Abstract
Case summary
A 9-year-old neutered male domestic shorthair cat was presented for multiple deep lesions on all four limbs and a nodule on the right pinna. The limb lesions ranged from nodules with necrotic surfaces to full-thickness ulcerations with exposure of muscles and tendons. The cat lived indoors only in a single-pet household and had no prior history of trauma. The owner reported that the lesions appeared abruptly and that the cat was not apparently painful or pruritic. Histopathology of the limb lesions and pinnal nodule confirmed severe lesions of the eosinophilic granuloma complex. Resolution of lesions was achieved with a combination of antibiotics, prednisolone, topical therapies, diet change and ciclosporin.
Relevance and novel information
This case report demonstrates a severe, aggressive presentation of eosinophilic granuloma complex. It will expose practitioners to atypical clinical signs of this commonly diagnosed disease.
Keywords: Eosinophilic granuloma complex, EGC, feline eosinophilic dermatoses, feline allergic disease, feline cutaneous reaction patterns, hypersensitivity disorder
Introduction
Feline eosinophilic granuloma complex (EGC) is a common finding in veterinary dermatology.1 It comprises a group of reaction patterns that affects the skin, oral cavity and mucocutaneous junctions of cats.1,2 EGC can be caused by a variety of factors but is most commonly thought to be the cutaneous manifestation of feline allergic disease. The three primary clinical lesions of EGC include indolent (also referred to as eosinophilic or rodent) ulcer, eosinophilic plaque and eosinophilic granuloma. All three lesion types share an inflammatory etiology and a pathogenesis involving an influx of eosinophils into dermal tissues.3 This case describes an unusually severe clinical presentation of EGC that does not fit into one of the aforementioned clinical entities. Despite the novel appearance, the lesions responded to the therapies traditionally used to treat EGC.
Case description
A 9-year-old neutered male domestic shorthair cat was presented for evaluation of full-thickness wounds on its limbs and a nodular lesion on the right convex pinna. These lesions developed acutely and did not appear to affect the cat. Historically, the cat only suffered from occasional pruritus manifested by licking and secondary mild hypotrichosis. The cat received an injection of cefovecin and a dose of topical fluralaner prior to referral.
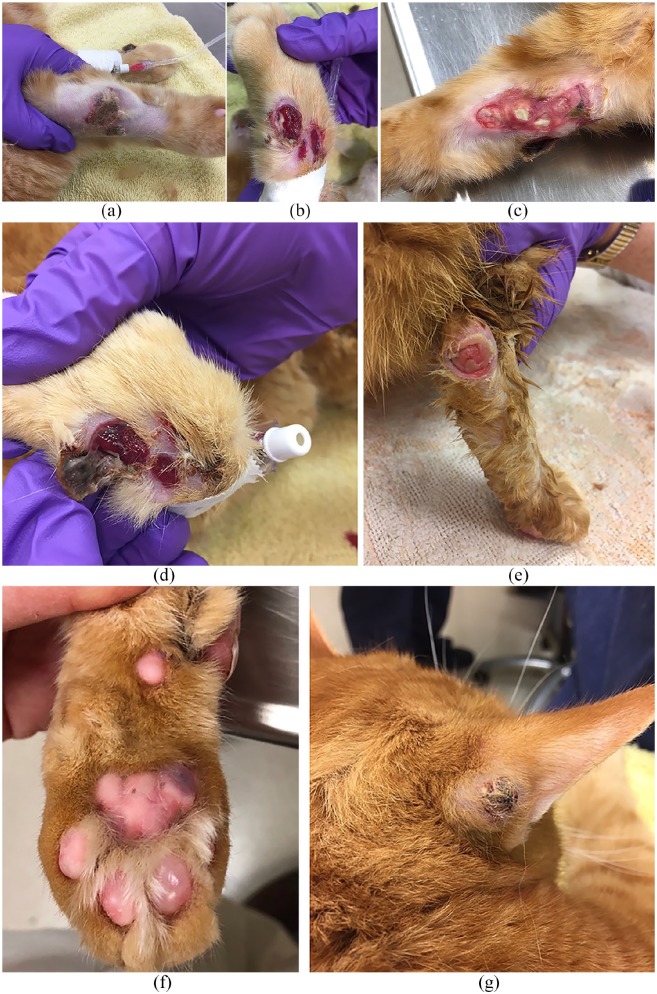
Physical examination revealed a nodule on the right forelimb with an ulcerated necrotic surface and adjacent full-thickness ulceration. The lateral left carpus had multiple full-thickness ulcerations with muscle and tendon exposed. Both hocks had similar lesions comprised of deep ulcerations. The left metacarpal pad had a central erosion, while the third digital pad was mildly swollen with pigmentary change. A purpuric macule also was noted on the lateral aspect of the right metacarpal pad. The left metatarsal pad had one erosion present. A semi-soft nodule was located at the base of the convex right pinna (Figure 1). All body systems other than dermatological were found to be unremarkable.
Figure 1.
Initial presentation: (a) nodule on right forelimb; (b) full-thickness ulceration on left carpus; (c) full-thickness ulceration on left forelimb; (d) left carpus; (e) ulcerated lesion on right hock; (f) metacarpal pad discoloration; and (g) right pinnal nodule
A preliminary list of differential diagnoses based on the history and clinical presentation included infection (viral, bacterial, fungal, mycobacterial) and neoplasia. Initial diagnostics included a chemistry panel, complete blood count (CBC), cytology, aerobic and fungal cultures, and histopathology. Cytology of an impression smear from the forelimb lesions revealed mixed inflammatory cells, including neutrophils and macrophages without obvious infectious organisms. The chemistry panel was within normal limits. The CBC revealed eosinophilia (3562/µl, range 0–1500/µl). Deep tissue culture showed no aerobic growth after 72 h. Fungal culture of deep tissue obtained via biopsy revealed no growth of pathogenic fungus after 3 weeks.
Skin biopsies were performed to obtain samples of the affected areas using 6 mm punches. Three samples were collected from the lesions on the forelimbs and a fourth sample was obtained from the right pinnal nodule. Histopathological examination revealed necro-ulcerative and severe, multifocal eosinophilic dermatitis (Figure 2). A second opinion was requested due to the initial diagnosis being seemingly inconsistent with the clinical presentation. A second pathologist reported severe ulcerative and necrotizing eosinophilic dermatitis, consistent with severe lesions of the eosinophilic granuloma complex. This pathologist did make note of the fact that there were also lymphocytes amongst the eosinophils, so lymphoma could be a differential, although this was less likely.
Figure 2.
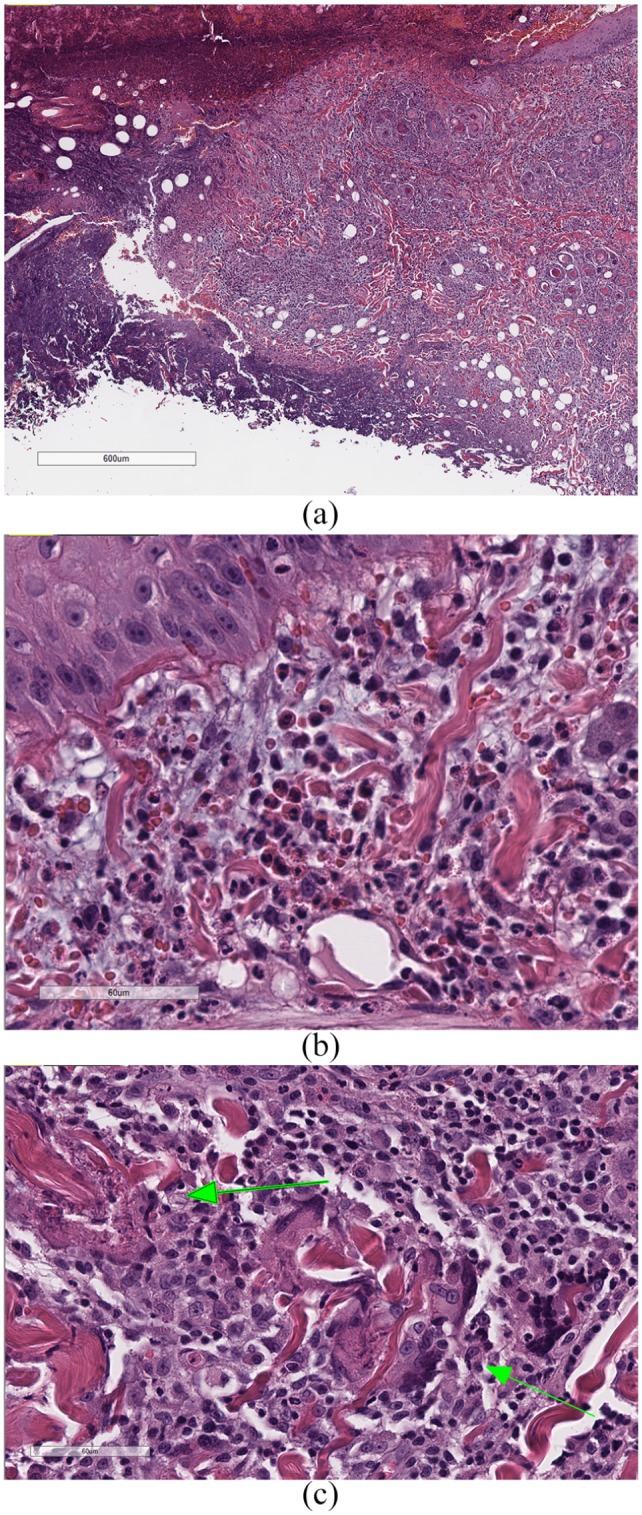
(a) Ulcerative dermatitis with necrosis of the underlying dermis, and heavy dermal infiltrates of eosinophils; (b) dermal infiltrates of eosinophils, inflammatory mast cells and small lymphocytes, with mild-to-moderate edema and mucinosis; and (c) ‘flame figures’ (arrows), consisting of collagen bundles surrounded by degranulated eosinophils, which, in turn, are surrounded by macrophages and multinucleated giant cells. The bars in (a), (b) and (c) are 600 μm, 60 μm and 60 μm, respectively
Treatments prescribed at presentation (prior to histopathology or culture results) included pradofloxacin (6 mg/kg/day [Veraflox; Bayer]) and sublingual buprenorphine (0.01 mg/kg q12h). Antibiotics were prescribed due to the severe clinical appearance and large amount of tissue necrosis evident on presentation. Pradofloxacin was considered an appropriate antibiotic choice based on mycobacterial infection being a differential.4,5 Buprenorphine was prescribed for analgesia post-biopsy procedure. After all diagnostic results were reviewed, prednisolone was added to the regimen at 2 mg/kg q24h.6 The pradofloxacin course was completed but not refilled. A food trial was also initiated with a hydrolyzed salmon prescription diet (Blue Natural Veterinary Diet HF).
Additional diagnostics were performed to rule out underlying systemic causes of the dermatologic abnormalities. Thoracic radiographs revealed no evidence of intrathoracic disease. Abdominal ultrasound showed mild cystic sand (deemed likely incidental) and prominent medial iliac lymph nodes. The radiologist noted this lymphadenopathy may be normal or mildly reactive to the cutaneous lesions in the hindlimbs, with no additional diagnostics (such as fine-needle aspirate) deemed necessary. A urinalysis was submitted due to the cystic sand finding, and was unremarkable. Retroviral testing confirmed the cat was negative for feline leukemia virus and feline immunodeficiency virus. A lymphoma panel (PCR for antigen receptor rearrangement [PARR]) was recommended but was ultimately declined by the owner.
The cat was re-evaluated 2 weeks later. The lesions were stable: there was no worsening of existing lesions and no new lesions. Four-week follow-up showed improvement of all limb lesions. Weight loss was noted but was accounted for by the owner not feeding an adequate amount of the new diet. A gradual steroid taper was initiated. Topical application of a spray containing hypochlorous acid (Vetericyn; Innovacyn) to the lesions was recommended. A chemistry panel with CBC was repeated. The eosinophil count normalized, but there was a mild lymphopenia present (855 µ/ml). This abnormality was attributed to the use of the steroid.
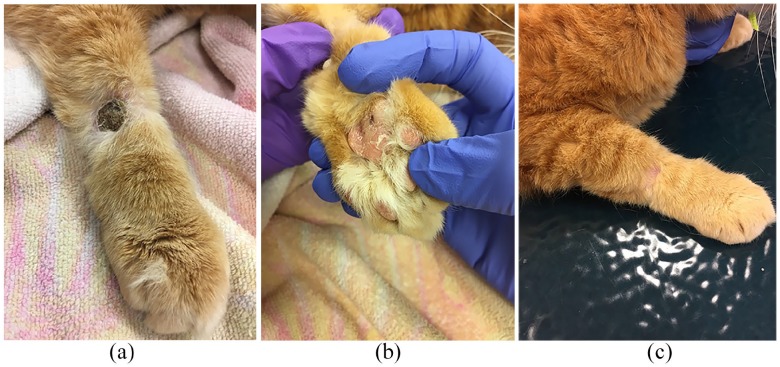
Unfortunately, the client failed to bring the cat in for the next scheduled recheck and discontinued the prednisolone when the supply was finished. When the cat did return for re-evaluation, it had several new lesions (primarily foot pads and pinna). The original lesions were heavily crusted with underlying purulent discharge (Figure 3). Cytology revealed cocci and therefore a culture was submitted. The steroid was resumed and a cefovecin injection (Convenia; Zoetis) was administered. Cefovecin was chosen due to its documented efficacy in treating staphylococcal infections and ease of administration in the cat.7 The client was instructed to soak the affected feet in dilute chlorhexidine solution once daily.
Figure 3.
(a) Exacerbation of pad lesions after steroid discontinuation; and (b) crusting of lesions with underlying purulent discharge
Aerobic culture from the cat’s limb lesions revealed heavy growth of methicillin-resistant Staphylococcus pseudintermedius, sensitive to only amikacin and chloramphenicol. An extended susceptibility panel was performed and the only antibiotic options were linezolid, vancomycin and rifampicin. Vancomycin was not chosen due to its cost and route of administration (injectable). Rifampin was also a poor choice due to its known adverse effects in cats. Owing to limited systemic antibiotic options, a pharmacologist was consulted. He voiced reluctance to use chloramphenicol owing to potential bone marrow suppression and instead recommended linezolid. Linezolid is typically well tolerated in cats, is easily administered and is fast-acting. The cat was started on linezolid at 10 mg/kg q24h for 30 days. Mupirocin was also applied topically to affected areas, q12h, and prednisolone was continued.
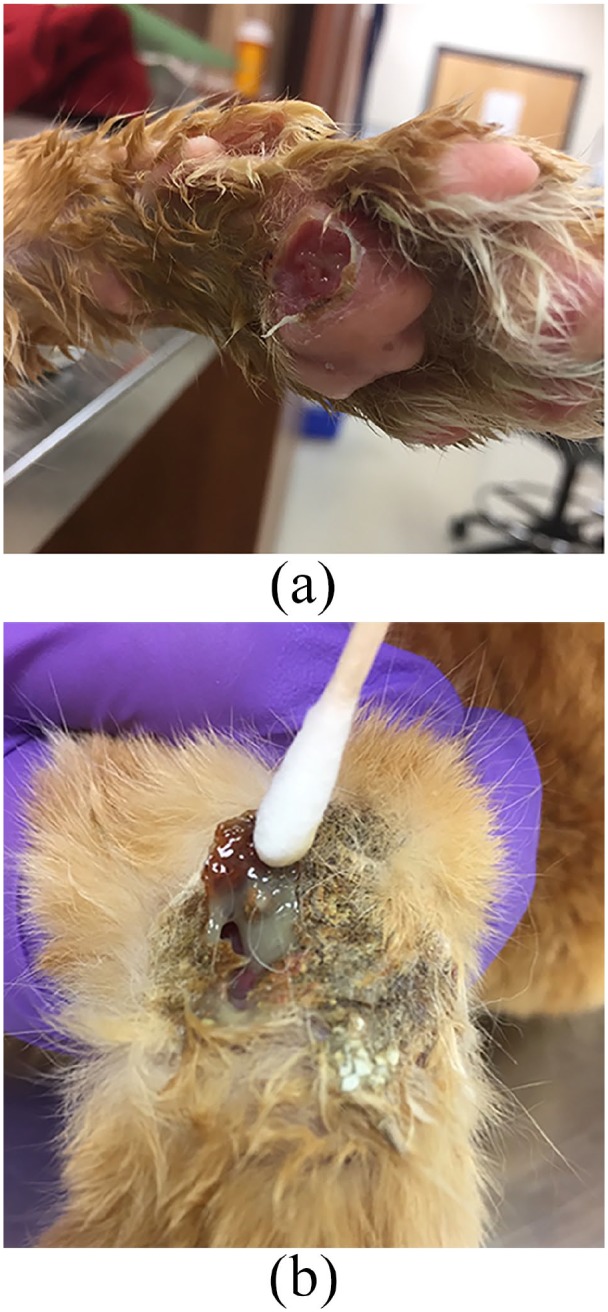
The 2-week recheck showed improvement of all limb and foot pad lesions. Repeat blood work showed neutrophilia (17,513/µl), lymphopenia (1055/µl) and eosinophilia (2100/µl). The nodule at the base of the right pinna was larger than previously noted. Fine-needle aspirate and cytology of the nodule showed eosinophils (1+) and occasional neutrophils.
The pinnal nodule continued to grow and rupture intermittently (Figure 4). The client was concerned with quality of life as the cat was required to wear an Elizabethan collar and yet still excessively bled from the pinnal mass. At this time, the cat was started on ciclosporin (Atopica; Elanco) at 7 mg/kg q24h. The prednisolone was gradually tapered over the course of 4 weeks.
Figure 4.
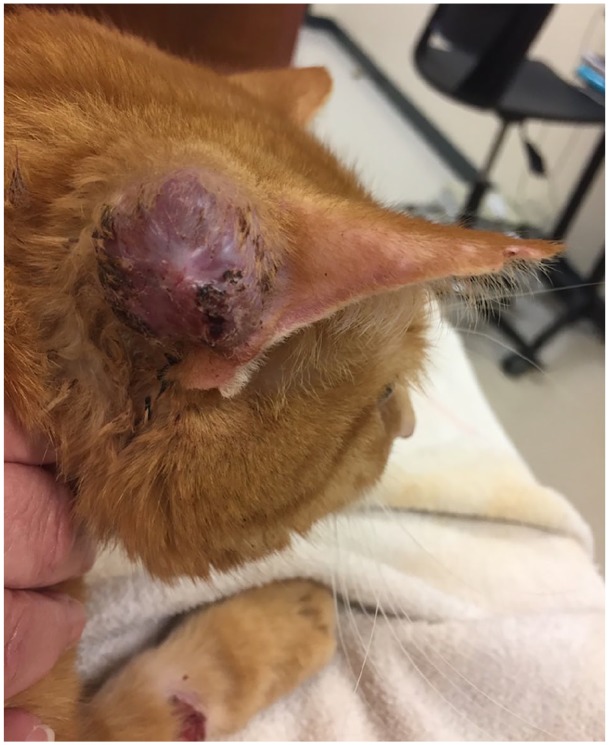
Enlargement of pinnal nodule
Surgical excision of the pinnal nodule was pursued due to lack of improvement with medical management. Owing to the size of the mass and the limited skin available for closure, a pinnectomy was performed (Figure 5). Although this same nodule was biopsied 4 months earlier, it was suspected to be a different entity than EGC. Therefore, the entire pinna was submitted. Histopathological examination confirmed cutaneous hemangiosarcoma. The surgical margins were clean but narrow (1–2 mm). Repeat metastatic work-up (thoracic radiographs, abdominal ultrasound) was recommended, along with an oncology consultation. The owner declined further diagnostics.
Figure 5.
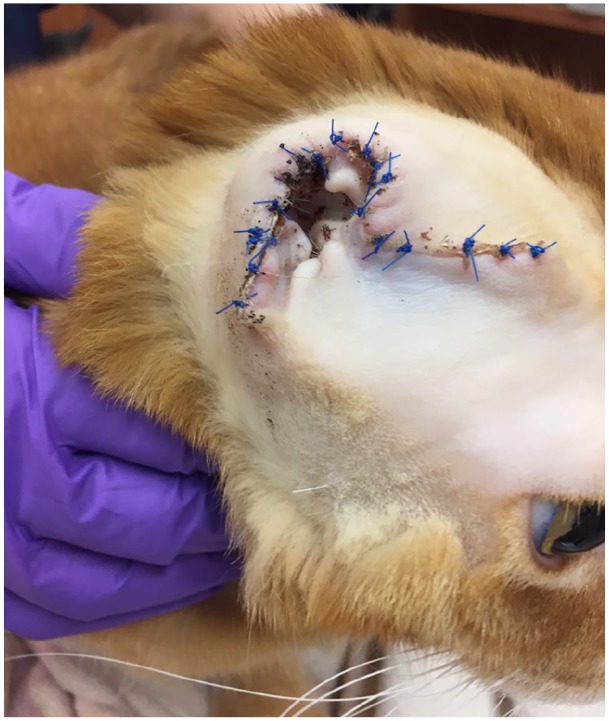
Fourteen days post-pinnectomy
At the recheck 6 months after referral, the pinnectomy lesion was healed and the forelimb lesions were significantly improved (Figure 6). The foot pad lesions resolved. The slowest healing lesions were the erosions on the hocks, but even these had granulation tissue. The delayed improvement of these lesions was attributed to their location (directly over the hocks) and the potential glucocorticoid effects on wound healing. Blood work showed persistent neutrophilia (9828/µl), lymphopenia (550/µl) and hyperglycemia (226 mg/dl). The fructosamine level was normal (282 μmol/L), indicating no diabetes mellitus.
Figure 6.
(a) Residual lesion on right forelimb after 6 months of therapy; (b) healing metacarpal pad after 6 months of therapy; and (c) right distal forelimb 9 months after initial presentation
Over the next 3 months, the cat’s lesions continued to improve and the prednisolone was gradually tapered. At the 9-month recheck, the left forelimb was completely free of lesions and the right forelimb had only residual scarring in the previous lesion site. The healing of the hock lesions was progressing. The cat remained on a low dose of prednisolone (0.1 mg/kg q48h), as well as ciclosporin (7 mg/kg q24h). The cat continued to have fluralaner applied every 3 months and continued to eat solely the prescription hydrolyzed salmon diet (the owner declined challenging the diet).
Discussion
This case demonstrates a novel, aggressive presentation of EGC. The commonly recognized lesions associated with EGC include eosinophilic plaque, eosinophilic granuloma and indolent ulcer.1,3,6,8,9 Eosinophilic plaques are raised, well-demarcated erythematous lesions typically found on the ventral abdomen.6 Eosinophilic granulomas can appear as raised or nodular lesions in the oral cavity, on the lower lip or on the footpads.3 Pinpoint white particles representing eosinophil degranulation are often noted in the center of eosinophilic granulomas.3,6 Eosinophilic granulomas may also present as linear lesions on the caudal limbs. Indolent ulcers are well-circumscribed ulcers located on the upper lip adjacent to the maxillary canine teeth.1 The cat in this case presented with unique lesions involving full-thickness ulcerations of the limbs. One may argue that the nodular appearance of some of the lesions most closely resembles eosinophilic granulomas. However, the severe ulceration associated with the lesions has not been previously documented, to our knowledge.
EGC can resemble several other disease processes such as viral infections, bacterial or fungal infections, and neoplasia.6 The cat was retrovirus negative. Histopathology was not consistent with herpes or caliciviral infections. The deep tissue bacterial and fungal cultures were negative. Neoplasia was not noted on histopathology. There were, however, lymphocytes among the eosinophils in the biopsy specimens, suggesting perhaps an inflamed lymphoma. Ideally, a precautionary lymphoma panel (PARR) would have enabled us to distinguish between monoclonal neoplastic lymphoid cells and polyclonal, inflammatory lymphoid cells. However, the owner declined this additional testing. The final diagnosis was still reported as severe EGC by more than one pathologist.
The cutaneous hemangiosarcoma on the cat’s right pinna was an unexpected finding. The nodule was initially biopsied and diagnosed as part of the EGC. However, that one lesion continued to enlarge despite treatment and despite improvement of all other lesions. Cutaneous hemangiosarcomas in cats are infrequently reported.10 Aggressive surgical excision was elected due to the tumor affecting the cat’s quality of life and the knowledge that complete surgical excision is typically associated with a favorable prognosis.10 It is still unknown whether the hemangiosarcoma was always present but not represented in the initial biopsy, or if the neoplasia was a later development.
Initial treatment of EGC involves addressing secondary infection and inflammation. Long-term management entails immunosuppression and control of the hypersensitivity response.9A glucocorticoid was prescribed initially in order to provide rapid dampening of the immune response and reduction in the severe inflammation. Ciclosporin was later added in this case in the hope of being steroid-sparing and to allow for reduction and possibly discontinuation of the glucocorticoid. Common causes of EGC include flea, food and non-flea, non-food induced hypersensitivity dermatitis. Routine application of fluralaner addressed a potential flea allergy, while a strict prescription diet trial addressed a potential food allergy. The client was educated about skin and/or serum tests for environmental allergies and formulation of allergen-specific immunotherapy. However, the cat responded to the food trial and immunosuppression so that further allergy work-up was temporarily postponed.
The antibiotic choice of linezolid may be received with some controversy. The International Society for Companion Animal Infectious Diseases lists linezolid as a ‘third-tier’ antimicrobial agent.11 In some countries in Europe (including France), the use of third-tier antibiotics is prohibited by law, as such molecules are reserved exclusively for the treatment of humans and not animals. However, it is important to note that linezolid is not prohibited universally and is permitted for use in animals in the USA (where this case was clinically managed). Its use should be reserved for cases in which first- and second-tier antimicrobials are not appropriate and culture indicates susceptibility. Both of these criteria were met in this case. An independent expert on antimicrobial resistance was consulted on this case. This expert suggested linezolid owing to its favorable pharmacokinetics, safety profile in cats and lack of documented resistance in bacteria isolated from animals (M Papich, 2019, personal communication). Therefore, there was no violation of responsible antimicrobial stewardship in the treatment of this case.
Conclusions
This case introduces a presentation of EGC that has not been previously documented. It expands our knowledge of this common dermatologic condition. It also stresses the need for a thorough work-up and management of allergies in feline patients presenting with severe skin lesions.
Acknowledgments
The authors would like to thank Erin Locke DVM, DVSc, Dipl ACVP for her interpretation and guidance on the histopathology of this case. They also thank Mark Papich DVM, MS, Dipl ACVCP for his advice on this case’s culture results.
Footnotes
Accepted: 2 November 2019
Conflict of interest: The authors received funding from Blue Buffalo. The hydrolyzed diet prescribed in this case was Blue Natural Veterinary Diet HF.
Funding: The authors received funding for publication from Blue Buffalo.
Ethical approval: This work involved the use of non-experimental animals only (owned or unowned), and followed established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care. Ethical approval from a committee was not necessarily required.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work for the procedure(s) undertaken. For any animals or humans individually identifiable within this publication, informed consent for their use in the publication (verbal or written) was obtained from the people involved.
ORCID iD: Kaitlin P Hopke  https://orcid.org/0000-0002-5334-286X
https://orcid.org/0000-0002-5334-286X
References
- 1. Oliveira A, Van den Broek A. The feline eosinophilic granuloma complex. UK Vet Comp Anim 2006; 11: 48–55. [Google Scholar]
- 2. Forsythe P. Feline eosinophilic dermatoses part 2: further investigation and long-term management. UK Vet Comp Anim 2011; 16: 31–35. [Google Scholar]
- 3. Miller WH, Griffin CE, Campbell KL. Muller Kirk’s small animal dermatology. 7th ed St Louis, MO: Elsevier Press, 2013. [Google Scholar]
- 4. Lees P. Pharmacokinetics, pharmacodynamics and therapeutics of pradofloxacin in the dog and cat. J Vet Pharmacol Ther 2013; 36: 209–221. [DOI] [PubMed] [Google Scholar]
- 5. Vishkautsan P, Reagan KL, Keel MK, et al. Mycobacterial panniculitis caused by Mycobacterium thermoresistibile in a cat. JFMS Open Reports 2016; 1–7. DOI: 10.1177/2055116916672786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckley L, Nuttall T. Feline eosinophilic granuloma complex(ities). Some clinical clarification. J Feline Med Surg 2012; 14: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stegemann MR, Sherington J. The efficacy and safety of cefovecin in the treatment of feline abscesses and infected wounds. J Small Anim Pract 2007; 48: 683–689. [DOI] [PubMed] [Google Scholar]
- 8. Forsythe P. Feline eosinophilic dermatoses part 1: etiology, clinical signs and investigation. management. UK Vet Comp Anim 2011; 16: 40-45. [Google Scholar]
- 9. O’Dair H. Clinical refresher – eosinophilic granuloma complex. UK Vet Comp Anim 2009; 14: 55–58. [Google Scholar]
- 10. McAbee KP, Ludwig LL, Bergman PJ, et al. Feline cutaneous hemangiosarcoma: a retrospective study of 18 cases (1998–2003). J Am Animal Hosp Assoc 2005; 41: 110–116. [DOI] [PubMed] [Google Scholar]
- 11. International Society for Companion Animal Infectious Diseases. http://iscaid.org