Abstract
Objective: Polycystic ovary syndrome (PCOS) is a condition of anovulation causing infertility. Many kinds of therapy have been used to treat PCOS. However, the results have not been satisfactory. Acupuncture is a trusted way to repair the reproductive system. Yet, there is not enough evidence of the effectiveness of acupuncture to induce ovulation or to treat infertility in patients who have PCOS. The objectives of this study were to find out how successfully electroacupuncture (EA) could complete conventional therapy for patients with PCOS-related infertility, to analyze the effect of EA on these patients, and if EA could repair folliculogenesis to create quality oocytes so that these patients could become pregnant.
Materials and Methods: A case controlled study was conducted in Sekar Dr. Moewardi General Hospital, Surakarta, Central Java, Indonesia. There were 44 patients with PCOS who were included according to Rotterdam criteria and exclusion criteria. The patients' characteristics studied were age, height, weight, and duration of infertility. Subjects were divided randomly into 2 groups (22 subjects in a PCOS+Lifestyle Management as a control group and 22 subjects in a PCOS Lifestyle Management+EA case group. EA was performed for 15 minutes twice per week for a total of 12 sessions. The main outcome measure was transvaginal ultrasonographic detection of follicle size in ovulation on days 2, 6, 8, 10 and 12, starting from the first day of each patient's last menstruation.
Results: There were significant differences in follicle growth on days 2, 6, 8, 10, and 12. Follicle growth in the PCOS+Lifestyle Management group versus the PCOS Lifestyle Management+EA group was, respectively, on day 2: 5.59 ± 0.73 versus 6.45 ± 1.22, p = 0.012; on day 6: 7.40 ± 1.14 versus 9.45 ± 1.94, p = 0.012; on day 8: 9.50 ± 1.40, versus 11.63 ± 2.25, p = 0.002; on day 10: 11.59 ± 1.36, versus 13.77 ± 2.22, p = 0.001, and on day 12: 13.72 ± 1.20; versus 16.13 ± 2.43; p = 0.001.
Conclusions: EA improves oocytes' growth in patients with PCOS.
Keywords: electroacupuncture, polycystic ovary syndrome, oocytes' growth
Background
Polycystic ovary syndrome (PCOS) is a clinical condition caused by oligo-ovulation or anovulation, hyperandrogenism (either chemical or biochemical), and the presence of polycysytic ovaries.1 This condition has caused 40% of infertility cases, among whom the most common disorder was anovulatory infertility. Anovulation is a condition in which there are few ovum fertilized by sperm, thus the chance of achieving pregnancy is low.
Women with PCOS undergo therapy because of their infertility. After being married for 1 year and having regular intercourse without contraception, these women find that they cannot become pregnant. There are ∼90%–95% women with anovulation who come to an infertility clinic; these patients are diagnosed as having PCOS.2 PCOS has many kinds of symptoms and underlying problems, such as menstrual disorders, hyperandrogenism, and polycystic ovaries caused by a disorder of the endocrine system in women of reproductive age.3
It has been proven that acupuncture can increase menstrual frequency and decrease testosterone circulation in women with PCOS. There are many studies showing a prevalence of acupuncture used in patients undergoing infertility therapy. Prevalent studies in the United States—including in 8 infertility centers in 2010—reported that 29% of their patients were using complementary and alternative therapy to treat their infertility and 22% of these patients had acupuncture therapy.4,5 Clinical experiments in many countries showed that acupuncture could repair reproductive function. However, a trial of electroacupuncture (EA) did not produce enough evidence to determine the effectiveness of EA to induce ovulation or to treat infertility in patients.6 Based on that study, more studies are needed to analyze the effect of EA on patients with PCOS, with respect to repairing the folliculogenesis process.
Materials and Methods
This was an analytical observational study, using a prior case control study that was conducted in the infertility clinic of Sekar Dr. Moewardi General Hospital, Surakarta, Central Java, Indonesia, for 12 months. Subjects were divided into 2 groups, with 22 subjects in a PCOS+Lifestyle Management group and 22 subjects in a PCOS+Lifestyle Management+EA group. Lifestyle management for PCOS ranges from actual lifestyle management to pharmacologic interventions. Actual lifestyle management is associated with diet, exercise, and weight loss; patients are given information about the importance of weight management, a healthy diet, and a need for physical activity (WHO recommendations).7
To establish a PCOS diagnosis, the Rotterdam criteria were used; this enabled a choice of 2 of 3 conditions, including clinical or biochemical signs of:
-
(1)
Oligo-ovulation or anovulation—Oligomenorrhea is defined as an intermenstrual interval >35 days and <8 menstrual bleeding cycles in the past year. Amenorrhea is defined as <3 cycles per year.
-
(2)
Hyperandrogenism (hirsutism or acne)—Acne is defined by a positive response to the question. “Do you have acne?” Meanwhile, hirsutism is defined as a self-reported Ferriman–Gallwey (FG) score ≥8 (≥ 5 in Asian subjects).
-
(3)
Polycystic ovaries (PCO)—PCO is shown on ultrasonography (US). PCO is detected by a transvaginal ultrasound (TVUS) finding of ≥12 follicles of 2–9 mm, and an ovarian volume of ≥10 mL of one or both ovaries.7,8
Subjects were ages 20–45 and were infertile.
Exclusion criteria included nonclassical congenital adrenal hyperplasia due to deficiency of 21-hydroxylase, Cushing's syndrome; hyperprolactinemia; hyperthyroidism or prolactinoma; primary hypothyroidism; acromegaly; obesity; virilizing adrenal neoplasms of the ovaries; and use of such pharmaceuticals as androgen, valproic acid, and cyclosporin.8,9
Controls were subjects chosen by applying fixed diseases sampling based on selecting subjects related to disease status. Meanwhile, exposure status was related to disease status, which had fixed characteristics.
EA therapy was performed with stainless-steel acupuncture needles (Huanqiu, China) that were sterile and used only once. The needles were 0.25 × 25 mm or 0.25 × 40 mm, depending on each subject's body mass index (BMI). The EA involved use of a stimulator (Hwato SDZ V, Shanghai China) producing 15 minutes of continuous wave stimulation at 2 Hz. The treatments were applied twice per week until 12 treatments were provided. Points used were CV 3 (Zhongji), CV 6 (Qihai), ST 29 (Guilai) bilateral, SP 6 (Sanyinjiao) bilateral, LI 4 (Hegu) bilateral, Dan ST 36 (Zusanli) bilateral. The locations treated were: CV 3 on the midline, 4 cun caudal to the umbilicus; CV 6 on the midline, 1.5 cun caudal to the umbilicus; ST 29, 1 cun cranial to the pubic bone and 2 cun lateral to the midline; SP 6, 3 cun proximal to the medial malleolus; LI 4, on the highest point at the musculi interosseus dorsalis; and ST 36, on the anterior lateral side of the leg 3 cun below Dubi (ST 35), which is one (middle) finger width from the anterior crest of the tibia.10
Follicle growth was measured on days 2, 6, 8, 10, and 12 after menstruation. The resulting data were analyzed with the Mann-Whitney-U test. The Statistical Package for Social Science (SPSS) 24.0 for Windows was used.
All subjects were instructed to read the informed consent forms carefully and to sign agreement on them.
Outcome Measures/Assessment
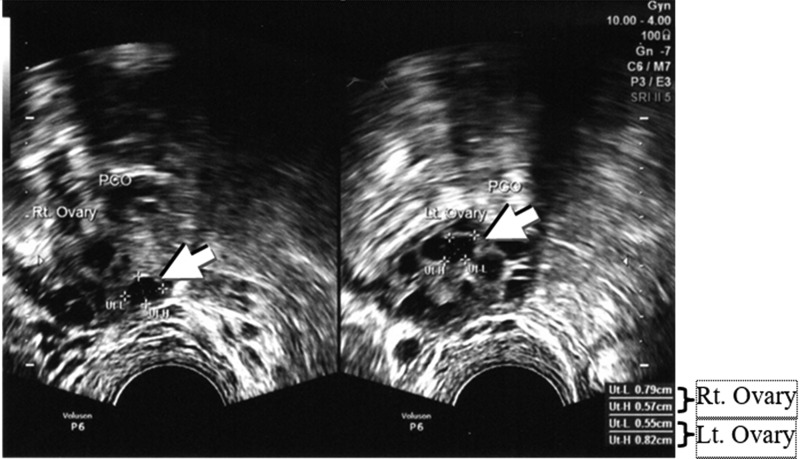
Both groups had their follicle sizes measured via TVUS (VolusonTM P6, General Electric Healthcare). Furthermore, when ovulation was detected on days 2, 6, 8, 10, 12, the largest diameter of the follicles from both ovaries of the subjects were recorded, regardless of group assignments. These data were analyzed with a Mann-Whitney-U test with a significance of P < 0.05.Figure 1 shows the follicles in PCOS.
FIG. 1.
Follicle growth in polycystic ovary syndrome (arrows). Follicle sizes were measured for Ut-L (follicle length), diameter 1; and Ut-H (follicle height), diameter 2. This transvaginal ultrasound was taken on day 2 of menstruation. A diameter of 0.68 cm in the right ovary was seen clearly (0.79 + 0.57)/2 = 0.68, and there was a diameter of 0.69 cm in the left ovary (0.55 + 0.82)/2 = 0.69.
Ethical Clearance
Ethical clearance was approved by the commission of ethical health research of Dr. Moewardi General Hospital in Central Java, and the medical faculty of Sebelas Maret University, Surakarta, Central Java, Indonesia, Number: 646/VIII/HREC/2018, on August 8, 2018.
Results
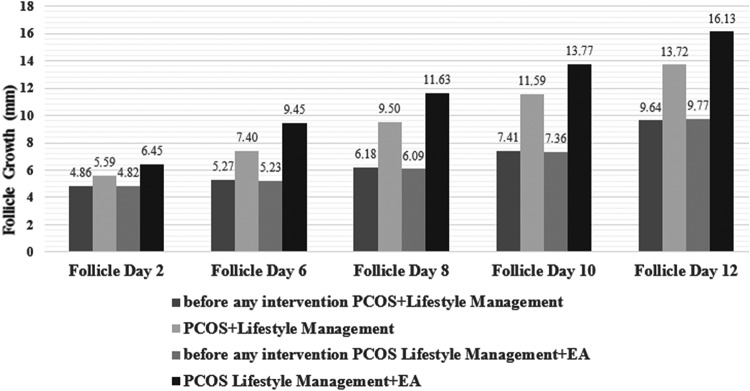
Figure 2 shows the results.
FIG. 2.
Comparison of oocytes' growth in the menstrual cycle on days 2, 6, 8, 10, and 12 before any intervention (PCOS+Lifestyle Management), PCOS+Lifestyle Management, before any intervention (PCOS Lifestyle Management+EA), and PCOS Lifestyle Management+EA. PCOS, polycystic ovary syndrome; EA electroacupuncture.
Follicle growth between before any intervention (PCOS+Lifestyle Management), compared to before any intervention (PCOS Lifestyle Management+EA) on days 2, 6, 8, 10, and 12 was not significant. Detection of ovulation on day 2 was, respectively, 4.86 ± 0.63 versus 4.82 ± 0.75, P = 0.907; on day 6 was, respectively, 5.27 ± 0.55 versus 5.23 ± 0.81, P = 0.824; on day 8 was, respectively, 6.18 ± 0.58 versus 6.09 ± 0.75, P = 0.534; on day 10 was, respectively, 7.41 ± 0.59 versus 7.36 ± 0.72, P = 0.764; and| on day 12 was, respectively, 9.64 ± 0.65; versus 9.77 ± 0.61, P = 0.431. See Table 1.
Table 1.
Correlations Among Age, Height, Weight, BMI, & Duration of Infertility Between PCOS+Lifestyle Management & PCOS Lifestyle Management+EA Groups
| Variables | n | Mean | SD | P |
|---|---|---|---|---|
| Age | ||||
| PCOS+Lifestyle Management (yr) | 22 | 29.50 | 4.31 | 0.991 |
| PCOS Lifestyle Management+EA (yr) | 22 | 29.36 | 4.25 | |
| Height | ||||
| PCOS+Lifestyle Management (cm) | 22 | 157.63 | 4.29 | 0.516 |
| PCOS Lifestyle Management+EA (cm) | 22 | 157.27 | 5.40 | |
| Weight | ||||
| PCOS+Lifestyle Management (kg) | 22 | 58.09 | 10.82 | 0.074 |
| PCOS Lifestyle Management+EA (kg) | 22 | 63.90 | 11.83 | |
| BMI | ||||
| PCOS+Lifestyle Management (kg/m2) | 22 | 22.95 | 3.99 | 0.105 |
| PCOS Lifestyle Management+EA (kg/m2) | 22 | 24.90 | 4.37 | |
| Duration of infertility | ||||
| PCOS+Lifestyle Management (yr) | 22 | 3.54 | 2.70 | 0.559 |
| PCOS Lifestyle Management+EA (yr) | 22 | 3.79 | 2.82 |
BMI, body mass index; PCOS, polycystic ovary syndrome; EA, electroacupuncture; SD, standard deviation, yr, years.
Follicle diameter growth on days 2, 6, 8, 10, and 12 was larger in the PCOS Lifestyle Management+EA group, compared to the PCOS+Lifestyle Management group. This result was significantly different. Detection of ovulation on day 2 for PCOS+Lifestyle Management was 5.59 ± 0.73, and, for PCOS Lifestyle Management+EA, was 6.45 ± 1.22; P = 0.012. On day 6, detection of ovulation for PCOS+Lifestyle Management was 7.40 ± 1.14 and, for PCOS Lifestyle Management+EA, was 9.45 ± 1.94; P = 0.012. On day 8, detection of ovulation for PCOS+Lifestyle Management was 9.50 ± 1.40, and for PCOS Lifestyle Management+EA, was 11.63 ± 2.25; P = 0.002. On day 10, detection of ovulation for PCOS+Lifestyle Management was 11.59 ± 1for.36, and for PCOS Lifestyle Management+EA, was 13.77 ± 2.22; P = 0.001. On day 12, detection of ovulation for PCOS+Lifestyle Management was 13.72 ± 1.20, and for PCOS Lifestyle Management+EA was 16.13 ± 2.43; P = 0.001.
Discussion
Analysis of the resulting follicle sizes between the PCOS+Lifestyle Management and PCOS Lifestyle Management+EA groups with P < 0.05 (Fig. 2) showed that there were significant differences in follicle sizes on days 2, 6, 8, 10, and 12.
This result was similar to another result that was noted by Stener-Victorin and Wu,11 who used EA to treat 24 women with PCOS. There were actually decreases of PCOS symptoms in that study. Of the 24 who were treated with EA 9 had increased ovulation, as shown in a study by Qu et al.12
In another study, acupuncture resulted in higher ovulation frequencies in overweight women with PCOS, and these treatments were more effective. However, ovarian and adrenal sex-steroid serum levels were reduced but luteinizing hormone (LH) secretion remained unaffected.9 Acupuncture could increase recovery of menstrual cycles significantly and decrease BMI and LH in women with PCOS. Furthermore, acupuncture could improve the level of ovulation and regulation of the menstrual cycle.
In addition, acupuncture had significant statistical advantages for therapy, with respect to LH, LH/follicular stimulating hormone (FSH), testosterone, fasting insulin, and pregnancy stage, although the evidence in both studies was considered to be low-level confidence in a systematic review and meta-analysis.13
In yet another study, EA, at 2 Hz, was given to subjects at CV 3, CV 6, ST 29, SP 6, and SP 9, and manual acupuncture was given another group at LI 4 or PC 6. Treatments were given twice weekly for 2 weeks, then once weekly for 6 weeks, and once every other week for 8 week, for a total of 14 treatments over 16 weeks. EA and physical exercise in this study reduced hyperandrogenism and improved menstrual frequency more effectively than no intervention in women with PCOS. Low-frequency EA was superior to physical exercise and might be useful for treating hyperandrogenism and oligo/amenorrhea.14
Acupuncture decreased symptoms of PCOS, in a study, through modulation of the endogen regulation system, including the sympathetic nervous system (SNS), endocrine system, and neuroendocrine system. This change might have occurred through the endogen opioid system.15 Anovulatory women showed high β-endorphin plasma levels, and the women's low skin temperature revealed an increase of sympathetic nervous activity that could be repaired by EA. This study demonstrated that EA could inhibit the SNS, which had a possible role in PCOS.11
Intramuscular needle insertion caused activity of afferent muscles' peripheral nerves. Depending on the intensity, stimulation with acupuncture needles could activate afferent muscles to the spinal cord and central nervous system. In EA, electrical stimulation at a low frequency (1–15 Hz) would stimulate ergoreceptors in muscles that are activated physiologically during muscle contractions. Low-frequency EA caused a greater number of releasing neoropeptides, serotonin, endogeneous opioids, and oxytocin, which were crucial to inducing functional changes in different organ systems.11,16
β-Endorphin was produced and released from the nucleus, hypothalamus, arcuate nucleus, and nucleus tractus solitarius in the brain. The hypothalamus system of central β-endorphin became a key mediator of auotonomic functional changes after acupuncture. This change was possibly caused by the inhibition of vasomotor centers, which could decrease sympathetic tonus and blood pressure. β-Endorphin was also released into the peripheral blood flow from the hypothalamus through the anterior hypophysis in a process managed by corticotropin-releasing factor (CRF) secreted from the paraventricular nucleus of the hypothalamus.
Plasma β-endorphin is supposed to be associate with the hyperinsulinemia response and stress. Stress could increase the activity of the hypothalamus–pituitary–adrenal (HPA) axis and decrease reproductive function. Thus, hormones from the HPA axis has strong correlations with the hypothalamic–pituitary–gonadal (HPG) axis, CRF, adrenocorticotropic hormone, β-endorphin, and adrenal corticosteroids that modulates stress effects on reproductive function. Acupuncture has influenced the HPA axis by decreasing cortisol concentration and also influenced the HPG axis by modulating production and secretion of central and peripheral endorphins. Therefore, this treatment could influence releasing of gonadotropin-releasing hormone in the hypothalamus and secretion of gonadotropin hormone in the hypophysis.17,18
Acupuncture effects depend on the kinds of stimulation (manual or electrical stimulation) applied, the frequency of stimulation (number of manual manipulations or frequency of electrical stimulations), the number of inserted acupuncture needles, how often the treatments are applied, the duration of acupuncture treatments, and environmental and psychologic factors. Therefore, many variables could influence the results of a study of acupuncture.11
Conclusions
EA produced significant effects on follicle sizes on days 2, 6, 8, 10, and 12; this was shown when comparing the PCOS+Lifestyle Management and PCOS Lifestyle Management+EA groups. Moreover, EA influenced improvements of oocytes' growth in patients with PCOS.
Acknowledgments
The authors thank Suharto Wijanarko, MD, PhD, director of the Sekar, Dr. Moewardi General Hospital for his permission and cooperation to conduct this research in this hospital.
Author Disclosure Statement
No financial conflicts of interest exist.
Funding Information
This article is the output of research funded by Penerimaan Negara Bukan Pajak (PNBP) number 516/UN27.21/PP/2019.
References
- 1. Fauser BC, Tarlatzis BC, Rebar RW, et al. . Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25 [DOI] [PubMed] [Google Scholar]
- 2. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3(1):25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JF, Eisenberg ML, Millstein SG, et al. ; Infertility Outcomes Program Project Group. The use of complementary and alternative fertility treatment in couples seeking fertility care: Data from a prospective cohort in the United States. Fertil Steril. 2010;93(7):2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pachiappan S, Matheswaran S, Saravanan PP, Muthusamy G,. Medicinal plants for polycystic ovary syndrome: A review of phytomedicine research. Int J Herb Med. 2017;5(2[ptB]):78–80 [Google Scholar]
- 6. Chen J, Feng S, Zeng J, et al. . Effectiveness of electroacupuncture for polycystic ovary syndrome: Study protocol for a randomized controlled trial. Trials. 2016;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stener-Victorin E, Zhang H, Li R, et al. . Acupuncture or metformin to improve insulin resistance in women with polycystic ovary syndrome: Study protocol of a combined multinational cross sectional case-control study and a randomised controlled trial. BMJ Open. 2019;9(1):e024733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azziz R. Diagnosis of polycystic ovarian syndrome: The Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91(3):781–785 [DOI] [PubMed] [Google Scholar]
- 9. Johansson J, Stener-Victorin E. Polycystic ovary syndrome: Effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Alternat Med. 2013;2013:762615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Hung EHY, Stener-Victorian E, et al. . Comparison of acupuncture pretreatment followed by letrozole versus letrozole alone on live birth in anovulatory infertile women with polycystic ovary syndrome: A study protocol for a randomised controlled trial. BMJ Open 2016;6(10):e010955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stener-Victorin E, Wu X. Effects and mechanisms of acupuncture in the reproductive system. Auton Neurosci Basic Clin. 2010;157(1–2):46–51 [DOI] [PubMed] [Google Scholar]
- 12. Qu F, Wu Y, Hu X-Y, et al. . The effects of acupuncture on polycystic ovary syndrome: A systematic review and meta-analysis. Eur J Integr Med. 2016;8(1):12–18 [Google Scholar]
- 13. Jo J, KMD, Lee YJ, Lee H. Acupuncture for polycystic ovary syndrome: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(23)e7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jedel E, Labrie F, Odén A, et al. . Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: A randomized controlled trial. Am J Physiol Endocrinol Metab. 2011;300(1):E37–E45 [DOI] [PubMed] [Google Scholar]
- 15. Stener-Victorin E, Jedel E, Mannerås L. Acupuncture in polycystic ovary syndrome: Current experimental and clinical evidence. J Neuroendocrinol. 2008;20(3):290–298 [DOI] [PubMed] [Google Scholar]
- 16. Andersson S, Lundeberg T. Acupuncture- from empiricism to science: Functional background to acupuncture effects in pain and disease. Med Hypotheses 1995;45(3):271–281 [DOI] [PubMed] [Google Scholar]
- 17. Stener-Victorin E, Waldenström U, Tāgnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2000;79(3):180–188 [PubMed] [Google Scholar]
- 18. Stener-Victorin E, Lindholm C. Immunity and beta-endorphin concentrations in hypothalamus and plasma in rats with steroid-induced polycystic ovaries: Effect of low-frequency electroacupuncture. Biol Reprod. 2004;70(2):329–333 [DOI] [PubMed] [Google Scholar]