Abstract
Significance: Maternal health and diet during gestation are critical for predicting fetal outcomes, both immediately at birth and in adulthood. While epigenetic modifications have previously been tightly linked to carcinogenesis, recent advances in the field have suggested that numerous adulthood diseases, including those characteristic of metabolic syndrome, could be programmed in utero in response to maternal exposures, and these “programmable” diseases are associated with epigenetic modifications of vital genes. Recent Advances: While little is currently known about the epigenetic regulation of the antioxidant (AOX) defense system, several studies in animals show that AOX defense capacity may be programmed in utero, making it likely that the critical genes involved in this pathway are epigenetically regulated, either by DNA methylation or by the modification of histone tails. Critical Issues: This article presents the most current knowledge of the in utero regulation of the AOX defense capacity, and will specifically focus on the potential epigenetic regulation of this system in response to various in utero exposures or stimuli. The ability to appropriately respond to oxidative stress is critical for the health and survival of any organism, and the potential programming of this capacity may provide a link between the in utero environment and the tendency of certain individuals to be more susceptible toward disease stimuli in their postnatal environments. Future Directions: We sincerely hope that future studies which result in a deeper understanding of the in utero programming of the epigenome will lead to novel and effective therapies for the treatment of epigenetically linked diseases. Antioxid. Redox Signal. 17, 237–253.
Introduction
The antioxidant (AOX) defense system plays an integral part in an organism's ability to deal with environmental toxins and disease conditions, so any dysfunction within this system is detrimental to health and well-being. The fetus is sensitive and responsive to the maternal milieu, and there is evidence that the oxidative stress that accompanies many pregnancy-associated disorders has the potential to affect fetal development. Additionally, since the placenta does not prevent the passage of most harmful substances from mother to fetus, studies have also shown that numerous environmental toxins that a mother is exposed to during pregnancy can be transferred directly to the offspring (86), resulting in the activation and potential programming of the AOX defense system.
The role of epigenetics in the programming of gene expression has been confirmed in various cell and animal models. While epigenetic modifications do not change the genetic code itself, events such as DNA methylation and histone modification alter the conformation of the DNA, which has a substantial effect on gene transcription. Cancer development and progression has been and continues to be a major focus of epigenetic studies, as numerous cell-cycle genes have been shown to be controlled by DNA methylation and histone modification. However, recent studies from the field of “Developmental Origins of Human Disease” have suggested that the in utero environment also has the potential to program gene expression through epigenetic modifications. While studies are limited, it has been suggested that genes involved in numerous postnatal diseases, including cancer, diabetes, heart disease, and liver disease, may be programmed prenatally, potentially through the action of DNA methylation and histone modification.
Disease states are accompanied by an increase in oxidative damage and a decrease in the AOX capacity, so the potential programming of this crucial system during the prenatal period is vital for the long-term health of the offspring. However, studies that focus on the in utero programming of the AOX defense system are limited, as are those that focus on the epigenetic control of AOX genes. This article aims at presenting current data related to the oxidative balance that occurs during pregnancy, as well as to the consequences of maternal oxidative stress on fetal development. Additionally, we intend to introduce the most current knowledge related to the epigenetic control of the AOX defense system. Finally, since data related to the epigenetic programming of AOX genes in utero are sparsely available, this article aims at identifying potential future directions in the field. Women are exposed to an ever-increasing barrage of stressors during pregnancy, both from their environmental surroundings, as well as by the world-wide increase in metabolic syndrome and other diseases, so they may have direct control over the programming of disease development in future generations. Therefore, unraveling the sources and mechanisms behind the programming of adult-onset diseases may help assure that offspring are armed with the best possible defense against their potentially unhealthful environment.
Oxidative Balance During Pregnancy
Pregnancy alters maternal physiology, including hemodynamics such as increased cardiac output, blood volume and composition, resting pulse rate, and decreased systemic vascular resistance. Maternal metabolic processes are also modified to ensure a readily available supply of substrate to support placental and fetal growth and development. Metabolic adaptations and hormonal changes during pregnancy increase weight gain, insulin resistance, fat deposition, and hyperlipidemia (94). Maternal hyperlipidemia during pregnancy facilitates the availability of lipids and fuel to the fetus but can also contribute to lipid peroxidation and the reduction of AOX capacity. Oxidative stress occurs when the production of reactive oxygen species (ROS) exceeds their reduction by the AOX defense system. Pregnancy is a time of increased oxidative stress within both maternal and fetal tissues. It is clear that an appropriate balance between ROS production and AOX function is required for all aspects of mammalian reproduction. The corpus luteum is essential for the priming of the uterus for pregnancy in humans and for the maintenance of pregnancy in other species, and it has been shown that the AOX enzymes (superoxide dismutase 1 [SOD1], glutathione peroxidase (GPx), glutathione-s-transferase [GST]) prevent luteal apoptosis and regulate its function (3, 112, 116). Additionally, AOX enzymes appear to regulate fertilization (65), implantation (9), decidualization (34), and uterine contractions (130).
The placental AOX system is also activated during the earliest stages of development (54), which is not surprising, as this rapidly developing tissue is infiltrated with numerous ROS-producing compounds and a high volume of oxygenated blood. It has been suggested that during the first trimester of pregnancy, the placenta may function to limit the oxygen supply to the fetus to maintain a relatively hypoxic environment in utero, which may explain the activation of the AOX defense system within even the earliest period of placental development (53). The placental AOX capacity is maintained throughout pregnancy, as numerous AOX enzymes have been shown to be upregulated during all stages of fetal development (41, 54, 56, 115).
While excess ROS are generally thought to be damaging to cells and tissues (44), they are also required for appropriate fetal angiogenesis fetal development (32). During normal development, the early embryo has little AOX capacity and appears to be exceptionally sensitive to oxidative stress (37), but as its AOX capacity increases, low levels of oxidation become bearable and even absolutely required for development (21). In fact, oxidation has been shown to be required for differentiation and cell-cycle control (4, 5, 36, 107, 126), so it appears that there is a fine balance between oxidation and reduction (also referred to as the redox state) during the early stages of fetal development.
Oxidative Stress in Maternal Disorders
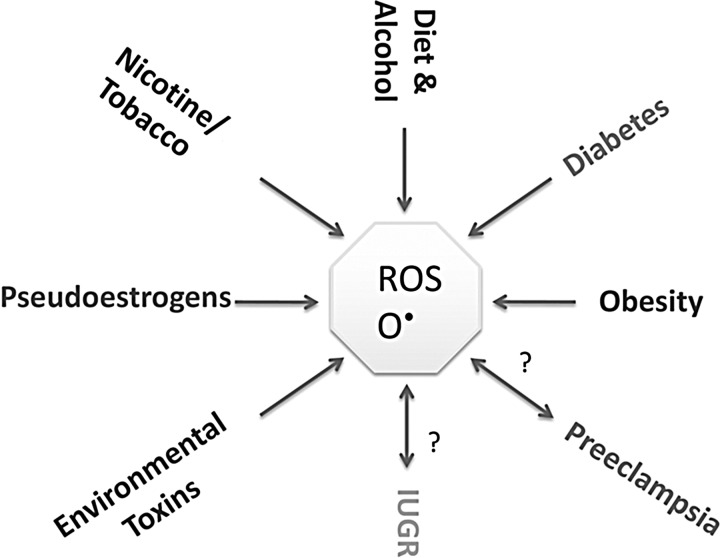
Maternal health is a definitive predictor of fetal outcomes, and it appears that many of the maternal disorders associated with poor pregnancy outcomes are also accompanied by increased oxidative stress or an inability to appropriately respond to the increased oxidative burden of pregnancy. Additionally, as discussed next, numerous environmental factors have been shown to dysregulate the fine balance between pro- and antioxidants during pregnancy (Fig. 1). Therefore, while it is unclear whether maternal or placental oxidative stress is directly passed to the developing fetus or simply acts as a signal for the fetus to alter their own physiology and metabolism, the consequences of increased oxidative stress and decreased AOX capacity appear to have severe negative effects on fetal development.
FIG. 1.
Sources of ROS during pregnancy. Under standard conditions, pregnancy is a time of elevated oxidative stress. However, numerous physiological and environmental stressors can further increase ROS production and oxidative stress, which can be detrimental for both the mother and the fetus. IUGR, intrauterine growth restriction; ROS, reactive oxygen species.
Preeclampsia and oxidative stress
Preeclampsia, a pregnancy-associated disease characterized by hypertension and proteinuria, is considered one of the leading causes of maternal mortality, and its rates continue to rise (108). Numerous studies have shown that preeclampsia is accompanied by increased inflammation and oxidative stress as well as a decrease in AOX capacity (99), maternal plasma biomarkers of which have included increased leptin (8), MCP-1 (59), interleukin (IL)-6 (91), IL-8 (59), malondialdehyde (MDA); thiobarbituric acid reactive substances (TBARS) (75), H2O2 (124), and tumor necrosis factor-α (14). Preeclampsia has also been associated with placental pathologies, and a concurrent increase in placental oxidative stress (7, 75, 84, 105, 140), which is thought to decrease placental efficiency and thereby impair fetal growth capacity (52). Despite the extensive data connecting preeclampsia and placental oxidative stress, there is still no definitive cause and effect established between the disease, oxidative balance, and fetal outcomes. It is likely that numerous factors tip the balance toward increased placental oxidative stress, including general maternal inflammation and excessive ROS production, and these factors likely contribute to the development of preeclampsia, which, in turn, feeds the cycle of increased ROS production.
Intrauterine growth restriction and oxidative stress
In addition to preeclampsia, intrauterine growth restriction (IUGR) is now widely accepted as being associated with poor birth outcomes and an increased risk for many adult-onset diseases. Additionally, many (but not all) cases of IUGR are thought to occur due to placental insufficiency (61) [some due to preeclampsia (89)], which is also accompanied by placental oxidative stress (114). As just bdiscussed, the placenta is subjected to high volumes of oxygenated blood to support fetal development (23), and is, therefore, at risk for increased ROS production. A study comparing women who gave birth to either normal-weight or IUGR babies showed that the levels of MDA and xanthine oxidase were higher in maternal plasma, umbilical cord plasma, and the placenta when compared with normal mothers, and this corresponded to a decrease in the AOX potential within these samples (15). In cord blood from IUGR neonates, AOXs as well as the activity of AOX enzymes, including SOD, catalase, and glutathione peroxidase, were significantly decreased when compared with normal-weight babies, and this was accompanied by increased lipid peroxidation (50). Another study comparing small, average, and large-for-gestational-age infants showed that while oxidative stress was higher and AOX status was lower in small and large infants when compared with those of an average size, the AOX status of mothers who gave birth to small but not large babies was lower when compared with mothers of average-sized infants (100), suggesting that IUGR pregnancies are accompanied by whole-system oxidative stress that is potentially passed to the infant from maternal circulation.
Diabetes and oxidative stress
While patients with diabetes often exhibit signs of whole-system oxidative stress (78), several recent studies have suggested that in utero exposure to hyperglycemia, either in pregnancies complicated by gestational diabetes mellitus (GDM) or in women with uncontrolled Type 1 or Type 2 diabetes, results in oxidative stress in the embryo or fetus. This likely occurs, because the metabolism of excess glucose results in the increased production of ROS. Cell culture studies using rat embryos showed that incubation with glucose led to an increase in the activity of SOD, the mRNA expression of Cu/ZnSOD, MnSOD, and GPx (40), as well as a decrease in the activity of the Glutathione (GSH) AOX enzyme (123). In humans, placentas of women with GDM had increased isoprostane release, SOD activity, and protein carboyl activity (30), and an analysis in placental explants after exposure to oxidative stress demonstrated that placentas of women with gestational diabetes were less responsive in terms of isoprostane release and NF-kappaB DNA-binding than women without GDM, suggesting a decreased capacity to respond to oxidative stress in these tissues (29). These molecular alterations in response to oxidative stress could be potentially linked to embryonic malformations (90), and due to this, elevated gestational glucose has been classified by some as a teratogen (134).
Maternal obesity and oxidative stress
Obesity is a state of increased inflammation and oxidative stress, and there is evidence that gestational obesity not only affects maternal oxidative status, but can also impact the offspring. It has been suggested that the reason for this may be the fact that preeclampsia in obese women has been shown to occur two- to fourfold more frequently than in normal-weight women (64, 102), and as discussed earlier, preeclampsia is associated with increased oxidative stress, in both the mother and offspring. However, several studies in animals suggest that the oxidative stress during obese pregnancy may modulate oxidative stress independently of preeclampsia. In a mouse model of diet-induced obesity, oocytes and zygotes of obese dams had significantly increased rates of ROS generation as well as a depletion in glutathione (51), and in offspring of diet-induced obese rats, oxygen radical absorbance capacity (ORAC) levels and catalase (CAT) activity were decreased from birth through adulthood, and SOD activity was increased by the time offspring reached adulthood (17). Additional studies in animals and humans will be needed to tease out the effects of obesity-induced preeclampsia and maternal obesity alone on fetal outcomes and oxidative balance.
Consequences of Environmental Toxins During Pregnancy
Exposure to environmental toxins and endocrine disruptors in utero has long ago been demonstrated to regulate embryonic as well as fetal development and adulthood phenotypes (31). Many of these compounds are known to induce ROS production and oxidative stress (63); therefore, their deleterious action during pregnancy has been proposed to correspond to their capacity to generate oxidative stress in either mother or fetus.
Xenobiotics, heavy metals, and oxidative stress
In rats, a gestational exposure to lead and cadmium led to a gender-specific activation of hepatic phase 1 and 2 xenobiotic-metabolizing enzymes in adult offspring, and cadmium had the strongest effect on depressing the activities of AOX enzymes, including Cu/Zn-SOD, Mn-SOD, CAT, GPx, and Glutathione Reductase (GR) (95). In mice, an injection with the endotoxin lipopolysaccharide (LPS) during gestation resulted in fetal malformations, and these were associated with an increase in lipid peroxidation and decrease in glutathione content in maternal liver, embryo, and the placenta. Additionally, a free-radical trapping agent reversed these outcomes, including reducing the number of external malformations (136), suggesting that the teratogenic effects of LPS are, at least in part, mediated by oxidative stress. Exposure to polycyclic aromatic hydrocarbons (PAHs), from either environmental pollutants or maternal tobacco use, during pregnancy has been shown to influence both physical (96) and cognitive development (92) in the offspring. PAH exposure is associated with increased oxidative stress, and a study comparing PAH exposure, pregnancy oxidative stress, and fruit/vegetable intake found that an association between markers of PAH exposure and oxidative stress, and interestingly, the increased intake of fruits and vegetables during pregnancy eliminated this association (60), suggesting not only that PAH exposure in pregnancy is directly associated with maternal oxidative stress, but also that this outcome can be modulated by diet.
Bisphenol A and oxidative stress
Bisphenol A (BPA) exposure has been shown to be toxic to numerous organ systems, in both humans and animals, presumably due to its activity as a pseudo-estrogen (45). Few studies are available to clearly establish the relationship between BPA and oxidative stress, except that it has been proposed that estrogens cause ROS-induced DNA damage, and BPA may behave in a similar manner to induce cellular damage (27). In mice, a 5-day injection of BPA induced SOD and decreased catalase activity in the liver, and led to an increase in reduced glutathione and glutathione disulfide in the brain, kidney, liver, and testes, and these outcomes were hypothesized to be due to the BPA-mediated increase in hydrogen peroxide production in mouse organs (58). Due to its teratogenic effects, the in utero exposure to BPA has received considerable attention (42), and recent animal studies have attempted to provide the molecular basis for the deleterious effects of BPA exposure on fetal outcomes. BPA exposure limited to the periods of gestation and lactation in a mouse model resulted in a decrease in the weight of the brain, kidneys, and testes in 4-week old offspring, with a concurrent increase of TBARS in these tissues and an increase in the activity of catalase in the liver and glutathione peroxidase in the kidneys (57). While confirming the toxic nature of BPA has become of critical importance, primarily due to its ubiquitous nature, other environmental endocrine disruptors should also be closely monitored and studied to determine their effects on oxidative stress, especially if these also play a role in modulating fetal outcomes and health in adulthood.
Maternal cigarette or nicotine exposure and oxidative stress
The exposure to tobacco smoke during pregnancy has been linked to negative pregnancy outcomes (101), and numerous studies have suggested that these outcomes may be directly associated with the capacity of cigarette smoke or nicotine to induce oxidative stress, both in the mother and the offspring. A recent transcriptome analysis of pregnant smokers and non-smokers showed that smoking was associated with altered expression of numerous oxidative stress-related genes in peripheral blood, placenta, and cord blood, suggesting that smoking significantly affects oxidative balance in maternal and fetal tissues (128). In fetal explant cultures, treatment with cigarette smoke extract resulted in increased markers of oxidative stress and a decrease in anti-apoptotic markers (81). While poor pregnancy outcomes have been historically associated with cigarette smoke itself, studies suggest that nicotine, independent of smoking, has dire consequences during pregnancy, potentially by inducing oxidative stress. In rats, nicotine administration increased arterial contractions and hypertension in the offspring, and this was associated with decreased SOD activity, and increased superoxide, MDA, and nitrotyrosine protein levels in the vascular walls of adult offspring (133). Additionally, offspring of rats that were prenatally exposed to nicotine had pancreatic islet oxidative stress, mitochondrial abnormalities, glucose intolerance, and reduced glucose-stimulated insulin secretion, indicating that prenatal nicotine exposure induced a diabetic phenotype in offspring (20). Specifically, the markers of pancreatic oxidative stress in offspring in response to nicotine exposure included increased Gpx-1, MnSOD, islet ROS, and protein carbonyl production (19). These studies unquestionably support the notion that maternal smoking induces oxidative damage, in both the mother and the fetus, and future studies are needed to determine whether addressing the oxidative effects of smoking and nicotine can ameliorate the damage caused by maternal smoking.
Consequences of Maternal Diet
Numerous dietary as well as physiological pregnancy manipulations have been utilized in animals to study the potential for the in utero programming of adult-onset chronic diseases, and have included maternal calorie restriction, protein restriction, high fat (HF) feeding, as well as the induction of obesity, hypertension, and diabetes. Since dysregulations of the AOX capacity are associated with almost all chronic diseases, it is not surprising that all of these disruptions during pregnancy were shown to result in oxidative stress, in both the mother as well as the offspring, either at birth or in adulthood. Tables 1 and 2 present an overview of outcomes from the most commonly studied models discussed next as well as several others.
Table 1.
Studies Focusing on the Relationship Between Poor Maternal Nutrition and Fetal Oxidative Balance
| Experimental model | Oxidative balance-related outcomes in offspring | Additional relevant observations in offspring | References |
|---|---|---|---|
| Rats exposed to a gestational LP diet | ↑ eNOS mRNA, ↓ HO-1 mRNA in liver in males | ↓ Endothelial responsiveness to acetylcholine in resistance arteries | Rodford et al. (97) |
| Rats exposed to an LP diet during gestation compared with those exposed to a LP diet postnatally | ↑ Urinary 8-oxo-dG; ↓ MnSOD | Tarry-Adkins et al. (118) | |
| Rats exposed to a gestational and lactational LP diet | ↑ TBARS in plasma and liver; ↓ CAT and SOD activity | Hypohomocysteinemia | Fetoui et al. (39) |
| Rats exposed to a gestational or gestational and lactational LP diet | ↓ GPx activity in fetal islets; ↓ SOD activity in fetal livers; ↑ SOD activity in 3 months old islets of both groups | ↓ Birth weight but ↑ body weight gain; ↓ Insulin in the gestational/lactational group and a trend of ↓ in gestational group at 3 months | Theys et al. (120) |
| Rats exposed to an LP diet during gestation compared with those exposed to an LP diet postnatally | ↓ Kidney HO-1 and Sod1 mRNA | ↑ Albuninuria | Chen et al. (26) |
| Rats exposed to an LP diet during gestation compared with those exposed to an LP diet postnatally | ↑ Xanthine oxidase; ↓ MnSOD, Cu/Zn-SOD, HO-1 | Pancreatic fibrosis | Tarry-Adkins et al. (117) |
| Rats exposed to a “junk food” diet during gestation and lactation | ↑ Sod1 (males and females) and CAT (males) mRNA | Hepatic steatosis and lipid accumulation | Bayol et al. (12) |
| Mice exposed to an HF diet before and during gestation and during lactation compared with those also exposed to an HF diet after weaning | ↑ Hepatic Nos3, Nos2, Gstm6, and Lcn2 mRNA at 15 weeks of age | NAFLD at 30 weeks of age | Bruce et al. (18) |
| Nonhuman primates of lean or obese mothers exposed to a chronic HF diet | ↑ 8-oxo-dG and 4-hydroxy-2-nonenol; Activation of the c-Jun N-terminal Kinase pathway as a marker of oxidative stress in fetal offspring of obese mothers on HF diet | ↑ Liver TG | McCurdy et al. (80) |
| Rats exposed to an HF diet during gestation and a control diet for 12 weeks after birth | ↑ TBARS; ↓ Gpx-1, Sod1, Pon1, Pon2, Pon3 | ↑ Hepatic TG, TBARS | Zhang et al. (135) |
| Rats exposed to a Zinc restriction diet during gestation and lactation | ↓ CAT and GPx activity and glutathione levels; ↑ Lipid peroxidation | Renal apoptosis | Tomat et al. (121) |
| Rats exposed to a marginal gestational Zinc restriction | ↑ Protein cysteine oxidation in fetal brains | ↓ Tubulin polymerization in fetal brains | Mackenzie et al. (74) |
CAT, catalase; GPx, glutathione peroxidase; HF, high fat; LP, low protein; NAFLD, nonalcoholic fatty liver disease; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TG, triglycerides.
Table 2.
Studies Focusing on the Relationship Between Maternal Nutrient Supplementation in Ameliorating Fetal Oxidative Stress or Other Outcomes
| Experimental model and supplementation type | Oxidative balance-related improvements in offspring | Additional relevant improvements in offspring | References |
|---|---|---|---|
| Rats exposed to a gestational HSF diet supplemented with quercetin compared with an HSF diet | ↑ Total mineralized tissues, crown-rump length, total bone volume | Liang et al. (68) | |
| Rats exposed to a gestational HF diet supplemented with antioxidants compared with an HF diet | ↓ TBARS in neonate; ↓ ROS and ↑ GSX in embryos; | ↓ Adiposity in adult offspring; | Sen et al. (103) |
| Mice exposed to a gestational HF diet supplemented with fiber compared with an HF diet | ↑ Total hepatic SOD activity; ↑ MnSOD (liver, heart), Cu/Zn-SOD (liver, heart), Hif-1a (liver, heart), Trx1 (liver, heart), Trx2 (liver), Gpx1 (liver). | Lin et al. (73) | |
| Rats exposed to DHA during pregnancy followed by induction of hypoxic-ischemic encephalopathy | ↓ 8-oxo-dG. | ↓ Apoptotic neuronal cells. | Suganuma et al. (111) |
| Rats exposed to gestational alcohol supplemented with folate or selenium or a combination compared with alcohol alone | ↑ GPX activity, ↓ GR activity, ↓ protein peroxidation (with double supplementation) | Ojeda et al. (88) | |
| Mice exposed to gestational and life-long soy supplementation | ↑ Mitochondrial glutathione; ↑ eNOS, MnSOD, cytochrome c oxidase mRNA. | ↑ Endothelial function, ↓ Blood Pressure | Mahn et al. (76) |
GR, glutathione reductase; HSF, high-saturated fat; ROS, reactive oxygen species.
Maternal HF diet and oxidative stress
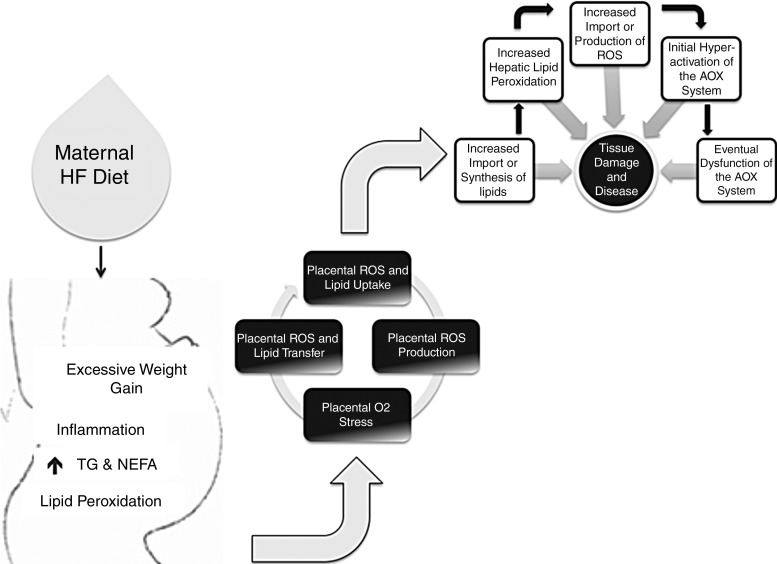
A HF diet is thought to alter oxidative balance by increasing lipid peroxidation and the production of ROS, and our group recently showed that a gestational HF in rats repressed the expression of AOX genes in livers of adult offspring. Despite being on a standard control diet for 12 weeks after birth, offspring exposed to a gestational HF diet had increased plasma triglycerides, and their livers had increased TBARS, as well as decreased transcription of Gpx-1, Sod1, Pon1, Pon2, and Pon3 (135). Our data demonstrates that the maternal environment is a critical programmer of the AOX capacity, and that molecular events programmed in utero can last into adulthood. Unpublished observations from our group have also found that a gestational HF diet resulted in the gender-specific increased mRNA expression of AOX enzymes in livers of neonatal rats. These results suggest that while the fetus initially responds to a maternal HF diet by increasing its AOX capacity, this response may be short lived. Under a chronic stress, the AOX system no longer functions with time, which eventually leads to oxidative stress-induced tissue damage (Fig. 2). Additionally, what remains to be clarified is whether the dysregulation in oxidative balance in the offspring is due to the increased in utero supply of maternal lipids itself, or the innate increase in the production of lipids or ROS due to the offspring's programmed altered lipid handling and metabolism.
FIG. 2.
Maternal HF diet and the offspring's AOX defense system. Studies have suggested that a maternal HF contributes to numerous diseases in the offspring, including nonalcoholic liver disease, and this may be accompanied by a dysregulation of the AOX defense system in the offspring. The exact mechanism behind these observations remains undefined and is likely complicated and multi-faceted. Potentially, a maternal HF diet dysregulates maternal lipid metabolism, which increases maternal oxidative stress. The increased ROS produced during maternal lipid peroxidation are sensed by the placenta, which either increases its own ROS production, or transports maternal peroxidation products to the offspring. This is also accompanied by an increased transport of free fatty acids from the mother to the fetus. The excess fatty acids may lead to endogenous ROS production, and these, along with those from the mother, initially activate the AOX defense system. However, it is possible that with time, and under a chronic ROS burden, the AOX system no longer functions at capacity, which leads to oxidative damage and eventual tissue damage. AOX, antioxidant; HF, high fat; NEFA, non-esterified fatty acids; TG, triglycerides.
Maternal protein restriction and oxidative stress
Our group has shown that gestational protein restriction in rats has severe consequences for the growth capacity of offspring (109), and Ozanne et al. have repeatedly demonstrated that protein restriction during gestation or lactation influences oxidative balance in the offspring. A gestational low-protein (LP) diet, when compared with post-natal protein restriction, was shown to increase renal albuminuria in young mice, and this was accompanied by altered expression of genes that function to protect the kidney against oxidative stress (26). Additionally, a gestational LP diet resulted in an increase in markers of fibrosis in pancreatic islets of adult offspring, and this was accompanied by increased islet expression of xanthine oxidase, and decreased MnSOD, Cu/Zn-SOD, and heme oxygenase-1 (117), demonstrating increased oxidative stress and diminished AOX capacity in pancreatic cells of offspring exposed to a gestational LP diet. Using the same model, aortas of offspring exposed to a gestational LP diet had lower MnSOD protein content than the postnatally LP-fed group, and there was a significant decrease of MnSOD between 3 and 12 months of age in the gestational LP group, suggesting a decrease in AOX capacity in these animals. These changes were also associated with increased urinary 8-oxo-dG, a marker of oxidative stress, in the gestational LP group by 12 months of age when compared with both the control and the postnatal LP groups (118). How gestational protein restriction modulates the AOX defense system in the offspring remains unclear. Potentially, protein malnutrition can be sensed by several molecular signaling pathways as a cellular stressor, and this activates the AOX response, which diminishes with age. Alternatively (or concurrently), inadequate protein supply during pregnancy may increase the metabolism of other substrates, such as lipids and carbohydrates, by both the mother and fetus, therefore increasing free fatty acids, ketones, and glucose, all of which have been shown to be capable of dysregulating oxidative balance.
Gestational dietary interventions that decrease oxidative stress
Certain maternal dietary interventions have been shown to positively influence oxidative status. When a HF diet supplemented with dietary AOXs (Vitamins A, C, and E, as well as selenium) was fed to pregnant rat dams, the offspring of these animals had significantly improved outcomes when compared with offspring of dams fed the HF diet without AOX supplementation. These beneficial changes included decreased adiposity at 2 weeks and 2 months of age, decreased TBARS in the fetus and neonate, and decreased ROS and increased GSH in the preimplantation embryos (103). A high fiber diet fed to pregnant mice that were also consuming a HF diet improved the hydroxyl radical scavenging capacity in the placenta as well as maternal plasma when compared with the HF diet alone. Additionally, the livers of offspring whose mothers were supplemented with fiber, in conjunction with a HF diet, had increased total SOD, Cu/ZnSOD, and MnSOD protein, as well as increased mRNA expression of these genes, Hif-1α, Trx1, Trx2, and Gpx1. A similar increase of Hif-1α, Trx1, Cu/ZnSOD, and MnSOD mRNA was also observed in fetal hearts when compared with offspring of dams consuming a HF diet alone without fiber supplementation (73). In a rat model of gestational high-saturated fat feeding, fetuses of HF-fed dams had lower total mineralized tissues, crown-rump length, and total bone volume than controls, and quercetin supplementation improved these outcomes. The authors proposed that the HF diet increased oxidative stress, and quercetin, which has been shown to have strong AOX capacity, decreased it, thus improving fetal outcomes (67).
While soy has been shown to have some beneficial effects, the intake of soy during pregnancy has been somewhat controversial, as some animal studies have shown that the intake of phytoestrogenic genistein, the major isoflavone in soy, during either pregnancy or lactation has the ability to interfere with fertilization, oocyte maturation, and general development (10, 25, 28, 98). However, there is also evidence that soy isoflavones may positively affect endothelial tissues by mediating the oxidative response. A study of aged male rats that were fed a soy protein-rich diet during gestation and adult life showed that animals fed the soy diet had improved endothelial function, reduced blood pressure, and these improvements were associated with decreased oxidative stress (76). At the molecular level, these differences in oxidative status between animals fed soy-rich and soy-deficient diets were likely due to the observed increase in mitochondrial glutathione, as well as the increased mRNA expression of endothelial nitric oxide synthase (eNOS), MnSod, and cytochrome c oxidase. Additionally, when the soy-deficient group was re-fed the soy-rich diet for 6 months, they showed improvements in blood pressure, endothelial function, and the expression of AOX genes in vascular tissues. As previously discussed, estrogens have been shown to induce ROS production, and, interestingly, further studies with isoflavone-rich diets in animals have suggested that dietary estrogens improve vascular function by inducing nitric oxide and, therefore, the AOX defense system, potentially through the activation of Nrf2, thus improving endothelial function and defense capacity (106). While these studies suggest that soy intake may be cardioprotective, it is critical to reiterate that ROS production and overproduction exist in a fine balance, and further studies with dietary phytoestrogens will be needed to set intake safety levels, especially during pregnancy, when it is critical to maintain oxygen and ROS levels within a narrow range.
Role of Epigenetics in Oxidative Balance
Epigenetic modifications refer to events that alter the DNA without changing the genetic code itself. In this way, epigenetic modifications are environment responsive and can be either transient or persist throughout the lifetime of the organism (77). Nutritional epigenetics, especially during the critical prenatal period, has become a vast new frontier for studying the way in which our environment influences not only our immediate health, but also how it may leave a mark on the way we in which we respond to our future surroundings.
Classification and functions of epigenetic modifications
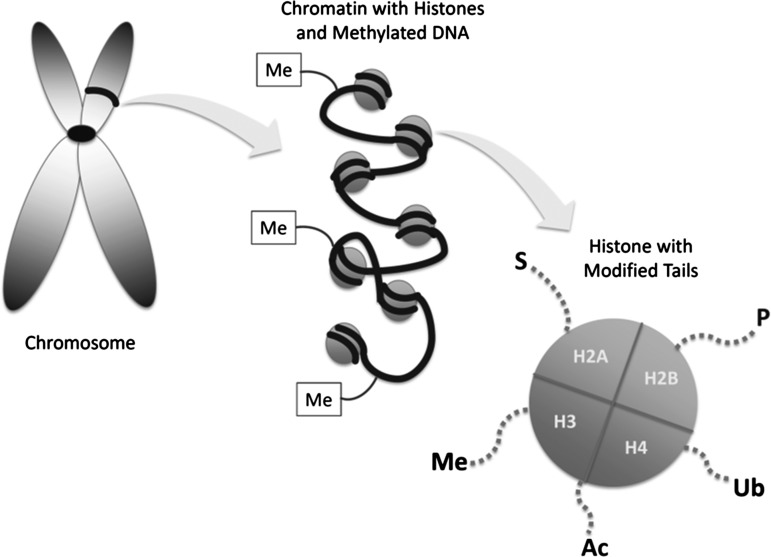
Both DNA methylation and the covalent modification of histone tails are epigenetic modifications that have been shown to be powerful regulators of gene expression (Fig. 3). DNA methylation most often occurs at “CG”-rich regions, so called “CpG Islands,” and is thought to be relatively permanent (110). Under normal conditions, the genome undergoes several regulated bouts of methylation and demethylation during the earliest stages of development (69); however, in adulthood, any considerable change in the methylation of DNA is primarily associated with abnormal pathologies, such as carcinogenesis (79). Clinical, animal, and cell studies have demonstrated the role of DNA methylation in the development and progression of numerous cancers, including colon, breast, hepatic, ovarian, prostate, and others. While DNA methylation is relatively permanent and results in the silencing of genes, the modification of histone tails has been thought to be less static and more responsive to diet and the environment. Additionally, the complicated interplay or crosstalk between the various histone modifications has made it more difficult to classify these as absolute activators or inhibitors of transcription. Histone modifications act by modifying the physical configuration of the DNA, either loosening or tightening the chromatin, and these arrangements either activate or inhibit gene transcription by regulating the binding of transcription factors and the transcription-initiation machinery (62). Therefore, while certain histone modifications, such as acetylation, are thought to be activators, and others, such as sumoylation, have been shown to be inhibitors of transcription, these are general observations and have many exceptions. Adding to the complexity of epigenetic modifications is the proposed importance of the interaction between them. In addition to the complicated interplay between the many histone modifications, it has also been shown that DNA methylation and histone modifications can regulate each other and/or be regulated by the same factors in response to various stimuli (85). Therefore, while epigenetics has become a new and exciting frontier for the study of gene transcription, the context within which these modifications occur should be considered when making any concrete conclusions about their regulation of specific genes.
FIG. 3.
Epigenetic modifications. The two most common epigenetic modifications include DNA methylation and histone modifications. Methylation of the DNA strand and CG dinucleotides is considered an inhibitor of transcription, while histone modifications can either activate or inhibit gene transcription. Covalent modifications to the tails of histones regulate transcription by either loosening or tightening the DNA helix, and include acetylation (Ac), methylation (Me), sumoylation (S), phosphorylation (P), and ubiquitination (Ub).
The role of epigenetics in the programming of gene expression in utero
Maternal physiology and diet are potent regulators of fetal outcomes, and there is mounting evidence suggesting that these changes occur in response to the in utero programming of gene expression. Epigenetics was suggested to contribute to these outcomes, because early epidemiological studies related to the “Fetal Origins Of Disease Hypothesis” showed that the prevalence of certain diseases was associated with environmental events rather than evolutionary transformations in gene expression (11). Since then, numerous studies have shown that maternal diet results in alterations in fetal birth outcomes and may predict adult disease development (38). Additionally, in animals, studies that manipulate gestational diet have confirmed that these physiological outcomes in the offspring are accompanied by molecular alterations to vital pathways that regulate almost all chronic diseases (2, 13, 22, 49, 55, 82). In an attempt to discover the molecular mechanisms behind the in utero programming of disease, recent studies have focused on connecting the physiological response to maternal diet and any epigenetically induced changes in gene transcription. Table 3 presents an overview of outcomes from studies discussed next as well as from several others.
Table 3.
Studies Focusing on the Relationship Between Maternal Diet and Epigenetic Modifications in the Offspring That May Also Be Associated with Altered Physiological Outcomes
| Experimental model | Epigenetic modification | Expression change | Physiological outcomes or predicted consequences | References |
|---|---|---|---|---|
| Mice exposed to gestational genistein | DNA hypermethylation | ↓ Agouti mRNA | Coat color change, ↓ Adiposity | Dolinoi et al. (35) |
| Rats exposed to gestational LP diet supplemented with folate compared with LP alone | DNA hypermethylation by folate | ↓ Hepatic GR mRNA by folate | Impaired lipid homeostasis and cardiovascular disease discussed | Lillycrop et al. (70) |
| Rats exposed to a gestational LP diet | DNA hypomethylation; ↑ H3K9 acetylation, H4K9 acetylation, H3K9 methylation; ↓ H3K9 dimethylation | ↑ Hepatic GR mRNA | Gluconeogenesis and metabolism discussed | Lillycrop et al. (72) |
| Rats exposed to gestational protein restriction | ↑ H3K9Me3, H3K27Me3; ↓ H3K4Me3, H4K20Me3 | ↓ Hepatic Igf2 mRNA | IUGR and liver development discussed | Sharif et al. (104) |
| Rats exposed to a gestational LP diet supplemented with folate compared with LP alone | Differential methylation at several CpG islands by folate | ↓ Hepatic Ppar-α mRNA by folate | Impaired lipid homeostasis and cardiovascular disease discussed | Lillycrop et al. (71) |
| Rats exposed to gestational food restriction and consequent IUGR | ↓ H3K4Me2; ↑ H3K4Me3 | ↑ Igf1 mRNA in obese adult offspring | IUGR, catch-up growth ↓ Liver weights | Tosh et al. (122) |
| Rats exposed to a gestational LP diet supplemented with folate compared with LP alone | Hypomethylation of imprinting control region by folate | ↓ Hepatic Igf2/H19 mRNA by folate | Metabolism and hepatic development discussed | Gong et al. (43) |
| Rats exposed to a gestational LP diet | ↑ Promoter AcH3 and AcH4 | ↑ C/EBPβ mRNA in female muscle | Diabetes and energy metabolism discussed | Zheng et al. (138) |
| Rats exposed to a gestational LP diet | ↓ Promoter AcH4 and H3K4Me2 | ↓ p16 mRNA in mammary | Breast cancer discussed | Zheng et al. (137) |
| Macaques exposed to a gestational HF diet | Hyperacetylation of H3K14, trend of ↑ acetylation at H3K9 and at H3K18 in fetal tissues | Differential mRNA expression of epigenetic determinants | ↑ TG and markers of NAFLD in fetal liver | Aagard-Tillery et al. (1) |
| Mice exposed to an HF diet during gestation and lactation | DNA hypomethylation | ↑ DAT, MOR, PENK mRNA (dopamine/opiod system) | ↑ Preference for sucrose and fat | Vucetic et al. (129) |
| Rats exposed to a gestational HF diet independent of obesity | ↓ Promoter H3Ac, H3K4Me2, H3K9Me3 and H3K27Me3; a trend in ↓ H3Ac, ↓ H3K9Me3; ↑ H4Ac and H3K4Me2 in the coding region; ↑ H4Ac and H3K4Me2 in an upstream region | ↑ Hepatic Pepck mRNA in fetal offspring | ↑ Plasma glucose in fetal offspring | Strakovsky et al. (109) |
| Macaques exposed to a gestational HF diet and gestational obesity | ↑ H3K14Ac within the RORE | ↑ Npas mRNA | Obesity and circadian regulation of metabolism | Suter et al. (113) |
| Rats exposed to choline deficiency or supplementation | Hypermethylation of G9a and Suv39h1 by deficiency; ↓ H3K9Me2 and H3K27Me3; ↑ H3K4Me2 | ↓ G9a and Suv39h1 by deficiency in liver and brain | Liver development and brain memory processes discussed | Davison et al. (33) |
IUGR, intrauterine growth restriction; H3Ac, acetylated histone H3; H4Ac, acetylated histone H4.
The hallmark example of the effect of maternal diet on the fetal epigenome came from studies in Avy mice, which were shown to have epigenetic modifications to the Agouti gene that altered coat color, as well as increased their susceptibility toward obesity and tumor development (132). The DNA methylation that led to the altered expression of the Agouti gene was shown to be modified by the addition of methyl donors to the maternal diet, showing a direct link between genotype, phenotype, and maternal intake. In later studies, Jirtle et al. showed that the supplementation of the maternal diet with genistein (a plant isoflavone from soy) increased the methylation of the Agouti gene, which not only changed the coat color in Avy mice, but also protected them from developing obesity, and these effects persisted into adulthood (35). Consequently, further studies of these “Agouti” mice [those carrying the viable yellow agouti A(vy) epiallele] showed that supplementation with other dietary methyl donors, which are known to influence DNA methylation, induced the pseudoagouti phenotype (131). These initial discoveries paved the way for the study of the developmental origins of disease from the epigenetic perspective, specifically focusing on the interaction between maternal nutrition and the fetal epigenome in programming adult-onset diseases.
While research in the field is currently somewhat limited, there are several other animal models of maternal nutritional manipulation that directly connect gestational dietary intake and alterations within the fetal epigenome. Lillycrop et al. have demonstrated that maternal protein restriction results in considerable DNA methylation in the offspring, and affects the transcription of genes critical for hepatic metabolic processes, such as Pppar-α (71) and glucocorticoid receptor (72). Using a similar model of gestational protein restriction, our group also showed that a gestational low-protein diet resulted in a significant increase of hepatic Igf2 transcription, and this was correlated with the methylation of the gene (43). A gestational HF diet in mice was shown to lead to the hypomethylation of dopamine and opioid-related genes in brains of offspring that also had a preference for sucrose and fat (129). Either supplementation or deficiency of folic acid and other methyl donors during pregnancy has also been implicated in regulating methylation events in the offspring (129), as the process of methylation requires a constant supply of one-carbon molecules. As discussed earlier, histone modifications, rather than DNA methylation, may represent a more immediate response of the fetal epigenome to the maternal nutrient supply. A maternal low-protein diet resulted in gender-specific histone modifications within the Cebp/β gene in skeletal muscle of adult offspring that corresponded to the transcription of this critical metabolic regulator (138). The same treatment also resulted in histone modifications and the repression of p16/Cdkn2a, a key cell-cycle control gene, in mammary glands of adult female offspring, an event that has been linked to breast carcinogenesis (137). Gestational choline supplementation led to an increase in H3K9Me2 and H3K27Me2, while choline-deficiency increased the levels of H3K4Me2 in offspring rats (33). In another study in rats, gestational food restriction led to the dimethylation of histone H3 at lysine residue 4 (H3K4Me2) within the Igf1 promoter, which affected the mRNA expression of the gene (122), and a gestational low-protein diet in mice resulted in a decrease of H3K4Me3 and H4K20Me3, and an increase of H3K9Me3 and H3K27Me3 within Igf2, which was accompanied by a decrease in the mRNA expression of the gene (104). Although it is clear that a maternal diet that is high in fat is deleterious to fetal development, the consequences of a gestational HF diet on the histone code have not been thoroughly elucidated. We recently reported that a maternal HF diet, independent of maternal obesity development, results in a decreased association of acetylated histone H3 (H3Ac), H3K4Me2, H3K9Me3, and H3K27Me3 within the promoter of the hepatic gluconeogenic Pck1 gene. Additionally, our analysis of the Pck1 coding region showed that a maternal HF diet led to a decrease in H3K9Me3, and an increase in acetylated histone H4 (H4Ac) and H3K4Me2, and that these modifications were associated with an increased transcription and transcriptional rate of the Pck1 gene (109). In primates, the chronic consumption of a maternal HF diet led to an increase in H3K14Ac, a trend of increase in H3K9Ac, H3K18Ac, H3K9Me2, H3K9Me3, and H3K27Me3, and these fetal hepatic tissues had an increase in triglycerides and non-alcoholic fatty liver disease (1). The studies just mentioned suggest that a maternal diet in which either macro- or micro-nutrients are manipulated alters fetal chromatin structure in rodents as well as in primates via covalent histone modifications, and this contributes to changes to gene expression as well as to the phenotype of the offspring.
Epigenetic modifications of the AOX defense system
Due to the AOX defense system's ability to quickly and efficiently respond to numerous environmental events, it is not surprising that it has been shown to be regulated at the epigenetic level (Table 4). There is a strong support from human, animal, and cell studies showing that epigenetic events are critical for the regulation of cancer development and progression, and since cancer patients often exhibit increased oxidative stress, several studies have focused on the role of epigenetics in oxidative balance during carcinogenesis. While ROS themselves are potent epigenetic modifiers of critical carcinogenesis-related genes (139), genes involved in oxidative balance have themselves also been shown to be epigenetically modified. In breast cancer cells, which often have decreased MnSod activity, the gene that encodes the enzyme (SOD2) was hypermethylated, suggesting that its expression was epigenetically regulated (48). Additionally, the decrease in SOD2 transcription was accompanied by a decrease in dimethylation of histone H3 at K4 (a transcriptional activator) and hypoacetylation of histone H3 at K9, and reversing the hypoacetylation increased SOD2 expression (47), suggesting that the gene is at least in part regulated by histone modifications that influence the condensation state of the chromatin. In prostate cancer, the epigenetic regulation of glutathione-s-transferase P1 (GSTP1) has also been shown to be responsible for this AOX's decreased transcription (127) and an increase in oxidative damage (125). The GSTP1 promoter is hypermethylated during prostate cancer (83), thus decreasing its transcription and AOX activity, which has been linked to the development of high-grade intraepithelial neoplasia (87).
Table 4.
Studies Focusing on the Relationship Between Epigenetic Modifications and the Regulation of the Antioxidant Defense System
| Experimental model | Epigenetic modification | Expression change | Disease state implicated | References |
|---|---|---|---|---|
| Breast cancer cell lines compared with normal breast cells | ↑ Promoter methylation; ↓ Acetylated histones; | ↓ SOD2 | Breast cancer | Hitchler et al. (48) |
| Breast cancer cell lines compared with normal breast cells | ↓ Dimethyl H3K4; ↓ Acetylated H3K9 | ↓ SOD2 | Breast cancer | Hitchler et al. (47) |
| Prostate cancer compared with normal prostate samples | DNA hypermethylation of both alleles | ↓ Gstp1 | Prostate cancer | Millar et al. (83) |
| Primary human endothelial cells and vascular smooth muscle cells compared with iNOS-inducible cell lines | DNA hypermethylation; ↑ Histone H3 lysine 9 methylation | ↓ iNOS | Vascularization | Chan et al. (24) |
| Atherosclerotic aorta compared with normal arteries | DNA hypomethylation | ↓ EC-SOD | Atherosclerosis | Laukkanen et al. (66) |
| Thickened arterial smooth muscle cells compared with normal arteries | DNA hypomethylation | ↑ transcriptional activity | Atherogenesis | Hiltunen et al. (46) |
| Pulmonary artery smooth muscle cells in humans and rats with PAH compared with healthy samples | DNA hypermentylation | ↓ SOD2 | Pulmonary Artery Hypertension | Archer et al. (6) |
| Buccal mucosal cells in vegetarians compared with omnivores | DNA hypomethylation | ↑ SOD2 | Oxidative status | Thaler et al. (119) |
EC-SOD, extracellular superoxide dismutase; iNOS, inducible nitric oxide synthase; PAH, pulmonary artery hypertension.
Atherosclerosis is marked by increased oxidative stress and a decrease in AOX capacity, and epigenetic events appear to regulate genes in atherosclerotic patients. In the atherosclerotic aorta, inducible nitric oxide synthase (iNOS) has been shown to be upregulated (93), which could potentially be due to its hypomethylation (24). Additionally, both extracellular SOD (66) and 15-Lipoxygenase (46) are hypomethylated in atherosclerotic lesions, which likely regulates their activation in response to oxidative stress. In an animal model of spontaneous pulmonary artery hypertension (PAH), SOD2 was significantly decreased in the pulmonary artery smooth muscle cells [which has also been shown in humans with PAH (16)], and this decrease was accompanied by a significant increase of SOD2 promoter methylation (6), suggesting that the decreased transcription and activity of this AOX during pulmonary hypertension is a result of its epigenetic status.
In utero epigenetic programming of the AOX system: The need for further research
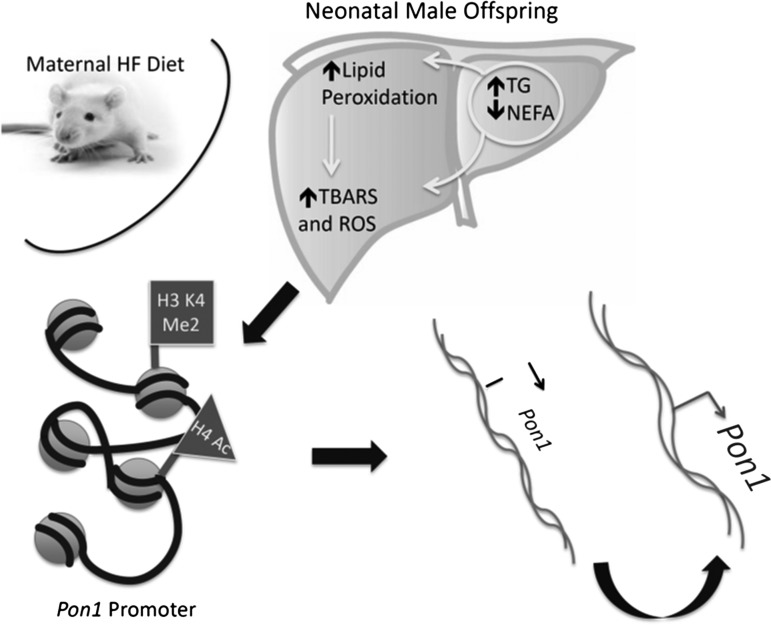
While little data are available regarding the epigenetic modifications occurring within the AOX system in response to maternal diet, the response of AOX genes to maternal nutrition is likely similar to that of other vital pathways, in that they are regulated in utero, which likely occurs at the epigenetic level. A few studies are available relating to nutrition and epigenetics in humans, but a study of mucosal swabs showed that MnSOD was differentially expressed in vegetarians versus meat-eaters, and that this was associated with differential global DNA methylation as well as methylation of CpG islands within the MnSod gene (119). Unpublished observations from our lab have shown that a maternal HF diet impacts the expression of the Pon genes in liver, and these changes are gender-specific and may occur through the epigenetic modifications of these genes. We showed that Pon1, Pon2, and Pon3 were increased after a maternal HF diet in livers of male but not female neonates. Histone modifications within the Pon1 gene promoter corresponded to the mRNA expression of the gene, with increased H4Ac, H3K4Me2 and a decrease in H3K9Me3. These modifications were associated with increased oxidative stress in the liver, suggesting that the fetal AOX system responds to maternal nutrition, which occurs at the epigenetic level (Fig. 4).
FIG. 4.
Epigenetic regulation of hepatic Pon1 by maternal HF diet. Although the direct mechanism behind the regulation of the AOX system remains unknown, unpublished data from our lab suggests that a maternal HF diet can induce epigenetic changes in the fetal epigenome and affect AOX genes, including hepatic Pon1. We observed that fetal livers of offspring whose mothers consumed an HF diet during gestation but did not develop obesity (image represents OR-CD [Obese Resistant] rats from Charles River Laboratories) had increased TG and TBARS, which was accompanied by the increased mRNA expression of the Pon1 gene, a hepatic antioxidant. Chromatin immunoprecipitation, a technique utilized to test proteins associated with the chromatin, showed that the Pon1 promoter was enriched with H3K4Me2 as well as H4Ac, both of which are associated with active transcription and act by loosening the DNA to allow for the binding of various transcription factors. H3K4Me2, dimethylated histone H3 at Lysine residue 4; H4Ac, acetylation of histone H4.
Perspectives and Future Directions
While it is unquestionably valuable to determine specific epigenetic modifications governing the regulation of the AOX system, it is more imperative to clarify how these changes contribute to the overall AOX capacity of the organism. The overall balance of pro-oxidants and AOXs is the determinant of oxidative balance, and it remains unclear whether maternal diet regulates one or both of these. Additionally, more research is needed to clearly establish whether maternal physiology or the nutrients themselves are responsible for altered AOX balance observed in offspring from the studies discussed here. This article has highlighted numerous reports of maternal programming of gene expression and focused on the epigenetic mechanisms behind these changes. However, while recent advancements in the field make it possible to conclude that epigenetic modifications play a critical role in regulating fetal health in response to maternal diet, a few studies have addressed how these modifications can be reversed and whether the resulting adult diseases or symptoms can be ameliorated. Epigenetics has become an intriguing new approach toward medicine, because unlike genetic mutations or polymorphisms, epigenetic modifications are reversible, which makes epigenetic therapy a promising new approach in dealing with numerous human diseases. Since the AOX defense system is critical for mediating the relationship between the environment and human disease, it is essential to determine whether the AOX system can be modified epigenetically and whether reversing these marks can lead to improved health outcomes. Additionally, since studies in animals and humans have been convincing in showing that maternal nutrition is a potent regulator of fetal health, future epigenetic data in the field will be a powerful tool for showing the promise of disease prevention or therapy during the earliest developmental periods.
Abbreviations Used
- AOX
antioxidant
- BPA
bisphenol-A
- CAT
catalase
- EC-SOD
extracellular superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- GDM
gestational diabetes mellitus
- H3K(x)Me/Ac(y)
mono/di/tri (y) methylation or acetylation of histone H3 at lysine residue (x)
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSTP1
glutathione-s-transferase P1
- H3Ac
acetylated histone H3
- H4Ac
acetylated histone H4
- HF
high fat
- HSF
high-saturated fat
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IUGR
intrauterine growth restriction
- LP
low protein
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- NAFLD
nonalcoholic fatty liver disease
- NEFA
non-esterified fatty acid
- PAHs
polycyclic aromatic hydrocarbons
- PON
paraoxonase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- TG
triglycerides
References
- 1.Aagaard-Tillery KM. Grove K. Bishop J. Ke X. Fu Q. McKnight R. Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akyol A. Langley-Evans SC. McMullen S. Obesity induced by cafeteria feeding and pregnancy outcome in the rat. Br J Nutr. 2009;102:1601–1610. doi: 10.1017/S0007114509990961. [DOI] [PubMed] [Google Scholar]
- 3.Al-Gubory KH. Bolifraud P. Germain G. Nicole A. Ceballos-Picot I. Antioxidant enzymatic defence systems in sheep corpus luteum throughout pregnancy. Reproduction. 2004;128:767–774. doi: 10.1530/rep.1.00389. [DOI] [PubMed] [Google Scholar]
- 4.Allen RG. Balin AK. Oxidative influence on development and differentiation: an overview of a free radical theory of development. Free Radic Biol Med. 1989;6:631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- 5.Allen RG. Venkatraj VS. Oxidants and antioxidants in development and differentiation. J Nutr. 1992;122:631–635. doi: 10.1093/jn/122.suppl_3.631. [DOI] [PubMed] [Google Scholar]
- 6.Archer SL. Marsboom G. Kim GH. Zhang HJ. Toth PT. Svensson EC. Dyck JR. Gomberg-Maitland M. Thebaud B. Husain AN. Cipriani N. Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aris A. Benali S. Ouellet A. Moutquin JM. Leblanc S. Potential biomarkers of preeclampsia: inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta. 2009;30:342–347. doi: 10.1016/j.placenta.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Atamer Y. Kocyigit Y. Yokus B. Atamer A. Erden AC. Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;119:60–66. doi: 10.1016/j.ejogrb.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Baiza-Gutman LA. Flores-Sanchez MM. Diaz-Flores M. Hicks JJ. Presence of uterine peroxidase activity in the rat early pregnancy. Int J Biochem Cell Biol. 2000;32:255–262. doi: 10.1016/s1357-2725(99)00061-8. [DOI] [PubMed] [Google Scholar]
- 10.Ball ER. Caniglia MK. Wilcox JL. Overton KA. Burr MJ. Wolfe BD. Sanders BJ. Wisniewski AB. Wrenn CC. Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm Behav. 2010;57:313–322. doi: 10.1016/j.yhbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 12.Bayol SA. Simbi BH. Fowkes RC. Stickland NC. A maternal "junk food" diet in pregnancy and lactation promotes nonalcoholic Fatty liver disease in rat offspring. Endocrinology. 2010;151:1451–1461. doi: 10.1210/en.2009-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayol SA. Simbi BH. Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol. 2005;567:951–961. doi: 10.1113/jphysiol.2005.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardi F. Guolo F. Bortolin T. Petronilho F. Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res. 2008;34:948–951. doi: 10.1111/j.1447-0756.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- 15.Biri A. Bozkurt N. Turp A. Kavutcu M. Himmetoglu O. Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest. 2007;64:187–192. doi: 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- 16.Bonnet S. Michelakis ED. Porter CJ. Andrade-Navarro MA. Thebaud B. Haromy A. Harry G. Moudgil R. McMurtry MS. Weir EK. Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 17.Bouanane S. Benkalfat NB. Baba Ahmed FZ. Merzouk H. Mokhtari NS. Merzouk SA. Gresti J. Tessier C. Narce M. Time course of changes in serum oxidant/antioxidant status in overfed obese rats and their offspring. Clin Sci (Lond) 2009;116:669–680. doi: 10.1042/CS20080413. [DOI] [PubMed] [Google Scholar]
- 18.Bruce KD. Cagampang FR. Argenton M. Zhang J. Ethirajan PL. Burdge GC. Bateman AC. Clough GF. Poston L. Hanson MA. McConnell JM. Byrne CD. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 19.Bruin JE. Petre MA. Lehman MA. Raha S. Gerstein HC. Morrison KM. Holloway AC. Maternal nicotine exposure increases oxidative stress in the offspring. Free Radic Biol Med. 2008;44:1919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Bruin JE. Petre MA. Raha S. Morrison KM. Gerstein HC. Holloway AC. Fetal and neonatal nicotine exposure in Wistar rats causes progressive pancreatic mitochondrial damage and beta cell dysfunction. PLoS One. 2008;3:e3371. doi: 10.1371/journal.pone.0003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caluwaerts S. Lambin S. van Bree R. Peeters H. Vergote I. Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism. 2007;56:1431–1438. doi: 10.1016/j.metabol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Carter AM. Placental oxygen consumption. Part I: in vivo studies—a review. Placenta. 2000;21(Suppl A):S31–S37. doi: 10.1053/plac.1999.0513. [DOI] [PubMed] [Google Scholar]
- 24.Chan GC. Fish JE. Mawji IA. Leung DD. Rachlis AC. Marsden PA. Epigenetic basis for the transcriptional hyporesponsiveness of the human inducible nitric oxide synthase gene in vascular endothelial cells. J Immunol. 2005;175:3846–3861. doi: 10.4049/jimmunol.175.6.3846. [DOI] [PubMed] [Google Scholar]
- 25.Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod Toxicol. 2009;28:52–58. doi: 10.1016/j.reprotox.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Chen JH. Tarry-Adkins JL. Matharu K. Yeo GS. Ozanne SE. Maternal protein restriction affects gene expression profiles in the kidney at weaning with implications for the regulation of renal function and lifespan. Clin Sci (Lond) 2010;119:373–384. doi: 10.1042/CS20100230. [DOI] [PubMed] [Google Scholar]
- 27.Cho SH. Choi MH. Kwon OS. Lee WY. Chung BC. Metabolic significance of bisphenol A-induced oxidative stress in rat urine measured by liquid chromatography-mass spectrometry. J Appl Toxicol. 2009;29:110–117. doi: 10.1002/jat.1387. [DOI] [PubMed] [Google Scholar]
- 28.Cimafranca MA. Davila J. Ekman GC. Andrews RN. Neese SL. Peretz J. Woodling KA. Helferich WG. Sarkar J. Flaws JA. Schantz SL. Doerge DR. Cooke PS. Acute and chronic effects of oral genistein administration in neonatal mice. Biol Reprod. 2010;83:114–121. doi: 10.1095/biolreprod.109.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlan MT. Permezel M. Georgiou HM. Rice GE. Repression of oxidant-induced nuclear factor-kappaB activity mediates placental cytokine responses in gestational diabetes. J Clin Endocrinol Metab. 2004;89:3585–3594. doi: 10.1210/jc.2003-031953. [DOI] [PubMed] [Google Scholar]
- 30.Coughlan MT. Vervaart PP. Permezel M. Georgiou HM. Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. 2004;25:78–84. doi: 10.1016/S0143-4004(03)00183-8. [DOI] [PubMed] [Google Scholar]
- 31.Crinnion WJ. Maternal levels of xenobiotics that affect fetal development and childhood health. Altern Med Rev. 2009;14:212–222. [PubMed] [Google Scholar]
- 32.Daikoku T. Matsumoto H. Gupta RA. Das SK. Gassmann M. DuBois RN. Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278:7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- 33.Davison JM. Mellott TJ. Kovacheva VP. Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284:1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devasagayam TP. Sivabalan R. Tarachand U. Lipid peroxidation in the rat uterus during deciduoma induced cell differentiation. Biochem Int. 1990;21:27–32. [PubMed] [Google Scholar]
- 35.Dolinoy DC. Weidman JR. Waterland RA. Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 37.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Fall C. Maternal nutrition: effects on health in the next generation. Indian J Med Res. 2009;130:593–599. [PubMed] [Google Scholar]
- 39.Fetoui H. Garoui M. Zeghal N. Protein restriction in pregnant- and lactating rats-induced oxidative stress and hypohomocysteinaemia in their offspring. J Anim Physiol Anim Nutr (Berl) 2009;93:263–270. doi: 10.1111/j.1439-0396.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 40.Forsberg H. Borg LA. Cagliero E. Eriksson UJ. Altered levels of scavenging enzymes in embryos subjected to a diabetic environment. Free Radic Res. 1996;24:451–459. doi: 10.3109/10715769609088044. [DOI] [PubMed] [Google Scholar]
- 41.Garrel C. Fowler PA. Al-Gubory KH. Developmental changes in antioxidant enzymatic defences against oxidative stress in sheep placentomes. J Endocrinol. 2010;205:107–116. doi: 10.1677/JOE-09-0362. [DOI] [PubMed] [Google Scholar]
- 42.Golub MS. Wu KL. Kaufman FL. Li LH. Moran-Messen F. Zeise L. Alexeeff GV. Donald JM. Bisphenol A: developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol. 2010;89:441–466. doi: 10.1002/bdrb.20275. [DOI] [PubMed] [Google Scholar]
- 43.Gong L. Pan YX. Chen H. Gestational low protein diet in the rat mediates Igf2 gene expression in male offspring via altered hepatic DNA methylation. Epigenetics. 2010;5:619–626. doi: 10.4161/epi.5.7.12882. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7:645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- 45.Hengstler JG. Foth H. Gebel T. Kramer PJ. Lilienblum W. Schweinfurth H. Volkel W. Wollin KM. Gundert-Remy U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiltunen MO. Turunen MP. Hakkinen TP. Rutanen J. Hedman M. Makinen K. Turunen AM. Aalto-Setala K. Yla-Herttuala S. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med. 2002;7:5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- 47.Hitchler MJ. Oberley LW. Domann FE. Epigenetic silencing of SOD2 by histone modifications in human breast cancer cells. Free Radic Biol Med. 2008;45:1573–1580. doi: 10.1016/j.freeradbiomed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hitchler MJ. Wikainapakul K. Yu L. Powers K. Attatippaholkun W. Domann FE. Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics. 2006;1:163–171. doi: 10.4161/epi.1.4.3401. [DOI] [PubMed] [Google Scholar]
- 49.Howie GJ. Sloboda DM. Kamal T. Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hracsko Z. Orvos H. Novak Z. Pal A. Varga IS. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. 2008;13:11–16. doi: 10.1179/135100008X259097. [DOI] [PubMed] [Google Scholar]
- 51.Igosheva N. Abramov AY. Poston L. Eckert JJ. Fleming TP. Duchen MR. McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansson T. Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 53.Jauniaux E. Gulbis B. Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus—a review. Placenta. 2003;24(Suppl A):S86–S93. doi: 10.1053/plac.2002.0932. [DOI] [PubMed] [Google Scholar]
- 54.Jauniaux E. Watson AL. Hempstock J. Bao YP. Skepper JN. Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones HN. Woollett LA. Barbour N. Prasad PD. Powell TL. Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones ML. Mark PJ. Lewis JL. Mori TA. Keelan JA. Waddell BJ. Antioxidant defenses in the rat placenta in late gestation: increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol Reprod. 2010;83:254–260. doi: 10.1095/biolreprod.110.083907. [DOI] [PubMed] [Google Scholar]
- 57.Kabuto H. Amakawa M. Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–2940. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 58.Kabuto H. Hasuike S. Minagawa N. Shishibori T. Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res. 2003;93:31–35. doi: 10.1016/s0013-9351(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 59.Kauma S. Takacs P. Scordalakes C. Walsh S. Green K. Peng T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol. 2002;100:706–714. doi: 10.1016/s0029-7844(02)02169-5. [DOI] [PubMed] [Google Scholar]
- 60.Kim H. Hwang JY. Ha EH. Park H. Ha M. Lee SH. Hong YC. Chang N. Fruit and vegetable intake influences the association between exposure to polycyclic aromatic hydrocarbons and a marker of oxidative stress in pregnant women. Eur J Clin Nutr. 2011;65:1118–1125. doi: 10.1038/ejcn.2011.77. [DOI] [PubMed] [Google Scholar]
- 61.Korteweg FJ. Gordijn SJ. Timmer A. Holm JP. Ravise JM. Erwich JJ. A placental cause of intra-uterine fetal death depends on the perinatal mortality classification system used. Placenta. 2008;29:71–80. doi: 10.1016/j.placenta.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Kovacic P. Somanathan R. Integrated approach to immunotoxicity: electron transfer, reactive oxygen species, antioxidants, cell signaling, and receptors. J Recept Signal Transduct Res. 2008;28:323–346. doi: 10.1080/10799890802305217. [DOI] [PubMed] [Google Scholar]
- 64.Kumari AS. Pregnancy outcome in women with morbid obesity. Int J Gynaecol Obstet. 2001;73:101–107. doi: 10.1016/s0020-7292(00)00391-x. [DOI] [PubMed] [Google Scholar]
- 65.Lapointe J. Bilodeau JF. Antioxidant defenses are modulated in the cow oviduct during the estrous cycle. Biol Reprod. 2003;68:1157–1164. doi: 10.1095/biolreprod.102.007476. [DOI] [PubMed] [Google Scholar]
- 66.Laukkanen MO. Mannermaa S. Hiltunen MO. Aittomaki S. Airenne K. Janne J. Yla-Herttuala S. Local hypomethylation in atherosclerosis found in rabbit ec-sod gene. Arterioscler Thromb Vasc Biol. 1999;19:2171–2178. doi: 10.1161/01.atv.19.9.2171. [DOI] [PubMed] [Google Scholar]
- 67.Liang C. Decourcy K. Prater MR. High-saturated-fat diet induces gestational diabetes and placental vasculopathy in C57BL/6 mice. Metabolism. 2010;59:943–950. doi: 10.1016/j.metabol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Liang C. Oest ME. Jones JC. Prater MR. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res B Dev Reprod Toxicol. 2009;86:362–369. doi: 10.1002/bdrb.20204. [DOI] [PubMed] [Google Scholar]
- 69.Liang P. Song F. Ghosh S. Morien E. Qin M. Mahmood S. Fujiwara K. Igarashi J. Nagase H. Held WA. Genome-wide survey reveals dynamic widespread tissue-specific changes in DNA methylation during development. BMC Genomics. 2011;12:231. doi: 10.1186/1471-2164-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lillycrop KA. Phillips ES. Jackson AA. Hanson MA. Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 71.Lillycrop KA. Phillips ES. Torrens C. Hanson MA. Jackson AA. Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lillycrop KA. Slater-Jefferies JL. Hanson MA. Godfrey KM. Jackson AA. Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin Y. Han XF. Fang ZF. Che LQ. Nelson J. Yan TH. Wu D. Beneficial effects of dietary fibre supplementation of a high-fat diet on fetal development in rats. Br J Nutr. 2011;106:510–518. doi: 10.1017/S0007114511000614. [DOI] [PubMed] [Google Scholar]
- 74.Mackenzie GG. Salvador GA. Romero C. Keen CL. Oteiza PI. A deficit in zinc availability can cause alterations in tubulin thiol redox status in cultured neurons and in the developing fetal rat brain. Free Radic Biol Med. 2011;51:480–489. doi: 10.1016/j.freeradbiomed.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madazli R. Benian A. Aydin S. Uzun H. Tolun N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J Obstet Gynaecol. 2002;22:477–480. doi: 10.1080/0144361021000003573. [DOI] [PubMed] [Google Scholar]
- 76.Mahn K. Borras C. Knock GA. Taylor P. Khan IY. Sugden D. Poston L. Ward JP. Sharpe RM. Vina J. Aaronson PI. Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 77.Margueron R. Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin-Gallan P. Carrascosa A. Gussinye M. Dominguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med. 2003;34:1563–1574. doi: 10.1016/s0891-5849(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 79.Maunakea AK. Chepelev I. Zhao K. Epigenome mapping in normal and disease States. Circ Res. 2010;107:327–339. doi: 10.1161/CIRCRESAHA.110.222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCurdy CE. Bishop JM. Williams SM. Grayson BE. Smith MS. Friedman JE. Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menon R. Fortunato SJ. Yu J. Milne GL. Sanchez S. Drobek CO. Lappas M. Taylor RN. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32:317–322. doi: 10.1016/j.placenta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 82.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. Adv Exp Med Biol. 2009;646:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 83.Millar DS. Ow KK. Paul CL. Russell PJ. Molloy PL. Clark SJ. Detailed methylation analysis of the glutathione S-transferase pi (GSTP1) gene in prostate cancer. Oncogene. 1999;18:1313–1324. doi: 10.1038/sj.onc.1202415. [DOI] [PubMed] [Google Scholar]
- 84.Mise H. Sagawa N. Matsumoto T. Yura S. Nanno H. Itoh H. Mori T. Masuzaki H. Hosoda K. Ogawa Y. Nakao K. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998;83:3225–3229. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- 85.Murr R. Interplay between different epigenetic modifications and mechanisms. Adv Genet. 2010;70:101–141. doi: 10.1016/B978-0-12-380866-0.60005-8. [DOI] [PubMed] [Google Scholar]
- 86.Myllynen P. Pasanen M. Pelkonen O. Human placenta: a human organ for developmental toxicology research and biomonitoring. Placenta. 2005;26:361–371. doi: 10.1016/j.placenta.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama M. Bennett CJ. Hicks JL. Epstein JI. Platz EA. Nelson WG. De Marzo AM. Hypermethylation of the human glutathione S-transferase-pi gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ojeda ML. Nogales F. Jotty K. Barrero MJ. Murillo ML. Carreras O. Dietary selenium plus folic acid as an antioxidant therapy for ethanol-exposed pups. Birth Defects Res B Dev Reprod Toxicol. 2009;86:490–495. doi: 10.1002/bdrb.20211. [DOI] [PubMed] [Google Scholar]
- 89.Olsson MG. Centlow M. Rutardottir S. Stenfors I. Larsson J. Hosseini-Maaf B. Olsson ML. Hansson SR. Akerstrom B. Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger alpha(1)-microglobulin in preeclampsia. Free Radic Biol Med. 2010;48:284–291. doi: 10.1016/j.freeradbiomed.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 90.Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Ouyang YQ. Li SJ. Zhang Q. Cai HB. Chen HP. Interactions between inflammatory and oxidative stress in preeclampsia. Hypertens Pregnancy. 2009;28:56–62. doi: 10.1080/10641950802233064. [DOI] [PubMed] [Google Scholar]
- 92.Perera FP. Rauh V. Whyatt RM. Tsai WY. Tang D. Diaz D. Hoepner L. Barr D. Tu YH. Camann D. Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perrotta I. Brunelli E. Sciangula A. Zuccala V. Donato G. Tripepi S. Martinelli GL. Cassese M. Inducible and endothelial nitric oxide synthase expression in human atherogenesis: an immunohistochemical and ultrastructural study. Cardiovasc Pathol. 2009;18:361–368. doi: 10.1016/j.carpath.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr. 2003;133:1997S–2002S. doi: 10.1093/jn/133.6.1997S. [DOI] [PubMed] [Google Scholar]
- 95.Pillai P. Patel R. Pandya C. Gupta S. Sex-specific effects of gestational and lactational coexposure to lead and cadmium on hepatic phase I and phase II xenobiotic/steroid-metabolizing enzymes and antioxidant status. J Biochem Mol Toxicol. 2009;23:419–431. doi: 10.1002/jbt.20305. [DOI] [PubMed] [Google Scholar]
- 96.Polanska K. Hanke W. Sobala W. Brzeznicki S. Ligocka D. Exposure to polycyclic aromatic hydrocarbons and newborn biometric indicators. Int J Occup Med Environ Health. 2010;23:339–346. doi: 10.2478/v10001-010-0028-1. [DOI] [PubMed] [Google Scholar]
- 97.Rodford JL. Torrens C. Siow RC. Mann GE. Hanson MA. Clough GF. Endothelial dysfunction and reduced antioxidant protection in an animal model of the developmental origins of cardiovascular disease. J Physiol. 2008;586:4709–4720. doi: 10.1113/jphysiol.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ross AE. Marchionni L. Phillips TM. Miller RM. Hurley PJ. Simons BW. Salmasi AH. Schaeffer AJ. Gearhart JP. Schaeffer EM. Molecular effects of genistein on male urethral development. J Urol. 2011;185:1894–1898. doi: 10.1016/j.juro.2010.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sagol S. Ozkinay E. Ozsener S. Impaired antioxidant activity in women with pre-eclampsia. Int J Gynaecol Obstet. 1999;64:121–127. doi: 10.1016/s0020-7292(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 100.Saker M. Soulimane Mokhtari N. Merzouk SA. Merzouk H. Belarbi B. Narce M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gynecol Reprod Biol. 2008;141:95–99. doi: 10.1016/j.ejogrb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 101.Salmasi G. Grady R. Jones J. McDonald SD. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89:423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 102.Sebire NJ. Jolly M. Harris JP. Wadsworth J. Joffe M. Beard RW. Regan L. Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 103.Sen S. Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010;59:3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharif J. Nakamura M. Ito T. Kimura Y. Nagamune T. Mitsuya K. Okamura K. Food restriction in pregnant mice can induce changes in histone modifications and suppress gene expression in fetus. Nucleic Acids Symp Ser (Oxf) 2007:125–126. doi: 10.1093/nass/nrm063. [DOI] [PubMed] [Google Scholar]
- 105.Sikkema JM. van Rijn BB. Franx A. Bruinse HW. de Roos R. Stroes ES. van Faassen EE. Placental superoxide is increased in pre-eclampsia. Placenta. 2001;22:304–308. doi: 10.1053/plac.2001.0629. [DOI] [PubMed] [Google Scholar]
- 106.Siow RC. Mann GE. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31:468–477. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 107.Sohal RS. Allen RG. Nations C. Oxidative stress and cellular differentiation. Ann N Y Acad Sci. 1988;551:59–73. doi: 10.1111/j.1749-6632.1988.tb22320.x. discussion 74. [DOI] [PubMed] [Google Scholar]
- 108.Steegers EA. von Dadelszen P. Duvekot JJ. Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 109.Strakovsky RS. Zhang X. Zhou D. Pan YX. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase (Pck) expression and histone modification in neonatal offspring rats. J Physiol. 2011;589(Pt 11):2707–2717. doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Straussman R. Nejman D. Roberts D. Steinfeld I. Blum B. Benvenisty N. Simon I. Yakhini Z. Cedar H. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16:564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- 111.Suganuma H. Arai Y. Kitamura Y. Hayashi M. Okumura A. Shimizu T. Maternal docosahexaenoic acid-enriched diet prevents neonatal brain injury. Neuropathology. 2010;30:597–605. doi: 10.1111/j.1440-1789.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- 112.Sugino N. Takiguchi S. Kashida S. Karube A. Nakamura Y. Kato H. Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol Hum Reprod. 2000;6:19–25. doi: 10.1093/molehr/6.1.19. [DOI] [PubMed] [Google Scholar]
- 113.Suter M. Bocock P. Showalter L. Hu M. Shope C. McKnight R. Grove K. Lane R. Aagaard-Tillery K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011;25:714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takagi Y. Nikaido T. Toki T. Kita N. Kanai M. Ashida T. Ohira S. Konishi I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004;444:49–55. doi: 10.1007/s00428-003-0903-2. [DOI] [PubMed] [Google Scholar]
- 115.Takehara Y. Yoshioka T. Sasaki J. Changes in the levels of lipoperoxide and antioxidant factors in human placenta during gestation. Acta Med Okayama. 1990;44:103–111. doi: 10.18926/AMO/30438. [DOI] [PubMed] [Google Scholar]
- 116.Takiguchi S. Sugino N. Kashida S. Yamagata Y. Nakamura Y. Kato H. Rescue of the corpus luteum and an increase in luteal superoxide dismutase expression induced by placental luteotropins in the rat: action of testosterone without conversion to estrogen. Biol Reprod. 2000;62:398–403. doi: 10.1095/biolreprod62.2.398. [DOI] [PubMed] [Google Scholar]
- 117.Tarry-Adkins JL. Chen JH. Jones RH. Smith NH. Ozanne SE. Poor maternal nutrition leads to alterations in oxidative stress, antioxidant defense capacity, and markers of fibrosis in rat islets: potential underlying mechanisms for development of the diabetic phenotype in later life. FASEB J. 2010;24:2762–2771. doi: 10.1096/fj.10-156075. [DOI] [PubMed] [Google Scholar]
- 118.Tarry-Adkins JL. Martin-Gronert MS. Chen JH. Cripps RL. Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008;22:2037–2044. doi: 10.1096/fj.07-099523. [DOI] [PubMed] [Google Scholar]
- 119.Thaler R. Karlic H. Rust P. Haslberger AG. Epigenetic regulation of human buccal mucosa mitochondrial superoxide dismutase gene expression by diet. Br J Nutr. 2009;101:743–749. doi: 10.1017/S0007114508047685. [DOI] [PubMed] [Google Scholar]
- 120.Theys N. Clippe A. Bouckenooghe T. Reusens B. Remacle C. Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction. PLoS One. 2009;4:e6110. doi: 10.1371/journal.pone.0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomat AL. Inserra F. Veiras L. Vallone MC. Balaszczuk AM. Costa MA. Arranz C. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol. 2008;295:R543–R549. doi: 10.1152/ajpregu.00050.2008. [DOI] [PubMed] [Google Scholar]
- 122.Tosh DN. Fu Q. Callaway CW. McKnight RA. McMillen IC. Ross MG. Lane RH. Desai M. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid versus delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trocino RA. Akazawa S. Ishibashi M. Matsumoto K. Matsuo H. Yamamoto H. Goto S. Urata Y. Kondo T. Nagataki S. Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose-induced rat embryo culture. Diabetes. 1995;44:992–998. doi: 10.2337/diab.44.8.992. [DOI] [PubMed] [Google Scholar]
- 124.Tsukimori K. Yoshitomi T. Morokuma S. Fukushima K. Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens. 2008;21:1343–1346. doi: 10.1038/ajh.2008.289. [DOI] [PubMed] [Google Scholar]
- 125.Valko M. Izakovic M. Mazur M. Rhodes CJ. Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 126.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Vanaja DK. Ehrich M. Van den Boom D. Cheville JC. Karnes RJ. Tindall DJ. Cantor CR. Young CY. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27:549–560. doi: 10.1080/07357900802620794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Votavova H. Dostalova Merkerova M. Fejglova K. Vasikova A. Krejcik Z. Pastorkova A. Tabashidze N. Topinka J. Veleminsky M., Jr. Sram RJ. Brdicka R. Transcriptome alterations in maternal and fetal cells induced by tobacco smoke. Placenta. 2011;32:763–770. doi: 10.1016/j.placenta.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 129.Vucetic Z. Kimmel J. Totoki K. Hollenbeck E. Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Warren AY. Matharoo-Ball B. Shaw RW. Khan RN. Hydrogen peroxide and superoxide anion modulate pregnant human myometrial contractility. Reproduction. 2005;130:539–544. doi: 10.1530/rep.1.00437. [DOI] [PubMed] [Google Scholar]
- 131.Waterland RA. Travisano M. Tahiliani KG. Rached MT. Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wolff GL. Kodell RL. Moore SR. Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 133.Xiao D. Huang X. Yang S. Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zabihi S. Loeken MR. Understanding diabetic teratogenesis: where are we now and where are we going? Birth Defects Res A Clin Mol Teratol. 2010;88:779–790. doi: 10.1002/bdra.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang X. Strakovsky RS. Zhou D. Zhang Y. Pan YX. A maternal high-fat diet represses the expression of antioxidant defense genes and induces the cellular senescence pathway in the liver of male offspring rats. J Nutr. 2011;141:1254–1259. doi: 10.3945/jn.111.139576. [DOI] [PubMed] [Google Scholar]
- 136.Zhao L. Chen YH. Wang H. Ji YL. Ning H. Wang SF. Zhang C. Lu JW. Duan ZH. Xu DX. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol Sci. 2008;103:149–157. doi: 10.1093/toxsci/kfn027. [DOI] [PubMed] [Google Scholar]
- 137.Zheng S. Pan YX. Histone modifications, not DNA methylation, cause transcriptional repression of p16 (CDKN2A) in the mammary glands of offspring of protein-restricted rats. J Nutr Biochem. 2011;22:567–573. doi: 10.1016/j.jnutbio.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 138.Zheng S. Rollet M. Pan YX. Maternal protein restriction during pregnancy induces CCAAT/enhancer-binding protein (C/EBPbeta) expression through the regulation of histone modification at its promoter region in female offspring rat skeletal muscle. Epigenetics. 2011;6:161–170. doi: 10.4161/epi.6.2.13472. [DOI] [PubMed] [Google Scholar]
- 139.Ziech D. Franco R. Pappa A. Panayiotidis MI. Reactive Oxygen Species (ROS)—Induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 140.Zusterzeel PL. Rutten H. Roelofs HM. Peters WH. Steegers EA. Protein carbonyls in decidua and placenta of pre-eclamptic women as markers for oxidative stress. Placenta. 2001;22:213–219. doi: 10.1053/plac.2000.0606. [DOI] [PubMed] [Google Scholar]