Abstract
Approximately 50% of HER2 positive breast cancer cases are also estrogen receptor (ER) positive. Data supports a role for close cross-talk between the ER and HER2 signaling pathways as an important contributor to the development of de novo or acquired resistance to hormone therapies. Therefore a strategy that simultaneously blocks both signaling pathways is a reasonable approach to prevent or overcome either endocrine or anti-HER2 therapy resistance. Moreover, preclinical data support the idea that PI3K inhibitors and CDK4/6 could be an attractive target that functions downstream of both ER and HER2 pathways. We conducted a literature review of the results of phase II and III studies testing targeted therapies in metastatic breast cancer with HER2-positive and hormonal-receptor-positive disease. The analyses included efficacy and toxicity data from earlier studies with a single anti-HER2 drug combined with hormonal therapy up to more recent studies testing new molecules targeting these signaling pathways. The aims of this review are to summarize current knowledge and to discuss research development including the possibility to spare chemotherapy in this subgroup of HER2-positive breast cancer patients.
Keywords: breast cancer, HER2+ breast cancer, hormone-receptor-positive breast cancer, metastasis, molecular oncology
Introduction
The human epidermal growth factor receptor 2 (HER2) is a member of the membrane tyrosine kinase receptor family (i.e. HER1-4), amplified or overexpressed in 20–25% of breast cancers.1
Unlike other receptors of the family, HER-2 has unique receptor features: it binds no known specific ligand, but seems to exert its biological activity by serving as a preferential partner for the other HER receptors. Indeed, it forms heterodimers with other members of the erbB family after binding to their specific ligands, thus enhancing and prolonging cell signaling.2–4 Interestingly, overexpression of HER-2 also induces its spontaneous homodimerization, thereby leading to activation of the tyrosine kinase moiety of the intracytoplasmic domain without the need for a ligand.5
HER2 positivity is correlated with aggressive behavior and reduced progression-free survival (PFS) and overall survival (OS).6–8 HER2 is also a treatment target, predictive of response of anti-HER2 therapy. The efficacy of anti-HER2 drugs has been confirmed in several settings (adjuvant, neoadjuvant and metastatic) and the use of anti HER2 drugs has dramatically modified the course of the disease.9
Approximately 50% of HER2-positive breast cancers are also estrogen receptor (ER)-positive, dividing HER2 positive patients into two main subgroups (ER-negative plus HER2-positive, and ER-positive and HER2-positive) with different patterns of growth and response to treatment.10
For the nonendocrine-responsive and HER2-positive disease, the treatment strategy includes the use of chemotherapy combined with anti-HER2 drugs. In the subgroup of HER2- and ER-positive metastatic breast cancer (MBC), different strategies are being investigated.
Several lines of data support a role for close crosstalk between the ER and HER2 signaling pathways (Figure 1). In the preclinical setting, it has been shown that the expression of ER and its downstream targets is increased in cells with acquired resistance to anti-HER2 therapy.11,12 Reactivation of ER expression and signaling, including a switch from ER-negative to ER-positive status, were observed in clinical HER2-positive tumors after neoadjuvant lapatinib treatment.12 These results suggest the significance of ER expression and signaling as an alternative survival mechanism in these tumors.
Figure 1.
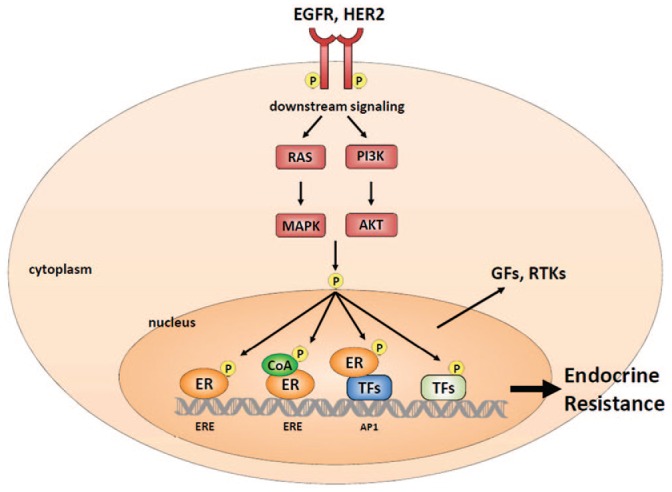
Crosstalk between ER and HER family receptors.
ER, estrogen receptor; HER human epidermal growth factor receptor.
Moreover, this crosstalk contributes to the development of both intrinsic and acquired resistance to hormonal treatment.12–16 HER2 overexpression or amplification affects endocrine therapy responsiveness both to tamoxifen and to estrogen deprivation by aromatase inhibitors (AI).17,18 Indeed, in neoadjuvant therapy, ER- and HER2-positive breast cancer patients treated with letrozole or tamoxifen, showed less Ki67 suppression when compared with ER-positive and HER2-negative patients, despite similar short-term clinical efficacy.19 High levels of HER signaling, working via the PI3K and MAPK pathways, can reduce ER levels. Moreover, activation of the pathway by posttranslational modifications (e.g. phosphorylation) of ER, can contribute to the reduction of tumor estrogen-dependency; consequently, the receptor could be activated in the absence of estrogen.13
Understanding of the intrinsic link between the ER and HER2 leads to the development of a strategy that simultaneously blocks both signaling pathways. This is a reasonable approach to prevent or overcome either endocrine or anti-HER2 therapy resistance in some ER- and HER2-positive breast cancer patients.
We conducted a review of the results of phase II and III studies testing targeted therapies in HER2 positive, hormonal-receptor-positive MBC. Studies were identified by a computerized search on PubMed using the following text words: ‘breast cancer and (c-erbB-2 or c-erbB2 or Neu or HER2).’
To be included in the analysis, retrieved studies had to fulfill the following simple inclusion criteria: advanced or metastatic breast cancer, endocrine therapy (any line of treatment), and evaluation of HER-2 expression (any method). The analyses included the efficacy and toxicity data of the studies. The future development of this strategy for this subtype of breast cancer are also discussed.
Studies of hormonal therapy with single HER2 blockade in metastatic breast cancer patients
To prevent or overcome either endocrine or anti-HER2 therapy resistance in ER- and HER2-positive metastatic breast cancer patients, some studies have valuated the possibility of treating with endocrine therapy (i.e. AI) and a single anti HER2 drug. These studies are summarized in Table 1.
Table 1.
Studies of hormonal therapy with single HER2 blockade in metastatic breast cancer patients.
| Trial | No of patients | Phase study | Study end point | Treatment arm | Outcome |
|---|---|---|---|---|---|
| TAnDEM | 207 | Randomized III | PFS | Anastrozole plus trastuzumab or placebo | PFS 4.8 months versus 2.4 months p 0.0016 median OS 28.5 months versus 23.9 months |
| Study of Marcom. | 31 | II | ORR | Letrozole and trastuzumab | ORR 26% OS non reported |
| eLEcTRA | 57 | Randomized III | TTP | Letrozole alone or letrozole plus trastuzumab | TTP3.3 months versus 14.1 months p 0.23 OS non reported |
| Study of Johnston | 219 | Randomized III | PFS | Letrozole plus lapatinib or placebo | PFS 8.2 months versus 3.0 months p 0.019 median OS was 33.3 months versus 32.3 months |
| MINT | 359 | Randomized II | PFS | anastrozole plus AZD8931 20 mg bid anastrozole plus AZD8931 40 mg bid anastrozole plus placebo |
PFS 10.9 months p 0.135 13.8 months p 0.485 14.0 months 16, 20, and 12 deaths had occurred in the AZD8931 20, 40 mg, and placebo groups, respectively. |
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
Trastuzumab is a humanized monoclonal antibody that recognizes the extracellular domain of HER2, and was the first anti-HER2 drug developed in a clinical setting. The development of trastuzumab as a HER2-targeting agent represented a paradigm shift in breast cancer from nonspecific chemotherapy to a molecular targeted approach.
In the TAnDEM trial, a randomized phase III study, Kauffman and colleagues compared the PFS in the trastuzumab plus anastrozole arm versus the anastrozole alone arm in MBC with HER2-positive and endocrine-responsive disease. Previous treatment with tamoxifen as adjuvant or hormonal therapy for MBC or with anastrozole, if begun up to 4 weeks before random assignment, was permitted. Prior chemotherapy for MBC or adjuvant chemotherapy within 6 months was not permitted.
The authors reported that the 103 patients in the trastuzumab plus anastrozole arm experienced significantly improved PFS, with a median PFS of 4.8 months (95% CI, 3.7–7.0 months) versus 2.4 months (95% CI, 2.0–4.6 months) in the anastrozole alone arm (104 patients).20 The results of TAnDEM trial are consistent with those from a phase II study assessing letrozole plus trastuzumab in 31 evaluable postmenopausal women with HER2- and ER-positive advanced breast cancer. This trial showed an objective response rate of 26% and a clinical benefit rate (CBR) of 52%. The median time to progression (TTP) was 5.8 months and the median duration of response was 20.6 months, suggesting that durable responses can be seen with combined hormonal and anti-HER2 therapy.21
The phase III eLEcTRA trial investigated the efficacy and safety of trastuzumab plus letrozole as first-line treatment in 57 HER2-positive and hormone-receptor-positive MBC. Median TTP increased from 3.3 months with letrozole to 14.1 months with trastuzumab plus letrozole; however, this was not statistically significant (HR, 0.67; 95% CI, 0.35–1.29; p = 0.23).22
The combination of trastuzumab and AI improved the outcome compared with hormonal therapy alone, suggesting that the use of HER2-targeted therapy with an AI can substantially delay chemotherapy in some patients who experience clinical benefit.
A different therapeutic approach to inhibiting HER2-mediated signaling is to use lapatinib, a reversible small molecule tyrosine kinase inhibitor targeting the HER2 and EGFR intracellular kinase domain.23,24 In fact, the endocrine resistance development could also be involved the EGFR pathway. Two randomized trials in ER-positive MBC suggested that gefitinib, a EGFR tyrosine kinase inhibitor, may improve PFS when added to endocrine therapy with either tamoxifen or anastrozole.25,26
In a phase III trial, the combination of letrozole plus lapatinib with letrozole plus placebo was compared as first-line treatment in patients with ER-positive MBC.27
In the HER2-positive population, the median PFS increased from 3.0 months for letrozole-placebo to 8.2 months for letrozole-lapatinib, with a significant reduction in the risk of progression for the combination (HR 0.71; 95% CI, 0.53–0.96; p = 0.019); however, of the 60 patients (10%) who had grade 3 or 4 diarrhea in the combination arm, 15% required discontinuation.
AZD8931 is an orally active, reversible, equipotent tyrosine kinase inhibitor of EGFR, HER2, and HER3 signaling that has shown anticancer activity in a range of in vitro and in vivo preclinical models.28 In the phase II MINT study, 359 MBC patients were randomized 1:1:1 to receive daily anastrozole (1 mg) in combination with AZD8931 20 mg twice daily, AZD8931 40 mg bid, or placebo. Median PFS in the AZD8931 20, 40 mg, and placebo arms was 10.9 (1.37; 0.91–2.06, p = 0.135), 13.8 (1.16; 0.77–1.75, p = 0.485), and 14.0 months, respectively. Safety findings showed a greater incidence of diarrhea, rash, dry skin in patients treated with AZD8931 versus placebo.29 Consequently, the use of AZD8931 does not enhance endocrine responsiveness and is associated with greater skin and gastrointestinal toxicity.
The results of the above-mentioned studies showed that the combination of hormonal and single HER2 (trastuzumab or lapatinib) blockade leads to a mild improvement in PFS. This suggest that interruption of the crosstalk between HER2 and ER-α might be effective only in a few cases or for a short duration This strategy could be relegated either to patients who could not tolerate chemotherapy or to postchemotherapy empirical maintenance strategies
Studies of hormonal therapy with dual anti HER2 blockade in metastatic breast cancer patients
Pertuzumab is a monoclonal antibody that binds to different epitopes on HER2 compared with trastuzumab.
The combination of pertuzumab and trastuzumab is more active than a single anti HER2 drug because of a more comprehensive signaling blockade.30,31 Moreover, several studies have demonstrated that dual block of HER2 combined with chemotherapy is superior in both the metastatic, neoadjuvant and adjuvant settings compared with a single HER2 blockade.32–34
The studies evaluating the efficacy of dual block anti HER2 and hormonal therapy in MBC HER2-positive and endocrine-responsive disease are summarized in Table 2. In all studies, the hormonal therapy was an AI.
Table 2.
Studies of hormonal therapy with double HER2 blockade in metastatic breast cancer patients.
| Trial | No of patients | Phase study | Study end Point | Treatment arm | Outcome |
|---|---|---|---|---|---|
| PERTAIN | 258 | Randomized II | PFS | Trastuzumab AI +/– pertuzumab | PFS 18.8 months and 15.8 months p 0.0070 OS not reported |
| ALTERNATIVE | 355 | Randomized III | PFS | Trastuzumab and lapatinib and AI Trastuzumab and AI Lapatinib and AI |
PFS 11 versus 5.7 months p 0.0064 (lapatinib + trastuzumab + AI versus trastuzumab + AI) median OS, 46.0 versus 40.0 months |
AI, aromatase inhibitor; OS, overall survival; PFS, progression-free survival.
In the PERTAIN study, 129 patients were randomly assigned to dual block with trastuzumab and pertuzumab and AI or trastuzumab and AI.35 The patients could receive induction chemotherapy with taxane according to physician choice. An interesting observation about PERTAIN was the fact that investigators opted not to include taxanes in nearly 50% of cases, even though it was permitted by the protocol. The median PFS was 18.89 months (95% CI, 14.09–27.66 months) in the anti-HER2 dual block arm and 15.80 months (95% CI, 11.04–18.56 months) in the trastuzumab arm (stratified hazard ratio, 0.65; 95% CI, 0.48–0.89; p = 0.0070). Among patients who did not receive induction chemotherapy, the median PFS was 21.72 months (95% CI, 12.42–32.95 months) in the pertuzumab plus trastuzumab arm and 12.45 months (95% CI, 6.21–18.53 months) in the trastuzumab arm. The safety profile was consistent with previous trials of pertuzumab plus trastuzumab.
Consequently, the dual block combination with pertuzumab and trastuzumab significantly improved PFS compared with trastuzumab plus an AI.
The combination of trastuzumab and lapatinib is based on the different mechanisms of action of these drugs that simultaneously target the intracellular and extracellular HER2 domains.
In the phase III study of Blackwell and colleagues, patients with HER2-positive MBC whose disease progressed during prior trastuzumab-based therapies were randomly assigned to receive lapatinib monotherapy or lapatinib in combination with trastuzumab. Of the 128 patients with both HER2-positive and ER-positive breast cancer, no differences were observed in OS between the combination arm compared with the monotherapy arm (12 versus 11.2 months, respectively; HR, 0.85; 95% CI, 0.57–1.26).36 Further new data comes from a phase III trial, ALTERNATIVE. The eligible patients, in second line or further, were randomly assigned (1:1:1) to treatment with lapatinib plus trastuzumab and AI, trastuzumab plus AI or lapatinib and AI. Investigators’ choice of AI included letrozole, anastrozole, or exemestane.37 Median PFS was 11.0 months in the combination with dual HER2 block plus AI group versus 5.7 months in the trastuzumab group (hazard ratio = 0.62, p = 0.0064). Median PFS in the lapatinib group was 8.3 months (hazard ratio versus trastuzumab group = 0.71, p = 0.0361). The incidence of grade 3 or 4 serious adverse events (SAEs) was similar in the three treatment groups, with the exception of grade 3 diarrhea, which was higher in the lapatinib plus trastuzumab arm (13%).
All these trials in the MBC setting confirmed that hormonal therapy, including an AI and dual anti-HER2 therapy, can be safely and effectively combined.
New strategies in metastatic HER2-positive, endocrine-responsive disease
CDK4/6 pathway activation is a well-known mechanism of resistance to endocrine therapy, consequently CDK4/6 inhibitors have shown activity in cellular models of acquired resistance to endocrine therapies.38,39
Moreover, preclinical studies have demonstrated that ER-positive or HER2-positive cell lines are the most sensitive to inhibition by CDK4/6 inhibitors, and that the combination of CDK4/6 inhibitors and hormonal therapy or CDK4/6 inhibitors and anti-HER2 agents have synergistic effects.40
These data collectively indicate that CDK4/6 could be a therapeutic target that functions downstream of both ER and HER2 pathways. The potential efficacy of multiple blocks including CDK4-5 inhibitor in HER positive disease were recently confirmed in the neoadjuvant setting while results in the metastatic setting are not yet available.
NA-PHER2 (NCT 02530424) is a multicohort, open-label, exploratory, phase II study. In this study, the eligible patients received as neoadjuvant therapy a combination of dual anti HER therapy (trastuzumab and pertuzumab) for six cycles plus a CDK4/6 inhibitor (palbociclib) and a hormonal therapy [fulvestrant (500 mg) every 4 weeks for five cycles].41 The coprimary endpoints were change from the baseline in Ki67 expression at 2 weeks of treatment and at surgery, and changes in apoptosis from baseline to surgery. A total of 30 patients were assessed for primary and secondary endpoints. At surgery, eight (27%; 95% CI 12–46) patients had a pathological complete response in breast and axillary nodes. The most frequent grade 3 adverse events were neutropenia [10 (29%)] and diarrhoea [5 (14%)].
In the MBC setting, several studies are ongoing with CDK 4-6 inhibitors, anti HER2 therapy, and hormonal therapy. These studies are summarized in Table 3 and include a phase I/II trial assessing the side effects and best dose of anastrozole, palbociclib, trastuzumab, and pertuzumab in treating participants with ER- and HER2-positive MBC.
Table 3.
Ongoing trials with CDK 4/6 inhibitors and anti HER2 blockade and hormonal therapy in metastatic breast cancer.
| CDK 4/6 inhibitor | Phase study | Population | Study end point | Treatment arm | ClinicalTrials.gov number |
|---|---|---|---|---|---|
| Palbociclib | I/II | Up to 1 previous line of anti HER2 therapy | The number of patients in the phase Ib part of the study with any adverse events (AE). PFS (phase II part) |
Tucatinib + Palbociclib + letrozole | NCT 3054363 |
| Palbociclib | II | 2–4 previous lines of HER2 therapy | PFS at 6 months | Palbociclib + trastuzumab +/– letrozole | NCT 02448420 |
| Palbociclib | I/II | First line advanced HER2 positive | DLT MTD CBR |
Palbociclib + trastuzumab + pertuzumab + anastrozole | NCT 03304080 |
| Ribociclib | Ib/II | Cohort A: at least one previous line of anti HER2 therapy B at least trastuzumab, pertuzumab, TDM1 |
MTD or Recommended phase II Dose (RP2D) CBR |
A Ribociclib + TDM1 B Ribociclib + trastuzumab |
NCT 02657343 |
| Abemaciclib | II | At least 2 lines anti HER2 therapy | PFS | Abemaciclib + trastuzumab + fulvestrant versus
Abemaciclib + trastuzumab versus trastuzumab + chemotherapy |
NCT 02675231 |
CBR, clinical benefit rate; DLT, dose-limiting toxicity; MTD, maximum tolerated dose; PFS, progression free survival.
There is a phase Ib/II trial that studies the side effects and best dose of ribociclib (a CDK4/6 inhibitor) with trastuzumab or trastuzumab emtansine in MBC.
MonarcHER (NCT02675231) is a phase II, open-label, study in postmenopausal women with hormonal-receptor-positive and HER2-positive locally advanced or MBC previously treated with ⩾2 HER2-directed therapies in the advanced disease setting, with T-DM1 and a taxane in any disease setting.
This trial aims to evaluate the effectiveness of abemaciclib (a selective inhibitor of CDK4 and CDK6) plus trastuzumab with or without fulvestrant or chemotherapy.
The results of these studies will contribute to clarifying the role of target treatment in a subgroup of HER2-positive breast cancer.
A resistance to anti-HER2 therapy may arise through PI3K/Akt/mTOR pathway activation.42 The PI3K/AKT pathway is downstream from the site of action of HER2-targeted drugs, making its activation an important resistance pathway.
A pooled analysis of a large number (>950) of HER2-positive patients from five neoadjuvant trials that tested the efficacy of single or dual anti-HER2 therapy with chemotherapy reported that PIK3CA mutant tumors are associated with lower pCR rates compared with wild-type tumors, especially within the ER-positive subgroup.43
The BOLERO 1 and 3 trials evaluated the mTOR inhibitor everolimus in HER2-MBC, but showed only a small benefit at the cost of significant toxicity.44,45
Conversely, treatment with alpelisib (an orally bioavailable, small-molecule, α-specific PI3K inhibitor) and fulvestrant prolonged PFS among patients with PIK3-mutated, ER-positive, HER2-negative advanced breast cancer who had received endocrine therapy previously.46
Recently, a small study reported that the combination of alpelisib and T-DM1 is tolerable and demonstrates activity in trastuzumab-resistant HER2-positive MBC. Furthermore, activity was observed in T-DM1-resistant disease.47 These data suggest that PIK3CA inhibition targets an important resistance pathway to anti-HER2 therapy and provides the rationale for further studies of PI3K inhibition in refractory HER2-positive MBC to validate these results.
Discussion
The currently recommended first-line treatment for HER2-positive MBC is trastuzumab, pertuzumab, and a taxane. If the cancer progresses during or after first-line therapy, T-DM1 is recommended for 2nd-line treatment.48 However, these guidelines do not propose distinct management strategies for ER+ and ER− HER2+ BC.
HER2-positive, hormonal-receptor-positive breast cancer represents a heterogeneous disease that has a unique feature, namely the presence of three targets.
The results of early studies that focused on the HER2-positive and hormonal-receptor-positive disease (triple-positive), showed that the combination of hormonal therapy plus an anti-HER2 drug is more effective compared with hormonal therapy alone. However, the improvement in PFS is moderate, suggesting that interruption of the crosstalk between HER2 and ER-α might be effective only in a few cases or for a short duration. These trials did not have a trastuzumab alone arm, thus it is not known whether the addition of endocrine therapy to HER2-targeted therapy actually improves PFS; however, trastuzumab by itself, with no chemotherapy, is much less effective, probably due to the incomplete inhibition of the HER receptor.49
The subsequent step was to evaluate whether the dual HER2 blockade combined with hormonal therapy (AI) was superior to a single block in interfering with crosstalk between the ER and HER2. These data suggest that maintenance strategies after taxane in first-line therapy are safe; although no randomized studies prove the strategy is superior to dual blockade alone, it remains valid in ER-positive patients following induction chemotherapy. Moreover, the use of dual anti-HER2 inhibition represents a promising strategy to further improve the clinical outcomes of these patients. This suggests that a niche of patients with HER2-positive and hormone-receptor-positive disease may be treated only with endocrine and anti HER 2 therapies, therefore sparing chemotherapy. The possibility of avoiding chemotherapy leads to more manageable treatment with the possibility of prolonging it for a long period. This is a relevant aspect because, although advanced breast cancer is a treatable disease, it is still generally incurable and the goals of care are to optimize both length and quality of life.48
Taken together, these data suggest that, among highly selected patients with ER-positive and HER2-positive breast cancer, it is possible to avoid chemotherapy using only targeted therapies that include the dual anti-HER2 blockade (with either pertuzumab and trastuzumab or lapatinib and trastuzumab) with hormonal therapy or anti-HER2 monotherapy. The dual blockade can be used since it provides a benefit in PFS. However, the greater side effects, higher costs, and lack of OS benefit so far, when compared with monotherapy, should be discussed with the patient weighing the pros and cons of each treatment.
In endocrine-responsive disease and HER2-negative MBC, all currently available CDK4, six inhibitors combined with hormonal therapy (AI or fulvestrant) radically modified the course of disease, showing large improvement in PFS compared with hormonal therapy alone.50–52 It is possible that the CDK 4/6 inhibitor will play a role also in HER-positive disease. Indeed, the possibility of revert the resistance of trastuzumab using CDK4/6 inhibition will lead to the development of CDK4/6 inhibition in HER2-positive disease.
The toxicity profile of CDK4/6 inhibition limits its use in combination with chemotherapy, thereby rendering it a good candidate for the combination with endocrine therapy plus biological agents.
In conclusion, better knowledge of the mechanism of anti-HER2 resistance could permit the development of new drugs and treatment. Current research fields include the role of the microenviroment (e.g. immune-system) or the use of CDK4/6 and PI3K inhibitors.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: Emilia Montagna has received a speaker honorarium from Pierre Fabre.
Marco Colleoni has received a speaker honorarium from Pierre Fabre, Novartis, Astrazeneca, Cell Dex, Obipharma, Pfizer.
Contributor Information
Emilia Montagna, Division of Medical Senology, European Institute of Oncology, Via Ripamonti 435, Milan, 20141, Italy.
Marco Colleoni, Division of Medical Senology, IEO, European Institute of Oncology IRCCS, Milan, Italy.
References
- 1. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712. [DOI] [PubMed] [Google Scholar]
- 2. Karunagaran D, Tzahar E, Beerli RR, et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J 1996; 15: 254–264. [PMC free article] [PubMed] [Google Scholar]
- 3. Beerli RR, Hynes NE. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J Biol Chem 1996; 271: 607–616. [DOI] [PubMed] [Google Scholar]
- 4. Graus-Porta D, Beerli RR, Daly JM, et al. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 1997; 16: 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lonardo F, Di Marco E, King CR, et al. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol 1990; 2: 992–1003. [PubMed] [Google Scholar]
- 6. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182. [DOI] [PubMed] [Google Scholar]
- 7. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 8. Ross JS, Fletcher JA. The HER-2/neu oncogene, in breast cancer: prognostic factor, predictive factor and target for therapy. Stem Cells 1998; 16: 413–428. [DOI] [PubMed] [Google Scholar]
- 9. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011; 365: 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomized controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014; 15: 640–647. [DOI] [PubMed] [Google Scholar]
- 11. Wang YC, Morrison G, Gillihan R, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers– role of estrogen receptor and HER2 reactivation. Breast Cancer Res 2011; 13: R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaz-Luis I, Winer EP, Lin NU. Lin Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol 2013; 24: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol 2005; 23: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 14. Schiff R, Massarweh SA, Shou J, et al. Estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 2004; 10: 331S–336S. [DOI] [PubMed] [Google Scholar]
- 15. Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res 2001; 7: 4429s–4435s; discussion 4411s–4412s. [PubMed] [Google Scholar]
- 16. Zhang H, Berezov A, Wang Q, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest 2007; 117: 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 2004; 96: 926–935. [DOI] [PubMed] [Google Scholar]
- 18. Martin LA, Farmer I, Johnston SR, et al. Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 2003; 278: 30458–30468. [DOI] [PubMed] [Google Scholar]
- 19. Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat 2007; 105(Suppl. 1): 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009; 27: 5529–5537. [DOI] [PubMed] [Google Scholar]
- 21. Marcom PK, Isaacs C, Harris L, et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 2007; 102: 43–49. [DOI] [PubMed] [Google Scholar]
- 22. Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2- positive, hormone-receptor-positive metastatic breast cancer: results of the eLEcTRA trial. Breast 2012; 21: 27–33. [DOI] [PubMed] [Google Scholar]
- 23. Soto-Perez-De-Celis E, Loh KP, Baldini C, et al. Targeted agents for HER2-positive breast cancer in older adults: current and future perspectives. Expert Opin Investig Drugs 2018; 9: 1–15. [DOI] [PubMed] [Google Scholar]
- 24. Badache A, Gonçalves A. The ErbB2 signaling network as a target for breast cancer therapy. J Mammary Gland Biol Neoplasia 2006; 11: 13–25. [DOI] [PubMed] [Google Scholar]
- 25. Cristofanilli M, Valero V, Mangalik A, et al. A phase II multicenter, double-blind, randomized trial to compare anastrozole plus gefitinib with anastrozole plus placebo in postmenopausal women with hormone receptor-positive (HR) metastatic breast cancer (MBC). J Clin Oncol 2008; 26: (Suppl. 44): abstract: 1012. [Google Scholar]
- 26. Blackwell KL, Kaplan EH, Franco SX, et al. A phase II, open-label, multicenter study of GW572016 in patients with trastuzumab-refractory metastatic breast cancer. Ann Oncol 2009; 20: 1026–1031.19179558 [Google Scholar]
- 27. Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009; 27: 5538–5546. [DOI] [PubMed] [Google Scholar]
- 28. Hickinson M, Klinowska T, Speake G, et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB3 receptor blockade in cancer. Clin Cancer Res 2010; 16: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 29. Johnston S, Basik M, Hegg R, et al. Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: phase II randomized study in women with endocrine-therapy-naïve advanced breast cancer. Breast Cancer Res Treat 2016; 160: 91–99. [DOI] [PubMed] [Google Scholar]
- 30. Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004; 64: 2343–2346. [DOI] [PubMed] [Google Scholar]
- 31. Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009; 69: 9330–9336. [DOI] [PubMed] [Google Scholar]
- 32. Baselga J, Corte’s J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012; 366: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016; 17: 791–800. [DOI] [PubMed] [Google Scholar]
- 34. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017; 377: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rimawi M, Ferrero JM, de la Haba-Rodriguez J, et al. PERTAIN study group. First- line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J Clin Oncol 2018; 36: 2826–2835. [DOI] [PubMed] [Google Scholar]
- 36. Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2- positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol 2012; 30: 2585–2592. [DOI] [PubMed] [Google Scholar]
- 37. Johnston SRD, Hegg R, Im SA, et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: ALTERNATIVE. J Clin Oncol 2018; 36: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wardell SE, Ellis MJ, Alley HM, et al. Efficacy of SERD/SERM Hybrid-CDK4/6 inhibitor combinations in models of endocrine therapy-resistant breast cancer. Clin Cancer Res 2015; 21: 5121–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gianni L, Bisagni G, Colleoni M, et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol 2018; 19: 249–256.29326029 [Google Scholar]
- 42. LoRusso PM. Inhibition of the PI3K/AKT/mTor pathway in solid tumors. J Clin Oncol 2016; 34: 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Loibl I, Majewski V, Guarneri V, et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol 2016; 27: 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015; 16: 816–829. [DOI] [PubMed] [Google Scholar]
- 45. André F, Regan RO, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014; 15: 580–591. [DOI] [PubMed] [Google Scholar]
- 46. André F, Hurvitz S, Fasolo A, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 47. Jain S, Shah AN, Santa-Maria CA, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat 2018; 171: 371–381. [DOI] [PubMed] [Google Scholar]
- 48. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018; 29: 1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med 2015; 66: 111–128. [DOI] [PubMed] [Google Scholar]
- 50. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 51. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 52. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]


