Abstract
Recent work provides evidence that the infant brain is able to make top-down predictions, but this has been explored only in limited contexts and domains. We build upon this evidence of predictive processing in infants using a new paradigm to examine auditory repetition suppression (RS). RS is a well-documented neural phenomenon in which repeated presentations of the same stimulus result in reduced neural activation compared to non-repeating stimuli. Many theories explain RS using bottom-up mechanisms, but recent work has posited that top-down expectation and predictive coding may bias, or even explain, RS. Here, we investigate whether RS in the infant brain is similarly sensitive to top-down mechanisms. We use fNIRS to measure infants’ neural response in two experimental conditions, one in which variability in stimulus presentation is expected (occurs 75% of the time) and a control condition where variability and repetition are equally likely (50% of the time). We show that 6-month-old infants exhibit attenuated frontal lobe response to blocks of variable auditory stimuli during contexts when variability is expected as compared to the control condition. These findings suggest that young infants’ neural responses are modulated by predictions gained from experience and not simply by bottom-up mechanisms.
Keywords: Infant, fNIRS, Learning, Prediction, Auditory, Language, Lexical, Words, Language development, Development, Expectation, Repetition suppression, Repetition enhancement, Context, Task
1. Introduction
A crucial question for the field of developmental cognitive neuroscience is how the developing brain adapts to the statistics or the structure of its environment. We know that young infants have an incredible learning capacity where brief exposures to statistical information (which reflect the structures or patterns in their environment) result in behavioral changes (audition: Saffran et al., 1996; vision: Kirkham et al., 2002). It is believed that these behavioral changes reflect an incremental developmental process. However, little is known about how this process occurs neurally and understanding the neural underpinnings of this process will help uncover how experience with statistical information shapes development. The main view of how statistical information shapes the developing brain is as a bottom-up, weighting process. Specifically, this view proposes that increases in weight are given to the internal representations that have been experienced more frequently. In this way, new sensory input that matches frequently encountered input is more easily processed, and sensory input that doesn’t match these frequent experiences triggers a novelty preference. Importantly, this mechanism would always lead to greater responses to highly variable stimuli than to less variable stimuli because each of the latter is more frequent. Drawing from a related domain, this is the proposed mechanism used to explain how face perception is shaped in the context of the other-race effect. In this well-known developmental phenomenon, the types of faces that an infant has experience with are better processed and remembered than faces that they don’t have experience with (e.g., Kelly et al., 2007). Parallel examples to the other-race effect are found in language comprehension (e.g. speech perception, Werker and Tees, 1984) and crossmodal processing (e.g. recognizing audiovisual monkey calls, Lewkowicz and Ghazanfar, 2009). In these examples, it is the case that experience is posited to bias perceptual processing through a bottom-up mechanism.
However, in the field of adult cognitive neuroscience, there has been recent interest in how the brain can adjust to experience using top-down or feedback connections. In the theory of predictive coding, for example, perceptual cortices combine feedforward sensory signals and top-down or feedback signals which convey the current expectations or predictions about the upcoming sensory input, and it is the match or mismatch of these responses that drives the cortical activity that we observe in neuroimaging experiments (Clark, 2013; Friston, 2005). Specifically, the better the prediction or expectation matches the sensory input, the less cortical activity will be observed in sensory input. The larger the mismatch between expectation and sensory input, the larger the cortical response. In this way, the brain is able to adapt to the structure or statistics in the environment in a top-down fashion through the feedback of expectations.
This difference between bottom-up and top-down mechanisms of learning could have large consequences for both what is learned from experience as well as how quickly and readily infants can adapt to their environment. Bottom-up mechanisms can be thought of like a magnet that is following the structure of the environment by changing the weighting of an infant’s internal representations. Top-down mechanisms also result in changes in processing based on the environment but don’t require changing the internal representations themselves. Instead, these internal representations are weighted to anticipate changes based on the infant’s current understanding of what is likely in the environment. Moreover, top-down processes are likely
faster to adapt if the environment changes, as these changes do not require rebuilding internal structures of the system. Thus, under many circumstances, top-down systems are faster and more flexible in how they use experience to change their responses in a complex world.
Despite the importance of dissociating top-down vs. bottom-up mechanisms early in development, there is little work establishing whether young infants are capable of employing top-down strategies to learn about the environment. While there is good empirical support that the adult brain uses predictions to modulate perceptual cortices (e.g., Summerfield and Egner, 2009), determining whether this capacity is available early in life is an essential precondition for prediction or top-down modulation to play a role in development. Moreover, there are a number of reasons to think that the ability to use top-down or feedback connections to modulate perceptual cortices may be later developing. For example, in order for a brain to engage in top-down processing, it must have established connections between disparate brain regions so predictions or feedback information can be communicated to lower-level regions. We know that infants are born with poorly connected brains with an abundance of local, short-range connections and a paucity of long-range connections (Smyser et al., 2011). This capacity develops over the first several years of life, but given that it is difficult for the infant brain to send information between disparate brain regions, this suggests that the capacity to use predictions to modulate neural responses might be absent or at least strongly reduced early in development. Despite these apparent neuroanatomical limitations on predictive processing in the infant brain, there has been some recent evidence that infants can engage in top-down sensory prediction (Emberson et al., 2015; Kouider et al., 2015). Moreover, recent work has made a link between infants at-risk for poor developmental outcomes or developmental delays and deficits in top-down prediction (Emberson et al., 2017a). However, these initial pieces of evidence all rely on very similar paradigms (cross-modal associative cueing paradigms with responses measured during violations of the audiovisual association) and have always focused on visual prediction. By contrast, predictive processing has been established across sensory modalities in adults and in a number of different disparate paradigms. Thus, while there is some initial suggestion that top-down prediction may be available early in life, the evidence is quite limited.
In this paper, we aim to extend this initial evidence of top-down, sensory prediction in infants. Specifically, we extend previous findings to a different sensory modality (audition) and investigate whether top-down prediction affects a well-known neural phenomenon: Repetition suppression (RS). This expansion of previous findings to new modalities and phenomena is important to determine whether prediction or top-down mechanisms affect the developing brain more generally or whether prediction substantially modulates neural responses that only occur in specific contexts.
There is both a bottom-up and a top-down or predictive theory of repetition suppression (RS). RS is a phenomenon where the repetition of a stimulus results in a decreased neural response (Grill-Spector et al., 1999). The dominant theories as to why RS occurs involve purely bottom-up mechanisms (e.g., tuning, sharpening, fatigue, Grill-Spector et al., 2006). All of these models can be characterized as bottom-up because the differential pattern of neural responses to variable vs. repeated stimuli occurs through the tuning or weighting of these internal representations directly by sensory input. For example, the repetition of faces will produce an attenuated response to face stimuli because the neurons corresponding to faces become fatigued or the distribution of their responses becomes sharpened or tuned to the specific face stimuli being presented. Thus, these are purely bottom-up models of how and why RS occurs. By contrast, predictive models propose a top-down account of how RS occurs (Summerfield et al., 2008). These models simply posit that when a stimulus is correctly predicted (i.e., matches top-down signals), there is less neural activity in response to that stimulus (e.g., Friston, 2005). In the context of RS, repeated stimuli are expected, and this results in an attenuation of neural response to repeated stimuli over variable stimuli. Thus, predictive models of the cortex predict the presence of RS to depend on task-context as it is top-down signals relating to the prediction of upcoming stimuli that drive these changes in the magnitude of response to a given stimulus.
These top-down vs. bottom-up models of RS make different predictions about whether RS can be manipulated through task contexts (e.g., if participants are in a context where one is more or less likely to experience a repetition of a stimulus). All bottom-up models propose that the probability of seeing a repeated stimulus should not affect how much a given repetition affects the pattern of RS (i.e., a situation where repetition is rare will elicit the same degree of RS as a context where repetition is frequent). Moreover, you cannot have bottom-up tuning for variability because perceptual systems do not have representations or receptive fields for the abstract concepts of repetition and variability of stimuli. However, a top-down or predictive coding model can account for these effects: if repetitions shift from being common to being rare, then RS will be attenuated because participants are no longer predicting repeated stimuli (i.e., participants predict variable stimuli). In other words, since the expectation of stimulus repetition is the mechanism behind RS, according to a top-down model, reducing this expectation will affect RS.
Support for this top-down, predictive account of RS has already been found in adults. Summerfield et al. (2008) established the sensitivity of RS to task-context. Specifically, they found that adult RS was altered across blocks where repetition of stimuli is most likely vs. when variability (or non-repetition) is most likely. In blocks when repetition was likely, a canonical RS response was observed, but in blocks when variability was likely, they observed a reduction or absence of RS. This absence of RS was driven by both an increase in response to the repeated stimuli and a decrease in response to the variable stimuli. Summerfield et al. (2008) offer potential explanations for this observed reduction in RS (rather than a reversal to a repetition enhancement, RE, effect) including the fact that in the real world, in general, immediate repetitions of any given context are highly likely and thus expected even within artificially created contexts where variability occurs more frequently.
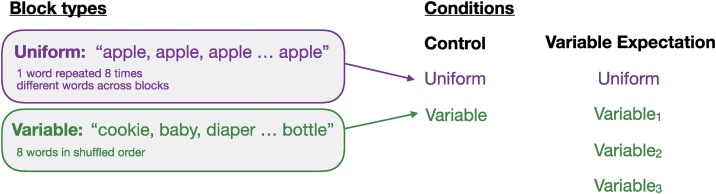
Here, we investigate whether the infants brain is also sensitive to the likelihood of repetition and whether the relative neural responses to repetition and variability are affected by the probability of variability or repetition. Building from our previous work investigating auditory and visual RS in young infants (Emberson et al., 2017b), we extend the Summerfield et al. (2008) paradigm to the auditory domain. Specifically, we investigate neural responses in two groups of infants across two conditions: One group received equal experience with repetition and variability (Control condition, 50% variable, 50% uniform, previously reported in Emberson et al., 2017b); The other group received stimuli that were biased towards variability (Variable Expectation condition, 75% variable, 25% uniform, see Fig. 1 for schematic of block types and these two conditions; note that the difference in probability between the conditions is quite subtle: for variability, 75% of the blocks are variable in the Variable Expectation condition but 50% of the blocks are variable in the Control condition).
Fig. 1.
Schematic of Block Types (Uniform or Variable, left panel) and Conditions (Control and Variable Expectation, right panel).
This between-group comparison will allow us to investigate whether top-down, predictive models of RS also apply to the infant brain. As summarized above, predictive models posit that when a stimulus is correctly predicted, there is less neural activity in response to that stimulus (e.g., Friston, 2005). If infants are able to use prediction to bias their neural responses, we expect that infants who received experience biased towards variability will exhibit either a reduction in RS or a flip from the canonical RS response to a repetition enhancement (RE). In other words, when variability is most likely, we hypothesize that neural responses to variability will be attenuated. These are the same predictions as Summerfield et al. (2008), although Summerfield et al (2008) find only a reduction in RS and not an RE effect (as summarized above). Following from Emberson et al. (2017b) where auditory RS was most strongly found in the frontal lobe and weakly present in the temporal lobe, we hypothesize that changes in RS according to expectation of variability will be most prominent in the frontal lobe but may also be present in the temporal lobe.
2. Methods
2.1. Participants
Participants were recruited from the same pool into one of two experimental groups: Control and Variable Auditory expectation. Data from the Control group was previously reported in Emberson et al (2017b) to establish the baseline for auditory RS for these stimuli and age group using fNIRS. We then compare this baseline finding to one where the expectation of variability is biased in a new (i.e., not previously reported) set of data. We have included the methods and analyses from the Control dataset for this comparison but see Emberson et al (2017b) for additional information (e.g., baselines for visual RS). We focused this research on 6-month-old infants as an example age within early infancy and one in the midst of perceptual narrowing and other important phases of perceptual development. This is the age of focus for a number of our studies and concentrating on this age allows for a cleaner comparison across studies (e.g., Emberson et al., 2015).
2.1.1. Control
Twenty-nine (29) participants were included in the analysis of Experiment 1, ages 5–7 months (M = 5.76, SD = 0.52, Min = 5.2, Max = 7.0 months; 12 female). Of the included infants, 75.9% heard only English at home. Of the seven remaining participants, one experienced another language 5% of the time, one 10% of the time, two 25% of the time, two 60% of the time, and one 100% of the time. Included participants were identified as Caucasian (75.9%), Black (10.3%), and mixed race (3.4%, White and Pacific Islander), with two additional participants unreported (6.9%). Twenty-five of the included infants were identified as non-Hispanic, three as Hispanic, and the remaining one was unreported. Infants who were tested could be excluded from analyses for two reasons: failure to sit through at least three blocks in both conditions (repeated and variable) or missing signal from too many channels in regions of interest. Two infants were excluded for failure to sit through the required number of trials.
2.1.2. Variable expectation
An additional eighteen (18) participants were recruited from the same subject pool as the Control group and using the same inclusion parameters: ages 5–7 months (M = 6.37, SD = 0.31, Min = 5.72, Max = 6.9); 8 male and 10 female. Of the included infants, 88.9% heard English exclusively at home. The two remaining participants heard another language at home 10% of the time. Included participants were identified as Caucasian (72.2%), Black (11.1%), mixed race (11.1%; one Black and White, one Black, White, and other), and other (5.6%). Seventeen included participants were identified as non-hispanic, with one infant not reported. As in the Control condition, infants who were tested could be excluded from analyses for two reasons: failure to sit through at least three blocks in the uniform condition and/or at least nine blocks in the variable condition or missing signal from too many channels in regions of interest. Four infants were excluded for failure to sit through the minimum number of blocks and two were excluded for too many channels missing data.
2.2. Stimuli, experimental design and procedure
All auditory stimuli were bisyllabic English words selected based on their appearance in infant-directed speech in the CHILDES database (MacWhinney, 1991). All words were spoken in infant-directed speech by a female, native English speaker with a local, Rochester accent. All stimuli were between 700–800 ms with a variable ISI applied so that all stimulus onset asynchronies (SOAs) are 1 s. Note, we selected familiar words to be engaging to infants compared to other auditory stimuli (Marcus et al., 2007) and not because we have specific hypotheses about infants’ early language comprehension.
2.2.1. Control
This condition employed a fifty percent (50%) probability of uniform or variable blocks. These two types of blocks were created using a set of 8 stimuli (i.e., the spoken words apple, baby, bottle, blanket, cookie, diaper, doggie, story). The Uniform blocks selected 1 of the words (without replacement, different word for each block) and presented it 8 times. The Variable blocks presented all 8 words in shuffled order. These auditory stimuli were presented along with a dim video of fireworks to maintain infant visual attention. Two additional visual blocks were included in the experimental design (again 50% uniform, faces presented in the same manner as the auditory words) but were not included in this analysis (see Emberson et al., 2017b for analyses of visual blocks). The combination of this video and the other visual blocks resulted in good compliance in this task.
2.2.2. Variable expectation
This condition was designed to enhance the expectation of variable stimulus presentation to see whether relative responses to uniform vs. variable blocks would change. To this end, we modified the procedure from Emberson et al (2017b). Most notably, we increased the total number of words to 32. These words were: apple, baby, birdie, blanket, bottle, bouncy, bunny, cookie, diaper, doggie, finger, fishy, funny, fussy, kitty, little, mirror, piggy, pretty, purple, rabbit, rattle, sleepy, story, sugar, talking, tickle, towel, turtle, yellow, yummy. For each infant, the words were randomly divided into 4 sets of 8.
Each group of 8 words was assigned to either 1 of 3 Variable blocks or 1 Uniform block. As in the Control condition, the Variable blocks presented all 8 words in shuffled order and the Uniform blocks presented 1 word 8 times, with a new word presented for each subsequent Uniform block.
Piloting with a separate group of infants revealed that presenting the baseline video of the dim fireworks (as used in the Control condition) was not sufficient to visually engage infants in the Variable Expectation condition. Since all blocks in the Variable Expectation condition presented similar types of stimuli (English words in different orders), infants became distracted by their environment (e.g., their parent, the curtains) and did not attend to the stimuli. To solve this problem, each block was accompanied with the presentation of a smiling face. These faces were presented for 1 s coincident with the onset of each word (i.e., 8 presentations of the same face throughout the block). The same face was presented for the entire block whether or not the words were uniform or variable. Thus, the visual stimuli were not manipulated across blocks and therefore should not influence neural responses or the infants’ task across block types. Moreover, each face was uniquely presented in each block (32 faces) so no cross-modal expectation could be acquired between a particular face (or type of face) and variable vs. uniform blocks. Faces were drawn from different genders and ethnicities in the NimStim database (Tottenham et al., 2009).
2.2.3. Presentation procedures
In the Control condition, the Uniform and Variable blocks (1 of each) were presented in random order to a maximum of 8 times. The same procedure was followed in the Variable Expectation condition with a few changes. First, since the number of Variable blocks was increased to 3 in this condition, each with their own set of 8 words, we can consider there to be 4 blocks (Uniform: 1; Variable: 3). These four blocks were presented in random order to a maximum of 8 times. The exception to this is the first 4 blocks in which the 3 Variable blocks were always presented first followed by the Uniform block. This was done to initiate an expectation for variable stimuli before the presentation of the first Uniform block.
In both conditions, in between blocks, a baseline video of the dim fireworks was presented along with soft music (length was pre-determined and randomly selected to be between 4 and 9 s based on a uniform distribution). Under ideal circumstances, the baseline would contain neither auditory nor visual stimuli. However, it is not possible to maintain infants’ attention in the absence of any stimulation, so the low-salience fireworks and music displays served as a minimally salient inter-block baseline. The experiment was conducted in a darkened room. During the experiment, the infant sat on a caretaker’s lap surrounded by a black curtain to reduce visual distraction and separate the infant from the experimenter. Participants watched the video display until they stopped looking consistently or all experimental blocks were viewed.
2.3. fNIRS recordings, preprocessing and analyses
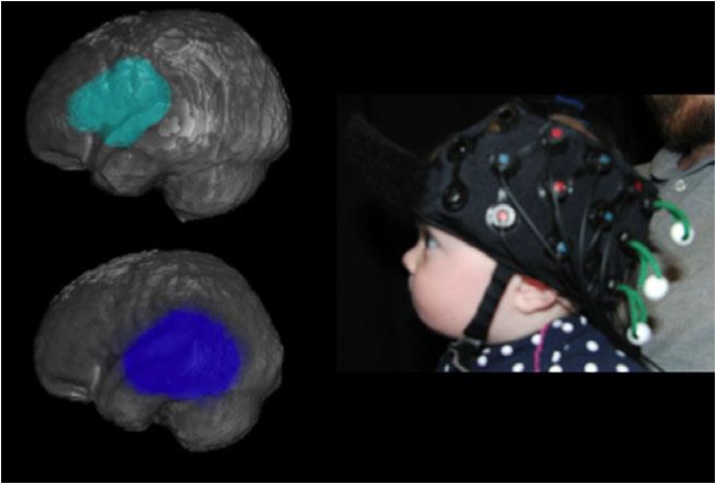
FNIRS recordings were collected using a Hitachi ETG-4000. Twenty-four channels were used in the NIRS cap, with 12 over the back of the head to record bilaterally from the occipital lobe, and 12 over the left lateral surface of the head to record from the left temporal and frontal lobes. The channels were organized in two 3 × 3 arrays, and the cap was placed so that, for the lateral array, the central optode on the most ventral row was centered over the left ear and, for the rear array, the central optode on the most ventral row was centered between the ears and over the inion. This cap position was chosen based on which fNIRS channels were most likely to record from temporal and occipital cortex in infants. Due to curvature of the infant head, a number of channels did not provide consistently good optical contact across infants (the most dorsal channels for each pad). We did not consider the recordings from these channels in subsequent analyses and only considered a subset of the channels (7 for the lateral pad over the ear and 5 for the pad at the rear array). Caretakers were instructed to refrain from influencing their infants, only providing comfort if needed and to keep their infant from either grabbing at the cap or rubbing their head against the caregiver (Fig. 2, right for cap placement).
Fig. 2.
Left: Location of 2 anatomically-defined regions of interest (ROI) for fNIRS analyses: Frontal and temporal ROIs. Right: position of the cap on one illustrative infant participant.
In order to provide clear, quantifiable anatomical localizations for our fNIRS recordings, we followed the methods reported in Lloyd-Fox et al. (2014) to co-register the fNIRS recordings for the infants with MR-templates. As this procedure has been done for this particular cap configuration for hundreds of infants at this age (reported in Emberson et al., 2015, 2017b,c, Fig. 2 left), we employed these robust, average localizations to select channels within our predetermined regions of interest (ROI). These ROIs correspond to our previous paper on RS (Emberson et al., 2017b) and are the temporal, frontal and occipital lobes. For a full description of these methods see Emberson et al. (2017b).
2.3.1. Preprocessing and analyses
The raw data were exported from the Hitachi ETG-4000 to MATLAB (version R2015a for Mac) for subsequent analyses with HomER 2 (Hemodynamic Evoked Response NIRS data analysis GUI, version 1.5) using the default preprocessing pipeline of the NIRS data. First, the raw (intensity) data were converted to optical density. Next, a PCA filter was applied as a first pass to remove motion artifacts. The data were then low-pass filtered with a cutoff frequency of 3 Hz to remove noise and the modified Beer-Lambert law was used to determine the oxy- and deoxy-hemoglobin concentrations for each channel (DOT.data.dc output variable was used for all subsequent analyses). A more detailed description can be found in the HomER 2 Users Guide (Huppert et al., 2009). Timing information (marks for block type and time received by the ETG-4000 relative to the fNIRS recordings) was also extracted from the ETG-4000 data using custom scripts run in MATLAB R2015a.
Subsequent analyses were conducted in MATLAB (R2015a) with custom analysis scripts. The first step consisted of removing any additional motion artifacts. A custom motion detection algorithm was written following Lloyd-Fox et al. (2009). Emberson et al. (2015) includes a detailed description of this algorithm. Next, the continuous data were segmented and sorted into individual block types based on the timing of marks. Because the experiment was ended when the infant became inattentive or fussy, we excluded trials at the end of the experiment that were not presented past the mean duration of the baseline (duration of stimulus presentation + 6.5 s). At this point in the analyses, infants were included or excluded based on their looking compliance.
The number of complete trials was determined for each block type and if the infant met the inclusion criteria of watching a minimum of 3 blocks of both types (see Participants for the number of infants excluded for not watching a sufficient amount of time for each experimental condition), their data were included in the final sample. Infants in the Variable Expectation condition looked on average for 15.94 Variable blocks (SD = 4.40, range = 10–24) and 5.44 Uniform blocks (SD = 1.50, range = 3–8, 2/18 infants had 3 uniform blocks in this condition). Infants included in the control condition saw an average of 5.34 Variable blocks (SD = 1.14, range = 3–7) and 5.28 Uniform blocks (SD = 1.19, range = 3–7, 3/29 infants had only 3 blocks of any block type in this condition). Note that the frequency of Variable blocks is much higher in the Variable Expectation condition but was equal across the Uniform blocks and thus, these numbers indicate that infants looked for a similar amount of time across the two conditions (5.44 vs. 5.28 Uniform Blocks, Variable Expectation vs. Control, respectively).
Then, for each infant, the average concentration of oxygenated and deoxygenated hemoglobin per channel was determined for each condition. Infants were excluded at this point if they were missing data from multiple critical channels (“bad” channels were identified using the output of the otparserex.m script and HomER 2). Specifically, if infants were missing data from >2 temporal channels, >1 occipital channels, or any frontal channels, they were excluded from analysis. Importantly, the decision to include or exclude infants was made before group averages were determined and was not revisited in order to minimize experimenter bias. Then, the average and variance of responses for oxygenated and deoxygenated hemoglobin were determined within each ROI for each infant. A single analysis time window, 6–14.5 seconds after stimulus onset and defined a priori, was used for all block types in both experimental conditions. Six seconds post block-onset was selected as it is the time delay at which we start to consistently detect the onset of response to the current block while still being past the decline period of the previous block. This time window is consistent with previous work using similar paradigms (Emberson et al., 2017b).
For direct contrasts (e.g., comparing Variable block activation across the two experimental conditions), we ran both standard hypothesis tests (e.g., ANOVA, t-tests) and non-parametric permutation tests. For examinations of effects over time, we employed linear mixed effect modeling as reported in Kersey and Emberson (2017).
3. Results
We hypothesized that the classic repetition suppression (RS) effect would be observed in the Control condition where Variable and Uniform blocks are presented at equal frequency (i.e., greater activation for Variable vs. Repeated blocks) and that when variable blocks are more frequent in the Variable Expectation condition, the RS effect would be attenuated. To this end, we compared responses to different block types (either Uniform or Variable) both within and between experimental conditions (Control and Variable Expectation) in both the frontal and the temporal ROIs. Responses in the occipital ROI, which are not believed to be involved in the current auditory experiment, are not reported.
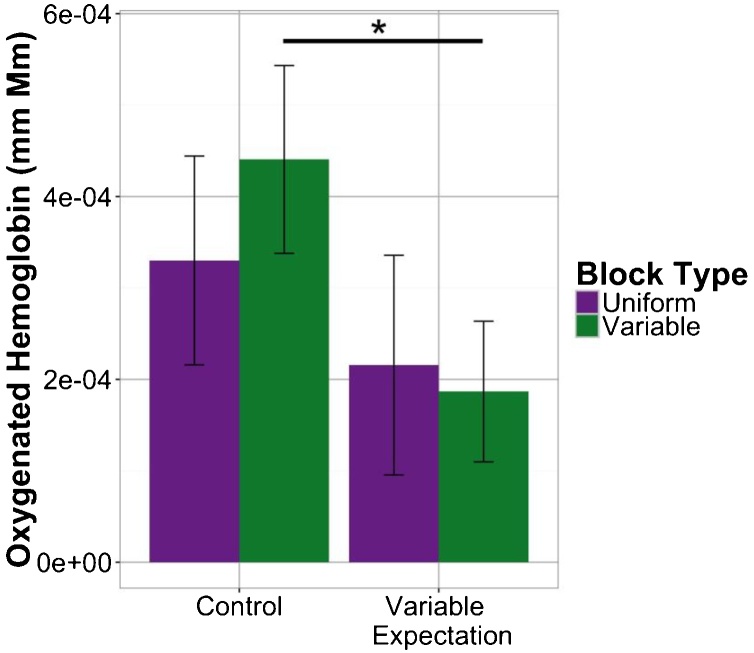
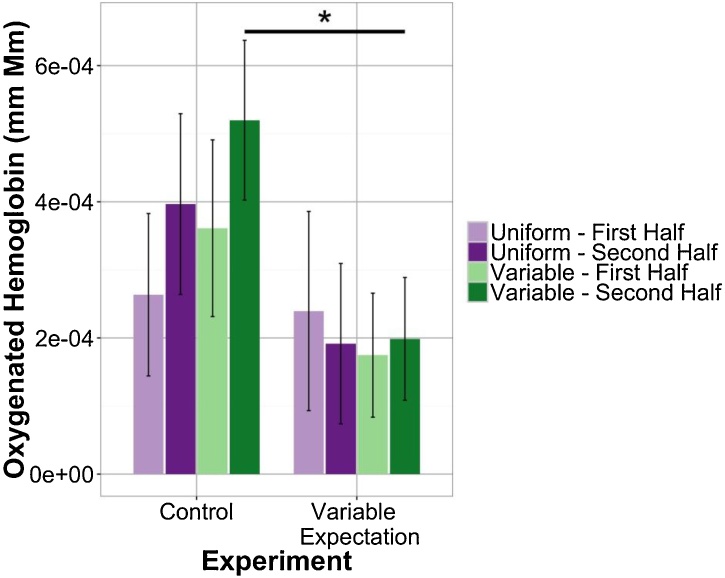
As reported in Emberson et al. (2017b) for the Control (50–50) condition, auditory RS effects were found most strongly in the frontal ROI. While numerically there was greater activation for Variable compared to Uniform blocks in this Control condition, the difference between the two block types was not significant (see Fig. 3). In the Variable Expectation (75–25) condition, there also was no evidence of RS in the frontal ROI (see Fig. 3). However, at issue is whether the RS effect in the Variable Expectation condition was less than (or even reversed compared to) the Control condition. Comparing across experimental conditions, there was a significant difference in response during Variable blocks between the two experiments (t(44.94) = 1.98, p = 0.05405; marginal for permutation test, p = 0.08446). This is consistent with our hypothesis that increased expectation for variable stimuli would result in decreases in the neural response to Variable blocks. We do not find corresponding changes in the Uniform blocks suggesting that increasing variable expectation did not increase the novelty of uniform blocks (see Fig. 3). Further, in a permutation-based 2-factor, mixed ANOVA there was a significant main effect of experiment TypeIIISS(1, 45) = 75.34, p = 0.03708; n.s. in parametric ANOVA). Overall, these results indicate that, while we may not have had enough power to observe trends in individual experimental conditions, we do see an effect of expectation that manifests in differences in frontal lobe response to variable stimulus presentation between conditions. See Supplementary Materials for an analysis equating the number of variable blocks included across the conditions (Fig. 7).
Fig. 3.
Mean response in frontal channels to different block types, uniform and variable, during both experimental conditions.
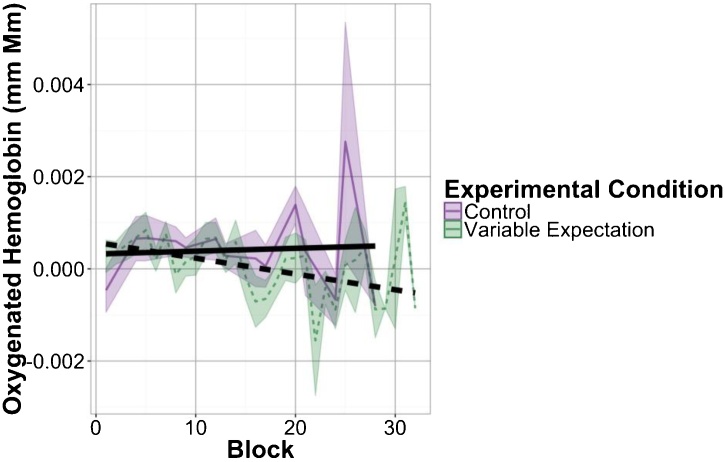
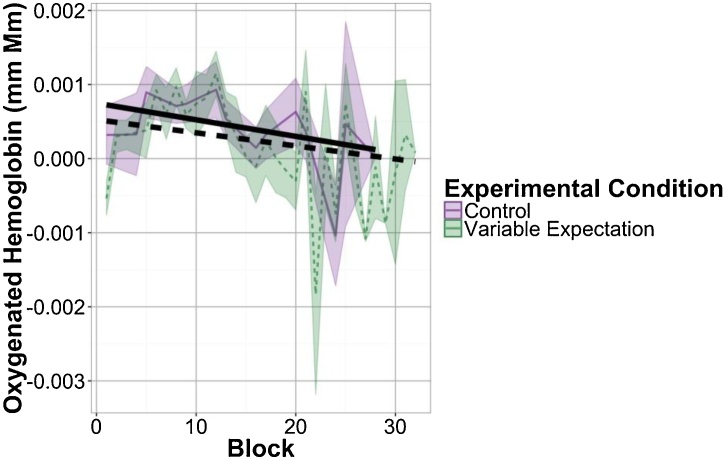
Fig. 7.
Linear fits to frontal activation during variable blocks over the course of both experiments. Colored lines show mean responses to each block with shaded regions showing standard error of the mean. Black lines show output of mixed effects models fitting all babies’ responses to individual variable blocks in each experimental condition.
Consistent with Emberson et al. (2017b), we find no strong effects in the temporal ROI (Fig. 4). Specifically, we find no significant differences across block types within experimental conditions. In other words, neural response to Variable blocks was statistically indistinguishable from response to Uniform blocks in both the Control and Variable Expectation experiments. Additionally, both block types had statistically indistinguishable responses between each experiment. There were also no main effects or interactions as measured by a 2-factor mixed ANOVA (parametric or non-parametric).
Fig. 4.
Mean response in temporal channels to different block types, uniform and variable, during both experimental conditions.
3.1. Dynamic responses to stimuli within individual blocks
To further investigate possible differences between block types, we examined within-block effects. Since it takes at least two stimulus presentations to determine whether a Uniform or Variable block is being presented, it follows that response levels would change after subsequent stimuli within a block either repeat or continue to vary. For instance, in the Variable Expectation condition, when subjects hear the first stimulus of a block they have no way of determining whether it is part of a Variable or a Uniform block. However, if this first stimulus is not repeated, their response might be attenuated given that variability is expected in this condition. Thus, we expect differences between experimental conditions and block types to increase later in the block compared to earlier in the block when block identity is clear and an infant’s expectations are able to modulate the responses to the sensory input. To explore this hypothesis, we divided the neural response from each block into two equal parts (first vs. second half). We chose this division to be as neutral as possible and to have equal amounts of data in each bin to maintain the best signal to noise ratio (e.g., comparing a very small bin very early in the block to a much longer bin later in the block conflates how much data is included in the comparison with where the data is being drawn from within a block). We also made this decision to divide the data in half once and did not revisit the decision to consider other divisions to avoid the problem of multiple comparisons. This exploratory analysis is motivated by the implications and overall conclusions from our main effects.
In each ROI (frontal, temporal), we determined the difference in response (if any) between the first and second halves of each block. These calculations were performed on each block type in each experimental condition (e.g. comparing response to the first half of variable blocks to the second half of variable blocks, all within the control condition). In addition, we assessed differences between block types and experimental condition within each half of the block (e.g., comparing Variable blocks across experimental conditions in the first half and then the second half of the block). We also performed 3-way, parametric ANOVAs to compare all portions of both trial types in both experimental conditions.
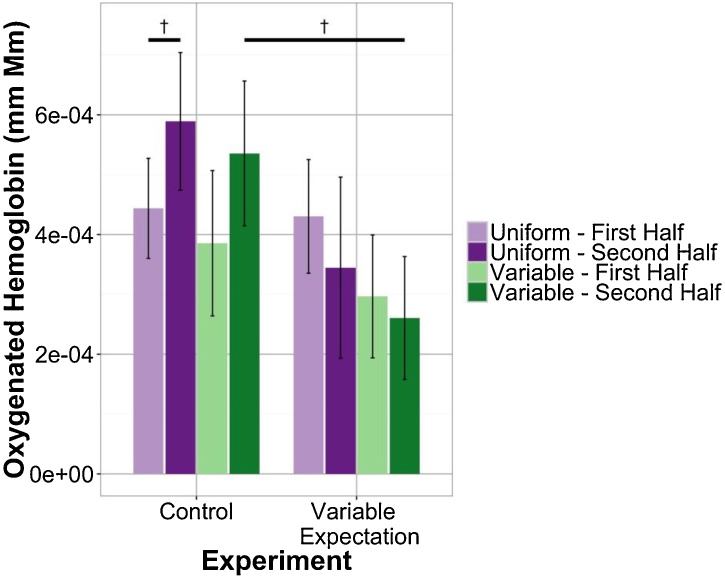
In the frontal ROI (Fig. 5), there were no significant differences in the Uniform blocks between first vs. second half or experimental condition. In contrast, we found that the neural response that we observe overall emerges during the second half of Variable blocks: The frontal response during the second half of variable blocks in the Control condition was significantly higher than in the Variable Expectation condition (t(44.99) = 2.17, p = 0.03529; permutation test p = 0.05397). Additionally, a 3-factor ANOVA shows a marginally significant interaction between experimental condition and portion of block in the frontal neural response (F(1, 176) = 3.57, p = 0.0604; permutation based TypeIIISS(1, 176) = 129.4, p = 0.07705). This result is further confirmation of the above results showing that patterns of activation in the frontal lobe are stronger in the second half vs. the first half of the blocks suggesting that these differences emerge as infants are able to confirm the block identity and compare their expectations to their sensory input.
Fig. 5.
Mean responses in frontal channels divided by portion of block. Here, each individual block’s neural signal is divided into two equal halves before further analysis is performed.
In the temporal ROI (Fig. 6), we found no significant differences. However, we found that the temporal response in the second half of Variable blocks is marginally reduced in the Variable Expectation experiment as compared to the Control experiment (t(44.68) = 1.73, p = 0.08998; n.s. in permutation test). This pattern is consistent with our hypothesis that response to Variable blocks should be lower in the Variable Expectation condition but that these effects, when present, emerge later in the block. We also find a marginally significant difference between the neural response in the first and second halves of Uniform blocks in the Control experiment (t(28) = 1.73, p = 0.09485; n.s. in permutation test). This finding suggests that the temporal response to Uniform blocks in the Control experiment increases through the course of the block. A similar pattern is observed in the frontal lobe but it didn’t reach even marginal significance and likely arises from the normal increases in activation across a block.
Fig. 6.
Mean responses in temporal channels divided by portion of block. Here, each individual block’s neural signal is divided into two equal halves before further analysis is performed.
3.2. Increases in expectation modulate neural response over the course of the experiment
In addition to the hypothesized dynamics within a single block (e.g., first vs. second half of a stimulus block) that we explored above, we also hypothesized that neural activation to blocks, in their entirety, would change over the course of the experiment. This analysis allows us to explore the possibility that there are not only overall differences in activation to Variable blocks dependent on variable expectation, but that these overall differences emerge as infants develop stronger expectations about their sensory input (i.e. over the course of the testing session). This hypothesis is best illustrated in the Variable Expectation condition. In this condition, 75% of all blocks that an infant experiences are Variable blocks (compared to 50% in the control condition). By design, as the experiment progresses infants become more familiar with this 75–25 distribution and, we postulate, learn to expect these Variable blocks, with this expectation modulating their neural responses. On the other hand, we do not expect to find this attenuation of Variable responses in the Control condition. Instead, infants should continue to show a strong response to the Variable blocks and possibly increased responses consistent with classic RS findings. Following from this, we expect that infants will show the opposite pattern over Variable blocks in the Control condition.
In order to examine the patterns of activation over the course of the experiment, we employed a technique developed by Kersey and Emberson (2017). We first calculated the average magnitude of the hemodynamic response for each infant during every block. We then used linear mixed effects models to elucidate the response to individual blocks over the course of the experiment. In this model, we include fixed effects of block number (i.e., where over the course of the experiment a given block occurred) and experimental condition (i.e., Control or Variable Expectation) as well as their interaction and a random effect of participant.
Following our findings that neural response in the Frontal ROI is modulated by expectation for variable stimuli and that this effect is specific to the Variable blocks, we examined responses to the Variable blocks in the Control and Variable Expectation conditions over the course of the experiment (Fig. 7). This analysis reveals that responses to the Variable blocks decrease in the Variable Expectation condition, t(5.92) = −2.60, p = 0.041, and remain static in the Control condition, t(26.7) = 0.17, p = 0.87. Moreover, we see a significant interaction between experimental condition and block number, t(348.6) = 2.45, p = 0.0147, in the frontal ROI response to Variable blocks. We find no such interaction in the Uniform blocks, t(232.2) = −1.15, p = 0.25. This finding is consistent with our hypothesis that these differences in response across experimental conditions emerge over the course of the experiment.
We additionally explored neural responses in the temporal ROI. Here, we see no significant main effects or interactions in neural response to the variable blocks (Fig. 8). This is consistent with the overall analyses that revealed no significant differences in temporal neural response to Variable blocks between experimental conditions. Finally, we explored response in both the frontal and temporal ROIs to uniform blocks and found no significant main effects or interactions in either region. This, too, is consistent with our previous results showing no significant differences in neural response to uniform blocks between conditions in any ROI.
Fig. 8.
Linear fits to temporal activation during variable blocks over the course of both experiments. Colored lines show mean responses across babies to each block with shaded regions showing standard error of the mean. Black lines show output of mixed effects models fitting all babies’ responses to individual variable blocks in each experimental condition.
4. Discussion
In early development, the infant brain becomes increasingly attuned to the structure of their environment. Here, we investigated whether this developmental process could occur, at least in part, by the infant brain using experience to form predictions about upcoming sensory input. To this end, we asked whether the expectation that stimuli will be variable (i.e., not repeat) will affect responses to subsequently presented variable vs. repeated stimuli. Typically, the brain exhibits larger responses to variable stimuli compared to repeated stimuli (i.e., repetition suppression or RS). However, results in adults has confirmed that regions which exhibit RS modulate responses to variability and repetition when the probability of variability in increased. Here, we find that if an infant experiences an increase in the probability of variable stimuli (from 50% variable to 75% variable), their neural responses shift and they exhibit reductions in their neural response to variability. In other words, when sensory input fulfills expectations, the infant brain exhibits an attenuated response. These findings provide evidence that prediction can modulate responses to sensory input starting very early in life.
Moreover, we conducted exploratory analyses of these effects as they unfolded over time both within a block of stimuli and over the entire experiment, uncovering additional, convergent evidence that the emergence of predictions and the comparison of predictions and sensory input are likely driving these changes in response in the frontal lobe. First, if prediction is modulating neural responses to input, this effect cannot occur starting at the beginning of the block. Specifically, whether a given block is Uniform or Variable is not disambiguated until the second stimulus of the block and predictions can either continue to be met (i.e., variable stimuli continue to be played as expected) or continue to be violated (i.e., stimuli continue to repeat) throughout the block. For these reasons, we hypothesized that any effect of prediction or expectation should be strongest in the second half of each block compared to the first half. Indeed, we found the strongest differences between experiments in the second half with reductions in the response to the Variable Expectation condition compared to the Control condition in the second half of the block. It should be noted this within-block analysis isn’t well suited to hemodynamic based methods of neuroimaging like fNIRS (also fMRI) and thus should be interpreted with caution. In particular, future work is needed to validate these within-block analyses to determine whether they reflect condition differences rather than other physiological factors. These findings provide further evidence that it is a predictive process that has resulted in the reduction of neural response to variable stimuli in the Variable Expectation condition.
While we found that the interplay between expectation and sensory input is modulated with emergence of information within a block, it is also very likely to emerge throughout the experiment. Specifically, the expectation for variable stimuli is learned by infants during the experimental session across blocks. If increases in variable expectation result in decreases in neural response to variable stimuli, we hypothesized that there should be decreases in the variable responses throughout the testing session in this group that would not be observed in the Control group. Linear mixed effects modeling confirmed these hypotheses and uncovered a significant interaction between block number (i.e., time in the experiment) and experimental condition (or context) for responses to the variable stimuli. Specifically, we find that responses to the variable block in the variable 75–25 context decrease, while the responses to the variable blocks in the control 50–50 context increase.
Thus, together, we find three convergent pieces of evidence that 6-month-old infants are able to take their experiences and form predictions to tune their neural responses (see previous paragraphs for summaries of these findings). This study expands on previous findings showing top-down, prediction effects in the visual system (Emberson et al., 2015). The present findings demonstrate that expectation can affect processing in response to auditory input as well. Interestingly, we find the effects of auditory expectation in the frontal lobe and not the temporal lobe. Finding these results in the frontal lobe, as opposed to the temporal lobe where auditory input is also processed, is consistent with previous work showing that modulation of neural responses to repetition and variability occurs most strongly in the frontal lobe as opposed to the temporal lobe and preferentially for auditory stimuli (Emberson et al., 2017b). While recent work has reported visual RS in the occipital lobe in young infants (Emberson, 2017), RS to visual stimuli is not present in the frontal lobe. It is unclear why the frontal lobe is most responsive to auditory repetition and variability at this age. Likely, this differential frontal lobe involvement is related to the early developing connectivity between the temporal and frontal lobes. Studies employing both anatomical (Dubois et al., 2009) and functional connectivity data (Sasai et al., 2011) reveal that the frontal and temporal lobes exhibit increases in connectivity in the first months of life and exhibit more connectivity than the frontal and occipital lobes. Indeed, Sasai et al. (2011) report significant decreases in fronto-occipital connectivity from birth to 6 months of life. It is possible that this early connectivity allows the temporal lobe to utilize the computational resources of the frontal lobe to respond to new patterns in the environment and to modulate neural responses in relation to an infant’s expectations.
Given this overall pattern of results across modalities and brain regions, we offer the following tentative hypothesis. RS occurs locally in brain regions that are involved in the primary processing of sensory input. As connections with other brain regions beyond these primary sensory areas increase during development, top-down effects are able to modulate the local sensory-based RS. This hypothesis accounts for the presence of visual RS in occipital cortex (Emberson et al., 2017b) and the top-down prediction effect from temporal to occipital cortex (Emberson et al., 2015). Similarly, this hypothesis accounts for the presence of auditory RS in the temporal cortex (Emberson et al., 2017a) and the top-down prediction effect observed in frontal cortex in the present findings. The absence of frontal cortex in visual RS may emerge later in development as long-range connections mature between frontal and occipital cortex. What seems clear is that frontal cortex cannot show RS effects unless there is connectivity with the sensory regions that process stimuli in a given modality.
We also find that changes across context are restricted to the Variable blocks in the Variable Expectation condition. Specifically, there are reductions in responses to Variable stimuli but not increases in responses to the now surprising Uniform stimuli. In previous findings with adults, effects of prediction or expectation appear to be driven by both a decrease in responses to variable stimuli and increases in response to uniform stimuli. These two directions of changes likely arise from both reductions of responses to expected stimuli and shifts of what types of stimuli are surprising. It appears as though infants do not exhibit either a shift in what is surprising or increases in activity due to the reduced likelihood of repetition in the variable context. Examining these possible differences between infants and adults is an important avenue of future work.
It is important to consider the relative subtly of the task manipulation. In the Control condition, infants have a 50% probability of a variable or uniform block. In the Variable Expectation condition, infants have a 75% probability of a variable block. This difference in probability of 75% vs 50% is not a large statistical difference, particularly if you consider statistical learning experiments where differences in probability are between 100% and 33% or 0% (e.g., Saffran et al., 1996; Kirkham et al., 2002). Manipulations of probability in future work could be more extreme (e.g., comparing 25% to 75% probability of variability) and might produce stronger results and potentially a modulation of temporal cortices in addition to frontal cortices.
While convergent analyses suggest that infants are able to modulate their neural responses to the overall probability of variable input (75% vs. 50% probability), it is not clear what the infant brain is learning or representing in the current paradigm. In particular, stimuli are highly variable across blocks so infants cannot learn that a particular word will be presented in the uniform blocks or that a particular sequence of stimuli are presented in the variable blocks. Thus, a higher-order or more abstract representation must be operating to produce this result. While the current study is not designed to investigate the nature of this higher-order representation of the stimuli, here we explore some interesting possibilities. Recent work has argued that 14-month-old infants (older than the current sample of 6 month olds) have a concept of “sameness” that can be abstracted and applied to many different stimuli and that they are able to employ this abstract representation in the context of a memory task (delayed matching to sample or non-matching to sample; Hochmann et al., 2016). While not the goal of the current study, one possibility is that infants, as young as 6 months, possess a concept of “variability” in line with Hochmann et al (2016). It is not clear how a concept of variability would result in the current pattern of neural findings however. Specifically, the linking hypothesis between a representation and a neural response is that when there is a strong match between the input and the representation, there is a stronger hemodynamic response. Here, we find that with more variability there are weaker hemodynamic responses. Another possibility is that the infant frontal lobe is able to track higher-order statistical properties such as volatility or change in the environment and when they are in an environment with a high degree of change, neural responses are shaped accordingly. The concept of volatility or higher-order change is represented in a higher-order elaboration of the Rescorla-Wagner model of reinforcement learning, a type of learning which relies on prediction and prediction error (Li et al., 2011). Thus, it is not clear what the frontal lobe is responding to in the current manipulation and whether the current work is evidence that infants have a concept of variability that they are able to employ to change their upcoming responses or whether they are able to track higher-order statistics about the environment that would determine the rate of change or stability and modulate neural responses accordingly. Future work comparing biased probabilities towards uniformity/repetition vs. variability/change could bear on this question.
In addition, future work could shed light on whether this ability to form predictions based on the proportion of variability is related to an infant’s emerging language abilities. The current study employed spoken words and selected words that are typically spoken to infants. Other possible stimuli include non-melodic tones or novel sounds. Spoken words were chosen to maximize infant engagement in the task as well as their ability to track changes in the input with little memory demand. We hypothesize that the same general effects would be found if non-familiar words were presented or non-language stimuli (provided they were sufficiently discriminable and interesting to infants). However, this is an important point for future investigation. Based on this assumption of the generality of the effect as well as the very early age of the infants, we recruited infants regardless of their exposure to English or bilingual status, thus infants have variable exposure to these specific exemplars of spoken words. While the question of whether prior language experience can affect these responses at this very young age is an important one and an interesting avenue for future investigation, this point is very likely to not be relevant for the current work as only 2/48 infants included in the final sample received substantial exposure to a non-English language (defined as 30% exposure). Thus, our sample is highly monolingual with substantial exposure to English. Future work will benefit from investigating these prediction effects with either non-language stimuli, a carefully selected bilingual sample, or visual stimuli.
In summary, these results support the view that young infants are developing expectations about their upcoming sensory input and using these expectations to modulate their neural responses based on whether or not this input meets their expectations. In three separate analyses of the data, we find convergent findings that as infants learn to expect variable input, their neural responses to variable input are attenuated. This is a shift in the typical pattern of repetition suppression where variable input produces a stronger response than repetitive input and suggests that even in young infants, neural responses to repetition and variability cannot be explained by exclusively bottom-up models. Moreover, this work complements previous findings demonstrating infants’ sensitivity to visual expectations and predictions in the occipital lobe and extends these findings to auditory stimuli and auditory expectations. Finding broad evidence that young infants are able to use their expectations to modulate responses based on predictions and task-context suggests that they are capable of employing top-down mechanisms to adapt to their experience during development.
Conflict of interest
None.
Acknowledgements
LLE was funded by a grant from NICHD (4R00HD076166-02) and the McDonnell foundation (AWD1005451), RNA was funded by a grant from NICHD (2R01HD037082-15A1).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.11.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013;36(3):181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Dubois J., Hertz-Pannier L., Cachia A., Mangin J.F., Le Bihan D., Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb. Cortex. 2009;19(2):414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Emberson L.L. How does experience shape early development? Considering the role of top-down mechanisms. In: Benson Janette., editor. vol. 52. Elsevier; Cambridge, MA: 2017. pp. 1–42. (Advances in Child Development and Behavior). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Boldin A.M., Riccio J.E., Guillet R., Aslin R.N. Deficits in top-down sensory prediction in infants at risk due to premature birth. Curr. Biol. 2017;27:1–6. doi: 10.1016/j.cub.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Cannon G., Palmeri H., Richards J.E., Aslin R.N. Using fNIRS to examine occipital and temporal responses to stimulus repetition in young infants: evidence of selective frontal cortex involvement. Dev. Cogn. Neurosci. 2017;23:26–38. doi: 10.1016/j.dcn.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Crosswhite S.L., Richards J.E., Aslin R.N. The lateral occipital cortex (LOC) is selective for object shape, not texture/color, at 6 months. J. Neurosci. 2017;37(13):3300–3316. doi: 10.1523/JNEUROSCI.3300-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Richards J.E., Aslin R.N. Top-down modulation in the infant brain: learning-induced expectations rapidly affect the sensory cortex at 6-months. Proc. Natl. Acad. Sci. U. S. A. 2015;112(31):9585–9590. doi: 10.1073/pnas.1510343112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2005;360(1456):815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Kushnir T., Edelman S., Avidan G., Itzchak Y., Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009;48(10):C280–C298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmann J.-R., Mody S., Carey S. Infants’ representations of same and different in match- and non-match-to-sample. Cogn. Psychol. 2016;86:87–111. doi: 10.1016/j.cogpsych.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D.J., Quinn P.C., Slater A.M., Lee K., Ge L., Pascalis O. The other-race effect develops during infancy: evidence of perceptual narrowing. Psychol. Sci. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey A.J., Emberson L.L. Tracing trajectories of audio-visual learning in the infant brain. Dev. Sci. 2017;20(6):e12480. doi: 10.1111/desc.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham N.Z., Slemmer J.A., Johnson S.P. Visual statistical learning in infancy: evidence for a domain general learning mechanism. Cognition. 2002;83(2):B35–B42. doi: 10.1016/s0010-0277(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Kouider S., Long B., Stanc L.L., Charron S., Fievet A.-c., Barbosa L.S., Gelskov S.V. Neural dynamics of prediction and surprise in infants. Nat. Commun. 2015;6:8537. doi: 10.1038/ncomms9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz D.J., Ghazanfar A.A. The emergence of multisensory systems through perceptual narrowing. Trends Cogn. Sci. 2009;13(11):470–478. doi: 10.1016/j.tics.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Li J., Schiller D., Schoenbaum G., Phelps E.A., Daw N.D. Differential roles of the human striatum and the amygdala in associative learning. Nat. Neurosci. 2011;14(10):1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Volein A., Everdell N., Elwell C.E., Johnson M.H. Social perception in infancy: a near infrared spectroscopy study. Child Dev. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Richards J.E., Blasi A., Murphy D.G.M., Elwell C.E., Johnson M.H. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1(2):025006. doi: 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWhinney B. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ, US: 1991. The CHILDES Project: Tools for Analyzing Talk. [Google Scholar]
- Marcus G.F., Fernandes K.J., Johnson S.P. Infant rule learning facilitated by speech. Psychol. Sci. 2007;18(5):387–391. doi: 10.1111/j.1467-9280.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- Saffran J.R., Aslin R.N., Newport E.L. Statistical learning by 8-month-old infants. Science. 1996;274(5294):1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Sasai S., Homae F., Watanabe H., Taga G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. NeuroImage. 2011;56(1):252–257. doi: 10.1016/j.neuroimage.2010.12.075. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Snyder A.Z., Neil J.J. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. NeuroImage. 2011;56(3):1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C., Egner T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 2009;13(9):403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Summerfield C., Trittschuh E.H., Monti J.M., Mesulam M.M., Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 2008;11(9):1004–1006. doi: 10.1038/nn.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare Ta., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J.F., Tees R.C. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behav. Dev. 1984;7(1):49–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.