Abstract
Background:
The aim of the study was to test the clinical relevance of neutralizing antibodies (NABs) in patients with cervical dystonia (CD) still responding to repeat injections with botulinum toxin type A (BoNT/A).
Methods:
Enzyme-linked immunosorbent assay (ELISA)-test evidence from a cross-sectional study on 221 CD-patients with treatment durations of between 2 and 21 years and still responding to repeat BoNT/A-injections showed the presence of antibodies against BoNT/A in 39 patients. A mouse hemi-diaphragm (MHDA) confirmation test was performed in these 39 ELISA-positive patients, and demographic (age, sex, age at onset of CD) and treatment-related (duration of treatment, mean dose of the last 10 injections, TSUI-score, patient’s subjective scoring of the treatment effect, patient’s scoring of quality of life by means of the CDQ24-questionnaire) data from these 39 patients were compared with data from ELISA-negative patients. Paralysis time, the MHDA outcome measure, was correlated with clinical data.
Results:
The ELISA-positive CD-patients had significantly higher TSUI-scores (p < 0.015), and had been treated for significant longer (p < 0.022) and with significantly higher doses (p < 0.001). Patient’s rating of BoNT/A-treatment effect and quality of life tended to be worse in ELISA-positive compared with ELISA-negative patients. The paralysis time of ELISA-positive patients was significantly correlated with the mean dose of the last 10 injections (p < 0.027) and the pain subscore of the CDQ24 (p < 0.012).
Conclusions:
Presence of NABs is clinically relevant in CD, leading to a significantly worse head position, therapy with significantly higher BoNT/A doses, and a correlation between the CDQ24 pain-subscore and antibody titers.
Keywords: cervical dystonia, clinical relevance, long-term botulinum toxin treatment, neutralizing antibodies, secondary treatment failure
Introduction
Intramuscular injections of botulinum toxin type A (BoNT/A) have become treatment of first choice for patients with cervical dystonia (CD).1 Repeat injections have to be performed to achieve permanent improvement.1,2 Therefore, the immune system of CD-patients under continuous BoNT/A-therapy is repetitively confronted with the 150 kD BoNT/A-polypeptide as well as with the much larger 600 kD BoNT/A-complex consisting of additional proteins as hemagglutinins and nonhemagglutinins.3,4 Antibodies can be induced not only against the complex proteins, but also against the BoNT/A-molecule itself,4–6 and may reduce the biological activity of BoNT/A,6,7 leading to reduced clinical efficacy [partial secondary therapy or treatment failure (PSTF)] or even a complete immunoresistance to BoNT-therapy [complete secondary therapy failure (CSTF)].7
On the other hand, there seem to be various other reasons for PSTF and CSTF. In the largest study on antibody formation in PSTF or CSTF to date, blood samples were collected between 1995 and 2000 from 65 centers across Germany from patients with ‘two unsuccessful treatments subsequent to treatments with satisfactory results’.8 A positive MHDA-test was found in only about 50% of the samples, confirming the presence of neutralizing antibodies (NABs). The authors therefore suggested that NABs were responsible for PSTF or CSTF in only about 50% of patients with PSTF or CSTF, and raised the question whether the analysis of NABs ‘is much ado about nothing’.8 This appears to be supported by a meta-analysis and systematic review on NABs and BoNT therapy,9 which also reports that ‘about half of the patients with secondary nonresponse do not have NABs’.9
But both these papers struggle with paucity and heterogeneity of the underlying clinical data.9 After comparison of the clinical and antibody status it is only mentioned that NAB prevalence is different in different patient groups,8,9 and that patients having received a cumulative dose of more than 6000 MU onabotulinum toxin have a higher risk of developing NABs.8
Thus, the clinical relevance of NABs still remains fairly unclear. Therefore, the present cross-sectional monocentric study on 221 still-responding, long-term BoNT/A-treated CD-patients was performed. Patients were recruited within 4 months, investigated clinically in detail, and blood samples were analyzed for the presence of antibodies all together at the same time. Thus, a detailed comparison between clinical and antibody status has become available, demonstrating for the first time a significant correlation between individual clinical findings and NAB titers.
Patients and methods
Patients and treatment-related data
All patients with idiopathic CD who had been treated at the botulinum toxin out-patient clinic of the Department of Neurology of the University of Düsseldorf (Germany) with BoNT-injections at least 10 times every 3–4 months (without disruption for the past 2–3 years) and who still experienced a treatment effect were asked to participate in the present study. Patients who did not have a subjective benefit were excluded from the study. A total of 221 patients agreed to participate and gave their written informed consent. A general approval from the local ethics committee allows us to take blood samples and publish anonymized clinical data and results of antibody testing of patients who have given informed consent (ethics committee number: 4085). The study was performed according to the Declaration of Helsinki.
Patients underwent a clinical examination just before blood samples were taken for NAB determination. Serum was separated by centrifugation and immediately frozen. Besides demographic data (age, gender, body weight, age at onset of CD), treatment-related data (duration of treatment, scoring of the severity of CD by means of the TSUI score)10 were determined by the attending physician. Patient’s subjective impression of the remaining severity of CD at time of the examination compared with severity of CD just before onset of BoNT-therapy was rated on a visual analogue scale (VAS 0–100; 0 = no more symptoms, 100 = CD severity just as bad as before start of BoNT-therapy). Furthermore, patients were asked to rate their quality of life using the well-established CDQ24-questionnaire.11
Patients had been treated with abobotulinumtoxinA (aboBoNT/A), onabotulinumtoxinA (onaBoNT/A), incobotulinumtoxinA (incoBoNT/A), or rimabotulinumtoxinB (rimaBoNT/B). To allow comparison, doses were transformed to ‘unified dose units’ (uDU): because most of the patients had been treated with aboBoNT/A, doses of onaBoNT/A and incoBoNT/A were multiplied by 4, doses of rimaBoNT/B were divided by 10, and aboBoNT/A-doses remained unchanged. These ratios have been used previously.12 The mean of the unified doses of the last 10 injections was used for data analysis.
Determination of antibody status
After all patients had been examined, all serum samples were sent off altogether. Samples were first sent to BioProof® AG (Munich, Germany) to determine the presence of antibodies using enzyme-linked immunosorbent assay (ELISA)-testing (fluoroimmunoassay). Thereafter, neutralizing antibody titers of ELISA-positive samples were determined by means of the mouse hemidiaphragm assay (MHDA) by Toxogen® GmbH (Hannover, Germany). Both laboratories received only coded samples and were blind to any clinical information except the time the samples were taken. ELISA-tests could be performed on 212 samples; 9 samples were either lost or spilt.
Statistical analysis
Data analysis was based on the 212 patients with known antibody status with stratification into the following subgroups: group I contained all ELISA-negative patients (n = 173), group II all ELISA-positive patients (n = 39). This group contained 8 MHDA-negative patients and 31 MHDA-positive patients; however, paralysis times of the 8 MHDA-negative patients were close to the threshold level of 2.31 mU/ml set by the Toxogen® laboratory.
All statistical analyses were carried out with the commercially available SPSS-package (version 23: IBM, Armonk, USA). After an ANOVA had yielded differences among subgroups in a first step, comparisons between subgroups were then performed nonparametrically using the Kendall tau B test. Results were confirmed by t test. Group size and parameters used always allowed the use of the t test. Both nonparametric and parametric testing yielded the same significant results (with slightly different levels of significance). The Pearson correlation coefficient was used for correlation analysis.
Results
An ANOVA revealed a significant group effect for both the demographic as well as treatment-related data.
Comparison of demographic data in ELISA-positive and ELISA-negative patients
In Table 1 (columns 3–6) demographical data of the Elisa-negative and the Elisa-positive subgroups and the entire cohort were presented. No significant difference between demographical data of the Elisa-negative and the Elisa-positive patients could be detected with only one exception. Because of a similar age at onset of CD, but a significant longer duration of treatment (p < 0.022), the mean age of the Elisa-positive patients was significantly higher (p < 0.018) than the mean age of the Elisa-negative patients (Table 1).
Table 1.
Demographical data as well as treatment related data of 212 long-term treated CD-patients in whom results of antibody testing were available.
| n = | Age (years) | Sex (f/m) | Weight (kg) | Onset of CD (years) | Duration of therapy (years) | TSUI-score | PSSTE subj.-score (VAS: 0–100) | CDQ24 total score | Dosis (uDU) 1:4:10 Bot:Dys:Neuro | |
|---|---|---|---|---|---|---|---|---|---|---|
| Group I ELISA-negative |
173 | MV: 59.8 SD: 12.0 |
102/71 | MV: 75.5 SD: 18.3 |
MV: 42.8 SD: 11.2 |
MV: 11.2 SD: 5.5 |
MV: 4.8 SD: 3.2 |
MV: 46.3 SD: 27.3 |
MV: 21.78 SD: 16.99 |
MV: 761 SD: 177 |
| Group II ELISA-positive |
39 | MV: 64.6 SD: 9.7 |
26/13 | MV: 74.4 SD: 15.4 |
MV: 44.1 SD: 10.8 |
MV: 13.5 SD: 4.2 |
MV: 6.1 SD: 3.7 |
MV: 48.3 SD: 29.8 |
MV: 22.49 SD: 18.70 |
MV: 850 SD: 164 |
| Entire cohort |
212 | MV: 61.0 SD: 11.8 |
128/84 | MV: 75.2 SD: 17.9 |
MV: 43.1 SD: 11.1 |
MV: 11.7 SD: 5.3 |
MV: 4.9 SD: 3.3 |
MV: 46.6 SD: 27.9 |
MV: 21.91 SD: 17.27 |
MV: 764 SD: 170 |
| Significance (I against II) |
0.018 | 0.114 n.s. |
0.76 n.s. |
0.532 n.s. |
0.022 | 0.015 | 0.696 n.s. |
0.534 n.s. |
0.001 |
Group I (ELISA-negative patients), Group II (ELISA-positive patients) and all patients (entire cohort). For subgroup definition see methods.
CD, cervical dystonia; ELISA, enzyme-linked immunosorbent assay; MV, mean value; PSSTE, severity of CD and associated treatment effect as scored by patients; SD, standard deviation.
Comparison of treatment related data in Elisa-positive and Elisa-negative patients
Table 1 also presents treatment-related data and clinical outcome measures (columns 7–10). Severity of CD was scored by the treating physician just before the blood samples for the determination of antibodies were taken. TSUI-score was significantly (p < 0.015) larger in ELISA-positive (MV: 6.1, SD: 3.7) than in Elisa-negative (MV: 4.8, SD: 3.2) patients (Figure 1a).
Figure 1.
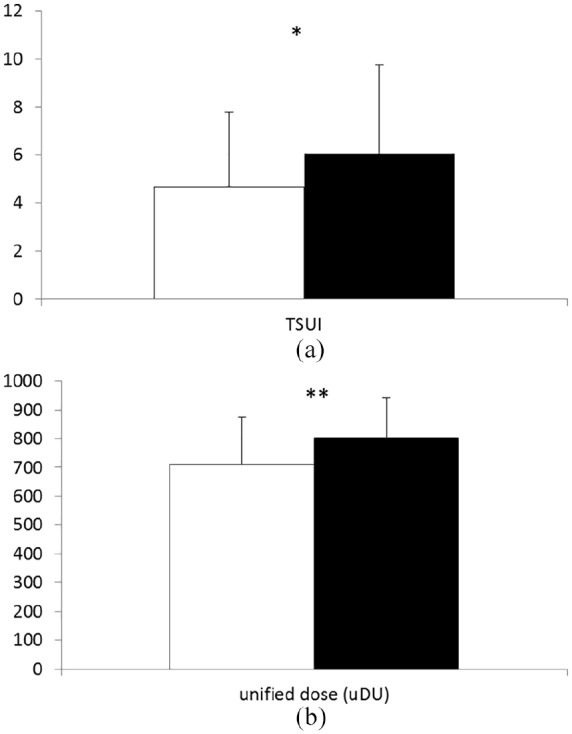
(a) Comparison of the clinical outcome (estimated by means of TSUI-score) in ELISA-negative (open bar) and ELISA-positive patients. The difference is significant (p < 0.015). (b) Comparison of the mean unified dose used for the treatment of ELISA-negative (open bar) and ELISA-positive patients. The difference is highly significant (p < 0.001).
ELISA, enzyme-linked immunosorbent assay.
When the remaining severity of CD, and associated treatment effect, was scored by the patients (PSSTE), no significant difference was found for PSSTE between ELISA-positive (MV: 48.3, SD: 29.8) and ELISA-negative patients (MV: 46.3, SD: 27.3). When patients scored their quality of life by means of the standardized CDQ24-questionnaire, also no significant difference was found between ELISA-positive (MV: 22.49, SD: 18.70) and ELISA-negative patients (MV: 21.78, SD: 16.99).
However, when the mean values of the doses of the last 10 BoNT-injections (Figure 1B) were compared, a highly significant (p < 0.001) difference was found between ELISA-positive (MV: 850 uDU, SD: 164 uDU) and ELISA-negative (MV: 761 uDU, SD: 177 uDU) patients.
Correlation of paralysis time of the MHDA and clinical data in ELISA-positive patients
For all 39 ELISA-positive patients, a MHDA-confirmation test was performed. In eight patients, the NAB-titer was just below 2.31 mU/ml, and, therefore, classified as not significant and negative. Nevertheless, the paralysis time of the MHDA-test for these eight patients was also available for correlation analysis with clinical data. The correlation between paralysis time of the MHDA-test and the mean unified dose of the last 10 injections (Figure 2a; r = 0.373; p < 0.027), as well as the pain subscore of the CDQ24 (Figure 2b; r = 0.398; p < 0.012), was significant. It is obvious from Figure 2(a,b) that, in the MHDA, 140 mins was the upper limit of the paralysis time. With expansion of the observation time, the correlations would probably have been even better.
Figure 2.
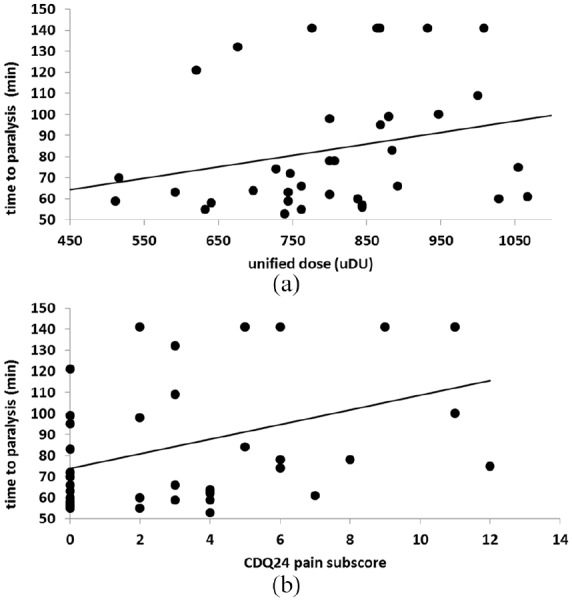
(a) Correlation of the unified dosis (mean dose of the last 10 injections) used for the treatment of the ELISA-positive patients (abscissa) with the paralysis time measured in the MHDA (ordinate). The correlation is significant (p < 0.027), patients with longer paralysis times were treated with higher doses. (b) Correlation of the pain subscore of the CDQ24 questionnaire of the ELISA-positive patients (abscissa) with the paralysis time measured in the MHD-assay (ordinate). The correlation is significant (p < 0.012), patients with longer paralysis times suffered from more intensive pain.
ELISA, enzyme-linked immunosorbent assay; MHDA, mouse hemi-diaphragm assay.
For other clinical data, a positive trend was found (TSUI-score: r = 0.310, n.s.; PSSTE: r = 0.284, n.s.; total CDQ24: r = 0.250, n.s.), which, however, did not reach the level of significance (p = 0.05). With prolongation of the MHDA observation time, these correlations might have reached the level of significance.
Discussion
General remarks on the clinical relevance of NABs in BoNT/A therapy
Soon after the licensing of ona- and abobotulinum toxin in the US and Europe, with subsequent broad clinical use of BoNT/A, it became obvious that a fairly large percentage of continuously treated CD-patients had developed resistance to botulinum toxin.5 This clinical experience has led, on the one hand, to the development of new BoNT/A preparations with much lower protein contents and a significant reduction of antibody rates.3,13–17 On the other hand, avoidance of booster injections, short intervals between injections, and high doses was strongly recommended.18 Both factors have led to the low incidences of antibody formation of between 0.5% and 3% reported nowadays.19 Therefore, management of BoNT-therapy is strongly influenced by the risk of inducing NABs.
This risk of NAB formation is still underestimated. In most studies presenting detailed information on antibodies, the duration of treatment does not exceed a few years. This implies that reported antibody rates estimate incidence of antibody formation rather than prevalence in long-term treated patients. The longer the duration of treatment, and the more patients are tested for the presence of NABs, the higher the NAB-rates reported.12,19–23
Relevance of NABs in secondary treatment failure
It has to be kept in mind that injection of BoNT in principle is a vaccination process. Because of the size of the BoNT-polypeptide, and the BoNT-complex, and the traumatic mode of application, induction of NABs cannot be avoided. Thus the remaining question is not whether antibodies are present, but whether clinically relevant titers are induced, and whether the NAB-test procedures are sensitive enough to detect clinically relevant titers. Instead of suggesting that analysis of NABs in PSTF or CSTF is ‘much ado about nothing’,8 and that ‘although NABs play a role in secondary treatment failure with BoNT/A it is not the main cause in about half of our patients, and the influence of other factors need to be investigated’,8 and to say that ‘about half of the patients with secondary non-response do not have NABs’,9 our perspective is that, in about 50% of patients with PSTF or CSTF, NAB-titers are below significance or the detection limit of the MHDA or mouse lethality assay (MLA).
Furthermore, it has to be taken into account that in the largest study on PSTF and CSTF to date,8 blood samples were collected over 6 years from 65 centers across Germany from patients who had been classified as secondary nonresponders to BoNT/A by their treating physician; the criterion for secondary treatment failure (STF) in this study was ‘at least two unsuccessful treatments subsequent to treatments with satisfactory results’,8 without any specification of what an unsuccessful treatment or a satisfactory result is. Depression and negative experience in social life may influence the success of BoNT treatment and patient satisfaction considerably.24 Therefore any criterion on PSTF or CSTF should not be based solely on the patient’s experience.
Relevance of NABs in still-responding CD-patients
Published data are consistent with the results of the present study clearly demonstrating that no significant difference was found between patient rating of treatment effect and quality of life in ELISA-positive and ELISA-negative patients (Table 1, columns 9 and 10). Significant differences were found in the scoring of CD severity by means of the TSUI-score, as determined by the treating physician, and the doses being documented in the course of BoNT-therapy (Table 1, columns 8 and 11).
Increase of dose to maintain clinical efficacy is a red flag for the presence of antibodies. Interestingly, in most of studies on long-term treatment of CD, an increase of dose with duration of therapy is reported.22,23 This is a clear hint that, also in other cohorts of CD-patients than ours, a considerable number of patients are going to develop PSTF.
Higher NAB-titers go along with higher pain subscores of the CDQ24. Therefore, a patient’s remark on pain should be documented whether or not there is a change in the character and intensity of pain reported by the patient. Thus, pain can be used as a third clinical hint for the presence of NABs in CD, in addition to severity of CD and the dose per session.
In patients who have been treated for more than 15 years, the treating physician should be aware that induction of relevant NAB-titers has to expected in at least 15% (probably up to 30%) of patients.12,25 Duration of therapy is a risk factor for NAB-induction (see Table 1, column 7). The problem is that, in most BoNT out-patient clinics, the treating physician changes during long-term treatment. Furthermore, neither the patient nor the treating physician will remember the severity of CD, including pain symptoms and doses used previously, except when these parameters have carefully been documented.
When severity of CD was compared between CD patients who had developed PSTF later during the course of BoNT-therapy and those CD patients who had not developed PSTF, a significant difference in outcome measured by means of the TSUI-score was detectable already after the second injection.26 This early reduction in efficacy of BoNT-injections indicates that NABs are induced early during the course of BoNT-treatment in CD. If a patient develops NABs early in the course of BoNT treatment, this patient and their treating physician become used to this reduced response behavior, and will hardly realize that this still-responding patient has already developed NABs, and initiate NAB testing.
Correlation of clinical data and MHDA test results
To emphasize the impact of antibody status on clinical presentation, the outcome measure of the MHDA (paralysis time) was correlated with clinical data. The MHDA is a complex assay with high sensitivity and specificity for the presence of NAB against BoNT/A in a blood sample. Stimulation of the phrenic nerve causes contractions of the mouse diaphragm in the test bath. When botulinum toxin is added to the bath, the time during which diaphragm contractions can be observed is reduced. When a blood sample with NABs is added to the bath prior to BoNT application, the time during which contractions can be observed depends on the NAB-titer neutralizing the BoNT effect. Therefore, the MHDA paralysis time measures NAB-titers (for details see Göschel and colleagues).6 Paralysis time was available not only for the 31 MHDA-positive patients but also for the 8 ELISA-positive patients with a titer just below the Toxogen® laboratory-defined threshold of 2.31 mU/ml.
There was a significant correlation between the mean dose of the last 10 injections and paralysis time (p < 0.027). This result by far extends the previous observation that the prevalence of NABs was higher in patients having received a cumulative dose of abobotulinum toxin of more than 6000 MUs compared with patients having received less than 6000 MUs,8 and is in line with the fact that the probability of NAB-induction increases with duration of treatment and dose per session in still-responding patients.12,26
But, a correlation between the paralysis time and the pain subscore of the CDQ24 was also detected. Pain is a highly relevant factor in the quality of life of CD-patients. This has been demonstrated in studies, not only in the short term,27 but also in long-term treated CD patients.28 Thus, NABs also have an impact on the quality of life of long-term BoNT-treated CD patients. Therefore, the development of high antibody titers should be avoided.
Conclusions and clinical implications
The present study demonstrates that NABs reduce efficacy of BoNT injections to a clinically relevant extent, and that higher paralysis times and, correspondingly, higher NAB titers go along with higher doses, indicating that the use of higher doses boosts NAB titers. Furthermore, NABs have an impact on patient’s quality of life.
Unfortunately, there is no clear-cut strategy to follow when induction of NABs has been proven. In previous years, it has been advised to stop therapy in case of antibody-induced treatment failure.29 Meanwhile, deep brain stimulation is an alternative for severely affected patients with poor response to BoNT. But for CD-patients with proven NABs who still respond to BoNT, the situation is more difficult. Continuous increase in dose, on the one hand, may lead to a transient improvement for a few injections, but, in the end, this approach will further booster the NAB titer. It could be demonstrated that NAB-titers developed under complex protein containing BoNT/A- preparations will decline under continuous treatment with the complex-protein-free inco-BoNT/A preparation.30 However, whether this decline in antibody titer is accompanied by a clinical improvement in parallel has not been demonstrated so far.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study has kindly been supported by two restricted grants of the Inge-Diesbach Stiftung (Weinheim, Germany) to H.H. Costs of the ELISA-tests and the mouse hemidiaphragm assay were covered by Merz/Pharmaceuticals (Frankfurt, Germany).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Harald Hefter  https://orcid.org/0000-0003-1069-7684
https://orcid.org/0000-0003-1069-7684
Contributor Information
Harald Hefter, Department of Neurology, Heinrich-Heine-Universitat Dusseldorf, Moorenstrasse 5, Düsseldorf, 40225, Germany.
Dietmar Rosenthal, Department of Neurology, Heinrich-Heine-Universitat Dusseldorf, Düsseldorf, Germany.
Hans Bigalke, toxogen GmbH, Hannover, Germany.
Marek Moll, Department of Neurology, Heinrich-Heine-Universitat Dusseldorf, Düsseldorf, Germany.
References
- 1. Simpson DM, Blitzer A, Brashear A, et al. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review). Report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology 2008; 70: 1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 2004; 75: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D 2010; 10: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 2010: 4: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord 1994; 9: 213–217. [DOI] [PubMed] [Google Scholar]
- 6. Göschel H, Wohlfarth K, Frevert J, et al. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies – therapeutic consequences. Exp Neurol 1997; 147: 96–102. [DOI] [PubMed] [Google Scholar]
- 7. Dressler D, Hallett M. Immunological aspects of Botox®, Dysport® and Myobloc™/NeuroBloc®. Eur J Neurol 2006; 13: 11–15. [DOI] [PubMed] [Google Scholar]
- 8. Lange O, Bigalke H, Dengler R, et al. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol 2009; 32: 213–218. [DOI] [PubMed] [Google Scholar]
- 9. Fabbri M, Leodori, Fernandes RM, et al. Neutralizing antibody and botulinum toxin therapy: a systematic review and meta-analysis. Neurotox Res 2016; 29: 105–117. [DOI] [PubMed] [Google Scholar]
- 10. Tsui JK, Eisen A, Stoessl AJ, et al. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet 1986; 2: 245–247. [DOI] [PubMed] [Google Scholar]
- 11. Müller J, Wissel J, Kemmler G, et al. Craniocervical dystonia questionnaire (CDQ-24): development and validation of a disease-specific quality of life instrument. J Neurol Neursurg Psychiatry 2004; 75: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hefter H, Rosenthal D, Moll M. High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Move Disord Clin Prac 2016; 3: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brin MF, Comella CL, Jankovic J, et al. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord 2008; 23: 1353–1360. [DOI] [PubMed] [Google Scholar]
- 14. Truong D, Brodsky M, Lew M, et al. Long-term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord 2010; 16: 316–323. [DOI] [PubMed] [Google Scholar]
- 15. Benecke R, Jost WH, Kanovsky P, et al. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005; 64: 1949–1951. [DOI] [PubMed] [Google Scholar]
- 16. Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (Xeomin®) in focal dystonia. Drugs 2007; 67: 669–683. [DOI] [PubMed] [Google Scholar]
- 17. Comella CL, Jankovic J, Truong DD, et al. Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®), botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci 2011; 308: 103–109. [DOI] [PubMed] [Google Scholar]
- 18. Moore P, Naumann M. General and clinical aspects of treatment with botulinum toxin. In: Moore P, Naumann M. (eds) Handbook of botulinum toxin treatment. 2nd ed. Malden, MA: Blackwell Science, 2003, pp. 28–75. [Google Scholar]
- 19. Naumann M, Boo LM, Ackerman AH, et al. Immunogenicity of botulinum toxins. J Neural Transm 2013; 120: 275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kessler KR, Skutta M, Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. J Neurol 1999; 246: 265–274. [DOI] [PubMed] [Google Scholar]
- 21. Kranz G, Sycha T, Voller B, et al. Neutralizing antibodies in dystonic patients who still respond well to botulinum toxin type A. Neurology 2008; 70: 133–136. [DOI] [PubMed] [Google Scholar]
- 22. Mohammadi B, Buhr N, Bigalke H, et al. A long-term follow-up of botulinum toxin A in cervical dystonia. Neurol Res 2009; 31: 463–466. [DOI] [PubMed] [Google Scholar]
- 23. Mejia NI, Vuong KD, Jankovic J. Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov Disord 2005; 20: 592–597. [DOI] [PubMed] [Google Scholar]
- 24. Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Economics 2012; 15: 419–423. [DOI] [PubMed] [Google Scholar]
- 25. Hefter H, Jansen A, Moll M, et al. High prevalence of neutralizing antibodies in BoNT/A long-term treated patients with focal dystonia and spasticity. Toxicon 2016; 123(Suppl.): S38–S39. [Google Scholar]
- 26. Hefter H, Spiess C, Rosenthal D. Very early reduction in efficacy of botulinum toxin therapy for cervical dystonia in patients with subsequent secondary treatment failure – a retrospective analysis. J Neural Transm 2014; 121: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hefter H, Benecke R, Erbguth F, et al. An open-label cohort study of the improvement of quality of life and pain in de novo cervical dystonia patients after injections with 500 U botulinum toxin A (Dysport). BMJ Open 2013; 3: e0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moll M, Rosenthal D, Hefter H. Quality of life in long-term botulinum toxin treatment of cervical dystonia: results of a cross sectional study. Parkinsonism Relat Disord 2018; 57: 63–67. [DOI] [PubMed] [Google Scholar]
- 29. Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord 2004; 19(Suppl. 8): S92–S100. [DOI] [PubMed] [Google Scholar]
- 30. Hefter H, Hartmann C, Kahlen U, et al. Prospective analysis of neutralising antibody titres in secondary non-responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins – a single cohort 4-year follow-up study. BMJ Open 2012; 2. pii: e000646. [DOI] [PMC free article] [PubMed] [Google Scholar]


