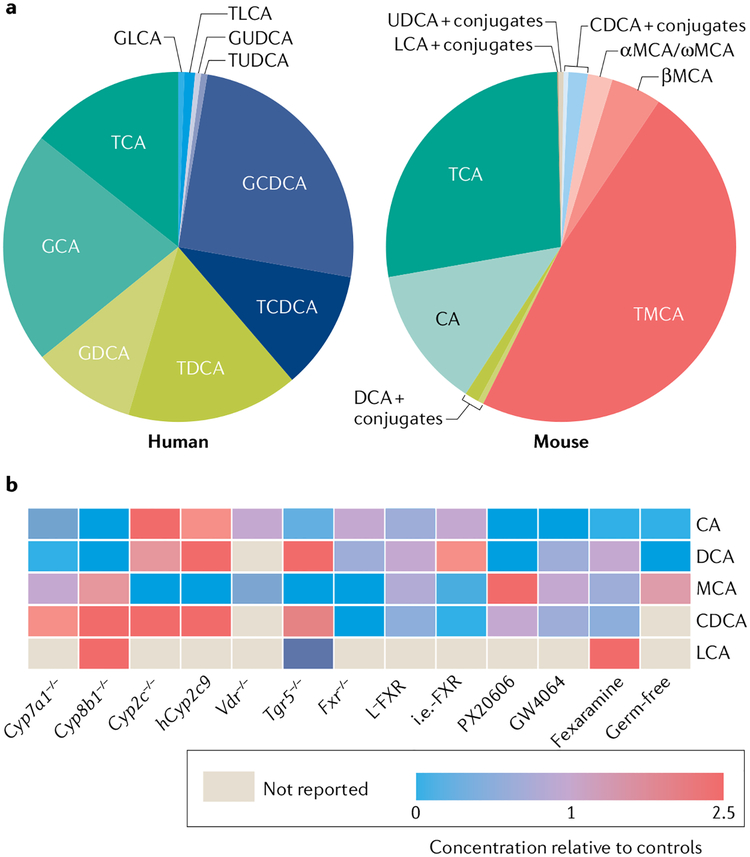
Fig. 2 |. Bile acid composition.
a | Average bile acid (BA) composition in human biliary bile (left) and in enterohepatic tissues (including bile) of wild-type mice (right). Human biliary bile data are averages from REFs142–144. Mouse BA pool data are averages from 58 wild-type mice across multiple studies including own published and unpublished studies. TMCA represents the sum of taurine-conjugated α-, β- and ω-muricholic acids (MCA). b | Effects of genetic knockouts38,182,187–190 and pharmacological treatments43,45,191,192 on mouse BA composition. Data for each BA species are the sum of conjugated and unconjugated BAs, and were calculated as (the percentage in the experimental pool/the percentage in the control pool). PX20606 and GW4064 are farnesoid X receptor (FXR) agonists. Fexaramine is a gut-restricted FXR agonist. In germ-free mice, no unconjugated BAs are detected. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; GUDCA, glycoursodeoxycholic acid; i.e.-FXR, intestine epithelium-specific FXR knockout; L-FXR, liver-specific FXR knockout; LCA, lithocholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid.
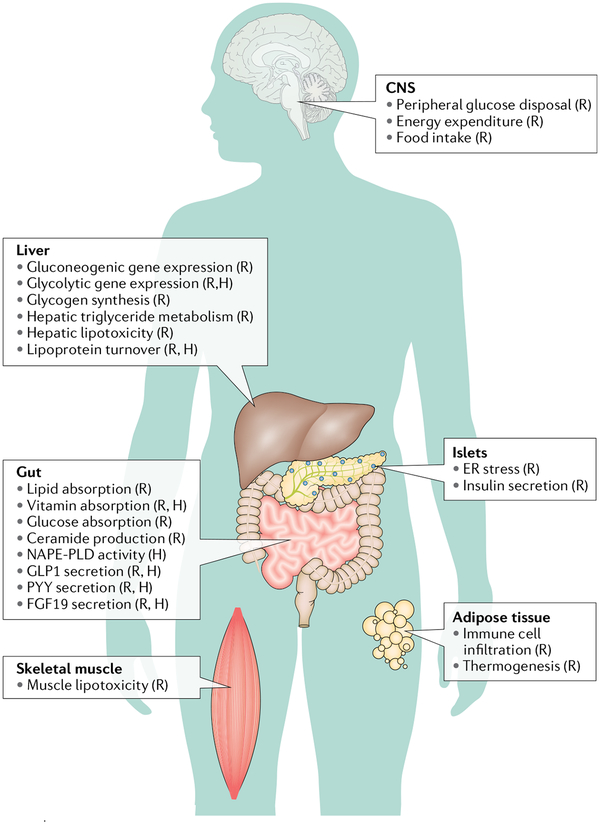
Fig. 3 |. Effects of bile acids on metabolic processes throughout the body.
The primary sites of bile acid (BA) function are the liver and intestine, which are enriched in BAs and BA receptors. Through their ability to facilitate secretion of hormones such as glucagon-like peptide 1 (GLP1), fibroblast growth factor 19 (FGF19) and others, BAs can indirectly affect other tissues, including the brain. Furthermore, low levels of BAs are found in the systemic circulation, potentially enabling direct effects of BAs in tissues throughout the body. (R) indicates that supporting data were mostly from rodents; (H) indicates that supporting data were from humans, human cells or purified human proteins. Data were originally presented in the following effects in the central nervous system (CNS): peripheral glucose disposal59 (R), energy expenditure58 (R), and food intake193 (R); effects in islets: endoplasmic reticulum (ER) stress33,173 (R), and insulin secretion194 (R); effects in the liver: gluconeogenic gene expression48,56 (R), glycolytic gene expression52,216 (R, H), glycogen synthesis57 (R), hepatic triglyceride metabolism195 (R), hepatic lipotoxicity48 (R), and lipoprotein turnover152,196 (R, H); effects in adipose tissue: immune cell infiltration91 (R), and thermogenesis58; effects in the gut: lipid absorption13,183,184 (R), vitamin absorption197 (R, H), glucose absorption49 (R), ceramide production50,198 (R), N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) activity36,37 (purified human protein), GLP1 secretion86–90,199 (R, H), peptide YY (PYY) secretion90,199 (R, H), and FGF19 secretion199 (R, H); and effects in skeletal muscle: lipotoxicity48 (R).
FXR.
The first BA-responsive receptor discovered40–42, FXR, is highly expressed in the liver, intestine and kidneys. Its role in glucose homeostasis has been investigated in multiple studies15,17,43–45. Some studies in mice have shown beneficial effects of FXR activation43,44, while others have shown beneficial effects of deleting or inhibiting FXR17,18. Contradictions in the results between studies could be due to differential effects in the liver versus the intestine, pharmacokinetics of agonists and/or antagonists, sex, age, diet and genetic background. It is also worth noting that nuclear receptor deletion, antagonism or absence of ligand are not necessarily equivalent, as endogenous nuclear receptors can have effects in the basal, unliganded state46.
The evidence that FXR activity is beneficial for glycaemia arose from studies in mice with FXR deficiency, as well as mice that were given FXR agonists. On normal chow diet, Fxr−/− mice showed worse intraperitoneal glucose tolerance and lower glucose disposal during hyperinsulinaemic-euglycaemic clamp than wild-type mice44,47,48. Furthermore, treating ob/ob and db/db mice with the FXR agonist GW4064 consistently lowered glucose excursions during intraperitoneal glucose and insulin tolerance tests44,47. The gut-restricted FXR agonist fexaramine improved glycaemia and reduced diet-induced weight gain in mice15,43. The proposed mechanisms of FXR beneficial effects on glucose metabolism include: suppression of gluconeogenic genes, due to FXR activation of the transcriptional repressor SHP48; protection from skeletal muscle lipotoxicity, via FXR-dependent liver lipid metabolism48; reduced weight gain due to adipose tissue browning, downstream of FXR-dependent alterations in BA composition43; increased GLP1 and insulin secretion, due to shifts in gut bacteria composition, which increase the TGR5 agonist taurolithocholic acid (TLCA)15; and increased secretion of fibroblast growth factor 15 (FGF15) and/or FGF19, described in detail below.
Conversely, other studies have shown that FXR inhibition improves glycaemia. Whole-body Fxr−/− mice and mice that lack FXR only in the intestinal epithelium had improved oral glucose tolerance, and this phenotype was frequently associated with reduced body weight17,18,49–51. Compared with vehicle-treated animals, when challenged with a high-fat diet, GW4064-treated mice displayed exacerbated weight gain, increased fasting glucose and insulin levels, and worsened glucose and insulin tolerance45. Furthermore, mice treated with glycine-β-muricholic acid (MCA) to antagonize FXR activity showed improved insulin tolerance and oral glucose tolerance and reduced fasting insulin levels compared with vehicle-treated control mice1.
The proposed mechanisms for the beneficial effects of FXR inhibition include: decreased hepatic gluconeogenesis due to decreased pyruvate carboxylase activity (this has been suggested to be downstream of lower FXR-dependent intestinal production of hepatotoxic serum ceramides)50; reduced weight gain due to increased thermogenesis (also downstream of FXR-dependent production of serum ceramides)17; release of FXR-dependent suppression of proglucagon, the GLP1 precursor, that leads to increases in glucose-stimulated GLP1 release51; delayed intestinal glucose absorption due to increased glucose phosphorylation in enterocytes49; and release of FXR-dependent suppression of hepatic glycolytic genes52.
FGF15 and/or FGF19.
By activating FXR, BAs induce robust transcription of the peptide hormone FGF15 and its human orthologue FGF19. FGF15 and/or FGF19, which are highly expressed in ileal enterocytes, have a key endocrine role in suppressing hepatic BA synthesis, which occurs through the FGFR4–β-Klotho receptor complex53. FGF15 and/or FGF19 are also important for maintaining normoglycaemia, as evidenced by the impaired glucose tolerance in Fgf15−/− mice and glycaemic improvements after transgenic expression or injection of FGF1954–57. The beneficial effects of FGF15 and/or FGF19 are potentially due to: reduced hepatic gluconeogenesis, downstream of FGF15- and/or FGF19-dependent dephosphorylation of the gluconeogenic transcription factor CREB56; increased hepatic glycogen synthesis, due to FGF15-/FGF19-dependent activation of an ERK-GSK3α/β phosphorylation cascade57; reduced body weight and adiposity54,58, due to increased metabolic rate by increasing β-Klotho-dependent sympathetic nerve activity in brown adipose tissue58; and increased insulin-independent peripheral glucose disposal59, downstream of FGF15 and/or FGF19 induction of ERK signalling in hypothalamic neurons58,60,61. Plasma FGF19 levels are reportedly reduced in patients with obesity and/or type 2 diabetes mellitus62–64 and are negatively correlated with BMI65. However, the endogenous functions of FGF19 have been called into question66,67.
The therapeutic prospects for FGF19 are potentially limited by the association of high levels of FGF15 and/or FGF19 with increased hepatocellular carcinoma in mice and humans68,69. However, non-tumorigenic variants of FGF19 have been generated and are now in development for the treatment of liver diseases. Variants M70 and M52 have been shown to protect against fibrosis, steatohepatitis and cholestasis in mice, effects that are expected to be secondary to suppression of BA synthesis70–72. M70 is also capable of suppressing BA synthesis in humans70 and in a phase II clinical trial, it markedly improved markers of liver damage, cholestasis and inflammation in patients with primary biliary cholangitis73. A phase II trial of M70 in patients with nonalcoholic steatohepatitis (NASH) is underway74.
Vitamin D receptor.
Some BAs, namely LCA and 3-keto-LCA, can activate the nuclear vitamin D receptor (VDR); however, these BAs are poorly taken up into cells. Although micromolar levels of LCA can activate VDR (comparable to FXR activation by CDCA)75, the active form of vitamin D, 1α,25-dihydroxyvitamin D3, activates VDR at nanomolar concentrations, making LCA about 1,000 times less potent than vitamin D76,77. Therefore, high doses of LCA are required to activate VDR in vivo, and activation occurs more strongly under conditions of vitamin D deficiency78. VDR has been reported to have a role in maintaining glycaemia, and this function could be carried out via effects in islets79, macrophages80 or endothelial cells81. However, few studies have specifically investigated the effects of LCA–VDR signalling on glucose homeostasis. In vitro studies suggest that the LCA derivative, LCA propionate, protects β cells against dedifferentiation82. Another LCA derivative, TLCA-3 sulfate, induces insulin resistance in cultured hepatocytes, although this was not specifically linked to VDR83. Whether physiological or pharmacological levels of LCA and its derivatives regulate glucose metabolism in vivo remains to be determined.
TGR5.
The most extensively studied G protein-coupled receptor for BAs, TGR5 (also known as GPBAR1), is expressed in a wide range of tissues84,85. Preclinical studies suggest that TGR5 has a protective role in glucose homeostasis. The most widely reported mechanism by which this occurs is by TGR5-mediated increases in GLP1 secretion, accompanied by increased insulin secretion86–90. Alternative mechanisms by which TGR5 may influence metabolism include C/EBPβ-dependent suppression of macrophage infiltration into white adipose tissue91 and increased energy expenditure92,93.
Conditions and treatments that affect BAs
Endogenous BAs are altered in multiple physiological, pathophysiological and therapeutic conditions, and it is possible that these alterations contribute to BA-driven glycaemic regulation12,13,15,94,95 (FIG. 4). Investigating the effects of human conditions on BA pool size and composition is inherently challenging. Stable isotope kinetic studies are a gold-standard method for assessing the synthesis and turnover of BAs in vivo, but require specialized expertise and are typically limited to small sample sizes. For studying larger populations, accessible specimens include plasma and faeces, but neither is a perfect representation of the concentrations and compositions of BA pools present in the liver, gallbladder or small intestine, the primary residences of BAs in the body. Nonetheless, in this section we Review the published literature, keeping these caveats in mind.
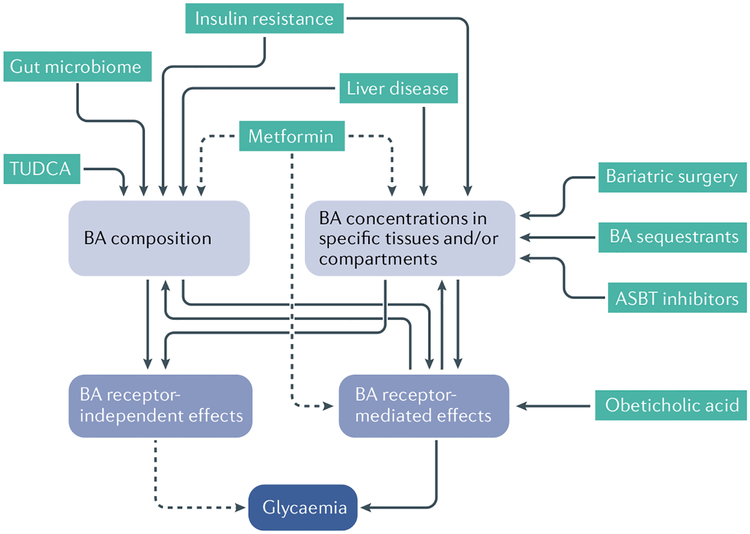
Fig. 4 |. Physiological and pathological conditions and therapies that influence bile acids.
An individual’s total levels of bile acids (BAs), levels in selected tissues such as the gut or plasma, and the composition of those bile acid species can each influence bile acid functions. These include functions mediated by receptors such as FXR and TGR5, as well as receptor-independent effects, such as nutrient absorption. Green boxes represent conditions, medications and interventions that can affect BAs. Solid lines represent known pathways that affect and/or are affected by BAs. Dotted lines represent potential pathways. ASBT, apical sodium-dependent bile acid transporter; TUDCA, tauroursodeoxycholic acid.
Impaired insulin signalling.
The first data indicating that insulin signalling regulates BA production and composition were from rodent models. Compared with healthy control animals, rodent models of insulinopenia and hyperglycaemia have a larger total BA pool size and a larger percentage of the BA pool consists of 12α-hydroxylated BAs96–98. The same is true in mice lacking hepatic insulin receptors99. The effects of hepatic insulin signalling on BA synthesis and composition are thought to be transcriptionally determined100. Evidence suggests that the transcription factor FOXO1, which is inactivated by insulin signalling, mediates the effect of insulin on BA composition101 (BOX 1).
Box 1 |. Hepatic insulin signalling regulates bile acid pool composition in mice and humans.
Evidence from mice
The insulin-repressible forkhead box protein o (FOXO) transcription factors increase mRNA expression of the sterol 12α-hydroxylase, Cyp8b1, in mouse liver. Hepatic FoxO1 ablation in mice reduces the levels of 12α-hydroxylated bile acids (BAs) in enterohepatic tissues101. Triple hepatic ablation of FoxO1, FoxO3 and FoxO4 exacerbates this phenotype [R.A.H., unpublished], demonstrating redundant functions of FOXO transcription factors on Cyp8b1.
Mouse and rat models of hyperglycaemia and insulinopenia are surmised to have higher FOXO activity, and they show increased levels of 12α-hydroxylated BAs. These models include rodents treated with the β-cell toxins streptozotocin and alloxan and NOD mice96–98,186. mice lacking hepatic insulin receptors also show increased levels of 12α-hydroxylated BAs101.
Evidence from humans
Stable isotope kinetic studies have shown increased synthesis of cholic acid, the primary 12α-hydroxylated BA, in patients with type 2 diabetes mellitus compared with controls matched for BMI107.
In non-obese human volunteers, insulin resistance (as assessed by gold-standard hyperinsulinaemic–euglycaemic clamp studies) is associated with increased plasma levels of 12α-hydroxylated BAs94.
Individuals with insulin resistance and obesity have higher CYP8B1 activity compared with non-obese control individuals, as determined by plasma levels of the CYP8B1 product, 7α,12α-dihydroxy-4-cholesten-3-one103.
Summary
These studies demonstrate the identification of a molecular pathway directly linking insulin signalling with BA pool composition in mice, which translates to human pathophysiology. The functional consequences of this pathway are being investigated by multiple research groups.
Several studies have analysed plasma BAs and markers of BA synthesis and how they relate to insulin sensitivity in humans. Insulin resistance has been reported to be positively correlated with the levels of plasma BAs, especially primary BAs and/or 12α-hydroxylated BAs16,94,102. Obesity is associated with increased BA synthesis103–105, 12α-hydroxylation103 and alterations in BA transport12,103. Patients with type 2 diabetes mellitus have been reported to have increased concentrations of taurine-conjugated BA species106. In addition, kinetic studies have shown increased synthesis of BAs, particularly CA, in patients with type 2 diabetes mellitus107. Thus, preclinical and human studies support the consensus that hepatic insulin resistance increases BA synthesis, and might also cause other alterations in BA composition, such as increased 12α-hydroxylation. The dual concepts that first, insulin resistance, obesity or diabetes mellitus influence BA concentration and composition and, second, that BA concentration and composition can influence energy metabolism, suggest the possibility of adaptive or maladaptive feedforward signals contributing to metabolic disease.
Bariatric surgery.
Since 2009, numerous studies have shown that BA concentrations in the systemic circulation are increased after Roux-en-Y gastric bypass, biliopancreatic diversion and possibly vertical sleeve gastrectomy, but not after adjustable gastric banding108. These findings have been recapitulated in animal models, including mice, rats and minipigs108–111. The mechanisms by which bariatric surgery cause increased circulating BA concentrations are not yet known, and could be different depending on the surgical procedure. Increased synthesis alone is not the explanation. BA synthesis is increased in patients after biliointestinal bypass and biliopancreatic diversion112,113, potentially because these procedures limit BA signalling in the ileum, which would decrease FGF19-dependent suppression of BA synthesis. In patients who have undergone Roux-en-Y gastric bypass or sleeve gastrectomy, BA synthesis is decreased in the short term and later returns to the normal range113–115. In sum, following metabolic surgery, there are probably alterations in BA transport that cause increased circulating BA concentrations. Preclinical models have suggested that concentrations of BA uptake transporters are increased in the ileum109 or decreased in the liver after surgery110,111. The effects of bariatric surgery on BA composition have not come to consensus, potentially because of differences in surgical procedures, differences between animals and humans, and environmental factors.
Whether or not alterations in BA levels are the cause of improved glycaemia after bariatric surgery is also still a matter of debate. Proposed mechanisms include BA-driven increases in the secretion of GLP1, insulin or FGF19 (REF.108). Some studies in humans have supported correlations between secretion of these hormones and the BA subtypes present in the plasma116, although BAs present in the plasma are not necessarily representative of BAs present in the relevant tissues. Evidence supporting a role for BAs in metabolic improvements following bariatric surgery has come from mice deficient in BA receptors. Mice lacking FXR117,118, the FXR target SHP119 or TGR5 (REFS120,121) have all been found to show resistance to the metabolic benefits of bariatric surgery. However, potential caveats arise due to differences between these knockout mice and their wild-type controls before surgery. Moreover, some have argued that the timing of elevated BA levels does not coincide with improved glycaemia after surgery115. Mechanistic studies are required.
Liver diseases.
It has been known for over 60 years that liver diseases differentially affect BA concentrations, distribution and composition122. One area that has been extensively examined is intrahepatic cholestasis of pregnancy (ICP). ICP is characterized by impaired bile flow as a consequence of genetic variation in hepatic BA transporters and high concentrations of pregnancy hormone metabolites, which competitively bind and reduce the activity of BA transporters and FXR123. Reduced activities of FXR and hepatic BA transporters cause elevations in maternal circulating plasma BA concentrations and altered BA composition, with a large increase in the proportion of CA in the pool124,125. Fetal plasma BAs, which are generated by the fetal liver, are also altered in ICP — concentrations are higher and CA predominates125. ICP is associated with an increased risk of adverse fetal outcomes123.
ICP is also associated with metabolic dysfunction. Compared with healthy pregnant women, women with ICP are more likely to have impaired glucose tolerance and gestational diabetes95,126. Babies born to women with the condition are more likely to be large for gestational age compared with babies born to healthy pregnant women95,127. Among children of women who were affected by ICP, adolescent boys show higher BMI and fasting insulin levels and girls show larger hip and waist girth than children of non-affected mothers128. Whether or not BAs are responsible for these effects is unclear. In mice, feeding pregnant dams a CA-rich diet results in offspring that are more susceptible to weight gain and glucose intolerance on a Western diet128. These findings suggest the possibility of in utero metabolic programming in response to BAs.
The development of nonalcoholic fatty liver disease (NAFLD) and NASH is strongly linked to insulin resistance and dysbiosis129. It has also been reported that in patients with NASH, liver tissue concentrations of BA are higher and composition is altered compared with disease-free control livers130,131. Plasma BA concentrations are also higher, with altered composition in patients with NASH compared with those in healthy control individuals132–137. BA concentrations have been found to be significantly higher in patients with NASH with and without type 2 diabetes mellitus than in controls136. Because patients with NAFLD and NASH are typically more obese and more insulin-resistant than control participants, Legry and colleagues compared patients with NASH and control participants matched for BMI and insulin resistance16. This analysis revealed that alterations in BA metabolism are associated with insulin resistance, rather than liver necroinflammation itself. These findings highlight the complex interactions among insulin resistance, BAs and NAFLD and NASH. Nonetheless, BA-dependent pathways are being vigorously investigated as targets for the treatment of NASH, and these have been reviewed extensively elsewhere129,138.
Gut microbiome.
The metabolism of BAs by gut microorganisms is an important determinant of BA composition. A common modification is the removal of the amino acid moiety of conjugated primary BAs, such as glycocholic acid and glycochenodeoxycholic acid, to create unconjugated or free BAs, such as CA and CDCA (FIG. 1). This deconjugation reaction depends on the action of bile salt hydrolases, which are expressed in a wide range of bacteria and archaea139. Unconjugated BAs can undergo dehydroxylation at the 7 position to form secondary BAs; for example, CA is converted to DCA and CDCA is converted to LCA. Other possible modifications include epimerization and oxidation140. Although these conversions occur primarily in the microorganism-abundant colon where there are no active BA uptake transporters, unconjugated and secondary BAs can be passively absorbed3. Once returned to hepatocytes, unconjugated primary and secondary BAs can be re-conjugated3,141. However, the human liver is not efficient at re-hydroxylating secondary BA species; this is evidenced by the substantial proportion (~30%) of the human BA pool made up of DCA and its conjugated forms142–144 (FIG. 2).
Agents that alter the microbiome can modify the composition of the BA pool. Individuals treated for 7 days with the gram-positive bacteria-directed antibiotic vancomycin showed significant reductions in the concentrations of secondary BAs in the plasma and faeces145. This effect occurred in response to vancomycin, which markedly altered the faecal microbiota composition, but not in response to amoxicillin. Indeed, different antibiotics have distinct effects on the composition of the BA pool146. Although it is outside the scope of this Review, it is worth noting that BAs can also influence the microbiome. A particularly intriguing example of this is presented by Clostridioides difficile: certain BAs (12α-hydroxylated species) promote the germination of C. difficile spores, while some BAs (secondary species) suppress vegetative growth147.
The consequences of bacteria-modified BAs on host metabolism are currently under investigation148 and new methodological advances hold the promise of new ways to investigate this. In 2018, Yao and colleagues colonized gnotobiotic mice with isogenic bacterial strains with or without bile salt hydrolase and found that eliminating BA deconjugation capacity was sufficient to attenuate high-fat diet-induced weight gain149. Such approaches are likely to further refine the effect of the microbiome–BA–energy metabolism axis.
Therapeutic agents.
Interventions that target BAs or BA signalling pathways are currently in use or being developed for metabolic indications150,151. BA sequestrants block intestinal BA reabsorption, consequently increasing BA faecal excretion and causing compensatory increases in BA synthesis, which lowers plasma cholesterol152,153. BA sequestrants are now also known to improve glycaemia in patients with type 2 diabetes mellitus. A meta-analysis of 17 randomized controlled trials showed that BA sequestrants reduced HbA1c by 0.55%154. The mechanisms by which BA sequestrants improve glycaemia remain under debate. One possibility is that these resins increase BA concentrations in the colon, thus activating BA-dependent secretion of GLP1 (REF.155), but this has not been confirmed in other studies156–158. One study has provided evidence that BA sequestrant actually reduces BA-induced GLP1 secretion159. Mechanistic studies suggest that TGR5 is localized on the basolateral membrane of enteroendocrine cells and thus BAs must be absorbed in order to activate it160,161, and this would preclude TGR5 activation by sequestrant-bound BAs. Another possibility is that sequestrants increase splanchnic glucose uptake and utilization156, potentially due to lower FXR signalling in the intestine49.
Another mechanism by which BA reabsorption from the intestine can be blocked is to inhibit the apical sodium-dependent bile acid transporter (ASBT), and ASBT inhibitors have been developed to lower LDL cholesterol150,162,163. Studies in rodent models of type 2 diabetes mellitus have suggested that, like BA sequestrants, ASBT inhibitors may also be able to improve glycaemia164–166. Consistent with this, inhibiting ASBT in patients with type 2 diabetes mellitus improves glycaemia167,168. In non-diabetic participants, ASBT inhibitors might increase GLP1, but do not affect plasma levels of glucose169,170.
Obeticholic acid (OCA, INT-747) is an FXR agonist that is a leading candidate in clinical trials for the treatment of NASH. In a small study that included patients with type 2 diabetes mellitus, OCA improved insulin sensitivity in hyperinsulinaemic–euglycaemic clamps171. However, in a larger study, OCA increased fasting insulin and thus worsened insulin resistance, as calculated by the homeostasis model of assessment (HOMA)172. Further studies are required to determine the effects of OCA on glycaemia in humans. We note that in addition to its direct effects on FXR-dependent energy metabolism pathways, OCA (and any other agonist or antagonist of FXR) will also have major consequences on BA concentrations and composition, because of the potent, FXR-mediated negative feedback loops on CYP7A1 and CYP8B1, thus potentially influencing other BA-dependent pathways.
The BA TUDCA has long been used in the treatment of liver diseases, due to its ability to increase bile flow. Studies in rodents have suggested that TUDCA might also improve glycaemia, potentially through its effects on ER stress in metabolic tissues and/or β cells33,173. Indeed, in obese individuals treatment with TUDCA (1,750 mg/day) for 4 weeks resulted improved hepatic and muscle insulin sensitivity174. However, this treatment did not affect ER stress markers, and another study with the related unconjugated molecule ursodeoxycholic acid (UDCA; 20 mg per kg per day) actually induced some ER stress markers in the liver175. Thus the mechanisms involved in the activity of TUDCA and UDCA continue to be elusive.
Metformin is the most widely used anti-diabetes drug and numerous mechanisms of action have been proposed, including several implicating BAs. One possibility is that metformin impairs intestinal BA uptake176,177, potentially increasing GLP1 secretion. Another study in humans has shown that metformin enhances BA-induced GLP1 secretion178. Metformin is known to activate AMPK, and it has been suggested that AMPK directly phosphorylates and inhibits FXR activity179. Other evidence indicates that metformin’s effect in altering the gut microbiome changes BA levels and/or composition, resulting in lower intestinal FXR activity180,181.
Future research needs
To fill the gaps in our understanding of the mechanisms linking BAs with glycaemia, especially those of clinical relevance, several laboratory approaches are available. First, many rodent models of BA receptor activation and/or inhibition are associated with alterations in BA composition (FIG. 2). Therefore, it is not possible to separate the direct effects of the receptor on glucose metabolism pathways per se from its indirect effects (via altered BA composition) on TGR5, or other receptor-mediated or non-receptor-mediated BA effects. One way to approach this concern is to use mice with controlled BA pools, such as those lacking the CYP2C family of enzymes, which generate the 6-hydroxylated MCAs182; those lacking CYP8B1, which generates 12α-hydroxylated BAs, and which is known to effect glycaemia via its effects on BA composition13,183,184; and those with designer microbiota reconstitution149. Second, lack of BA receptors or BA synthesis enzymes might engender long-term and compensatory phenotypes that make data interpretation challenging. Exemplifying this are the studies of bariatric surgery in BA receptor knockout mice, which have phenotypic differences from wild-type mice at baseline. Temporal control of genetic knockouts, using inducible systems, could temper these caveats.
Third, there are differences in BA composition between humans and mice (FIG. 2). Humans have predominantly glycine-conjugated BAs (compared with taurine in mice), abundance of DCA and conjugated forms (compared with rodents, which efficiently re-hydroxylate DCA into CA), and very low levels of MCAs (compared with high levels in mice and rats). One potential approach could be to use human liver chimeric mice, although these are known to retain a substantial proportion of murine hepatocytes. At a minimum, researchers should keep these differences in mind and consider comparing humanized versus murine BA pools where possible, such as in in vitro experiments. Fourth, there are differences in the primary structure of FXR between species that influence sensitivity to certain BA subtypes185. These differences call for using human cells when possible, and stem cell-derived organoid methods might provide novel experimental platforms. Finally, systems biology approaches might help resolve the complexity of the BA functionality network (FIG. 4). Ultimately, clinical and translational studies in human subjects will have the most impact in determining the mechanisms linking BAs with glucose metabolism.
Conclusions
BAs are unique in their ability to act as structural molecules, allosteric modulators and signalling molecules. It is through a combination of these mechanisms that BAs affect key aspects of metabolic homeostasis. Whether or not these are druggable targets in patients with type 2 diabetes mellitus is not yet clear. New opportunities and experimental tools will allow basic, translational and clinical researchers to answer this question.
Key points.
Pathways in multiple tissues have been reported to link bile acids (BAs) with glycaemia.
Physiological and disease settings, and several medications, influence BA levels and composition.
When interpreting studies with genetic and pharmacological modulations of BA receptors, one should take into consideration that these modulations affect BA concentration, distribution and composition.
Rodent models with humanized BA composition will increase the relevance of basic research findings to human health.
Human cells and organoid models should be used to address the interspecies differences in BA receptor structure.
Acknowledgements
The authors thank S. Higuchi and E. Bertaggia for helpful discussions. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants DK115825 and DK007328, American Diabetes Association grant 18-IBS-275, and the Russell Berrie Foundation.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hofmann AF The enterohepatic circulation of bile acids in mammals: form and functions. Front. Biosci. Landmark Ed 14, 2584–2598 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Dawson PA & Karpen SJ Intestinal transport and metabolism of bile acids. J. Lipid Res. 56, 1085–1099 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson PA in Physiology of the Gastrointestinal Tract 6th edn Ch. 41 (eds Said HM, Ghishan FK, Kaunitz JD, Merchant JL & Wood JD) 931–956 (Academic, 2018). [Google Scholar]
- 4.Fagerberg L et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom 13, 397–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagenbuch B & Stieger B The SLCO (former SLC21) superfamily of transporters. Mol. Asp. Med 34, 396–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alnouti Y Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol. Sci 108, 225–246 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Palmer RH & Bolt MG Bile acid sulfates. I. Synthesis of lithocholic acid sulfates and their identification in human bile. J. Lipid Res 12, 671–679 (1971). [PubMed] [Google Scholar]
- 8.Hofmann AF & Roda A Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J. Lipid Res 25, 1477–1489 (1984). [PubMed] [Google Scholar]
- 9.Heuman DM Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J. Lipid Res 30, 719–730 (1989). [PubMed] [Google Scholar]
- 10.Armstrong MJ & Carey MC The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J. Lipid Res 23, 70–80 (1982). [PubMed] [Google Scholar]
- 11.Di Ciaula A et al. Bile acid physiology. Ann. Hepatol 16, S4–S14 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Vincent RP et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann. Clin. Biochem 50, 360–364 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Kaur A et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes 64, 1168–1179 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Slätis K et al. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J. Lipid Res 51, 3289–3298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak P et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legry V et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J. Clin. Endocrinol. Metab 102, 3783–3794 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Jiang C et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun 6, 10166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun 4, 2384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgström B Influence of bile salt, pH, and time on the action of pancreatic lipase; physiological implications. J. Lipid Res 5, 522–531 (1964). [PubMed] [Google Scholar]
- 20.Wang DQ-H, Tazuma S, Cohen DE & Carey MC Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am. J. Physiol. Gastrointest. Liver Physiol 285, G494–G502 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Ikeda I & Sugano M Effects of glyco- and tauro-cholic and chenodeoxycholic acids on lymphatic absorption of micellar cholesterol and sitosterol in rats. Biosci. Biotechnol. Biochem 57, 2059–2062 (1993). [Google Scholar]
- 22.Wilson FA & Dietschy JM Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J. Clin. Invest 51, 3015–3025 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watt SM & Simmonds WJ The specificity of bile salts in the intestinal absorption of micellar cholesterol in the rat. Clin. Exp. Pharmacol. Physiol 3, 305–322 (1976). [DOI] [PubMed] [Google Scholar]
- 24.Lowe PJ & Coleman R Membrane fluidity and bile salt damage. Biochim. Biophys. Acta 640, 55–65 (1981). [DOI] [PubMed] [Google Scholar]
- 25.Garidel P, Hildebrand A, Knauf K & Blume A Membranolytic activity of bile salts: influence of biological membrane properties and composition. Molecules 12, 2292–2326 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt A et al. The bile acid-sensitive ion channel (BASIC) is activated by alterations of its membrane environment. PLOS ONE 9, e111549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odo MY, Cucé L, Odo L & Natrielli A Action of sodium deoxycholate on subcutaneous human tissue: local and systemic effects. Dermatol. Surg 33, 178–189 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Palumbo P et al. Effects of phosphatidylcholine and sodium deoxycholate on human primary adipocytes and fresh human adipose tissue. Int. J. Immunopathol. Pharmacol 23, 481–489 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Rotunda A, Suzuki H, Moy R & Kolodney M Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatol. Surg 30, 1001–1008 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Humphrey S et al. ATX-101 for reduction of submental fat: A phase III randomized controlled trial. J. Am. Acad. Dermatol 75, 788–797.e7 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Sousa T et al. Deoxycholic acid modulates cell death signaling through changes in mitochondrial membrane properties. J. Lipid Res 56, 2158–2171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Q et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36, 592–601 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Ozcan U Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piomelli D A fatty gut feeling. Trends Endocrinol. Metab 24, 332–341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiPatrizio NV & Piomelli D Intestinal lipid-derived signals that sense dietary fat. J. Clin. Invest 125, 891–898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magotti P et al. Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids. Structure 23, 598–604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margheritis E et al. Bile acid recognition by NAPE-PLD. ACS Chem. Biol 11, 2908–2914 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li-Hawkins J et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J. Clin. Invest 110, 1191–1200 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang JYL Negative feedback regulation of bile acid metabolism: impact on liver metabolism and diseases. Hepatology 62, 1315–1317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parks DJ Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Makishima M Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Chen J, Hollister K, Sowers LC & Forman BM Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Fang S et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med 21, 159–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem 286, 26913–26920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burris TP et al. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev 65, 710–778 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cariou B et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem 281, 11039–11049 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Ma K, Saha PK, Chan L & Moore DD Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest 116, 1102–1109 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Dijk TH et al. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr−/− mice. J. Biol. Chem 284, 10315–10323 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie C et al. An intestinal farnesoid X receptor–ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trabelsi M-S et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun 6, 7629 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caron S et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell. Biol 33, 2202–2211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inagaki T et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Tomlinson E et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143, 1741–1747 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Fu L et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Potthoff MJ et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 13, 729–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kir S et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lan T et al. FGF19, FGF21, and an FGFR1/β-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton GJ et al. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Invest 123, 4799–4808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcelin G et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab 3, 19–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S et al. A gut–brain axis regulating glucose metabolism mediated by bile acids and competitive fibroblast growth factor actions at the hypothalamus. Mol. Metab 8, 37–50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallego-Escuredo JM et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes 39, 121–129 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Gómez-Ambrosi J et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr 36, 861–868 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Roesch SL et al. Perturbations of fibroblast growth factors 19 and 21 in type 2 diabetes. PLOS ONE 10, e0116928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedrich D, Marschall H-U & Lammert F Response of fibroblast growth factor 19 and bile acid synthesis after a body weight-adjusted oral fat tolerance test in overweight and obese NAFLD patients: a non-randomized controlled pilot trial. BMC Gastroenterol. 18, 76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angelin B, Larsson TE & Rudling M Circulating fibroblast growth factors as metabolic regulators–a critical appraisal. Cell Metab. 16, 693–705 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Al-Khaifi A, Rudling M & Angelin B An FXR agonist reduces bile acid synthesis independently of increases in FGF19 in healthy volunteers. Gastroenterology 155, 1012–1016 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Nicholes K et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol 160, 2295–2307 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miura S et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 12, 56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo J et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci. Transl Med. 6, 247ra100 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Zhou M et al. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol. Commun 1, 1024–1042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gadaleta RM et al. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Sci. Rep 8, 17210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayo MJ et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol. Commun 2, 1037–1050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison SA et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 391, 1174–1185 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Makishima M Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Schräder M, Müller KM, Nayeri S, Kahlen JP & Carlberg C Vitamin D3-thyroid hormone receptor heterodimer polarity directs ligand sensitivity of transactivation. Nature 370, 382–386 (1994). [DOI] [PubMed] [Google Scholar]
- 77.Kahlen JP & Carlberg C Functional characterization of a 1,25-dihydroxyvitamin D3 receptor binding site found in the rat atrial natriuretic factor promoter. Biochem. Biophys. Res. Commun 218, 882–886 (1996). [DOI] [PubMed] [Google Scholar]
- 78.Nehring JA, Zierold C & DeLuca HF Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl Acad. Sci. USA 104, 10006–10009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeitz U et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 17, 509–511 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Oh J et al. Deletion of macrophage vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 10, 1872–1886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ni W, Glenn DJ & Gardner DG Tie-2Cre mediated deletion of the vitamin D receptor gene leads to improved skeletal muscle insulin sensitivity and glucose tolerance. J. Steroid Biochem. Mol. Biol 164, 281–286 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neelankal John A et al. Vitamin D receptor-targeted treatment to prevent pathological dedifferentiation of pancreatic β cells under hyperglycaemic stress. Diabetes Metab. 44, 269–280 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Mannack G et al. Taurolithocholic acid-3 sulfate impairs insulin signaling in cultured rat hepatocytes and perfused rat liver. Cell. Physiol. Biochem 21, 137–150 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Maruyama T et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun 298, 714–719 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Kawamata Y et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem 278, 9435–9440 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Katsuma S, Hirasawa A & Tsujimoto G Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun 329, 386–390 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Thomas C et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lasalle M et al. Topical intestinal aminoimidazole agonists of g-protein-coupled bile acid receptor 1 promote glucagon like peptide-1 secretion and improve glucose tolerance. J. Med. Chem 60, 4185–4211 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Cao H et al. Intestinally-targeted TGR5 agonists equipped with quaternary ammonium have an improved hypoglycemic effect and reduced gallbladder filling effect. Sci. Rep 6, 28676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhre RE et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab 11, 84–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perino A et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J. Clin. Invest 124, 5424–5436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Velazquez-Villegas LA et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun 9, 245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe M et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439, 484–489 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Haeusler RA, Astiarraga B, Camastra S, Accili D & Ferrannini E Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 62, 4184–4191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martineau MG et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care 38, 243–248 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Nervi FO, Severín CH & Valdivieso VD Bile acid pool changes and regulation of cholate synthesis in experimental diabetes. Biochim. Biophys. Acta 529, 212–223 (1978). [DOI] [PubMed] [Google Scholar]
- 97.Uchida K, Makino S & Akiyoshi T Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes 34, 79–83 (1985). [DOI] [PubMed] [Google Scholar]
- 98.Akiyoshi T, Uchida K, Takase H, Nomura Y & Takeuchi N Cholesterol gallstones in alloxan-diabetic mice. J. Lipid Res 27, 915–924 (1986). [PubMed] [Google Scholar]
- 99.Biddinger SB et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med 14, 778–782 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiang JYL Bile acids: regulation of synthesis. J. Lipid Res 50, 1955–1966 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD & Accili D Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 15, 65–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cariou B et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr. Metab 8, 48 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haeusler RA et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J. Clin. Endocrinol. Metab 101, 1935–1944 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steiner C et al. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLOS ONE 6, e25006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ståhlberg D et al. Hepatic cholesterol metabolism in human obesity. Hepatology 25, 1447–1450 (1997). [DOI] [PubMed] [Google Scholar]
- 106.Wewalka M, Patti M-E, Barbato C, Houten SM & Goldfine AB Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J. Clin. Endocrinol. Metab 99, 1442–1451 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brufau G et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 52, 1455–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Albaugh VL, Banan B, Ajouz H, Abumrad NN & Flynn CR Bile acids and bariatric surgery. Mol. Aspects Med. 56, 75–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei M et al. Bile acid profiles within the enterohepatic circulation in a diabetic rat model after bariatric surgeries. Am. J. Physiol. Gastrointest. Liver Physiol 314, G537–G546 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Bhutta HY et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLOS ONE 10, e0122273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chávez-Talavera O et al. Roux-en-Y gastric bypass increases systemic but not portal bile acid concentrations by decreasing hepatic bile acid uptake in minipigs. Int. J. Obes 41, 664–668 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Benetti A et al. Cholesterol metabolism after bariatric surgery in grade 3 obesity: differences between malabsorptive and restrictive procedures. Diabetes Care 36, 1443–1447 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferrannini E et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. Diabetes 64, 3377–3385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Escalona A et al. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis 12, 763–769 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Jørgensen NB et al. Improvements in glucose metabolism early after gastric bypass surgery are not explained by increases in total bile acids and fibroblast growth factor 19 concentrations. J. Clin. Endocrinol. Metab 100, E396–E406 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Mazidi M, de Caravatto PPP, Speakman JR & Cohen RV Mechanisms of action of surgical interventions on weight-related diseases: the potential role of bile acids. Obes. Surg 27, 826–836 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Ryan KK et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509, 183–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Albaugh VL et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 156, 1041–1051 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myronovych A et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity 22, 2301–2311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McGavigan AK et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut 66, 226–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ding L et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 64, 760–773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rudman D & Kendall FE Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J. Clin. Invest 36, 530–537 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pataia V, Dixon PH & Williamson C Pregnancy and bile acid disorders. Am. J. Physiol. Gastrointest. Liver Physiol 313, G1–G6 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Reyes H & Sjövall J Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Ann. Med 32, 94–106 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Geenes V et al. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid. PLOS ONE 9, e83828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martineau M, Raker C, Powrie R & Williamson C Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol 176, 80–85 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Wikström Shemer E, Marschall HU, Ludvigsson JF & Stephansson O Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: a 12-year population-based cohort study. BJOG 120, 717–723 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Papacleovoulou G et al. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J. Clin. Invest 123, 3172–3181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Friedman SL, Neuschwander-Tetri BA, Rinella M & Sanyal AJ Mechanisms of NAFLD development and therapeutic strategies. Nat. Med 24, 908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aranha MM et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur. J. Gastroenterol. Hepatol 20, 519–525 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Lake AD et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol. Appl. Pharmacol 268, 132–140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bechmann LP et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 57, 1394–1406 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Dasarathy S et al. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur. J. Gastroenterol. Hepatol 23, 382–388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalhan SC et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 60, 404–413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mouzaki M et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLOS ONE 11, e0151829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puri P et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67, 534–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferslew BC et al. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig. Dis. Sci 60, 3318–3328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanaka N, Aoyama T, Kimura S & Gonzalez FJ Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther 179, 142–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones BV, Begley M, Hill C, Gahan CGM & Marchesi JR Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. USA 105, 13580–13585 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ridlon JM, Kang D-J & Hylemon PB Bile salt biotransformations by human intestinal bacteria. J. Lipid Res 47, 241–259 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Hanson RF & Williams G Metabolism of deoxycholic acid in bile fistula patients. J. Lipid Res. 12, 688–691 (1971). [PubMed] [Google Scholar]
- 142.Rees DO et al. Comparison of the composition of bile acids in bile of patients with adenocarcinoma of the pancreas and benign disease. J. Steroid Biochem. Mol. Biol 174, 290–295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao M-F et al. Conjugated bile acids in gallbladder bile and serum as potential biomarkers for cholesterol polyps and adenomatous polyps. Int. J. Biol. Markers 31, e73–e79 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Stolk MF et al. Gallbladder emptying in vivo, bile composition, and nucleation of cholesterol crystals in patients with cholesterol gallstones. Gastroenterology 108, 1882–1888 (1995). [DOI] [PubMed] [Google Scholar]
- 145.Reijnders D et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 24, 63–74 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y, Limaye PB, Renaud HJ & Klaassen CD Effect of various antibiotics on modulation of intestinal microbiota and bile acid profile in mice. Toxicol. Appl. Pharmacol 277, 138–145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abt MC, McKenney PT & Pamer EG Clostridium difficile colitis: pathogenesis and host defence. Nat. Rev. Microbiol 14, 609–620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wahlström A, Sayin SI, Marschall H-U & Bäckhed F Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016). [DOI] [PubMed] [Google Scholar]
- 149.Yao L et al. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7, e37182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Slijepcevic D & van de Graaf SFJ Bile acid uptake transporters as targets for therapy. Dig. Dis 35, 251–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schaap FG, Trauner M & Jansen PLM Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol 11, 55–67 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Staels B, Handelsman Y & Fonseca V Bile acid sequestrants for lipid and glucose control. Curr. Diab. Rep 10, 70–77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Einarsson K et al. Bile acid sequestrants: mechanisms of action on bile acid and cholesterol metabolism. Eur. J. Clin. Pharmacol 40, S53–S58 (1991). [PubMed] [Google Scholar]
- 154.Hansen M et al. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: a systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complications 31, 918–927 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Beysen C et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia 55, 432–442 (2012). [DOI] [PubMed] [Google Scholar]
- 156.Smushkin G et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes 62, 1094–1101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marina AL et al. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care 35, 1119–1125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hansen M et al. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes Obes. Metab 18, 571–580 (2016). [DOI] [PubMed] [Google Scholar]
- 159.Brønden A et al. The bile acid-sequestering resin sevelamer eliminates the acute GLP-1 stimulatory effect of endogenously released bile acids in patients with type 2 diabetes. Diabetes Obes. Metab 20, 362–369 (2018). [DOI] [PubMed] [Google Scholar]
- 160.Brighton CA et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156, 3961–3970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Christiansen CB et al. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiol. Gastrointest. Liver Physiol 316, G574–G584 (2019). [DOI] [PubMed] [Google Scholar]
- 162.West KL, Zern TL, Butteiger DN, Keller BT & Fernandez ML SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis 171, 201–210 (2003). [DOI] [PubMed] [Google Scholar]
- 163.Root C et al. Ileal bile acid transporter inhibition, CYP7A1 induction, and antilipemic action of 264W94. J. Lipid Res. 43, 1320–1330 (2002). [PubMed] [Google Scholar]
- 164.Chen L et al. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am. J. Physiol. Endocrinol. Metab 302, E68–E76 (2012). [DOI] [PubMed] [Google Scholar]
- 165.Lundåsen T et al. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLOS ONE 7, e37787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rao A et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl Med 8, 357ra122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nunez DJ et al. Glucose and lipid effects of the ileal apical sodium-dependent bile acid transporter inhibitor GSK2330672: double-blind randomized trials with type 2 diabetes subjects taking metformin. Diabetes Obes. Metab 18, 654–662 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Tiessen RG et al. Safety, tolerability and pharmacodynamics of apical sodium-dependent bile acid transporter inhibition with volixibat in healthy adults and patients with type 2 diabetes mellitus: a randomised placebo-controlled trial. BMC Gastroenterol. 18, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rudling M, Camilleri M, Graffner H, Holst JJ & Rikner L Specific inhibition of bile acid transport alters plasma lipids and GLP-1. BMC Cardiovasc. Disord 15, 75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Palmer M, Jennings L, Silberg DG, Bliss C & Martin P A randomised, double-blind, placebo-controlled phase 1 study of the safety, tolerability and pharmacodynamics of volixibat in overweight and obese but otherwise healthy adults: implications for treatment of non-alcoholic steatohepatitis. BMC Pharmacol. Toxicol 19, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mudaliar S et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Neuschwander-Tetri BA et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee YY et al. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem. Biophys. Res. Commun 397, 735–739 (2010). [DOI] [PubMed] [Google Scholar]
- 174.Kars M et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mueller M et al. Ursodeoxycholic acid: effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 38, 523–531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carter D, Howlett HCS, Wiernsperger NF & Bailey CJ Differential effects of metformin on bile salt absorption from the jejunum and ileum. Diabetes Obes. Metab 5, 120–125 (2003). [DOI] [PubMed] [Google Scholar]
- 177.Napolitano A et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLOS ONE 9, e100778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brønden A et al. Single-dose metformin enhances bile acid–induced glucagon-like peptide-1 secretion in patients with type 2 diabetes. J. Clin. Endocrinol. Metab 102, 4153–4162 (2017). [DOI] [PubMed] [Google Scholar]
- 179.Lien F et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J. Clin. Invest 124, 1037–1051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu H et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med 23, 850–858 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Sun L et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med 24, 1919–1929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Takahashi S et al. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res 57, 2130–2137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bonde Y, Eggertsen G & Rudling M Mice abundant in muricholic bile acids show resistance to dietary induced steatosis, weight gain, and to impaired glucose metabolism. PLOS ONE 11, e0147772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bertaggia E et al. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am. J. Physiol. Endocrinol. Metab 313, E121–E133 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui J et al. The amino acid residues asparagine 354 and isoleucine 372 of human farnesoid X receptor confer the receptor with high sensitivity to chenodeoxycholate. J. Biol. Chem 277, 25963–25969 (2002). [DOI] [PubMed] [Google Scholar]
- 186.Kimura K & Ogura M Effect of streptozotocin-induced diabetes on the activity of 7 alpha-hydroxy-4-cholesten-3-one-specific 12 alpha-hydroxylase in rats. Biol. Chem. Hoppe. Seyler 369, 1117–1120 (1988). [DOI] [PubMed] [Google Scholar]
- 187.Ferrell JM, Boehme S, Li F & Chiang JYL Cholesterol 7α-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J. Lipid Res 57, 1144–1154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schmidt DR et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem 285, 14486–14494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Donepudi AC, Boehme S, Li F & Chiang JYL G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology 65, 813–827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kim I et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res 48, 2664–2672 (2007). [DOI] [PubMed] [Google Scholar]
- 191.Sayin SI et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013). [DOI] [PubMed] [Google Scholar]
- 192.de Boer JF et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152, 1126–1138.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Ryan KK et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vettorazzi JF et al. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 65, 54–63 (2016). [DOI] [PubMed] [Google Scholar]
- 195.Watanabe M et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest 113, 1408–1418 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Claudel T et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J. Clin. Invest 109, 961–971 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Probstfield JL, Lin T-L, Peters J & Hunninghake DB Carotenoids and vitamin A: the effect of hypocholesterolemic agents on serum levels. Metabolism 34, 88–91 (1985). [DOI] [PubMed] [Google Scholar]
- 198.Jiang C et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest 125, 386–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nielsen S et al. Chenodeoxycholic acid stimulates glucagon-like peptide-1 secretion in patients after Roux-en-Y gastric bypass. Physiol. Rep 5, e13140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Angelin B, Björkhem I, Einarsson K & Ewerth S Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J. Clin. Invest 70, 724–731 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jäntti SE et al. Quantitative profiling of bile acids in blood, adipose tissue, intestine, and gall bladder samples using ultra high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 406, 7799–7815 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Holzbach RT et al. Portal vein bile acids in patients with severe inflammatory bowel disease. Gut 21, 428–435 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reihnér E, Björkhem I, Angelin B, Ewerth S & Einarsson K Bile acid synthesis in humans: regulation of hepatic microsomal cholesterol 7 alpha-hydroxylase activity. Gastroenterology 97, 1498–1505 (1989). [DOI] [PubMed] [Google Scholar]
- 204.Takikawa H, Beppu T & Seyama Y Profiles of bile acids and their glucuronide and sulphate conjugates in the serum, urine and bile from patients undergoing bile drainage. Gut 26, 38–42 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perez de la Cruz Moreno M et al. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J. Pharm. Pharmacol 58, 1079–1089 (2006). [DOI] [PubMed] [Google Scholar]
- 206.Clarysse S et al. Postprandial evolution in composition and characteristics of human duodenal fluids in different nutritional states. J. Pharm. Sci 98, 1177–1192 (2009). [DOI] [PubMed] [Google Scholar]
- 207.Northfield TC & McColl I Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 14, 513–518 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hamilton JP et al. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol 293, G256–G263 (2007). [DOI] [PubMed] [Google Scholar]
- 209.Kakiyama G et al. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J. Lipid Res 55, 978–990 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Setchell KD et al. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 112, 226–235 (1997). [DOI] [PubMed] [Google Scholar]
- 211.Krasowski MD, Yasuda K, Hagey LR & Schuetz EG Evolution of the pregnane X receptor: adaptation to cross-species differences in biliary bile salts. Mol. Endocrinol 19, 1720–1739 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lew J-L et al. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem 279, 8856–8861 (2004). [DOI] [PubMed] [Google Scholar]
- 213.Sato H et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure–activity relationships, and molecular modeling studies. J. Med. Chem 51, 1831–1841 (2008). [DOI] [PubMed] [Google Scholar]
- 214.Yu J et al. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J. Biol. Chem 277, 31441–31447 (2002). [DOI] [PubMed] [Google Scholar]
- 215.Adachi R et al. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J. Lipid Res 46, 46–57 (2005). [DOI] [PubMed] [Google Scholar]
- 216.Duran-Sandoval D et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J. Biol. Chem 280, 29971–29979 (2005). [DOI] [PubMed] [Google Scholar]