Abstract
Background:
Reports have concluded that platelet-rich plasma (PRP) is an effective and safe biological approach in the treatment of knee osteoarthritis (OA). However, no consensus has been established regarding the number of injections required to observe a therapeutic effect.
Purpose:
To compare the clinical effectiveness reported in randomized controlled trials (RCTs) of single versus multiple PRP injections in the treatment of knee OA.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
A comprehensive search was conducted for RCTs published between 1970 and 2019 that compared the effect of single versus multiple PRP injections on pain and functionality in patients with knee OA. Searched databases included MEDLINE, Scopus, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials. A data extraction form was designed to obtain bibliographic information of the study as well as patient, intervention, comparison, and outcomes of interest data. A random-effects model was used to pool quantitative data from the primary outcomes.
Results:
We included 5 clinical trials with a low-moderate risk of bias that reported data for 301 patients. Meta-analysis showed that, at 6 months after the intervention, single and multiple (double or triple) injections had similar pain improvement, with no significant differences (standardized mean difference [SMD], 0.61 [95% CI, −1.09 to 2.31]; I 2 = 97%; P = .48). A significant improvement in knee functionality was observed in favor of multiple injections (SMD, 2.29 [95% CI, 0.45-4.12]; I 2 = 97%; P = .01). Subanalysis showed that the significant improvement was only evident for the results of single versus triple injections (SMD, 3.12 [95% CI, 0.64-5.60]; I 2 = 97%; P = .01).
Conclusion:
According to our results, a single injection was as effective as multiple PRP injections in pain improvement; however, multiple injections seemed more effective in joint functionality than a single injection at 6 months. We consider that the available evidence is still insufficient, and future research on this specific topic is needed to confirm our results.
Keywords: platelet-rich plasma, knee osteoarthritis, functionality, injection, meta-analysis
Osteoarthritis (OA) is the most common joint disorder in several countries, affecting approximately 10% of the population, more commonly in people older than 45 years.37 The prevalence of OA is rising, and in the coming decades, it will rise even more because of longevity and the increasing prevalence of obesity.
The knee is one of the most affected joints with OA.16 Knee OA typically leads to a serious decline in quality of life if no intervention is performed. Although knee replacement surgery provides an effective solution for severe knee OA,3 nonoperative therapies are proposed as management options to help relieve symptoms and improve function for middle-aged patients with less severe stages of OA.1
Injectable medications that can cause regenerative changes in tissue and manage and alleviate OA symptoms are very important. Among injectable options for symptom relief and functional improvement in patients with knee OA, platelet-rich plasma (PRP) has increased in popularity in recent years.25 Described as an easy, fast, and safe approach, autologous PRP has been the subject of increased clinical interest in the orthopaedic field. PRP consists of a centrifuged blood fraction that contains a concentration of platelets that is often many folds greater than physiological platelet concentrations at wound sites.18
Results from different studies suggest a possible chondroprotective activity of PRP. Platelets hold in their α-granules many growth factors such as transforming growth factor–beta, platelet-derived growth factor, epidermal growth factor, vascular endothelial growth factor, fibroblast growth factor, and insulin-like growth factor, which are proposed to have a regenerative capacity.31,33,38 Along with growth factors, other cytokines present in PRP inhibit inflammatory effects on chondrocytes by nuclear factor–kappa B, interleukin-1, and nitric oxide.26,32
Several reports, including systematic reviews and meta-analyses, have concluded that PRP is an effective and safe biological approach in the treatment of knee OA compared with other intra-articular injections.2,5,11,15,20,23,29 While some reviews assessed the effect of various injection therapies for knee OA, none of these studies have directly compared the outcome of the number of injections as well as the period of time between one application and another.
Regarding the number of injections, different therapeutic schemes have been evaluated. Typically, the number of interventions in clinical trials include 1 or 3 applications.24,27 Despite the increasing reports investigating the clinical effect of PRP, there is virtually no conclusive evidence on the dose or frequency of PRP in the setting of knee OA treatment. Because many of the clinical trials evaluating different PRP therapeutic approaches (≥1 applications) have reported clinical improvement in their patients, we hypothesized that 1 application would have similar clinical improvement to more than 1 PRP injection in the short term. This systematic review and meta-analysis aimed to assess and compare the clinical effectiveness reported in randomized controlled clinical trials of single versus several injections of PRP in the treatment of knee OA to help establish an adequate number of applications with therapeutic potential.
Methods
For this systematic review, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement22 and were guided by a registered protocol (PROSPERO registration: CRD42018106429).
Information Sources and Search Strategy
An experienced librarian designed the search strategy in collaboration with the main investigators of the study. A thorough and comprehensive search was executed to find articles that suited the review’s objective in several databases from 1970 to July 2019, as the use of PRP was first introduced in the 1970s. The general databases included MEDLINE via PubMed, Elsevier via Scopus, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials. We searched for additional references that addressed our study question in other systematic reviews and searched for unpublished clinical trials on clinicaltrials.gov and greylit.org so that any possible missing study was considered.
We used specific keywords and MeSH terms to assess studies evaluating the effect of single versus multiple PRP injections on pain and articular function improvement in patients with knee OA. After completing the data extraction phase, an extra search was performed to include any eligible study that could have been published during the previous months of data extraction. We present an example of a search strategy that was executed via Scopus in the Appendix.
Eligibility Criteria
Our review only included randomized controlled trials (RCTs) assessing the effect of the number of PRP injections (eg, autologous conditioned plasma, plasma rich in growth factors, and platelet-rich fibrin) on pain (visual analog scale [VAS] or visual numerical scale [VNS]) and functional improvement (Western Ontario and McMaster Universities Arthritis Index [WOMAC], International Knee Documentation Committee [IKDC] subjective knee evaluation form, and Lequesne index) in patients with knee OA. All observational studies (quasi-experimental, cohort, case-control, and cross-sectional studies) were excluded. A minimum of 1 review outcome was enough for a study to be included in the review. We considered studies enrolling patients aged ≥40 years who were clinically and radiographically diagnosed with knee OA defined by any recognized diagnosis criteria, regardless of its evolution time, and with a minimum follow-up of 3 months. We included RCTs that assessed the effectiveness of single and multiple PRP injections in patients with knee OA. Studies with missing data were excluded, and there was no language restriction.
Selection Process
A total of 3 (F.V.-C., V.M.P.-M., C.A.A.-O.) reviewers worked independently and in duplicate to review all titles and abstracts as well as selected full-text articles. First, a pilot phase of 50 studies was carried out until reaching a chance-adjusted interrater agreement (kappa) of 0.7 between reviewers. The pilot phase consisted of reviewers including and excluding studies by title and abstract with the purpose of certifying adequate comprehension and resolving any misunderstandings between reviewers.19 All studies required approval by at least 1 of the reviewers to proceed to the full-text phase.
After selecting the studies by title and abstract, a full-text phase was executed in which these 3 reviewers used the same methodology as previously mentioned by including or excluding the selected studies according to the full-text article. Disagreements were resolved by consensus with all reviewers. We used the Distiller Systematic Review Software (DistillerSR Evidence Partners) for the management of study data during the selection process mentioned above.
Data Collection Process
For the data collection process, a pilot model was conducted by the 3 reviewers in charge of performing data extraction to assess possible disagreements or approaches to extraction as well as calibrating the extraction. A data extraction form was designed to standardize data extraction. This form included the following items: a section addressing the bibliographic information of the study (year, authors, study design, etc), another section addressing the study question (patient, intervention, comparison, and outcomes of interest) showing the main results reported in each study, and a third section designed to address the risk of bias in each individual study. After the pilot model, the 3 reviewers (J.M.M.-A., N.A.-V., M.S.-M.) worked again independently and in duplicate to extract the data from each individual study; any kind of disagreement was resolved by consensus.
Outcomes and Prioritization
The outcomes of interest measured in this review were the following: (1) pain was assessed with the VAS or VNS and (2) functional improvement was assessed with the WOMAC, IKDC form, and/or Lequesne index.
Risk of Bias in Individual Studies
We assessed the risk of bias in individual studies by conducting an independent and duplicate review by 3 authors (J.M.M.-A., J.B.-S., M.S.-M.) who used the Cochrane risk of bias tool to assess the quality of the RCT.10 The items used for the assessment of each study were as follows: adequacy of sequence generation, allocation concealment, blinding, addressing of dropouts (incomplete outcome data), selective outcome reporting, and other potential sources of bias. According to the recommendations of the Cochrane Handbook, a judgment of “yes” indicated a low risk of bias, while “no” indicated a high risk of bias. Labeling an item as “unclear” indicated an unclear or unknown risk of bias. Any disagreement in assessing the quality of a study was resolved by consensus between the reviewers.
Quantitative Data Synthesis
Meta-analysis was conducted using the Review Manager Statistical Software (RevMan version 5.3; Cochrane Collaboration). For each study, a summary of the intervention effect was estimated by calculating standardized mean differences (SMDs) for numerical outcomes. For the VAS and VNS, all values were collected in centimeters. For the WOMAC, all scores were collected as a 5-point Likert scale. IKDC scores were obtained using the transformed total value to a scaled number that ranged from 0 to 100. When only the standard error of the mean (SEM) was reported, the standard deviation (SD) was estimated using the following formula: SD = SEM × sqrt (n), where n is the number of participants. When not able to obtain the SD of a record after trying to contact the study authors, we used the range-rule-of-thumb method to estimate the missing SD. This method estimates that the SD is one-fourth of the range of a determined variable.35 SDs of the mean differences were calculated using the following formula: SD = square root [(SDpretreatment)2 + (SDposttreatment)2 − (2R × SDpretreatment × SDposttreatment)], assuming a correlation coefficient (R) of 0.5. Net changes in measurements (mean difference) were calculated as follows: (measure at end of follow-up) − (measure at baseline).
Meta-analysis was conducted using a random-effects model (using the DerSimonian-Laird method) and the generic inverse variance method. Effect sizes were expressed as the SMD and 95% CI. Consistency, specifically focusing on the heterogeneity of the studies to include, was examined by applying the Cochran Q test and considering a P value of <.05 as statistically significant. In turn, the I 2 statistic was used, considering 0% to 25% of heterogeneity between studies as unimportant, 26% to 50% as moderate, and >50% as important.
Results
Search Output
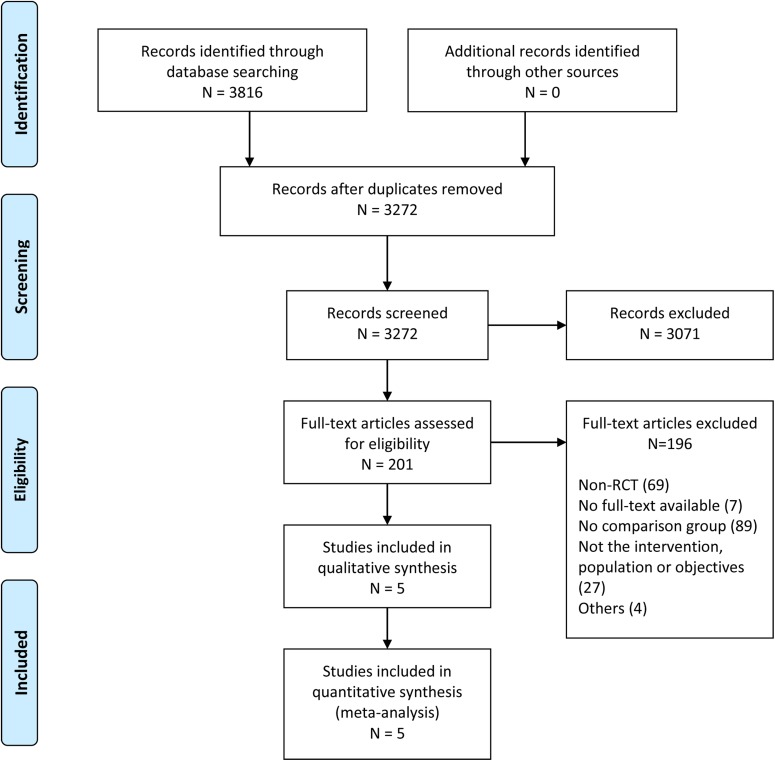
The search strategy identified 3816 publications (3272 records after duplicates were removed); of these, 3071 studies did not meet the inclusion criteria and were excluded. Subsequently, 201 full-text articles were reviewed for eligibility, and 196 were excluded for the following reasons: non-RCT, not using the comparator of interest, nonexistent full-text document, and duplicate reports. The resultant 5 clinical trials were selected and included in the present meta-analysis. The complete workflow is shown in Figure 1.
Figure 1.
Flowchart of the number of studies identified and included in this meta-analysis. RCT, randomized controlled trial.
Characteristics of Included Studies
The 5 included studies reported data for 301 patients. Patel et al24 had 2 PRP study groups (1 and 2 injections), and the study was performed in Chandigarh, India. Görmeli et al9 had 2 PRP study groups (1 and 3 injections), and the study was performed in Malatya, Turkey. Kavadar et al12 performed their study in Istanbul, Turkey, where they generated 3 PRP study groups (1, 2, and 3 injections). Uslu Güvendi et al34 performed their study in Erzurum, Turkey, where they evaluated 2 PRP study groups (1 and 3 injections). Last, Simental-Mendía et al30 had 2 PRP study groups (1 and 3 injections), and their study was performed in Monterrey, Mexico. All studies evaluated patients for a maximum of 6 months (with the exception of Simental-Mendía et al,30 who performed a maximum follow-up of 1 year) and included patients diagnosed with knee OA graded by either the Ahlback or Kellgren-Lawrence classification. Patel et al24 included patients with Ahlback grades 1 to 3, Görmeli et al9 with Kellgren-Lawrence grades 1 to 4, Kavadar et al12 and Uslu Güvendi et al34 with Kellgren-Lawrence grade 3, and Simental-Mendía et al30 with Kellgren-Lawrence grades 1 and 2. Additional information regarding study characteristics and patients is shown in Tables 1 and 2, respectively.
TABLE 1.
Characteristics of Included Studiesa
| Author (Year) | Study Design | Target Population | Treatment Duration, mo | n | Study Groups | Time Between Injections, wk | Type of PRP Used |
|---|---|---|---|---|---|---|---|
| Görmeli et al9 (2017) | Randomized, double-blind, placebo-controlled | Knee OA | 6 | (1) 44 (2) 39 |
(1) Single PRP injection (2) Triple PRP injection |
(1) NA (2) 1 |
2A,b P4-Aαc |
| Uslu Güvendi et al34 (2018) | Randomized, blinded, controlled | Knee OA | 6 | (1) 19 (2) 14 |
(1) Single PRP injection (2) Triple PRP injection |
(1) NA (2) 1 |
1B,b P3-Aαc |
| Kavadar et al13 (2015) | Randomized, blinded, controlled | Knee OA | 6 | (1) 33 (2) 32 (3) 33 |
(1) Single PRP injection (2) Double PRP injection (3) Triple PRP injection |
(1) NA (2) 2 (3) 2 |
2A,b P3-x-Aαc |
| Patel et al24 (2013) | Randomized, double-blind, placebo-controlled | Bilateral knee OA | 6 | (1) 27 (2) 25 |
(1) Single PRP injection (2) Double PRP injection |
(1) NA (2) 3 |
4B,b P4-x-Bβc |
| Simental-Mendía et al30 (2019) | Randomized, controlled | Knee OA | 12 | (1) 18 (2) 17 |
(1) Single PRP injection (2) Triple PRP injection |
(1) NA (2) 2 |
4B,b P2-x-Bβc |
TABLE 2.
Characteristics of Study Patientsa
| Author (Year) | Age, y | Female Sex, n (%) | BMI, kg/m2 | Pain Score | Functional Score |
|---|---|---|---|---|---|
| Görmeli et al9 (2017) | (1) 53.8 ± 13.4 (2) 53.7 ± 13.1 |
(1) 25 (56.8) (2) 23 (58.9) |
(1) 28.4 ± 4.4 (2) 28.7 ± 4.8 |
(1) ND (2) ND |
(1) 41.2 ± 6.1b
(2) 40.4 ± 5.0b |
| Uslu Güvendi et al34 (2018) | (1) 62.3 ± 1.6 (2) 60.4 ± 1.7 |
(1) 18 (94.7) (2) 13 (92.9) |
(1) 31.4 ± 0.7 (2) 31.0 ± 1.0 |
(1) 6.2 ± 0.8c
(2) 5.4 ± 1.1c |
(1) 58.1 ± 3.3d
(2) 62.9 ± 4.2d |
| Kavadar et al13 (2015) | (1) 53.6 ± 6.7 (2) 54.9 ± 5.4 (3) 55.2 ± 5.7 |
(1) ND (2) ND (3) ND |
(1) 24.9 ± 2.3 (2) 25.1 ± 1.6 (3) 25.5 ± 1.9 |
(1) 7.7 ± 0.6e
(2) 7.7 ± 6.8e (3) 8.4 ± 6.9e |
(1) 91.4 ± 11.5d
(2) 81.6 ± 17.0d (3) 89.9 ± 9.8d |
| Patel et al24 (2013) | (1) 53.1 ± 11.6 (2) 51.6 ± 9.2 |
(1) 16 (59.2) (2) 20 (80.0) |
(1) 26.3 ± 3.2 (2) 25.8 ± 3.3 |
(1) 4.6 ± 0.6e
(2) 4.6 ± 0.6e |
(1) 49.6 ± 17.8d
(2) 53.2 ± 16.2d |
| Simental-Mendía et al30 (2019) | (1) 54.6 ± 11.6 (2) 60.1 ± 10.6 |
(1) 17 (94.4) (2) 12 (70.6) |
(1) 29.6 ± 5.9 (2) 31.5 ± 4.8 |
(1) 7.3 ± 2.1e
(2) 6.6 ± 2.4e |
(1) 44.2 ± 19.7d
(2) 41.4 ± 15.5d |
aValues are expressed as mean ± SD unless otherwise specified. BMI, body mass index; ND, no data.
bInternational Knee Documentation Committee (IKDC).
cVisual numerical scale (VNS).
dWestern Ontario and McMaster Universities Arthritis Index (WOMAC).
eVisual analog scale (VAS).
Risk of Bias Assessment
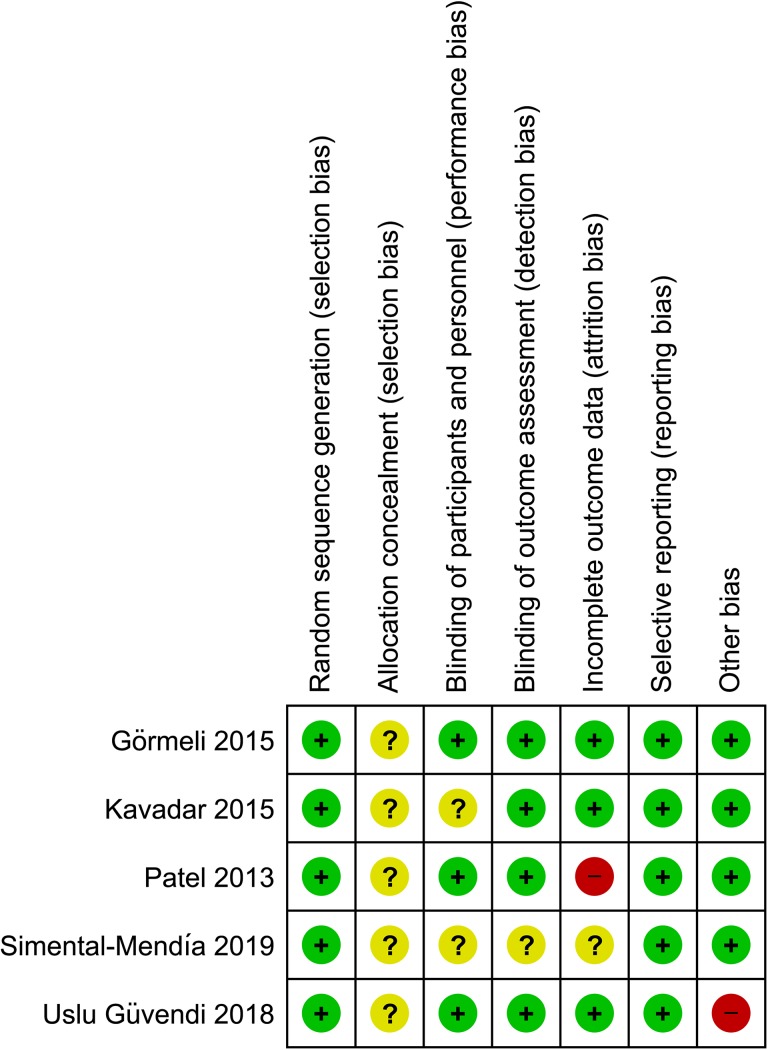
All studies were ranked as low risk of bias in the random sequence generation section; nevertheless, none of them reported the allocation concealment process, which was stated as unclear risk of bias. There were 2 studies that did not report information regarding blinding of participants and personnel, and 1 study did not report information regarding the blinded outcome assessment; therefore, they were ranked as unclear risk of bias for that section. Regarding the incomplete outcome data section, 1 study was ranked as high risk of bias because the SD for WOMAC scores at follow-up were not reported, and another study was ranked as unclear risk of bias because there was no information regarding the number of patients who were initially randomized. All studies had a low risk of bias for selective reporting, while 1 study had a high risk of bias for other biases because the injections were not ultrasound-guided and for performing a per-protocol analysis of data, although its loss-to-follow-up data was not statistically significant. The complete risk of bias assessment is shown in Figure 2.
Figure 2.
Quality of bias assessment of the included studies according to the Cochrane guidelines. “+” indicates low risk of bias; “−” indicates high risk of bias; “?” indicates unclear risk of bias.
Effectiveness of Single and Multiple PRP Injections
Pain
Essentially, 4 of the 5 included studies reported a pain assessment with the VAS/VNS12,24,30,34; 1 of these studies included one group treated with 2 PRP injections and another group treated with 3 PRP injections.12 We performed a meta-analysis between single versus multiple PRP injections and an additional subanalysis comparing single versus double PRP injections with single versus triple PRP injections (Figure 3). At 6 months, the results of the 2 analyses showed no significant differences in pain between single and double (SMD, −0.05 [95% CI, −1.97 to 1.87]; I 2 = 96%; P = .96), single and triple (SMD, 1.16 [95% CI, −0.53 to 2.86]; I 2 = 95%; P = .18), and single and multiple injections (SMD, 0.61 [95% CI, −1.09 to 2.31]; I 2 = 97%; P = .48).
Figure 3.
Forest plot displaying the standardized mean difference and 95% confidence interval for the impact of treatment with single or multiple platelet-rich plasma injections on pain (visual numerical scale and visual analog scale).
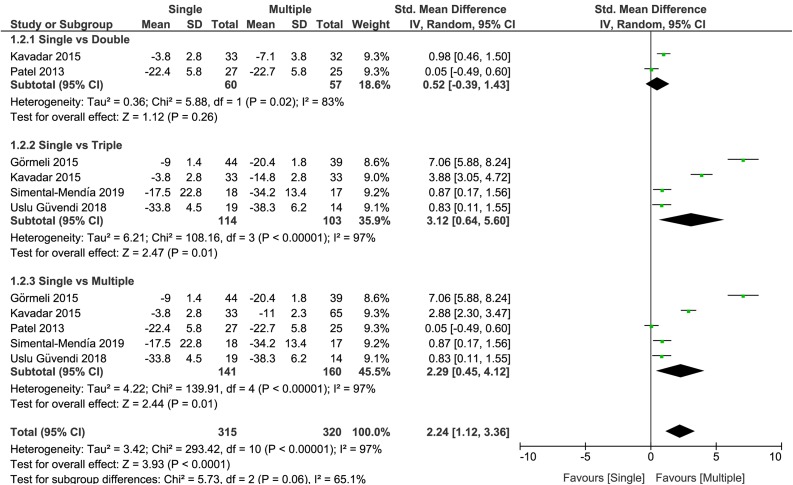
Functional Improvement
All included studies reported knee function based on patient-reported outcomes. There were 4 trials that reported the WOMAC score, whereas the other trial reported the IKDC score. For meta-analysis, we reported the SMD of the total WOMAC score and the IKDC score to compare single versus double PRP injections, single versus triple PRP injections, and single versus multiple PRP injections (Figure 4). At 6 months, the results showed no significant differences between single and double injections (SMD, 0.52 [95% CI, –0.39 to 1.43]; I 2 = 83%; P = .26); however, a significant improvement in knee functionality in favor of multiple injections for the results of single versus triple (SMD, 3.12 [95% CI, 0.64 to 5.60]; I 2 = 97%; P = .01) and single versus multiple PRP injections (SMD, 2.29 [95% CI, 0.45 to 4.12]; I 2 = 97%; P = .01) was detected. In this last scenario, the analysis was performed in all included studies.
Figure 4.
Forest plot displaying the standardized mean difference and 95% confidence interval for the impact of treatment with single or multiple platelet-rich plasma injections on joint function (Western Ontario and McMaster Universities Arthritis Index and International Knee Documentation Committee).
Discussion
As demonstrated in previous systematic reviews and meta-analyses, PRP is an effective choice of treatment for knee OA when compared to other intra-articular treatments.2,13,20 Although its clinical effectiveness for this condition has been demonstrated, there are still multiple unknown areas that must be addressed to establish a therapeutic scheme. The appropriate number of injections required to achieve clinical improvement as well as the appropriate time interval between applications are some of the aspects that have not yet been clarified. This systematic review and meta-analysis focused on exploring the most adequate number of PRP injections required to observe clinical improvement in pain and functionality with the currently available scientific evidence.
The main findings from this study suggest that within a 6-month interval, a single injection was as effective as multiple (n = 2 or 3) PRP injections in pain improvement (VAS or VNS) and that multiple injections (n = 3) were more effective than a single injection in functionality improvement (total WOMAC or IKDC). The effect of multiple PRP injections over joint functionality has been found to be not only significant (P = .0001) but also clinically important based on a threshold of 0.37 SD units, which has been established as a clinically important difference in patients with OA.17,36 In the current study, the effect size and 95% CI in favor of 3 injections versus a single injection were superior to this threshold of clinical importance. It is important to focus not only on statistical significance when reporting the effects of a treatment but also to provide plausible estimates of the magnitude of the effect in the population from which the data were analyzed. This aids in understanding how small or large the effect would be in the population of interest.28
The main objective in the study by Patel et al24 was to evaluate the role of PRP in the treatment of knee OA, focusing specifically on the number of injections and PRP concentration. They found that PRP was more effective than placebo and that 1 dose of PRP was as effective as 2 doses at 6-month follow-up. The study by Görmeli et al9 compared the efficacy of PRP injections against hyaluronic acid and placebo; it also explored the ideal number of PRP injections for treating knee OA. It concluded that 3 injections of PRP were the better choice of treatment for patients with early knee OA and that patients with advanced OA did not benefit from any of the treatments applied. Kavadar et al12 aimed to assess the effect of different numbers of PRP injections on pain and physical function. Their conclusion was that a minimum of 2 injections was necessary to observe an improvement in these parameters in patients with knee OA. While Uslu Güvendi et al34 hypothesized that PRP injections were as effective as corticosteroid injections, they found that PRP was more effective than corticosteroids and that there was no statistical difference in pain or functional improvement between applying 1 or 3 PRP injections. Simental-Mendía et al30 compared single versus triple PRP injections, finding that triple injections were clinically more effective.
Although our meta-analysis summarizes the currently available scientific evidence regarding this topic, there are some aspects that need to be clarified. There was high heterogeneity between the included studies in our meta-analysis, which can be explained by factors regarding the study population because, even though all the studies evaluated patients with knee OA, its severity tended to vary. Another factor that could have contributed to the heterogeneity is the fact that Patel et al24 and Simental-Mendía et al30 applied an activated PRP solution containing minimal or no white blood cells (WBCs), whereas the PRP solution used by Görmeli et al9 and Kavadar et al12 had an increased WBC count, and Uslu Güvendi et al34 used a nonactivated solution with increased WBCs. In addition, the platelet count also varied across PRP solutions. Another difference in the evaluated studies was the time between injections, in which the interventions varied between 1-, 2-, and 3-week intervals. The administration technique, volume injected, and methodological rigor of each study could have also influenced the heterogeneity. We handled the high heterogeneity by performing a meta-analysis with a random-effects model.
Although there are studies that have demonstrated the clinical effectiveness of PRP in knee OA, RCTs that directly compare the effectiveness of different numbers of injections on this condition are scarce. Even though a meta-analysis was performed, we consider that more research directly addressing this study question is needed to complement our results and to establish a specific therapeutic regimen for the application of PRP in knee OA. For this to be possible, it is important to discern the duration of the therapeutic effect of a single injection of PRP to establish the adequate number and time interval between injections. In addition, because knee OA has varying degrees of severity, future research studies should aim to clearly establish differences in results according to different stages of the condition. It has been reported that patients with OA in more severe stages have less clinical improvement than those in the earlier stages, independently of the number of injections applied.7-9,14
This study has some limitations. First, there were a limited number of studies included after a systematic review of the available scientific literature. Second, because of the small number of included studies, the number of studied participants was low (301 participants). Third, the follow-up of the patients was 6 months; therefore, the long-term effect of PRP injections could not be assessed. There are reports that indicate a worsening of positive therapeutic effects after 6 months.6 Finally, the aforementioned heterogeneity in patient OA severity and types of PRP used between studies is a factor to be considered.
The results of this study suggest that both single and multiple PRP injections showed pain improvement and that there was no difference between these 2 approaches, but triple PRP injections were more effective than 1 injection in improving joint functionality in patients with knee OA. However, we consider that the body of evidence supporting this assumption is still insufficient, and future research on this specific topic is needed to confirm our results.
Acknowledgment
English editing was performed by Dr Sergio Lozano (member of the American Translators Association and the American Medical Writers Association).
APPENDIX
Example of Search Strategy: Scopus
((((joints OR “Joint* Disease*” OR arthropathies OR arthroses OR arthrosis OR arthropathy OR arthralgia* OR “Joint Pain*” OR (pain W/3 joint) OR (pains W/3 joint ) OR arthritides OR “Degenerative Arthritides” OR (arthritides W/3 degenerative) OR “Degenerative Arthritis” OR osteoarthritides OR osteoarthroses OR osteoarthrosis OR (arthritis W/3 degenerative) OR “Osteoarthrosis Deformans” OR arthritis OR gonarthrosis OR “Articular Cartilage” OR “Articular Cartilages” OR (cartilages W/3 articular) OR chondromalacias OR chondromalacia)) AND ((knee OR (joint W/3 knee) OR tibiofemoral OR patellofemoral OR femoropatellar OR “Anterior Knee Pain Syndrome”))) AND ((“Conservative therapies” OR “Conservative Management*” OR “Conservative Therapy” OR “Conservative Therapies” OR (management* W/3 conservative) OR (therapies W/3 conservative) OR (therapy W/3 conservative) OR (treatment* W/3 conservative) OR “Conservative Treatment*”) OR (“Pain Management*” OR (management* W/3 pain)) OR (“multiple infiltrations” OR “multiple injections” OR “unique infiltration” OR “unique injection” OR “number of injections” OR “One application” OR “Two applications” OR “Three applications” OR “One injection” OR “Two injections” OR “Three injections” OR “One infiltration” OR “Two infiltrations” OR “Three infiltrations” OR injection* OR “Intra-Articular” OR intraarticular OR injectable*) OR (“Administration & dosage” OR “administration and dosage” OR “Drug Administration Schedule*” OR (“Drug Administration” W/3 schedules)) OR (“Blood Platelet*” OR thrombocyte OR “Blood Platelet Counts” OR “Blood Platelet Number*” OR “Platelet Number*” OR “Platelet Count*”) OR (pain OR ache OR “Burning Pain*” OR “Crushing Pain*” OR “Radiating Pain” OR “Physical Suffering*” OR “Refractory Pain” OR “Intractable Pains” OR “Pain measurement*” OR “Analog Pain Scale” OR “ Pain Assessment*” OR “McGill Pain Scale” OR “Visual Analog Pain Scale” OR “Analgesia Test” OR “Analog Pain Scales” OR “Analogue Pain Scales” OR “Pain Formalin Tests” OR “Nociception Test” OR “Pain Questionnaire” OR “Tourniquet Pain Test” OR “functional improvement” OR “Western Ontario and McMaster Universities Arthritis Index” OR “International Knee Documentation Committee” OR “IKDC” OR “functional score” OR “Recovery of Function” OR “Function Recoveries” OR “Function Recovery” OR “WOMAC” OR “visual analogue scale” OR “VAS” OR “lequesne index” OR “Treatment Outcome” OR “Clinical Effectiveness” OR “Clinical Efficacy” OR “Patient-Relevant Outcome” OR “Treatment Efficacy” OR “Clinical Effectivenesses” OR “Patient-Relevant Outcomes” OR “Treatment Effectiveness” OR “Rehabilitation Outcome*”))) AND (“Platelet Rich Plasma” OR “Platelet-Rich Plasma” OR “PRP” OR “platelet concentrate” OR “platelet derived growth factor” OR “Platelet-Derived Growth Factor” OR “PDGF” OR “platelet gel” OR “plasma rich in growth factors” OR “Centrifuged blood fraction” OR “Concentration of platelets”) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (SUBJAREA, “MEDI”) OR LIMIT-TO (SUBJAREA, “HEAL”)) AND (LIMIT-TO (SRCTYPE, “j”) OR LIMIT-TO (SRCTYPE, “p”)) AND (LIMIT-TO (EXACTKEYWORD , “Article”) OR LIMIT-TO (EXACTKEYWORD, “Human”) OR LIMIT-TO (EXACTKEYWORD, “Humans”) OR LIMIT-TO (EXACTKEYWORD, “Controlled Study”) OR LIMIT-TO (EXACTKEYWORD, “Adult”) OR LIMIT-TO (EXACTKEYWORD, “Thrombocyte Rich Plasma”))
Footnotes
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Brown GA. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition abstract. J Am Acad Orthop Surg. 2013;21(9):571–576. [DOI] [PubMed] [Google Scholar]
- 2. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213–2221. [DOI] [PubMed] [Google Scholar]
- 3. Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet. 2012;379(9823):1331–1340. [DOI] [PubMed] [Google Scholar]
- 4. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. [DOI] [PubMed] [Google Scholar]
- 5. Dold AP, Zywiel MG, Taylor DW, Dwyer T, Theodoropoulos J. Platelet-rich plasma in the management of articular cartilage pathology: a systematic review. Clin J Sport Med. 2014;24(1):31–43. [DOI] [PubMed] [Google Scholar]
- 6. Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):528–535. [DOI] [PubMed] [Google Scholar]
- 7. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Filardo G, Kon E, Pereira Ruiz MT, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082–2091. [DOI] [PubMed] [Google Scholar]
- 9. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. [DOI] [PubMed] [Google Scholar]
- 10. Higgin J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 London: Cochrane Collaboration; 2009. [Google Scholar]
- 11. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;24(5):1665–1677. [DOI] [PubMed] [Google Scholar]
- 12. Kavadar G, Demircioglu DT, Celik MY, Emre TY. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: a randomized prospective study. J Phys Ther Sci. 2015;27(12):3863–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khoshbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–2048. [DOI] [PubMed] [Google Scholar]
- 14. Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. J Arthrosc Relat Surg. 2011;27(11):1490–1501. [DOI] [PubMed] [Google Scholar]
- 15. Laudy ABM, Bakker EWP, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49(10):657–672. [DOI] [PubMed] [Google Scholar]
- 16. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):167–175. [DOI] [PubMed] [Google Scholar]
- 18. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. [DOI] [PubMed] [Google Scholar]
- 19. McGinn T, Wyer PC, Newman TB, et al. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). Can Med Assoc J. 2004;171(11):1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016. ;32(3):495–505. [DOI] [PubMed] [Google Scholar]
- 21. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185–1195. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(17):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ornetti P, Nourissat G, Berenbaum F, Sellam J, Richette P, Chevalier X. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine. 2016;83(1):31–36. [DOI] [PubMed] [Google Scholar]
- 24. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. [DOI] [PubMed] [Google Scholar]
- 25. Rachul C, Rasko JEJ, Caulfield T. Implicit hype? Representations of platelet rich plasma in the news media. PLoS One. 2017;12(8):e0182496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–191. [DOI] [PubMed] [Google Scholar]
- 27. Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. 2010;89(12):961–969. [DOI] [PubMed] [Google Scholar]
- 28. Schober P, Bossers SM, Schwarte LA. Statistical significance versus clinical importance of observed effect sizes. Anesth Analg. 2018;126(3):1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen L, Yuan T, Chen S, Xie X, Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simental-Mendía M, Acosta-Olivo CA, Hernández-Rodríguez AN, et al. Intraarticular injection of platelet-rich plasma in knee osteoarthritis: single versus triple application approach. Pilot study. Acta Reumatol Port. 2019;44(2):138–144. [PubMed] [Google Scholar]
- 31. Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34(4):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. [DOI] [PubMed] [Google Scholar]
- 33. Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35–41. [DOI] [PubMed] [Google Scholar]
- 34. Uslu Güvendi E, Aşkin A, Güvendi G, Koçyiǧit H. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Arch Rheumatol. 2018;33(3):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wandel S, Juni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;34 1(2):c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. [DOI] [PubMed] [Google Scholar]