SUMMARY
Background:
The Rnd proteins Rnd1, Rnd2, and Rnd3(RhoE) comprise a unique branch of Rho-family G-proteins that lack intrinsic GTPase activity, and consequently remain constitutively “active”. Prior studies have suggested that Rnd proteins play pivotal roles in cell regulation by counteracting the biological functions of the RhoA GTPase, but the molecular basis for this antagonism is unknown. Possible mechanisms by which Rnd proteins could function as RhoA antagonists include sequestration of RhoA effector molecules, inhibition of guanine nucleotide exchange factors and/or activation of GTPase-activating proteins (GAPs) for RhoA. However, effector molecules of Rnd proteins with such properties have not been identified.
Results:
Here we identify p190 RhoGAP (p190), the most abundant GAP for RhoA in cells, as an interactor with Rnd proteins and show that this interaction is mediated by a region in p190, which is distinct from the GAP domain. Using Rnd3-RhoA chimeras and Rnd3 mutants defective in p190-binding, as well as p190-deficient cells, we demonstrate that the cellular effects of Rnd expression are mediated by p190. We moreover show that Rnd proteins increase the GAP activity of p190 towards GTP-bound RhoA, and finally demonstrate that expression of Rnd3 leads to reduced cellular levels of RhoA-GTP by a p190-dependent mechanism.
Conclusions:
Our results identify p190 RhoGAPs as effectors of Rnd proteins and demonstrate a novel mechanism for regulating Rho signaling through which Rnd proteins function as antagonists of RhoA.
INTRODUCTION
Small G-proteins generally function as molecular switches by cycling between an inactive, GDP-bound, and an active, GTP-bound state. The G-protein cycle is tightly regulated spatially and temporally to permit precise G-protein-mediated activation of effector molecules. This is accomplished by two major classes of enzymes: guanine nucleotide exchange factors (GEFs) that activate G-proteins by catalyzing GDP-GTP exchange, and GTPase-activating-proteins (GAPs), which terminate G-protein function by promoting GTP hydrolysis [1]. However, the Rnd proteins Rnd1, Rnd2, and Rnd3 comprise an unusual branch of Rho family GTP-binding proteins in that at least Rnd1 and Rnd3 lack GTPase activity and are constitutively GTP-bound within the cell [2, 3]. Accordingly, Rnd proteins contain residues, which interfere with catalytic activity in GTPases [2, 3]. Moreover, no evidence has been provided to suggest that the nucleotide status of Rnd proteins can be influenced by GEFs and GAPs. Instead, the activity of Rnd proteins may be regulated at the level of expression as demonstrated by the finding that Ras and the Ras-activated Raf-MEK-ERK pathway induces expression of Rnd3 in Madin-Darby Canine Kidney (MDCK) cells [4].
Published studies demonstrate that overexpression of Rnd proteins elicits actin stress fiber collapse [3, 5]. In fibroblasts, overexpression of Rnd proteins moreover leads to cell rounding [3]. These studies indicate that these effects result from antagonizing RhoA function in cells, but little is known about the signaling pathways downstream of Rnd proteins. One candidate effector of Rnd proteins is a molecule termed Socius that has no significant homology to other proteins [6]. Socius was isolated in a yeast two-hybrid screen using Rnd1 as bait and was shown to also bind Rnd2 and Rnd3. It was demonstrated that Socius elicits actin stress fiber collapse similar to that of Rnd1 and Rnd3. However, expression of Socius by itself failed to elicit cell rounding. Moreover, when Socius was coexpressed with Rnd proteins, it was found to antagonize Rnd protein function. These results argue against Socius being an effector of Rnd proteins in mediating antagonism towards RhoA. Rnd1 was also found to interact with the Grb7 adaptor protein but the cellular effects of this interaction were not studied [7]. In addition, Rnd1 was shown to bind Plexin-1A, the signal-transducing subunit of the Semaphorin 3A receptor, resulting in actin rearrangements and growth cone collapse but the precise mechanism underlying this response is not understood [8]. Rnd2 was found to bind a molecule termed Rapostlin, which was implicated in neurite branching in PC12 cells [9]. Rapostlin shows extensive homology to the formin-binding protein FBP17, and to CIP4, a protein that binds to both microtubules and the Wiskott-Aldrich syndrome protein (WASP). Rnd2 was also reported to interact with the vacuolar protein sorting 4-A (Vps-4A) [10]. However, in spite of that several Rnd-interacting molecules have been identified, the mechanisms by which these molecules might antagonize RhoA signaling are not clear.
Rnd3, the first Rnd protein to be identified (then as RhoE), was isolated in a two-hybrid screen using p190A as bait [2]. p190 possesses an N-terminal GTP-binding domain and a C-terminal GAP domain [11, 12]. The region separating these two domains is referred to as the “middle domain”. With the exception of four FF-domains [13], and tandem SH2 domain-binding motifs that mediate interaction with p120 RasGAP [14], the p190 middle domain possesses an unknown domain structure. Here, we demonstrate that Rnd proteins bind directly to the middle domain of p190 and that this interaction is critical to the cellular effects elicited by expression of Rnd1 or Rnd3 in fibroblasts. Moreover, we show that Rnd proteins promote p190-mediated GAP activity toward RhoA, and that expression of Rnd proteins in cells elicits a p190-dependent decrease in RhoA-GTP levels. The present study thus provides the first evidence that Rnd proteins directly inhibit RhoA signaling by augmenting the RhoGAP activity of p190 molecules.
RESULTS
Rnd proteins interact with the middle domain of p190 in yeast two-hybrid assays.
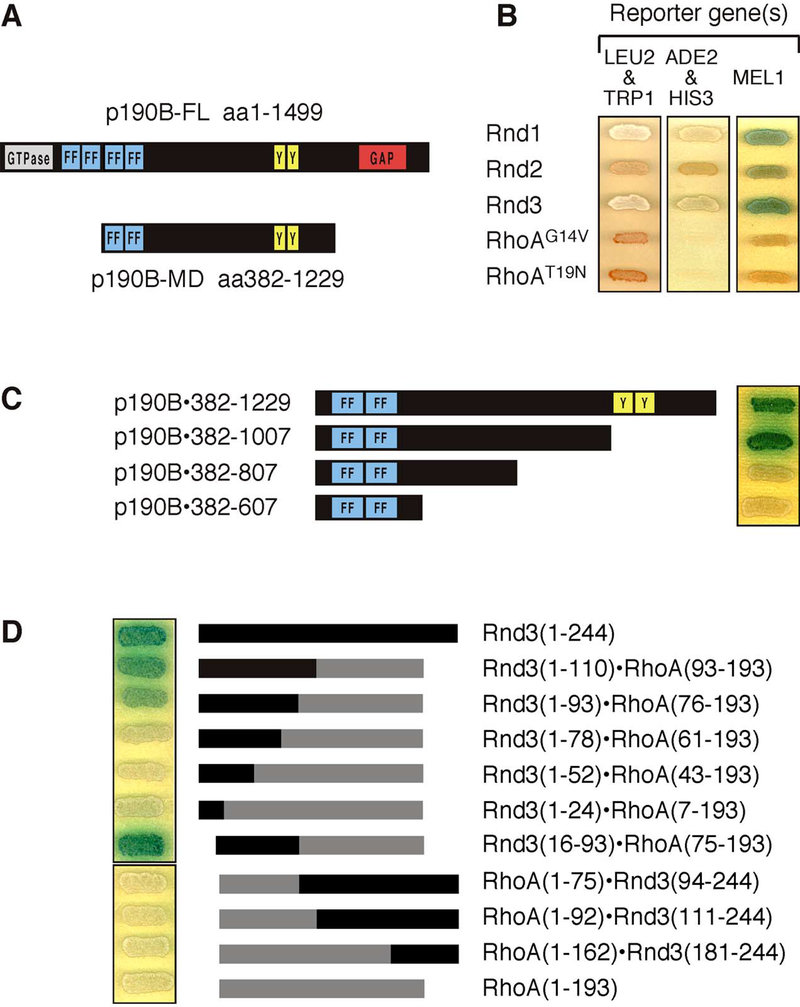
To identify effector molecules of Rnd proteins, we conducted a two-hybrid screen using the ubiquitously expressed Rnd3 as bait. We screened an oligo(dT) primed human fetal kidney cDNA library using a Gal4-based system with three reporters: HIS3, ADE2, and MEL1 of low, intermediate, and high stringency, respectively. More than 60 percent of the activators of all three reporter systems were found to contain inserts, which encode fragments of p190B [15], a closely related homologue of p190A. The smallest and most abundant fragment encoded amino acids 382–1229 of p190B, which largely corresponds to the middle domain of p190B (p190B-MD), thus excluding the N-terminal GTP-binding domain and C-terminal GAP domain (Figure 1A). p190B-MD also interacts with Rnd1 and Rnd2 but neither with constitutively active (G14V) nor dominant negative (T19N) RhoA mutants (Figure 1B). Truncation of p190B-MD from the C-terminus demonstrates that a fragment corresponding to amino acids 382–1007 is sufficient to interact with Rnd3 (Figure 1C). This fragment not only excludes the GTP-binding and the GAP domain of p190B, but it also eliminates the SH2 domain-binding motif that mediates binding to p120 RasGAP. Further truncation from either the N- or C-terminal end of this p190 fragment abolishes the interaction (Figure 1C and data not shown).
Figure 1: Rnd proteins interact with p190B-MD.
(A) Schematic presentation of full-length p190 and the fragment corresponding to p190B-MD isolated in the two-hybrid screen along with the known conserved domains found in these molecules, including the GTPase, FF, GAP, SH2 domain-binding (“Y”) domains.
(B) Rnd1, Rnd2, and Rnd3, but neither constitutively active (G14V) nor dominant negative (T19N) RhoA, interact with p190B-MD in the two-hybrid assay as evidenced by activation of the HIS3 and ADE2 reporter genes (growth) as well as the MEL1 reporter (blue color). The orange-red color of the yeast grown on drop-out plates results from the AH109 yeast strain being auxotroph for adenine.
(C) Truncation analysis of the Rnd3-interacting region of p190B-MD demonstrates that a fragment corresponding to amino acids 382–1007 is sufficient for mediating the interaction whereas further truncation of this fragment abrogates the activation of reporter genes.
(D) Results obtained with RhoA-Rnd3 chimeras demonstrate that amino acids 16–93 in Rnd3 are required, and in the background of RhoA, sufficient to mediate the interaction with p190B-MD as demonstrated by activation of the MEL1 reporter gene. Note that for (B) and (C), Rnd proteins are expressed as fusion proteins with the Gal4 DNA-binding domain and the p190 derivatives as a fusion with the Gal4 activation domain, whereas in (D), the assay is reversed.
Rnd3 is 49% identical to RhoA at the amino acid level [2]. To identify the region in Rnd3 that mediates binding to p190, we generated chimeras between Rnd3 and RhoA(G14V). These chimeras were based on the design of previously published RhoA-Rac1 or RhoA-Cdc42 chimeras, which were used successfully to identify domains in Rho proteins that specify effector binding [16–19]. We used RhoA(G14V) for our experiments because, like Rnd3, constitutively active RhoA has low catalytic activity and thus is more likely to mimic the conformation of Rnd3. The chimeras were fused to the Gal4 activation domain and their ability to interact with p190B-MD fused to the Gal4 DNA-binding domain was tested by two-hybrid assay as described above. These experiments show that a motif in Rnd3 contained within residues 16–93 is required and, in the context of a chimera, sufficient for interacting with p190B-MD (Figure 1D). This motif contains the P-loop as well as the switch I and switch II regions that in other Ras-like G-proteins are responsible for nucleotide-binding, as well as interacting with GAPs, GEF, and most effector molecules [1]. Although it remains formally possible that Rnd proteins can also bind the GAP domain of p190 molecules, the results with the chimeras rule out the possibility that Rnd proteins bind the middle and GAP domains concomitantly.
Interaction between Rnd proteins and p190 molecules in vitro and in mammalian cells.
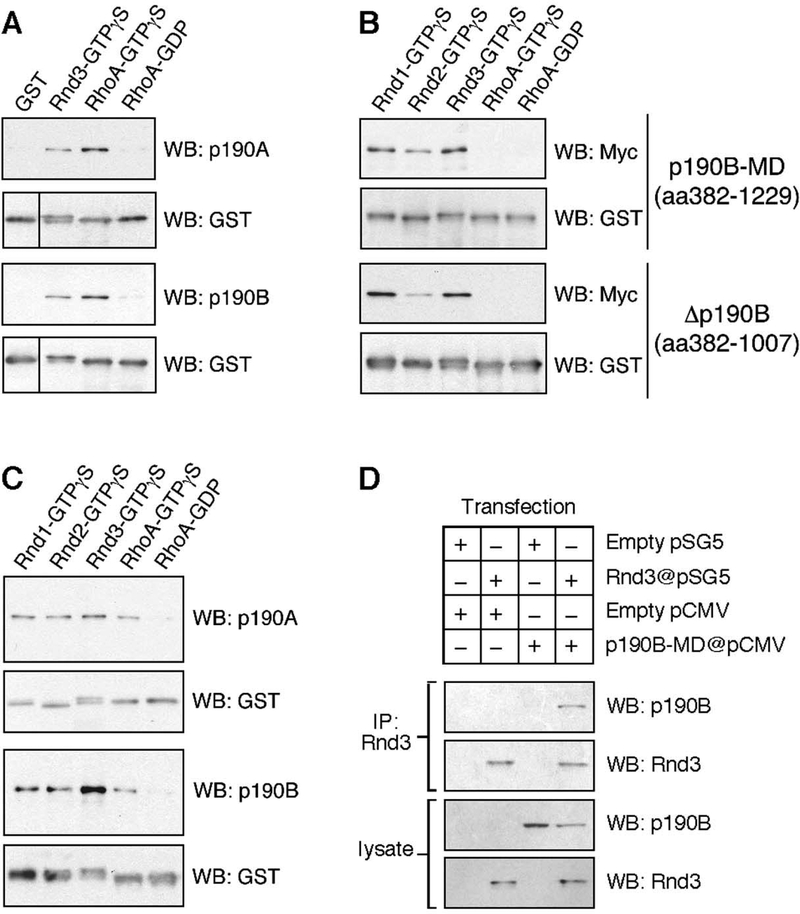
We next tested the interaction between Rnd proteins and p190 molecules using GST pull-down assays. We did not include GDP-bound GST-Rnd fusion proteins in these experiments because there is no evidence to support the existence of the GDP-bound form for either Rnd1 or Rnd3 in cells, thus questioning the physiological relevance of including such forms in the experiments described below. Both GTPγS-bound GST-Rnd3 and GST-RhoA, but not GDP-bound GST-RhoA, precipitate endogenous p190A and p190B from lysates of 3T3 fibroblasts (Figure 2A). Consistent with the fact that RhoA binds solely to the GAP domain of p190, we observe no interaction between GST-RhoA and p190B-MD from lysates of Cos7 cells transfected with a p190B-MD expression vector (Figure 2B). In contrast, GST fusion proteins of Rnd1, Rnd2, and Rnd3 all precipitate p190B-MD and the p190B fragment comprising amino acids 382–1007 from lysates of transfected Cos7 cells (Figure 2B). The interaction between Rnd proteins and p190 molecules is direct in that GST fusion proteins of Rnd proteins precipitate insect cell purified p190A and p190B with similar efficiency as GTPγS-bound GST-RhoA (Figure 2C).
Figure 2: Interaction between Rnd and p190 molecules in GST pull-down and co-immunoprecipitation experiments.
(A) GST pull-down experiments with immobilized GST fusion proteins of Rnd3 loaded with GTPγS, or RhoA loaded with GTPγS or GDP, as well as GST alone demonstrates an interaction between Rnd3 and endogenous p190A or p190B. In the western blot to detect GST fusion proteins, the left lane has been shifted upwards to compensate for the higher mobility of non-fusion GST.
(B) GST pull-down experiment from Cos7 cells transfected with expression constructs encoding Myc-epitope tagged p190B-MD (amino acids 382–1229), or a Myc-tagged fragment corresponding to amino acids 382–1007 of p190B. As shown, GTPγS-loaded Rnd1, Rnd2, and Rnd3, but not RhoA, bind p190B-MD or the 382–1007 fragment.
(C) GST pull-down experiment with insect cell purified p190A and p190B demonstrating that the interaction between Rnd proteins and p190 molecules is direct.
(D) Co-immunoprecipitation of p190B-MD with Rnd3 from lysates of Cos7 cells co-transfected with p190B-MD and Rnd3 expression vectors.
To determine whether the interaction between Rnd3 and p190-MD occurs in cells, we coexpressed Rnd3 and p190B-MD or appropriate control vectors in Cos7 cells and tested the interaction by co-immunoprecipitation analysis. p190B-MD consistently co-immunoprecipitates with Rnd3 (Figure 2D). In contrast, co-immunoprecipitation of full-length p190 molecules with Rnd3 yields variable results. At shorter incubation times, the p190 signal is weak, whereas with long incubations the p190 molecules tend to precipitate on their own (data not shown).
p190 molecules mediate Rnd function in cells.
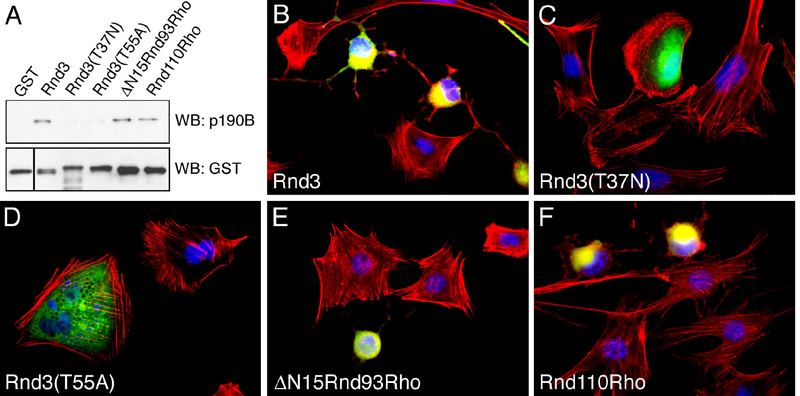
Overexpression of Rnd1 or Rnd3 in fibroblasts elicits a collapse of the actin stress fibers and extensive cell rounding, a phenotype similar to that of fibroblasts overexpressing p190 molecules [20]. To address whether p190-binding correlates with Rnd3 function in cells, we utilized derivatives of Rnd3, which either abrogate or promote p190 binding. These experiments included wild-type Rnd3, and the Rnd3(16–93)•RhoA(75–193) (“ΔN15Rnd93Rho”) and Rnd3(1–110)•RhoA(93–193) (“Rnd110Rho”) chimeras. In addition, we used a Rnd3(T37N) mutant, which is analogous to the well-characterized, persistently GDP-bound, RhoA(T19N) mutant, and a Rnd3(T55A) mutant that is analogous to the RhoA(T37A) mutant, which exhibits impaired effector binding. We expressed these Rnd3 derivatives as GST fusion proteins and tested their ability to precipitate p190B-MD from lysates of transfected Cos7 cells. We found that while the ΔN15Rnd93Rho and Rnd110Rho chimeras bind p190B-MD, the Rnd3(T37N) and Rnd3(T55A) mutations perturb the interaction (Figure 3A). Next, we transfected NIH 3T3 fibroblasts with mammalian expression vectors encoding Myc-epitope tagged versions of these constructs, and assessed their ability to promote cell rounding and actin stress fiber collapse as visualized by staining with fluorescent phalloidin (Figure 3B–F). Transfected cells were detected by labeling with 9E10 mAb to the Myc-epitope followed by Alexa488-conjugated secondary antibody. Expression of ΔN15Rnd93Rho or Rnd110Rho promotes collapse of actin stress fibers and cell rounding with similar efficiency as wild-type Rnd3 (Figure. 3B, 3E, and 3F), which is consistent with the finding that a motif contained within residues 16–93 of Rnd3 mediates these effects of Rnd3 expression. Moreover, while the ΔN15Rnd93Rho chimera retains the sequence corresponding to loop6 from RhoA, which contributes to effector activation [19], this sequence is absent from the Rnd110Rho chimera, thus arguing against the possibility that the cellular effects of these chimeras result from sequestration of RhoA effector molecules. In contrast, no morphological effects are observed in cells expressing the Rnd3(T37N) or Rnd3(T55A) mutants (Figure 3C and 3D), which do not bind p190B-MD (Figure 3A). Interestingly, DAPI staining reveals nuclear condensation in the Rnd3, ΔN15Rnd93Rho, as well as Rnd110Rho expressing cells, but not in cells expressing Rnd3(T37N) or Rnd3(T55A) (Figure 3B–F). This result likely reflects that cells overexpressing Rnd3 or p190-binding Rnd3 derivatives ultimately die and detach, possibly as a consequence of deprivation of cell-substratum dependent survival signals.
Figure 3: p190-binding correlates with phenotypic alterations elicited by Rnd3 derivatives.
(A) GST pull-down experiment from Cos7 cells transfected with p190B-MD expression construct. While GST-Rnd3, GST-ΔN15Rnd93Rho, and GST-Rnd110Rho all bind p190B-MD, no binding of GST-Rnd3(T37N), or GST-Rnd(T55A) to p190B-MD is observed. In the western blot to detect GST fusion proteins, the left lane has been shifted upwards to compensate for the higher mobility of non-fusion GST.
(B–F) Effects on 3T3 fibroblasts associated with expression of Rnd3 and derivatives thereof as follows: (B) Rnd3; (C) Rnd3(T37N); (D) Rnd3(T55A); (E) ΔN15Rnd93Rho; (F) Rnd110Rho. Note that expression of ΔN15Rnd93Rho (E) or Rnd110Rho (F) elicits actin stress fiber collapse and cell rounding, effects associated with Rnd3 expression in 3T3 fibroblasts (B). In contrast, no alterations are observed following expression of Rnd3(T37N) (C), or Rnd3(T55A) (D). Cells were imaged for expression of Myc-tagged Rnd protein (green), filamentous actin (red), and nuclei (blue).
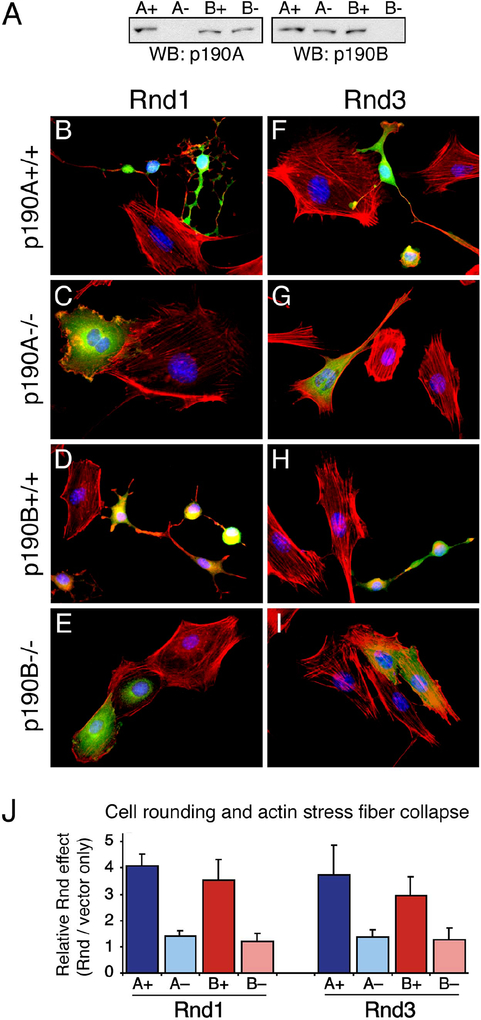
To test directly whether p190A and p190B are essential for Rnd function, we utilized p190A−/− and p190B−/− 3T3 fibroblasts derived from p190A and p190B knock-out mice, respectively, and the corresponding wild-type control cells from littermates (see Experimental Procedures). Knock-out and control cells (Figure 4A) were transfected with either Rnd1 or Rnd3 and analyzed for Rnd protein expression, cell rounding, and actin cytoskeletal organization (Figure 4B–I). In addition, for quantification, we determined a composite index for collapse of the actin cytoskeleton and cell rounding (Figure 4J). While expression of Rnd1 or Rnd3 in the control cells elicits a collapse of the actin stress fibers and extensive cell rounding as described above, these effects are substantially attenuated in both p190A−/− and p190B−/− cells (Figure 4B–J). The changes in nuclear morphology caused by Rnd1 or Rnd3 expression are also largely abrogated in the p190 knock-out cell lines. These results strongly support the conclusion that p190A and p190B are key effector molecules of Rnd1 and Rnd3 in eliciting actin stress fiber collapse and cell rounding. The differences between the control and p190 knock-out cells are most pronounced up to 36 h after transfection. At later time points the control cells start to detach and the biological effects associated with Rnd expression become evident also in p190A−/− and p190B−/− cells (data not shown). This observation is consistent with both p190A and p190B functioning as Rnd effector molecules in a dose-dependent manner.
Figure 4: Attenuated phenotype of Rnd protein expression in cells deficient in p190A or p190B.
(A) Western blot analysis of p190 expression in whole cell lysates of 3T3 fibroblasts derived from p190A−/− or p190B−/− knock-out or littermate wild-type mice using antibodies specific for p190A (left panel) or p190B (right panel).
(B–I) Phenotype elicited by expression of Rnd1 (B–E) or Rnd3 (F–I) in p190A control cells (B, F), p190A−/− cells (C, G), p190B control cells (C, H), or p190B−/− cells (E, I). The cells imaged for expression of Myc-tagged Rnd protein (green), filamentous actin (red), and nuclei (blue). While expression of Rnd1 or Rnd3 elicits cytoskeletal collapse and cell rounding in control 3T3 fibroblasts (B, D, F, H), the phenotype of Rnd1 or Rnd3 expression in p190A−/− as well as p190B−/− cells is substantially attenuated (C, E, G, I).
(J) Quantification of actin stress fiber collapse and cell rounding in response to Rnd1 or Rnd3 expression in 3T3 fibroblasts deficient in p190A (A−) or p190B (B−), as well as in cells derived from littermate wild-type mice (A+; B+). For the quantification, cells transfected with Rnd expression vectors or control plasmid were identified by co-transfection with an EGFP expression vector to control for differences between the four cell lines in the endogenous level of actin stress fibers and cell rounding as detailed in Experimental Procedures. The data represent the mean and SEM of four independent experiments. Both microscopy and quantification were performed 36 hours after transfection.
Rnd proteins augment the GAP activity of p190 to reduce cellular levels of RhoA-GTP.
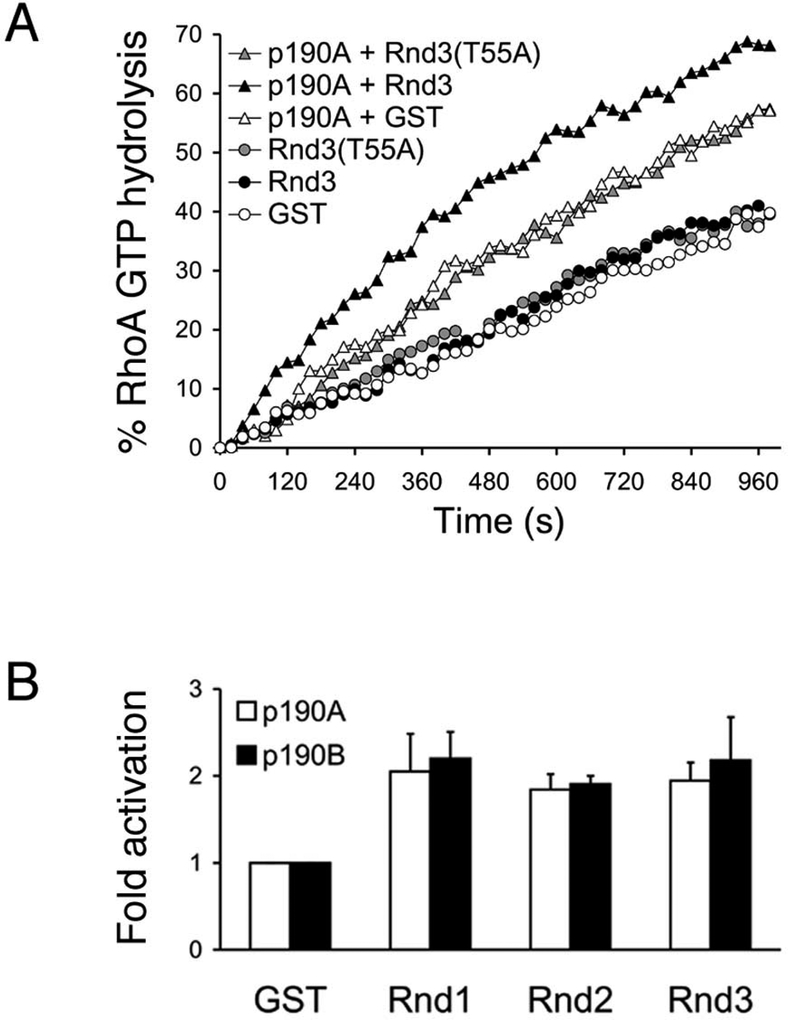
Given our finding that p190 molecules can function as effectors of Rnd proteins, we hypothesized that Rnd-binding stimulates p190-mediated RhoGAP activity. To test this hypothesis, we utilized a method that measures release of inorganic phosphate by binding to bacterial fluorophore-labeled phosphate-binding protein [21]. Using GTP-loaded GST-RhoA in combination with GTPγS-loaded GST-Rnd proteins and purified p190A or p190B from insect cells, we find that all three Rnd proteins increase both p190A- and p190B- dependent GAP activity towards RhoA-GTP by approximately two-fold (Figure 5A and 5B). The addition of GST alone or GST-Rnd3(T55A) does not affect p190-dependent GAP activity towards RhoA-GTP. Moreover, we do not detect any intrinsic GTP hydrolysis from Rnd proteins. Also, addition of GST or GST-Rnd proteins does not influence the GTPase activity of RhoA in the absence of p190 (Figure 5A, and data not shown).
Figure 5. Rnd proteins activate p190 molecules in vitro.
(A) In vitro GAP assays using fluorophore-labeled PBP to detect GTP hydrolysis. PBP and GTP-loaded RhoA was mixed with GST,GST-Rnd3, or GST-Rnd3(T55A) in the absence or presence of purified p190A (A), and the rates of RhoA GTP hydrolysis were measured as described in Experimental Procedures.
(B) Activation of p190A- or p190B-mediated RhoA GTP hydrolysis by addition of GST-Rnd1, GST-Rnd2, or GST-Rnd3 (calculated as described in Experimental Procedures). The data shown represent the mean and SD of three independent experiments.
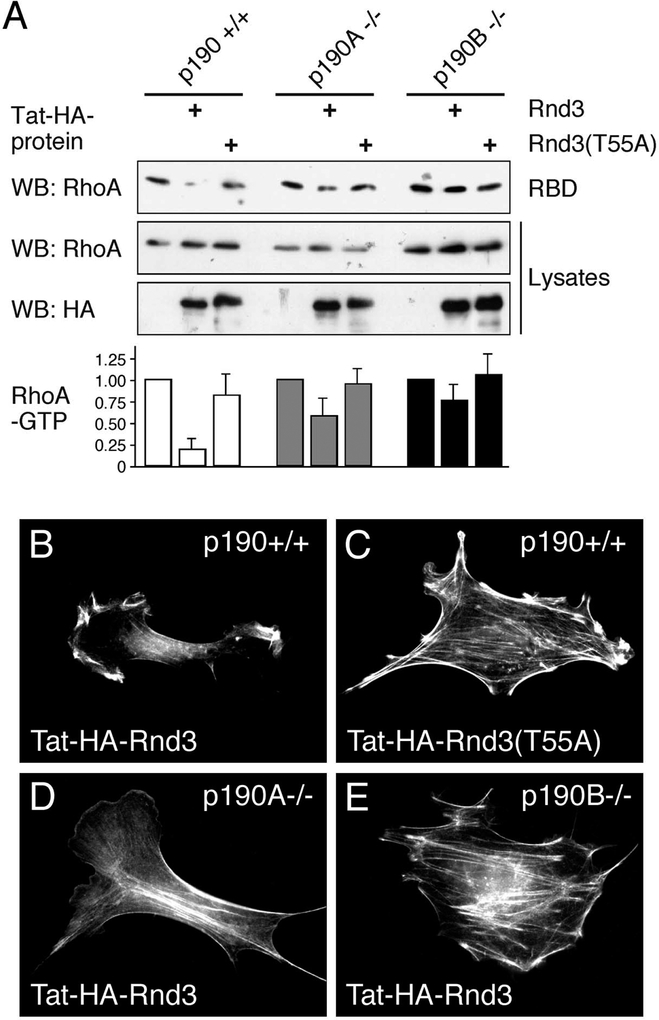
A prediction that follows from our finding that Rnd proteins stimulate the RhoGAP activity of p190 is that Rnd protein expression through activation of p190 molecules elicits a reduction in cellular levels of RhoA-GTP. To test this, we introduced Rnd3 or Rnd3(T55A) in p190A−/− and p190B−/− 3T3 fibroblasts as well as wild-type control cells by Tat-mediated protein transduction and determined the cellular levels of RhoA-GTP using the Rhotekin RBD assay [22]. In contrast to mouse embryo fibroblasts derived from p190 knock-out mice [41] (Settleman and co-workers, in preparation), 3T3 fibroblasts deficient in p190A or p190B did not exhibit substantially elevated constitutive levels of RhoA-GTP (Figure 6A), presumably as a result of adaptation. Transduction of Tat-HA-Rnd3, but not Tat-HA-Rnd3(T55A), substantially reduces RhoA-GTP levels in control 3T3 fibroblasts, whereas the reduction in p190A−/− and, in particular, in p190B−/− cells, is much less pronounced (Figure 6A). The reduction in RhoA-GTP levels correlates with the morphological effects elicited by Tat-Rnd proteins. While transduction of Tat-HA-Rnd3 (Figure 6B), but not Tat-HA-Rnd3(T55A) (Figure 6C), into control cells results in disappearance of actin stress fibers and a collapsed cell morphology, these effects are substantially attenuated in cells deficient in p190A (Figure 6D) or p190B (Figure 6E). These results further support the conclusion that Rnd proteins inhibit RhoA signaling by increasing the GAP activity of p190 towards RhoA-GTP. It should be noted that, due to instability of the protein, the effects of Tat-HA-Rnd3 on the actin cytoskeleton and cell morphology are transient, and consequently less pronounced than those observed following transfection with Rnd expression vectors.
Figure 6. Rnd proteins activate p190 molecules in cells.
(A) Rhotekin RBD pulldown assay to determine levels of GTP-bound RhoA in control (p190A/B+/+), p190A−/−, and p190B−/− cells following transduction of Tat-HA-Rnd3 or Tat-HA-Rnd3(T55A). GTP-bound RhoA (RBD) and total RhoA in 2% of cell lysate (Lysates) were determined by western blot analysis using an antibody specific for RhoA. Protein transduction efficiency was confirmed with anti-HA antibody to detect Tat-HA-Rnd3 protein. The histogram shows RhoA-GTP levels in Tat-HA-Rnd3 and Tat-HA-Rnd3(T55A) transduced control, p190A−/−, and p190B−/− cells expressed as a ratio of RhoA-GTP levels in the corresponding untransduced cell lines. The data represent the mean and SD of three independent experiments.
(B–E) Morphological effects of transduction of Tat-HA-Rnd3 (B, D, E) or Tat-HA-Rnd3(T55A) (C) into control cells (B and C) or cells deficient in p190A (D) or p190B (E).
DISCUSSION
Rho family GTP-binding proteins play pivotal roles in cell regulation and our knowledge of the specific functions of prototypical Rho proteins such RhoA, Rac1, and Cdc42 is substantial. By contrast, much less is known about the contributions of other Rho family members such as the Rnd proteins [23]. Previous studies suggested that Rnd proteins function as antagonists of RhoA [3–5, 24]. However, it has not been elucidated how this occurs. Given the strong overall sequence identity between Rnd proteins and RhoA, one possible mechanism by which Rnd proteins might antagonize Rho signaling would be through sequestration of effector molecules of RhoA. Yet our two hybrid, GST pull-down and co-immunoprecipitation experiments failed to demonstrate an interaction between Rnd3 and RhoA effectors such as Citron, ROCK, mDia2, and PKN (S.H.H, unpublished observation). An alternate possibility that we have investigated is that Rnd proteins might antagonize RhoGEF function. However, our survey of several RhoGEF molecules failed to support this hypothesis (K.W., S.M.E., K.B., and C.J.D., unpublished observation). A third mechanism that could account for the effects of Rnd proteins on cells is that they act by promoting GAP activity toward RhoA, thereby terminating downstream signaling. Indeed, we show here that Rnd proteins bind directly to p190 molecules. Both two-hybrid and GST pull-down assays as well as co-immunoprecipitation experiments indicated that Rnd proteins interact more strongly with the p190 middle domain than with full-length p190 molecules. While these differences may result from trivial technical problems, an alternative possibility is that the GTPase, FF, GAP, SH2 domain-binding, and/or other domains in p190 influence the binding of Rnd proteins. We are currently addressing this possibility. We further demonstrate that Rnd proteins augment the GAP activity of p190, the most abundant RhoGAP in cells [25], leading to a reduction in cellular levels of GTP-bound RhoA. Using cells deficient in p190A or p190B, we provide evidence that this mechanism accounts for the ability of Rnd1 or Rnd3 to elicit a collapse of actin stress fibers and cell rounding. Previous studies have shown that coexpression of constitutively active, GTPase-deficient RhoA prevents the cellular effects associated with expression of Rnd1 or Rnd3 [3, 4, 5, 24]. It was therefore speculated that Rnd proteins perturb upstream regulators of RhoA GDP/GTP cycling [3, 5], a hypothesis that is consistent with the mechanism identified in the present study.
We also found that Rnd2 in the GTP-bound state binds and activates p190 molecules in vitro even though Rnd2 does not cause stress fiber disassembly and cell rounding to the same extent as Rnd1 and Rnd3. This most likely results from that the GTPase activity of Rnd2, based on its amino acid composition, is predicted to be higher than that of Rnd1 and Rnd3 [3]. In addition, from our yeast two-hybrid analysis and GST pull-down experiments, it seems that Rnd2 binds p190 molecules with lower affinity than Rnd1 and Rnd3. Thus, in cells, Rnd2 may predominantly exist in the GDP-bound state and/or in complexes with effector molecules, which bind Rnd2 with higher affinity than p190.
Our findings demonstrate a Rnd-dependent mechanism for regulating p190 function. In this respect, it is interesting that previous studies have shown that p190 molecules respond to growth factor stimulation and integrin engagement [15, 26], which, at least in the case of p190A, increases GAP activity by a Src-dependent mechanism [26]. Src-mediated phosphorylation of tyrosines 1087 and 1105 in the SH2 domain-binding motifs of p190A promotes complex formation with p120 RasGAP [14, 27]. This finding likely extends to p190B, which also interacts with p120 RasGAP [15]. Hence, the intriguing possibility emerges that recruitment of p120 RasGAP as well as binding of Rnd proteins to the middle domain of p190 molecules may similarly promote RhoGAP activity, for instance, by eliciting conformational changes that expose the GAP domain. Thus, there appears to be both Src-dependent and Rnd-dependent mechanisms for augmenting the GAP activity of p190 molecules. The Src-dependent mechanism evidently is well suited for transient inhibition of RhoA effector activation in response to integrins, growth factors and possibly other acute stimuli [26, 28]. In contrast, we hypothesize that the Rnd-dependent mechanism permits sustained attenuation of RhoA signaling in processes where Rnd proteins and p190 molecules have been implicated, such as in morphogenetic movements during development [24, 29, 30] and cell transformation [4, 31].
There are numerous previous reports of functional interplay between small G-proteins. Within the Rho family, Hall and colleagues determined that in fibroblasts, activated Cdc42 promotes the activation of Rac1, which in turn stimulates the activation of RhoA [32–34]. How this regulatory cascade is mediated is not known but the fact that dominant negative mutants of Cdc42 and Rac1 block this cascade supports the involvement of GEFs. In an opposite scenario, activated Rac1 was shown to reduce cellular levels of GTP-bound RhoA in epithelial cells by a mechanism that was proposed to involve GEFs or GAPs [35]. GEFs also promote cross-talk between different families of small G-proteins. For instance, activated Ras binds and activates GEFs for Ral, Rab5, and Rac GTPases [36, 37] [38]. GAPs also serve as transducers between small G-proteins. One example is the Ral effector RalBP1, which functions as a GAP for Cdc42 [39]. The connection between Ras and Rho signaling through complex formation between p120 RasGAP and p190 RhoGAP is another [14]. A combination of a GEF and a GAP can also act within the same pathway such as the signaling cascade from Rac1 to Arf6, which is mediated by a complex consisting of the Rac effector Pak, the RacGEF PIX, and the ArfGAP PKL/GIT/Cat [40]. In contrast to these scenarios, the mechanism identified in the present study is not only simple and but also unprecedented in that one Rho family protein downregulates the activity of another through direct binding and activation of a RhoGAP. Further studies are required to address whether the antagonistic functions of other small G-proteins are mediated by similar mechanisms.
CONCLUSIONS
In this work we show that Rnd proteins bind to the RhoGAP p190 and increase its GAP activity towards GTP-bound RhoA. We demonstrate that the cellular effects of Rnd expression are mediated by p190 and correlate with p190-dependent reduced cellular levels of RhoA-GTP. Collectively, our results identify a novel mechanism in Rho protein signaling, which accounts for the functional antagonism between Rnd proteins and RhoA.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies were obtained as follows: anti-p190A mAb and anti-p190B mAb from BD laboratories; anti-HA (12CA5) mAb, anti-Myc (9E10) mAb, anti-GST pAb, and anti-RhoA mAb from Santa Cruz Biotechnology; rabbit antiserum reactive towards an N-terminal epitope in Rnd3 was generated at QCB; anti-Rnd3 antiserum, raised against the entire protein as well as secondary antibodies were previously described [4]. Alexa594-phalloidin was from Molecular Probes. Other reagents are detailed below or have been described previously [4].
Two-hybrid screening and mapping
An oligo(dT) primed human fetal kidney cDNA library was screened with the Gal4-based System 3 (Clontech) using Rnd3 fused to the Gal4 DNA-binding domain as bait. Interactors activating all three reporters (HIS3, ADE2, and MEL1) were subjected to yeast colony PCR. The products were digested with restriction enzymes and the interactors grouped according to their digestion pattern. One or two PCR products from each group was sequenced and identified using the NCBI BLAST tool.
Subsequent two-hybrid analyses were similarly carried out using System 3. To test the interaction between Rnd proteins or RhoA and p190B-MD or truncated derivatives of p190B, the orientation of the two-hybrid assay was maintained as in the screen. In contrast, for the experiments employing Rnd3-RhoA chimeras, the orientation was reversed such that p190B-MD was expressed as a fusion with the Gal4 DNA-binding domain and the chimeras, Rnd3, and RhoA(G14V) were expressed as a fusion with the Gal4 activation domain. Truncated p190B derivatives as well as Rnd3-RhoA chimeras were generated by PCR and verified by sequencing.
Expression of Rnd proteins and p190 molecules in mammalian cells
Mammalian expression constructs encoding Rnd proteins were generated in pCMV-Myc and in pCMV-HA (Clontech). Vectors utilized for mammalian expression of p190 molecules were described previously [2, 41]. Cos7 and 3T3 fibroblasts were transfected according to the manufacturer’s instructions using Lipofectamine and Lipofectamine 2000 (Invitrogen), respectively. Purification of Tat-HA-Rnd3 proteins and Tat-mediated proteins transduction was performed as previously described [42] by addition of 10 μg of protein in 3 ml of serum-free medium to one 100 mm dish of cells for 2 h.
GST pull-down experiments
GST-tagged derivatives of Rnd and RhoA proteins were prepared in pGEX-4T vectors (Amersham Biosciences). Rnd and RhoA GST fusion proteins were expressed in E. coli and purified using glutathione-Sepharose beads (Amersham Biosciences) according to Self and Hall [43]. To prevent proteolytic degradation of GST-Rnd fusion proteins during purification, 1 mM GTP was added to the E. coli suspension, prior to cell lysis [3].
GST fusion protein (1.5 μg) immobilized on glutathione beads were loaded with GTPγS or GDP and incubated with aliquots of 0.5 mg of cell lysates, for 1h at 4 °C, according to Tanaka et al. [10]. Next, the beads were washed extensively, the associated proteins eluted and subjected to SDS-PAGE and western blot analysis to detect p190 molecules and GST fusion proteins.
Rhotekin-RBD pull-down experiments to determine cellular levels GTP-bound RhoA were performed according to Ren et al. [22], and the results were quantified by densitometry. The RhoA-GTP level in a given sample was determined by dividing the densitometric value obtained for the RhoA band in the RBD pull-down by the value obtained for the RhoA band in the total cell lysate. To control for differences in constitutive RhoA-GTP levels among p190A−/−, p190B−/−, and wild-type control cells, changes in RhoA-GTP levels following Tat-mediated transduction of Rnd proteins were calculated as the ratio between the RhoA-GTP levels in transduced and untransduced samples for each cell line, separately.
Co-immunoprecipitation
Cos7 cells were co-transfected with expression constructs encoding p190B-MD and/or Rnd3, or with the appropriate control vectors. Cell lysates were prepared and then subjected to immunoprecipitation with rabbit anti-Rnd3 antiserum bound to protein A Sepharose beads, for 2 h at 4 °C. Following extensive washing, proteins were eluted from the beads and subjected to SDS-PAGE and transfer to nitrocellulose. The western blots were probed with mouse mAb to p190B and anti-Rnd3 N-terminal peptide rabbit antiserum to detect p190B-MD and Rnd3, respectively. Endogenous levels of Rnd3 and p190 in Cos7 cells are below the detection levels in this assay and thus do not interfere with the results.
Fluorescence microscopy
Immunofluorescence microscopy and staining of the actin cytoskeleton with fluorescent phalloidin were carried out essentially as previously described [4]. Samples were imaged on a Nikon fluorescence microscope equipped with a Hamatsu cooled CCD camera and processed with IPLab imaging software and Adobe Photoshop.
For quantification of phenotypic alterations elicited by expression of Rnd proteins in 3T3 fibroblasts derived from p190A−/− mice (Settleman and co-workers, in preparation) and p190B−/− mice [41] as well as wild-type littermate control mice, cells were co-transfected with vector only, Rnd1, or Rnd3 with an EGFP expression vector at a ratio of 7:1. Transfected cells were assigned 0, 1, or 2 points for no, partial, or complete cell rounding as well as 0, 1, or 2 points for no, partial, or complete absence of actin stress fibers. For each experiment, at least 50 cells from each condition were scored and the total score divided by the number of cells examined. No information about cell type or transfection was given to the person carrying out the quantification. The data were expressed as a ratio to vector only transfected cells to control for differences in the endogenous level of actin stress fibers and cell rounding in p190A−/− and p190B−/− 3T3 fibroblasts and corresponding control cell lines.
RhoGAP assays
Phosphate-binding protein carrying an A197C mutation was purified from E. coli ANCC75 bacteria carrying plasmid pSN5182/7 (a gift of Dr. Martin R. Webb, National Institute for Medical Research, London, UK) and labeled with N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide (Molecular Probes) as previously described [44]. His-tagged p190A and p190B was purified from SF9 cells 48h after infection with baculovirus by purification over Ni-NTA sepharose (Qiagen) followed by S sepharose cationic exchange chromatography (0–400 mM NaCl, pH 6.5) (Amersham Biosciences).
GAP assays were performed in a Spectramax Gemini XS fluorescence microplate reader (Molecular Devices) (excitation: 425 nm, emission: 465 nm) at 25 °C in a total volume of 100 μl buffer containing 20 mM Tris pH 7.6 and 1 mM MgCl2. 1 μM GTP-loaded GST-RhoA was incubated together with 2.5 μM fluorophore-labeled PBP in the absence or presence of 200 pM p190A or 1 nM p190B and with 400 nM GST or 200 nM GST-Rnd proteins. Upon hydrolysis of GTP, free Pi is bound rapidly (1.36×108 M−1 s−1 at 22 °C) and with high affinity (about 100 nM) by the PBP, resulting in a 13-fold increase in fluorescence at 465 nm [21, 44]. Consequently, the observed change in fluorescence corresponds to the rate and amount of Pi released from the GTPase.
All experiments were performed in duplicates. For each assayed condition presented, one control condition (without RhoA) was run and subtracted from the assayed data to account for any potential RhoA-independent effects on the fluorescence readings. Fluorescence readings were made with intervals of 20 seconds. Linear velocity of GTP hydrolysis was determined by calculating the percentage of RhoA-GTP hydrolysis at each time point and divided by elapsed time. Velocity was considered linear if the regression value of the exchange slope was greater than 0.97. The fold increase by addition of GST-Rnd proteins (X) over addition of GST alone were then calculated from the rates as follows: [Rate(RhoA + p190 + X)/Rate(RhoA + X)]/[Rate(RhoA + p190 + GST)/Rate(RhoA + GST)].
ACKNOWLEDGEMENTS
We are grateful to Drs. Arthur S. Alberts, Pierre Chardin, and Martin R. Webb for generously providing reagents. We are moreover thankful to Dr. Lynne Coluccio for helpful suggestions. This work was supported by a STINT postdoctoral fellowship to K.W., an ACS postdoctoral fellowship to S.M.E., and NIH grants R01 GM29860 to K.B., R01 CA62142 to J.S., R01 CA63071 to C.J.D., and R01 CA092354 to S.H.H.
REFERENCES
- 1.Takai Y, Sasaki T, Matozaki T (2001). Small GTP-binding proteins. Physiol. Rev 81, 153–208. [DOI] [PubMed] [Google Scholar]
- 2.Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J (1996). Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol. Cell. Biol 16, 2689–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P (1998). A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J. Cell Biol 141, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SH, Zegers MM, Woodrow M, Rodriguez-Viciana P, Chardin P, Mostov KE, McMahon M (2000). Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal- regulated kinase pathway. Mol. Cell. Biol 20, 9364–9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guasch RM, Scambler P, Jones GE, Ridley AJ (1998). RhoE regulates actin cytoskeleton organization and cell migration. Mol. Cell. Biol 18, 4761–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh H, Harada A, Mori K, Negishi M (2002). Socius Is a novel Rnd GTPase-interacting protein involved in disassembly of actin stress fibers. Mol. Cell. Biol 22, 2952–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vayssière B, Zalcman G, Mahé Y, Mirey G, Ligensa T, Weidner KM, Chardin P, Camonis J (2000). Interaction of the Grb7 adapter protein with Rnd1, a new member of the Rho family. FEBS Lett. 467, 91–96. [DOI] [PubMed] [Google Scholar]
- 8.Zanata SM, Hovatta I, Rohm B, Puschel AW (2002). Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J. Neurosci 22, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita H, Katoh H, Ishikawa Y, Mori K, Negishi M (2002). Rapostlin Is a novel effector of Rnd2 GTPase inducing neurite branching. J. Biol. Chem 277, 45428–45434. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Fujita H, Katoh H, Mori K, Negishi M (2002). Vps4-A (vacuolar protein sorting 4-A) is a binding partner for a novel Rho family GTPase, Rnd2. Biochem. J 365, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Settleman J, Narasimhan V, Foster LC, Weinberg RA (1992). Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell 69, 539–549. [DOI] [PubMed] [Google Scholar]
- 12.Foster R, Hu KQ, Shaywitz DA, Settleman J (1994). p190 RhoGAP, the major RasGAP-associated protein, binds GTP directly. Mol. Cell. Biol 14, 7173–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedford MT, Leder P (1999). The FF domain: a novel motif that often accompanies WW domains. Trends Biochem. Sci 24, 264–265. [DOI] [PubMed] [Google Scholar]
- 14.Bryant SS, Briggs S, Smithgall TE, Martin GA, McCormick F, Chang JH, Parsons SJ, Jove R (1995). Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J. Biol. Chem 270, 17947–17952. [DOI] [PubMed] [Google Scholar]
- 15.Burbelo PD, Miyamoto S, Utani A, Brill S, Yamada KM, Hall A, Yamada Y (1995). p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J. Biol. Chem 270, 30919–30926. [DOI] [PubMed] [Google Scholar]
- 16.Diekmann D, Nobes CD, Burbelo PD, Abo A, Hall A (1995). Rac GTPase interacts with GAPs and target proteins through multiple effector sites. EMBO J. 14, 5297–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R, Zhang B, Zheng Y (1997). Structural determinants required for the interaction between Rho GTPase and the GTPase-activating domain of p190. J. Biol. Chem 272, 32830–32835. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa K, Madaule P, Ishizaki T, Watanabe G, Bito H, Saito Y, Hall A, Narumiya S (1998). Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. J. Biol. Chem 273, 18943–18949. [DOI] [PubMed] [Google Scholar]
- 19.Zong H, Raman N, Mickelson-Young LA, Atkinson SJ, Quilliam LA (1999). Loop 6 of RhoA confers specificity for effector binding, stress fiber formation, and cellular transformation. J. Biol. Chem 274, 4551–4560. [DOI] [PubMed] [Google Scholar]
- 20.Tatsis N, Lannigan DA, Macara IG (1998). The function of the p190 Rho GTPase-activating protein is controlled by its N-terminal GTP binding domain. J. Biol. Chem 273, 34631–34638. [DOI] [PubMed] [Google Scholar]
- 21.Brune M, Hunter JL, Corrie JE, Webb MR (1994). Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry 33, 8262–8271. [DOI] [PubMed] [Google Scholar]
- 22.Ren XD, Kiosses WB, Schwartz MA (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall A (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- 24.Wünnenberg-Stapleton K, Blitz IL, Hashimoto C, Cho KW (1999). Involvement of the small GTPases XRhoA and XRnd1 in cell adhesion and head formation in early Xenopus development. Development 126, 5339–5351. [DOI] [PubMed] [Google Scholar]
- 25.Vincent S, Settleman J (1999). Inhibition of RhoGAP activity is sufficient for the induction of Rho- mediated actin reorganization. Eur. J. Cell. Biol 78, 539–548. [DOI] [PubMed] [Google Scholar]
- 26.Arthur WT, Petch LA, Burridge K (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol 10, 719–722. [DOI] [PubMed] [Google Scholar]
- 27.Hu KQ, Settleman J (1997). Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 16, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arthur WT, Burridge K (2001). RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 12, 2711–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J (2000). The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development 127, 4891–4903. [DOI] [PubMed] [Google Scholar]
- 30.Brouns MR, Matheson SF, Settleman J (2001). p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat. Cell. Biol 3, 361–367. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarty G, Roy D, Gonzales M, Gay J, Contreras A, Rosen JM (2000). p190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth. Differ 11, 343–354. [PubMed] [Google Scholar]
- 32.Ridley AJ, Hall A (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- 33.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. [DOI] [PubMed] [Google Scholar]
- 34.Nobes CD, Hall A (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- 35.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol 147, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolthuis RM, Bos JL (1999). Ras caught in another affair: the exchange factors for Ral. Curr. Opin. Gen. Dev 9, 112–117. [DOI] [PubMed] [Google Scholar]
- 37.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF (2001). Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82. [DOI] [PubMed] [Google Scholar]
- 38.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ (2002). Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat. Cell Biol 4, 621–625. [DOI] [PubMed] [Google Scholar]
- 39.Cantor SB, Urano T, Feig LA (1995). Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol. Cell. Biol 15, 4578–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner CE, Brown MC (2001). Cell motility: ARNOand ARF6 at the cutting edge. Curr. Biol 11, R875–877. [DOI] [PubMed] [Google Scholar]
- 41.Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Le Z, Takami H, Yamada Y, Settleman J (2002). Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell 2, 553–565. [DOI] [PubMed] [Google Scholar]
- 42.Vocero-Akbani A, Chellaiah MA, Hruska KA, Dowdy SF (2001). Protein transduction: delivery of Tat-GTPase fusion proteins into mammalian cells. Methods Enzymol. 332, 36–49. [DOI] [PubMed] [Google Scholar]
- 43.Self AJ, Hall A (1995). Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 256, 3–10. [DOI] [PubMed] [Google Scholar]
- 44.Brune M, Hunter JL, Howell SA, Martin SR, Hazlett TL, Corrie JE, Webb MR (1998). Mechanism of inorganic phosphate interaction with phosphate binding protein from Escherichia coli. Biochemistry 37, 10370–10380. [DOI] [PubMed] [Google Scholar]