Abstract
Background
The aging population is associated with increased multimorbidity and polypharmacy. Older adults are at a higher risk of adverse events and reduced therapeutic response. This phenomenon is partially explained by drug interactions and treatment adherence. Most randomized clinical trials have found no significant differences between morning and evening levothyroxine (LT4) administration in young adults, but there is little evidence regarding alternative LT4 regimens in older populations. Thus, the MONIALE trial aims to test an alternative schedule for LT4 administration in older adults.
Methods/design
This randomized crossover clinical trial will include participants aged 60 years or older with primary hypothyroidism. The trial groups will consist of morning LT4 intake (60 min before breakfast) or evening LT4 intake (60 min after supper). The primary outcome will be variation in serum thyrotropin (TSH) levels after 24 weeks of the LT4 protocol. The secondary outcomes will be the prevalence of drugs that potentially interact with LT4 and hypothyroidism control according to interaction status. The sample size was calculated to detect a minimum mean difference of 1 mUI/L in serum TSH level between the groups with 80% power and a 5% probability of type I error, resulting in 91 patients per group. The project was approved by the Hospital de Clínicas de Porto Alegre Ethics Committee.
Discussion
Considering the aging population, the increased prevalence of multimorbidity and polypharmacy, as well as potential drug interactions and treatment adherence difficulties, an alternative LT4 protocol could be useful for hypothyroidism treatment in the elderly. Prior studies comparing alternative LT4 administration protocols have mainly included young adult populations and have not addressed potential drug interactions.
Trial registration
ClinicalTrials.gov, NCT03614988. Registered 30 July 2018.
Keywords: Hypothyroidism, Aged, Levothyroxine
Background
Epidemiologic and demographic changes have resulted in aging of the population [1]. Between 1980 and 2017 the number of people aged 60 years or older worldwide has risen from 362 to 982 million, and by 2050 people in this age range will outnumber those in all other age ranges [2].
Older age is associated with a higher prevalence of multiple chronic diseases [3, 4] and polypharmacy, which is generally defined in the literature as the use of five or more concomitant medications [5]. Adverse events, such as drug–drug interactions [6], non-adherence [7], suboptimal therapeutic effectiveness, and poor clinical response [8] are related to multiple drug use. Both multimorbidity and polypharmacy are correlated with falls, hospitalizations, functional limitations, and mortality [9, 10].
The prevalence of thyroid dysfunction increases with age [11, 12]. The National Health and Nutrition Examination Survey, conducted between 1988 and 1994, found a hypothyroidism prevalence of 4.6% (0.3% clinical and 4.3% subclinical), being more common in women aged between 50 and 70 years (p < 0.001) [12]. Physiological changes due to the aging process could impact hypothyroidism treatment [13]. In older populations, pharmacokinetics might be modified by gastrointestinal aging and decreases in body water content, serum albumin, hepatic biotransformation, and renal clearance [14].
Levothyroxine is a synthetic derivative (levorotatory isomer) of thyroxine. Its ionization state and dissolution are influenced by gastric pH [15]. Although in healthy volunteers bioavailability can reach 60–80% [16, 17], there could be a 9.4% decrease in thyroxine absorption in patients over 70 years old (62.8% ± 13.5% SD vs 69.3% ± 11.9%; p < 0.001), as was found in a study of 45 euthyroid individuals [18]. The small bowel is the main site of absorption; the duodenum accounts for 15 ± 5% SD, the upper jejunoileum 29 ± 14% SD, and the lower jejunoileum 24 ± 11% SD of 24-h 131I-labeled thyroxine absorption [16]. The time necessary to reach the maximum serum concentration (Tmax) of the drug is approximately 2–3 h from ingestion, and plateaus occur at 18 and 48 h. Food and hypothyroidism delay Tmax [19, 20].
Drug bioavailability is responsible for most inter- and intra-individual therapeutic variation [6], which can result from (a) nonadherence, (b) physiological (weight, pregnancy, age) and paraphysiological (behavior, nutrition) conditions, (c) malabsorption diseases, and (d) concomitant medications [8].
In an in vitro study, Pabla et al. found that a higher pH impairs dissolution of thyroxine [21], and Centanni et al. observed a higher thyroxine requirement in ten euthyroid patients with multinodular goiter who were receiving concomitant omeprazole [22]. In a prospective study, however, the hormone levels of 19 hypothyroid subjects did not change when they were advised to take omeprazole 30 min after LT4 [23]. Besides the known interaction with proton pump inhibitors, interactions between LT4 and other drugs/supplements have been recognized [24], such as with iron [25], calcium supplements [26], aluminum hydroxide [27], raloxifene [28], sevelamer [29], cholestyramine [30], and ciprofloxacin [31]. In light of these findings, the American Thyroid Association recommends a 4-h interval between potentially interfering drugs, although this is based on a low grade of evidence [32].
A survey of referral centers about the appropriate use of LT4 revealed that, although the majority of patients understood that LT4 should not be taken with food, only 52.1% were aware that it should also not be taken with other medications [33]. Forgetting to take the tablets and a lack of understanding about the need for continued treatment were found to be the causes of low adherence in two-thirds of 100 uncontrolled hypothyroidism patients [34].
In 1977, in a sample of healthy volunteers, Wenzel and Kirschsieper found that LT4 absorption decreased from 79.3% ± 7.2% SD under fasting conditions to 63.9% ± 10.5% SD after a meal of two buttered rolls and a boiled egg (p < 0.001) [17]. Years later, Bevenga et al. demonstrated that postponing breakfast from 15 to 20 min to 60 min improved the thyroid function tests in hypothyroid individuals [20]. LT4 has also been reported to interact with coffee [35], soy [36], grapefruit [37], and milk [38].
Although some prospective studies on the timing of LT4 administration have been published, they were not designed to include older adults. In a pilot study, Bolk et al. [39] assessed the TSH levels of 19 hypothyroid women (mean age 48 years) for 24 h on two separate days. Evening LT4 intake (hours after supper) lowered TSH levels more than morning intake (30 min before breakfast) (1.2 ± 0.3 mUI/L vs 5.1 ± 0.9 mUI/L, p < 0.01). Despite the bedtime LT4 regimen, the circadian pattern of thyrotropin was maintained and did not interfere with morning blood sampling [39]. The same group was able to reproduce these results in a randomized crossover placebo-controlled trial with 90 hypothyroid patients (mean age 48 years), who were followed for 24 weeks. In the intergroup comparison, bedtime intake had a direct treatment effect, with a decrease in TSH levels of 1.25 mIU/L (95% confidence interval (CI) 0.60–1.89 mIU/L; p = 0.001) and an increase in free T4 of 0.07 ng/dL (95% CI 0.02–0.13, p = 0.01) compared to intake 30 min before breakfast [40]. Rajput et al. [41] randomized 152 drug-naive hypothyroidism patients (mean age 34.30 ± 11.82 years) into two parallel groups to receive LT4 2 h after dinner or 30 min before breakfast. At the end of 12 weeks, finding no significant difference between treatment strategies for both euthyroidism (96% vs 90%; p = 0,19) and mean serum TSH levels (3.27 mUI/L ± 4.19 vs 5.13 mUI/L ± 9.36; p = 0.31) [41]. Bach-Huynh et al. evaluated three timing strategies in 65 patients (48 ± 13 years) and found that taking LT4 an hour before breakfast resulted in significantly lower TSH levels (1.06 mIU/L 95% CI 0.60–1.52 mIU/L; p < 0.001) than when taking it 20 min before breakfast or 2 h after supper [42]. In agreement with these results, Perez et al. found higher serum TSH levels when LT4 was taken at the beginning of breakfast (2.89 mIU/L ± 2.82 vs 1.9 mIU/L ± 1.76; p = 0.028) than when taken 1 h before [43]. A recent study obtained data from 84 patients (71% aged ≤ 65 years old) on stable doses of LT4. They performed a three-period crossover trial of LT4 administration (30 min before breakfast, 1 h before lunch and 2 h after supper). No significant differences in thyroid profile results were found in the per-protocol analysis [44].
Trial rationale
Most published studies on the timing of LT4 administration have analyzed young populations and have not addressed the use of intervening medications. This trial aims to test an alternative schedule for LT4 administration in older adults with a randomized crossover clinical trial. Morning administration, which is more commonly used, will be compared with evening administration for 3 months of follow-up. This report follows the SPIRIT Statement guidelines [45]. We present a standardized checklist with recommended SPIRIT Statement items (Additional file 1).
Methods/design
Study design and setting
This prospective, randomized crossover trial will be conducted at the Endocrinology and Internal Medicine Clinic at the Hospital Clínicas de Porto Alegre, Brazil.
Eligible participants
The research team will identify outpatients ≥ 60 years old with primary hypothyroidism who have been using LT4 for at least 6 months and have been on stable doses for the last 3 months. The study procedures will be explained to those who meet the inclusion criteria, and those who provide written informed consent will be enrolled. Screening will continue until the target population is achieved.
Exclusion criteria
The exclusion criteria are severe organic syndrome, dementia, thyroid cancer, heart failure (functional class IV), or three or more hospital admissions in the last year due to heart failure decompensation, and refusal to participate.
Intervention
Participants will be instructed to take LT4 tablets according to random treatment allocation, either in the evening (60 min after supper) or in the morning (60 min before breakfast). After 3 months of follow-up, the treatment will be changed using a crossover strategy.
Outcomes
Primary outcome
Change in serum TSH levels after 24 weeks of follow-up.
Secondary outcomes
To identify concomitant drugs that affect LT4 absorption
To compare TSH control efficacy between the two treatment strategies according to age, drug interaction, and sex subgroups
Sample size calculation
SAS Studio 3.7 was used to calculate the sample size. The calculated sample size (182 participants, 91 in each group) can detect a difference of 1.0 mUI/L between the means as significant, considering a standard deviation of 2.0 for group 1 and 3.87 for group 2 (BOLK, 2010). A power of 80% and significance level of 5% were considered in the calculation. Adding 10% for possible losses and refusals, the sample size should be 200.
Random allocation
A randomization list stratified by sex was created using a web-based program (http://www.randomization.com/). An interchangeable random blocks/variable blocks randomization strategy was chosen to assure allocation concealment. An independent researcher is responsible for the randomization list and treatment allocation.
Blinding
Due to the crossover design, the trial participants, staff, and outcome assessors will be not blinded, although data analysis will be blinded.
Data collection methods
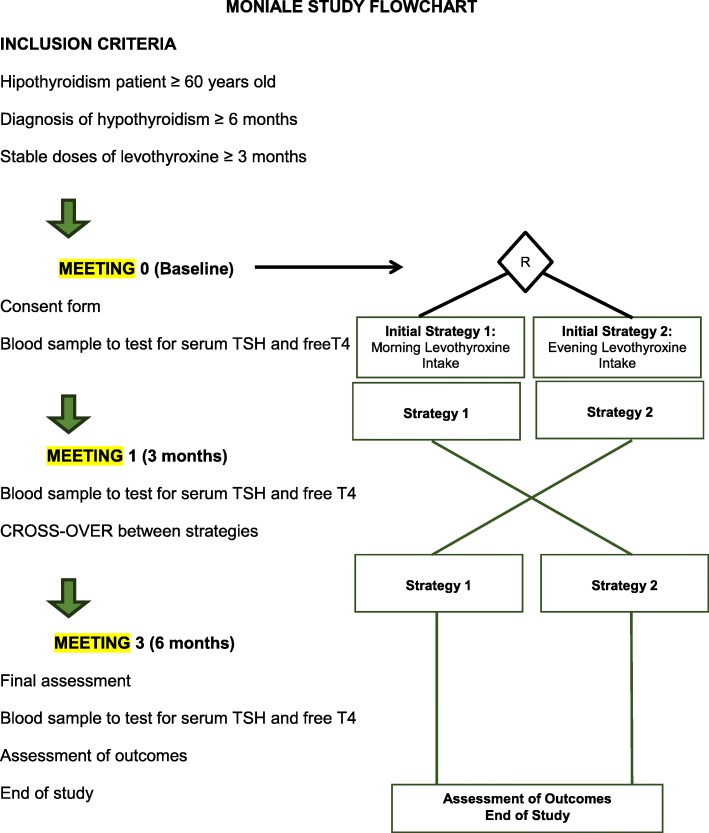
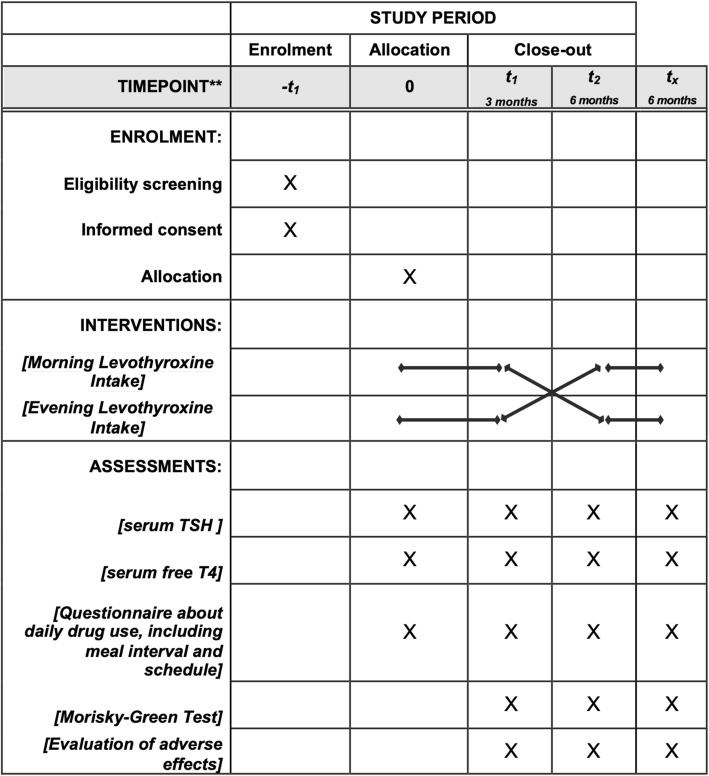
Eligible participants will attend meetings at 0, 12, and 24 weeks after enrollment. Each meeting will include application of standardized questionnaires to assess drug–food–thyroxine interactions, treatment adherence, and adverse events. Blood samples will be collected at baseline, 12 weeks, and 24 weeks after initial treatment allocation to measure free T4 and TSH levels. Figures 1 and 2 describe the follow-up procedure.
Fig. 1.
Flow chart of the MONIALE trial: inclusion criteria, randomization, and follow-up process
Fig. 2.
SPIRIT figure: Allocation, interventions and outcomes
Thyroid function (serum TSH and free T4 levels) will be measured by electrochemiluminescence assay; concentrations of 0.27–4.2 mUI/L and 0.93–1.7 ng/dL, respectively, are the method’s reference limits. Food–LT4 interactions will be analyzed according to time since last meal (< 30 min, 30–60 min, or > 60 min). Concomitant use (within 60 min) of calcium or iron supplements, proton pump inhibitors, other supplements/multivitamins, and daily medications will be considered in the drug–LT4 interaction assessment. The Morisky–Green test will be used to verify treatment adherence and comprehension at baseline and follow-up. Side effects will also be investigated regarding causality between LT4 treatment and severity. Patients will be discontinued from the allocated intervention upon individual request or worsening of their clinical condition due to decompensated hypothyroidism.
Statistical methods
To assess the treatment effect, ANOVA for 2 × 2 crossover studies will be performed. The carryover effect will be analyzed by an independent samples t-test of the sum of the variables for each patient at 12 and 24 weeks [46]. No interim analysis is planned. Primary and secondary outcomes will be presented as intention-to-treat analysis. Additionally, subgroup analysis comparing age groups of 60–74 years and ≥ 75 years will be performed.
Data monitoring and auditing
No data monitoring committee will be created due to the single-blind treatment assignment and the short follow-up and interval between thyroid function tests. Although the study coordinator will constantly audit the data and study conduct, no external auditing process is planned.
Logistics procedures are presented in Fig. 1.
Discussion
Aging is associated with an increased prevalence of multimorbidity (defined as two or more long-term diseases) [4], including a higher frequency of hypothyroidism [12]. Disease-centered, rather than patient-centered, clinical practice guidelines have led to polypharmacy and related adverse events [6, 8]. Due to these issues, in addition to pharmacokinetic changes, hypothyroidism treatment for older adults should be carefully managed [47].
Although alternative strategies of evening LT4 administration have been tested in clinical trials [39–44], the mean age of the included patients was under 60 years of age. In addition, most of the published trials have not addressed drug interaction [39–43] as a potential barrier to hypothyroidism control.
This study will evaluate the fastest growing population worldwide and will provide relevant clinical information for their medical care.
Trial status
Protocol version date 01/19/2018, Institutional Review Board number 2018–0209. This study is currently recruiting participants. The recruitment began in May 2018 and is expected to end by November 2019.
Supplementary information
Additional file 1. SPIRIT 2013 checklist: Recommended items to address in a clinical trial protocol and related documents.
Acknowledgements
The authors would like to thank the Internal Medicine and Endocrinology divisions of the Hospital de Clínicas de Porto Alegre, as well as the Federal University of Rio Grande do Sul Endocrinology Program for supporting clinical research, particularly this study.
Grants
The authors received grants from the Brazilian Ministry of Health, Division of Science and Technology (DECIT), and the Ministry of Science and Technology, FINEP, and CNPq.
Abbreviations
- ICMJE
International Committee of Medical Journal Editors
- LT4
Levothyroxine
- Tmax
Time until maximum serum concentration
- TSH
Thyrotropin
Authors’ contributions
KG: data collection, analysis, and manuscript writing. VP: data collection, manuscript writing, and manuscript revision. RGMB: study design, manuscript writing, revision, and final approval of the manuscript. TCR: study design, manuscript writing, revision, and final approval of the manuscript. All persons listed as authors have prepared and approved the manuscript and the ICMJE criteria for authorship have been met.
Funding
This project is being funded by the Hospital de Clínicas de Porto Alegre Fundo de Incentivo à Pesquisa and Eventos (FIPE). FIPE had no role in the study design, the data collection, analysis or interpretation, drafting the manuscript, or the decision to submit it for publication.
Availability of data and materials
The applied questionnaires will be stored at the research office with coded identification. The study data will remain unidentified. The final dataset will only be handled by the lead investigator, and the funding institution will not have access to it. The results of this clinical trial will be submitted to a peer-reviewed medical journal following the authorship guidelines of the ICMJE CONSORT Statement. Following study publication, the unidentified dataset will be made available by the corresponding author upon formal request.
Ethics approval and consent to participate
This project was approved by the Hospital de Clínicas de Porto Alegre Ethics Committee (83639318.2.0000.5327), which is accredited by the Office of Human Research Protection as an Institutional Review Board. Throughout the study, the local Ethics Committee will be notified of and will evaluate any protocol amendments. Informed consent will be obtained from all study participants.
Consent for publication
According to project number 83639318.2.0000.5327 of the Hospital de Clínicas de Porto Alegre Ethics Committee, written informed consent will be obtained from all individuals before participation in the study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Karina Giassi, Email: kgiassi@hcpa.edu.br.
Vanessa Piccoli, Email: piccoli.vanessa@gmail.com.
Ticiana da Costa Rodrigues, Email: trodrigues@hcpa.edu.br.
Renato Gorga Bandeira de Mello, Email: rgmello@hcpa.edu.br.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13063-019-3816-3.
References
- 1.World Health Organization . World report on ageing and health. 2015. [Google Scholar]
- 2.United Nations, Department of Economic and Social Affairs, Population Division . World population ageing 2017 - highlights (ST/ESA/SER.A/397) 2017. [Google Scholar]
- 3.Hajat Cother, Stein Emma. The global burden of multiple chronic conditions: A narrative review. Preventive Medicine Reports. 2018;12:284–293. doi: 10.1016/j.pmedr.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer C, et al. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016;354:1-23. 10.1136/bmj.i4843. [DOI] [PubMed]
- 5.Masnoon N, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):1–10. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelko E, Klemencketis Z, Tusekbunc K. Medication adherence in elderly with polypharmacy living at home: a systematic review of existing studies. Mater Sociomed. 2016;28(2):129–132. doi: 10.5455/msm.2016.28.129-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher Robert L, Hanlon Joseph, Hajjar Emily R. Clinical consequences of polypharmacy in elderly. Expert Opinion on Drug Safety. 2013;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yarnall AJ, et al. New horizons in multimorbidity in older adults. Age Ageing. 2017;46(6):882–888. doi: 10.1093/ageing/afx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlesworth CJ, et al. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J Gerontol A Biol Sci Med Sci. 2015;70(8):989–995. doi: 10.1093/gerona/glv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canaris GJ, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 12.Hollowell JG, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 13.Chaker L, et al. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6(9):733–742. doi: 10.1016/S2213-8587(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 14.Vrdoljak D, Borovac JA. Medication in the elderly - considerations and therapy prescription guidelines. Acta Med Acad. 2015;2(44):159–168. doi: 10.5644/ama2006-124.142. [DOI] [PubMed] [Google Scholar]
- 15.Janiro G, Mangiola F, diRienzo TA. Levothyroxine absorption in health and disease, and new therapeutic perspectives. European review for hypothyroidism. J Thyroid Res. 2011;2011:1–5. [Google Scholar]
- 16.Hays MT. Localization of human thyroxine absorption. Thyroid. 1991;1:241–248. doi: 10.1089/thy.1991.1.241. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 1977;26(1):1–8. doi: 10.1016/0026-0495(77)90121-4. [DOI] [PubMed] [Google Scholar]
- 18.Hays MT, Nielsen KR. Human thyroxine absorption: age effects and methodological analyses. Thyroid. 1994;4(1):55–64. doi: 10.1089/thy.1994.4.55. [DOI] [PubMed] [Google Scholar]
- 19.Berg JA, Mayor GH. A study in normal human volunteers to compare the rate and extent of levothyroxine absorption from Synthroid® and Levoxine®. J Clin Pharmacol. 1992;32(12):1135–1140. [PubMed] [Google Scholar]
- 20.Benvenga S, et al. Delayed intestinal absorption of levothyroxine. Thyroid. 1995;5(4):249–253. doi: 10.1089/thy.1995.5.249. [DOI] [PubMed] [Google Scholar]
- 21.Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72(1):105–110. doi: 10.1016/j.ejpb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787–1795. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 23.Abi-Abib RC, Vaisman M. Is it necessary to increase the dose of levothyroxine in patients with hypothyroidism who use omeprazole? Arq Bras Endocrinol Metabol. 2014;58(7):731–736. doi: 10.1590/0004-2730000002997. [DOI] [PubMed] [Google Scholar]
- 24.Hennessey JV. The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine. 2016;55(1):6–18. doi: 10.1007/s12020-016-1199-8. [DOI] [PubMed] [Google Scholar]
- 25.Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010–1013. doi: 10.7326/0003-4819-117-12-1010. [DOI] [PubMed] [Google Scholar]
- 26.Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483–486. doi: 10.1089/thy.2010.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183–184. doi: 10.1001/archinte.1992.00400130181024. [DOI] [PubMed] [Google Scholar]
- 28.Siraj ES, Gupta MK, Reddy SS. Raloxifene causing malabsorption of levothyroxine. Arch Intern Med. 2003;163:1367–1370. doi: 10.1001/archinte.163.11.1367. [DOI] [PubMed] [Google Scholar]
- 29.John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothryroxineabsorption. Thyroid. 2007;17:763–765. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 30.Northcutt RC, Stiel JN, Hollifield JW, Stant EG. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857–1861. doi: 10.1001/jama.1969.03160100047012. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg AS, Tirona RG, Asher LJ, et al. Ciprofloxacin and rifampin have opposite effects on levothtroxine absorption. Thyroid. 2013;23:1374–1378. doi: 10.1089/thy.2013.0014. [DOI] [PubMed] [Google Scholar]
- 32.Jonklaas J, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaisman F, et al. How good is the levothyroxine replacement in primary hypothyroidism patients in Brazil? Data of a multicentre study. J Endocrinol Invest. 2013;36(7):485–488. doi: 10.3275/8810. [DOI] [PubMed] [Google Scholar]
- 34.Bagattoli RM, Vaisman M, Lima JS, Ward LS. Estudo de Adesão ao Tratamento do Hipotiroidismo. Arq Braz Endocrinol Metab. 2000;44(2):483–487. doi: 10.1590/S0004-27302000000600006. [DOI] [Google Scholar]
- 35.Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18:293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 36.Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. 2004;89:37–40. doi: 10.1136/adc.2002.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60:337–341. doi: 10.1111/j.1365-2125.2005.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chon DA, et al. Concurrent milk ingestion decreases absorption of levothyroxine. Thyroid. 2018;28(4):454–457. doi: 10.1089/thy.2017.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolk Nienke, Visser Theo J., Kalsbeek Andries, van Domburg Ron T., Berghout Arie. Effects of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clinical Endocrinology. 2006;0(0):061019025934001-???. doi: 10.1111/j.1365-2265.2006.02681.x. [DOI] [PubMed] [Google Scholar]
- 40.Bolk N. Effects of evening vs morning levothyroxine intake. Arch Intern Med. 2010;170(22):1996–2003. doi: 10.1001/archinternmed.2010.436. [DOI] [PubMed] [Google Scholar]
- 41.Rajput R, Chatterjee S, Rajput M. Can levothyroxine be taken as evening dose? Comparative evaluation of morning vs. evening dose of levothyroxine in treatment of hypothyroidism. J Thyroid Res. 2011;2011:1–5. doi: 10.4061/2011/505239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bach-Huynh T-g, et al. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94(10):3905–3912. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez CLS, et al. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid. 2013;23(7):779–784. doi: 10.1089/thy.2012.0435. [DOI] [PubMed] [Google Scholar]
- 44.Skelin M, et al. Effect of timing of levothyroxine administration on the treatment of hypothyroidism: a three-period crossover randomized study. Endocrine. 2018;62(2):432–439. doi: 10.1007/s12020-018-1686-1. [DOI] [PubMed] [Google Scholar]
- 45.Chan An-Wen, Tetzlaff Jennifer M., Altman Douglas G., Laupacis Andreas, Gøtzsche Peter C., Krleža-Jerić Karmela, Hróbjartsson Asbjørn, Mann Howard, Dickersin Kay, Berlin Jesse A., Doré Caroline J., Parulekar Wendy R., Summerskill William S.M., Groves Trish, Schulz Kenneth F., Sox Harold C., Rockhold Frank W., Rennie Drummond, Moher David. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Annals of Internal Medicine. 2013;158(3):200. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senn S. Cross-over trials in clinical research. 2. London: Wiley; 2002. [Google Scholar]
- 47.Colucci P, et al. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur Endocrinol. 2010;9(1):40–47. doi: 10.17925/ee.2013.09.01.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. SPIRIT 2013 checklist: Recommended items to address in a clinical trial protocol and related documents.
Data Availability Statement
The applied questionnaires will be stored at the research office with coded identification. The study data will remain unidentified. The final dataset will only be handled by the lead investigator, and the funding institution will not have access to it. The results of this clinical trial will be submitted to a peer-reviewed medical journal following the authorship guidelines of the ICMJE CONSORT Statement. Following study publication, the unidentified dataset will be made available by the corresponding author upon formal request.