Abstract
Background:
Cytokeratins and Vimentin are intermediate filament proteins. Vimentin expression in tissue samples has been reported to be associated with a poor prognosis in non-small cell lung cancer patients who underwent surgery. CYFRA 21-1 (Cytokeratin 19 Fragment) is a well known tumor marker.
Objective:
This study aimed to investigate the usefulness of serum vimentin as a tumor marker and significance of CYFRA 21-1 and vimentin expression on prognosis of advanced lung cancer patients.
Methods:
One hundred and four advanced lung cancer patients and 19 non-lung cancer patients were included. A total of 157 clinical samples obtained from 113 patients was used for immunostaining of vimentin and measurements of CYFRA 21-1 and vimentin concentrations.
Results:
Compared to low concentration, high concentration of serum CYFRA 21-1 was associated with shorter overall survival in lung cancer patients. However, there was no difference in the serum vimentin concentration between the patients with lung cancer and those with non-lung cancer. No difference in vimentin concentration was observed between the malignant and non-malignant pleural effusions. Immunostaining revealed that of the 43 tumor samples, 21 were positive and 22 were negative for vimentin. No significant difference was found in overall survival between patients with positive and negative for vimentin.
Conclusion:
An elevated serum CYFRA 21-1 concentration was associated with shorter overall survival in advanced lung cancer patients. However, serum vimentin was not as useful as a tumor marker of lung cancer. The vimentin positivity in tumor samples might not predict patients’ prognosis in patients with advanced lung cancer.
Key words: Lung cancer, Pleural effusion, Serum, Tumor marker, Vimentin, Cytokeratin, CYFRA 21-1, Prognosis
1. INTRODUCTION
Cytokeratins are type I and II intermediate filament proteins, and cytokeratin 19 fragments (CYFRA 21-1) have been used as tumor markers for Non-Small Cell Lung Cancer (NSCLC). Vimentin is the type III intermediate filament protein that is widely expressed in mesenchymal cells. Vimentin is also expressed in a variety of cancer cells such as prostate, ovarian and lung cancers, and particularly in poorly differentiated, metastatic, or invasive cancers [1-5]. In studies of lung cancer, vimentin was detected in 145 of 295 NSCLC cases (49.2%) by immunohistochemistry [6]. Vimentin expression was associated with the histology (other types than adenocarcinoma and squamous cell carcinoma) and the poor differentiation status of NSCLCs, and with the occurrence of metastasis [6]. Vimentin expression was also shown to be associated with a poor prognosis in NSCLC patients who underwent surgery [5, 7]. It is also known that vimentin is a marker of epithelial to mesenchymal transition (EMT) [8]. Generally, cancer cells that have undergone an EMT show a high grade of malignancy and have invasive or metastatic potential [9]. These findings suggested that the expression of vimentin in tissue samples could be positively associated with a high grade of malignancies, including lung cancer. However, the significance of vimentin expression on the prognosis of patients with advanced lung cancer has never been studied.
When tested in the serum samples of patients with hepatocellular carcinoma, the vimentin level was significantly higher in small tumors (<2 cm) than in non-neoplastic controls (p<0.01), and was found to be more useful rather than serum alpha-fetoprotein [10]. Serum vimentin might be useful as a tumor marker in patients with lung cancer. However, the significance of the serum vimentin level in lung cancer patients has never been reported.
In the present study, we assessed the association of vimentin expression with survival in advanced lung cancer patients. In addition, we measured the vimentin concentrations in sera and pleural effusions in lung cancer patients and compared with known tumor markers such as CYFRA 21-1, to determine the significance of the serum vimentin level in lung cancer.
2. PATIENTS AND METHODS
2.1. Patients
This study was approved by the Institutional Review Board of Kagawa University (H25-053). Patients with pathologically confirmed advanced (stage III or IV) lung cancer who presented to the Department of Internal Medicine, Kagawa University Hospital between November 2013 to March 2018 and provided written informed consent were included. The lung cancers were staged according to the 7th edition of the TNM classification [11]. Patients who had undergone surgical treatment for lung cancer and those who had another concomitant active malignancy were excluded. For comparison, non-lung cancer patients who provided written informed consent for their materials to be used were also included. Relevant clinical and laboratory data were collected from the patients' medical records. Patient’s daily living activities were expressed by Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (available at https://ecog-acrin.org/resources.ecog-performance-status).
2.2. Measurement of Serum Vimentin, CEA, and CYFRA21-1 Concentrations
The concentration of serum vimentin was measured by a human vimentin Enzyme-Linked Immunosorbent Assay (ELISA) kit (#CSB-E08982h, Cusabio Biotech, College Park, MD, USA or #MBS721933, MyBioSource, San Diego, CA) according to the manufacturer’s instructions. The serum was diluted at 1:100 (#CSB-E08982h) or not diluted (#MBS721933) for the ELISA to prepare the appropriate measurable range of vimentin concentrations. Serum carcinoembryonic antigen (CEA) was measured by a chemiluminescence immunoassay kit (Architect 47-0522/R04; Abbott Japan Co., Tokyo, Japan). Serum CYFRA21-1 was measured by an electro-chemiluminescence immunoassay kit (ECLusys reagent CYFRA; Roche Diagnostics, Ritkreuz, Switzerland).
2.3. Vimentin Immunostaining
The immunostaining and the assessment of vimentin in tissue and cell block samples were performed as described [5]. Briefly, samples were fixed with 10% phosphate-buffered formalin, and paraffin-embedded sections were immunohistochemically examined using anti-vimentin antibody (Clone V9, #M0725; Dako, Glostrup, Denmark) using a Ventana BenchMark XT autostainer (Ventana Medical Systems, Tucson, AZ). All areas of each tumor were evaluated, and the percentage of tumor cells that expressed vimentin at any intensity level was calculated. A positive case was defined as the presence of >5% of cancer cells that express vimentin.
2.4. Detection of EGFR Mutations and ALK Fusions
Epidermal Growth Factor Receptor (EGFR) mutations were examined by the Cobas EGFR Mutation Test (Roche Diagnostics). Anaplastic Lymphoma Kinase (ALK) fusions were examined by immunohistochemistry (Nichirei’s N-Histofine ALK detection kit; Nichirei Biosciences inc., Tokyo, Japan) and fluorescence in situ hybridization (Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe; Abbott).
2.5. Statistical Analysis
Student’s t-test was used to compare two-group data (vimentin concentrations). The data are expressed as means ± standard error of the mean. Overall Survival (OS) was defined as the time between the date of diagnosis and the date of death from any cause. OS curves were constructed by the Kaplan-Meier method, and the differences in OS were assessed using the log-rank test. All statistical analyses were conducted using Excel 2013 and Ekuseru-Toukei 2015 software (Social Survey Research Information, Tokyo).
3. RESULTS
3.1. Patients and Clinical Samples
One hundred and four lung cancer patients and 19 non-lung cancer patients were included in this study. The characteristics of lung cancer patients are shown in Table 1. We obtained 157 clinical samples from 113 patients (94 with lung cancer and 19 with non-lung cancer) before the administration of treatment (chemotherapy and/or radiotherapy) for lung cancer. These samples include 113 peripheral blood samples and 44 pleural effusion samples. Tissue and cell block samples were prepared from liquid cell samples such as pleural effusions and the wash from a bronchoscopic examination.
Table 1. Lung cancer patients’ characteristics.
 |
3.2. Overall Survival of Lung Cancer Patients
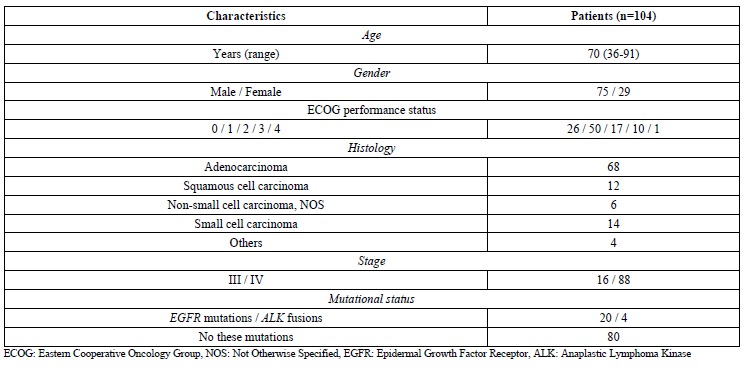
The Kaplan-Meier survival curves illustrate that the patients with poor ECOG PS showed shorter overall survival (Fig. (1A), median 115 vs 486 days, p<0.0001). Patients with driver gene mutations, that is, EGFR mutations or ALK fusions, had longer survival (Fig. (1B), median 507 vs 317 days, p=0.0140). An elevated serum CYFRA21-1 concentration was associated with poor survival (Fig. (1C), median 258 vs 614 days, p=0.0195). Serum Carcinoembryonic Antigen (CEA) concentration were not related to survival (Fig. (1D).
Fig. (1).
Kaplan-Meier curves of overall survival in patients with advanced lung cancer, dependent on the status of (A) ECOG performance status, (B) Presence or absence of EGFR mutations or ALK fusions, (C) Serum CYFRA21-1, and (D) Serum CEA.
3.3. Vimentin Expression in Tissue and Cell Block Samples
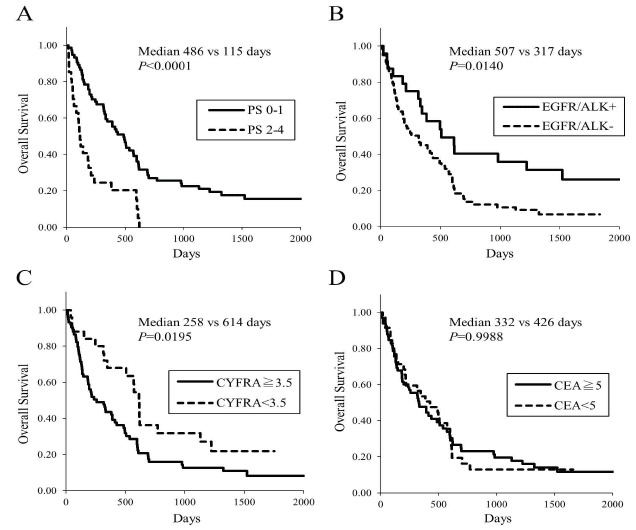
To confirm that some lung cancer cells express vimentin, we assessed the vimentin expression by immunostaining in 36 tissue samples and 15 cell block samples. Both tissue and cell block samples were available in 7 patients. Thus vimentin expressions in tumor samples were assessed in 43 patients. Twenty-one tumors were positive and 22 were negative for vimentin (a representative of positivity in cell block samples is shown in Figs. (2A) and (2B), demonstrating that vimentin is certainly expressed in some lung cancer cells. All 43 NSCLC patients underwent chemotherapy and/or molecular targeted therapy. No significant difference was found in the Kaplan-Meier survival curves for OS (Fig. (2C), median 507 vs. 392 days, p=0.2654) in patients with positive versus negative for vimentin. When analyzed in patients limited in stage IV, no difference in OS was still observed between vimentin positivity.
Fig. (2).
(A and B) A representative of vimentin positivity in lung cancer cells. (A) Hematoxylin-eosin staining and (B) Immunostaining of vimentin in a cell block sample obtained from a patient with adenocarcinoma. (C) Kaplan-Meier curves of overall survival in patients with advanced lung cancer, dependent on the status of vimentin positivity based on immunostaining.
3.4. The Serum Vimentin Concentrations
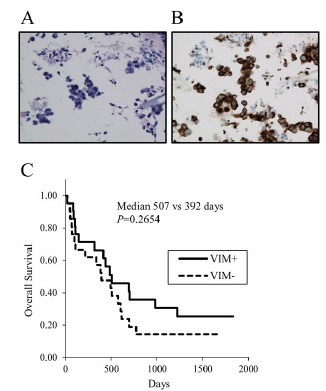
When used an ELISA kit (CSB-E08982h), no significant difference was revealed in the serum vimentin concentration between the patients with lung cancer (n=69) and those with non-lung cancer (n=15, including five infectious disease and three bronchial asthma patients) (Fig. 3A) or between the patients with NSCLC (n=57) and those with small-cell lung cancer (SCLC) (n=12) (Fig. 3B), although the standard curves were drawn without any problem (data not shown). We also measured the vimentin concentration in pleural effusions (n=22). No significant difference in the concentration was observed whether cancer cells were detected (malignant pleural effusion) or not in pleural effusions (Fig. 3C). In addition, there was no significant difference in the vimentin concentration between the serum and the malignant pleural effusion in six patients for whom both samples were available (data not shown).
Fig. (3).
The vimentin concentrations in clinical samples measured by ELISA kits: (A-C) CSB-E08982h (Cusabio Biotech) and (D-F) MBS721933, (MyBioSource). (A) Serum vimentin in patients with lung cancer vs. non-lung cancer. (B) Serum vimentin in patients with NSCLC vs. SCLC. (C) Vimentin in pleural effusions in which cancer cells are present vs. absent. (D) Serum vimentin in patients with lung cancer vs. non-lung cancer. (E): Serum vimentin in patients with NSCLC vs. SCLC. (F): Vimentin in pleural effusions in which cancer cells are present vs. absent.
To determine whether the non-positivity of serum vimentin in lung cancer patients is true, we additionally measured the concentrations of vimentin using another ELISA kit (#MBS 721933). Clear standard curves were acquired with this ELISA kit (data not shown). However, there was no significant difference in the concentration of serum vimentin between the patients with lung cancer (n=25) and those with non-lung cancer (n=4, including chronic obstructive pulmonary diseases and interstitial lung diseases) (Fig. 3D), or between the patients with NSCLC (n=20) and those with SCLC (n=5) (Fig. 3E).
In addition, no significant difference in vimentin concentrations was observed in the pleural effusions between the malignant pleural effusions (n=13) and the non-malignant pleural effusions (n=9) (Fig. 3F).
4. DISCUSSION
Our findings demonstrated that (1) the vimentin expression was not associated with survival in advanced lung cancer patients, (2) elevated serum CYFRA 21-1 concentration was associated with poor survival, (3) the vimentin concentration was not increased in the sera or malignant pleural effusions of patients with advanced lung cancer. The absence of a significant difference in vimentin concentrations between lung cancer and non-lung cancer was confirmed by two commercially available ELISA kits. These findings suggest that the serum vimentin concentration is not useful as a tumor marker of lung cancer.
Previous studies using mesenchymal cells cultured in vitro have indicated that vimentin is largely insoluble [12, 13]. Vimentin solubility was reported to be associated with substrate stiffness as well as aspects of the cellular environment such as serum starvation, cell-cell contacts, and other cytoskeletal assemblies including myosin and actin [13]. Although the expression of vimentin as well as, those of cytokeratin and actin would be dynamically dependent on the in vivo cellular environment, the vimentin release into sera seems to be too faint for use as a tumor marker of lung cancer.
In NSCLC patients who underwent surgery, the vimentin expression assessed by immunostaining was associated with a poor prognosis [5, 7]. In the present study, however, there was no significant difference in OS between the vimentin-positive and negative patients. Judged from small sample size (n=43), vimentin expression might not predict the prognosis of patients with advanced lung cancer.
The current study demonstrated that elevated serum CYFRA 21-1 concentration was associated with poor survival. Several studies have reported an association of CYFRA 21-1 with prognosis in NSCLC patients [14-21]. An elevated serum CYFRA 21-1 concentration showed shorter survival in patients treated with pemetrexed-based chemotherapy or EG FR-TKIs [15-18]. Another study reported that NSCLC patients with high serum CEA and normal serum CYFRA 21-1 concentrations had a longer survival [19]. Basically, cytokeratins negatively regulate the invasive potentials of several kinds of cancer cells including lung cancer [22-24]. Nevertheless, an elevated CYFRA 21-1 is commonly associated with poor prognosis. Such a negative correlation of CYFRA 21-1 and prognosis could reflect tumor burden [24]. In fact, a high CYFRA 21-1 concentration was correlated with the advanced stage as well as shorter OS [20]. Interestingly, it was recently reported that a higher serum CYFRA 21-1 concentration (more than 2.2 ng/mL) is a predictor of a favorable outcome in patients treated with nivolumab [21]. In the era of combination of chemotherapy and immune checkpoint inhibitors, the significance of serum CYFRA 21-1 as a predictor of prognosis would change.
CONCLUSION
The serum vimentin concentration is not as useful as a tumor marker of lung cancer. Vimentin expression in tumor samples might not predict patients’ prognosis in patients with advanced lung cancer. An elevated serum CYFRA 21-1 concentration is associated with poor overall survival in advanced lung cancer patients.
ACKNOWLEDGEMENTS
Nobuhiro Kanaji designed the study, collected clinical data, and wrote paper. Kyuichi Kadota collected and analyzed pathological data. Akira Tadokoro, Takuya Inoue, Naoki Watanabe, Reiji Haba, Norimitsu Kadowaki, and Tomoya Ishii revised paper.
LIST OF ABBREVIATIONS
- NSCLC
= Non-Small Cell Lung Cancer
- EMT
= Epithelial to Mesenchymal Transition
- OS
= Overall Survival
- ECOG
= Eastern Cooperative Oncology Group
- PS
= Performance Status
- CEA
= Carcinoembryonic Antigen
- EGFR
= Epidermal Growth Factor Receptor
- ALK
= Anaplastic Lymphoma Kinase
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board of Kagawa University (H25-053).
HUMAN AND ANIMAL RIGHTS
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 2013 Helsinki Declaration and its later amendments or comparable ethical standards.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all individual participants.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lang S.H., Hyde C., Reid I.N., Hitchcock I.S., Hart C.A., Bryden A.A., Villette J.M., Stower M.J., Maitland N.J. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52(4):253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 2.Gilles C., Polette M., Mestdagt M., Nawrocki-Raby B., Ruggeri P., Birembaut P., Foidart J.M. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63(10):2658–2664. [PubMed] [Google Scholar]
- 3.Korsching E., Packeisen J., Liedtke C., Hungermann D., Wülfing P., van Diest P.J., Brandt B., Boecker W., Buerger H. The origin of vimentin expression in invasive breast cancer: epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J. Pathol. 2005;206(4):451–457. doi: 10.1002/path.1797. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., Yan Q., Long X., Chen X., Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem. Funct. 2008;26(5):571–577. doi: 10.1002/cbf.1478. [DOI] [PubMed] [Google Scholar]
- 5.Tadokoro A., Kanaji N., Liu D., Yokomise H., Haba R., Ishii T., Takagi T., Watanabe N., Kita N., Kadowaki N., Bandoh S. Vimentin regulates invasiveness and is a poor prognostic marker in non-small cell lung cancer. Anticancer Res. 2016;36(4):1545–1551. [PubMed] [Google Scholar]
- 6.Dauphin M., Barbe C., Lemaire S., Nawrocki-Raby B., Lagonotte E., Delepine G., Birembaut P., Gilles C., Polette M. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81(1):117–122. doi: 10.1016/j.lungcan.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Al-Saad S., Al-Shibli K., Donnem T., Persson M., Bremnes R.M., Busund L.T. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br. J. Cancer. 2008;99(9):1476–1483. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuoriluoto K., Haugen H., Kiviluoto S., Mpindi J.P., Nevo J., Gjerdrum C., Tiron C., Lorens J.B., Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30(12):1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 9.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun S., Poon R.T., Lee N.P., Yeung C., Chan K.L., Ng I.O., Day P.J., Luk J.M. Proteomics of hepatocellular carcinoma: serum vimentin as a surrogate marker for small tumors (<or=2 cm). J. Proteome Res. 2010;9(4):1923–1930. doi: 10.1021/pr901085z. [DOI] [PubMed] [Google Scholar]
- 11.Goldstraw P., Crowley J., Chansky K., Giroux D.J., Groome P.A., Rami-Porta R., Postmus P.E., Rusch V., Sobin L. International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 12.Blikstad I., Lazarides E. Vimentin filaments are assembled from a soluble precursor in avian erythroid cells. J. Cell Biol. 1983;96(6):1803–1808. doi: 10.1083/jcb.96.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray M.E., Mendez M.G., Janmey P.A. Substrate stiffness regulates solubility of cellular vimentin. Mol. Biol. Cell. 2014;25(1):87–94. doi: 10.1091/mbc.e13-06-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y., Xu L., Qiu M., Wang J., Zhou Q., Xu L., Wang J., Yin R. Prognostic value of serum cytokeratin 19 fragments (Cyfra 21-1) in patients with non-small cell lung cancer. Sci. Rep. 2015;5:9444. doi: 10.1038/srep09444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sone K., Oguri T., Nakao M., Kagawa Y., Kurowaka R., Furuta H., Fukuda S., Uemura T., Takakuwa O., Kanemitsu Y., Ohkubo H., Takemura M., Maeno K., Ito Y., Sato H., Muramatsu H., Niimi A. CYFRA 21-1 as a predictive marker for non-small cell lung cancer treated with pemetrexed-based chemotherapy. Anticancer Res. 2017;37(2):935–939. doi: 10.21873/anticanres.11402. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K., Hata A., Kaji R., Fujita S., Otoshi T., Fujimoto D., Kawamura T., Tamai K., Takeshita J., Matsumoto T., Monden K., Nagata K., Otsuka K., Nakagawa A., Tachikawa R., Otsuka K., Tomii K., Katakami N. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation. J. Thorac. Oncol. 2013;8(7):892–898. doi: 10.1097/JTO.0b013e31828c3929. [DOI] [PubMed] [Google Scholar]
- 17.Fiala O., Pesek M., Finek J., Benesova L., Minarik M., Bortlicek Z., Topolcan O. Predictive role of CEA and CYFRA 21-1 in patients with advanced-stage NSCLC treated with erlotinib. Anticancer Res. 2014;34(6):3205–3210. [PubMed] [Google Scholar]
- 18.Takeuchi A., Oguri T., Sone K., Ito K., Kitamura Y., Inoue Y., Asano T., Fukuda S., Kanemitsu Y., Takakuwa O., Ohkubo H., Takemura M., Maeno K., Ito Y., Niimi A. Predictive and prognostic value of CYFRA 21-1 for advanced Non-small cell lung cancer treated with EGFR-TKIs. Anticancer Res. 2017;37(10):5771–5776. doi: 10.21873/anticanres.12018. [DOI] [PubMed] [Google Scholar]
- 19.Baek A.R., Seo H.J., Lee J.H., Park S.W., Jang A.S., Paik S.H., Koh E.S., Shin H.K., Kim D.J. Prognostic value of baseline carcinoembryonic antigen and cytokeratin 19 fragment levels in advanced non-small cell lung cancer. Cancer Biomark. 2018;22(1):55–62. doi: 10.3233/CBM-170885. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z., Zhang G., Yang M., Zhang S., Zhao B., Shen G., Chai Y. Systematic review of CYFRA 21-1 as a prognostic indicator and its predictive correlation with clinicopathological features in Non-small Cell Lung Cancer: A meta-analysis. Oncotarget. 2017;8(3):4043–4050. doi: 10.18632/oncotarget.14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirasu H., Ono A., Omae K., Nakashima K., Omori S., Wakuda K., Kenmotsu H., Naito T., Murakami H., Endo M., Nakajima T., Takahashi T. CYFRA 21-1 predicts the efficacy of nivolumab in patients with advanced lung adenocarcinoma. Tumour Biol. 2018;40(2):1010428318760420. doi: 10.1177/1010428318760420. [DOI] [PubMed] [Google Scholar]
- 22.Crowe D.L., Milo G.E., Shuler C.F. Keratin 19 downregulation by oral squamous cell carcinoma lines increases invasive potential. J. Dent. Res. 1999;78(6):1256–1263. doi: 10.1177/00220345990780061001. [DOI] [PubMed] [Google Scholar]
- 23.Bühler H., Schaller G. Transfection of keratin 18 gene in human breast cancer cells causes induction of adhesion proteins and dramatic regression of malignancy in vitroitalic> and in vivo. Mol. Cancer Res. 2005;3(7):365–371. doi: 10.1158/1541-7786.MCR-04-0117. [DOI] [PubMed] [Google Scholar]
- 24.Kanaji N., Bandoh S., Ishii T., Fujita J., Ishida T., Matsunaga T., Kubo A. Cytokeratins negatively regulate the invasive potential of lung cancer cell lines. Oncol. Rep. 2011;26(4):763–768. doi: 10.3892/or.2011.1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


