Abstract
OBJECTIVE
To evaluate whether increasing body mass index (BMI) alters the efficacy of ultrasound directed cerclage in women with a history of preterm birth.
METHODS
Planned secondary analysis of a multicenter trial. Women with prior spontaneous preterm birth were screened for short cervix and randomly assigned to cerclage or not for cervical length (CL) <25 mm.
RESULTS
Overall (n=986), BMI was not associated with CL (p=0.68), birth gestational age (GA) (p=0.12), or birth <35 weeks (p=0.68). For the cerclage group (n=148), BMI had no significant effect. For the no cerclage group (n=153), BMI decreased GA with an estimated slope of −0.14 weeks per kg/m2 (p=0.03). This result was driven primarily by several women with BMI >47 kg/m2.
CONCLUSION
In women at high risk for recurrent preterm birth, BMI was not associated with CL or birth GA. BMI did not appear to adversely affect ultrasound indicated cerclage.
Keywords: Body mass index, cerclage, cervical length, spontaneous preterm birth
INTRODUCTION
The rate of obesity continues to rise, with 34% of reproductive aged women in the United States considered obese [body mass index (BMI) ≥ 30] in 20081. Concurrent with rising obesity, the rate of preterm birth continues to increase (12.3% in 2008)2. While prior spontaneous preterm birth (SPTB) is considered one of the strongest historic risk factors for recurrent preterm birth,3,4,5 a woman’s BMI currently plays a controversial role as a predictor of preterm birth.6–11 This ambiguity is reflected in the American College of Obstetrics and Gynecology’s Committee Opinion on obesity in pregnancy, which reviewed two conflicting studies regarding the relationship of BMI with preterm birth without a final consensus.12 A recent meta-analysis pooled data from 84 studies and 1,095,834 women, and demonstrated that overweight and obese women have a higher risk of preterm birth before 33 weeks gestation (relative risk 1.26, 95% confidence interval 1.14–1.39).6 However, the risk of preterm birth prior to 37 weeks gestation was similar among normal weight, overweight, and obese women. Such inconclusive results may reflect the heterogeneous nature of the subjects in these studies.
The mechanisms by which obesity may lead to preterm birth have not been thoroughly elucidated; however, recent studies have suggested a heightened inflammatory response leading to increased adverse clinical outcomes. Expansion of adipose tissue, historically but inaccurately considered an inactive metabolic tissue, has been associated with increasing inflammation secondary to the release of multiple cytokines and the influx of inflammatory cells. These cytokines, which include such entities as TNF, IL6 and IL8, likely have both local and systemic effects, creating a chronic inflammatory state in the obese pregnant woman.19
Previously, we have shown that cerclage reduced recurrent previable preterm birth in women with both a prior SPTB 17 0/7–33 6/7 weeks and also a short CL < 25 mm, identified between 16 0/7–22 6/7 weeks, and did not prevent birth < 35 weeks unless the CL was < 15 mm.13 In the current investigation, our aim is to evaluate if increasing BMI alters the efficacy of ultrasound directed cerclage in women with cervical length < 25 mm with a history of preterm birth.
METHODS
This is a planned secondary analysis of the NICHD-sponsored randomized trial evaluating cerclage for women with singleton gestations, prior SPTB (17–33 6/7 wks), and cervical length < 25 mm, measured with serial transvaginal ultrasound evaluations between 16 and 22 6/7 weeks. This trial was performed at 15 U.S. Clinical Centers between January, 2003 and November, 2007. Each center obtained Institutional Review Board approval. The methods and materials are described in detail in the report of the parent trial.13 Importantly, healthy, multiparous women with at least one prior SPTB between 17 and 33 6/7 weeks of gestation were recruited. Our protocol included confirmation of the obstetrical history by a review of the subject’s medical records. When efforts to retrieve the records of the prior birth were unsuccessful, we accepted women as eligible if the events surrounding the prior birth included spontaneous causes such as preterm labor or preterm membrane rupture, and the reported birth weight was less than 2 kg. Exclusion criteria for the parent trial were fetal anomaly, planned history-indicated or prophylactic cerclage, medically indicated preterm birth, and clinically significant maternal-fetal complications (e.g. insulin-dependent diabetes mellitus). Gestational age was confirmed by standard sonographic biometric measurements at less than 20 weeks’ gestation. Sonologists underwent a uniform certification process by a single investigator (J.O.) to ensure uniformity in sonographic measurements of transvaginal ultrasound cervical length screening.13
Women with prior SPTB were screened with transvaginal ultrasound for cervical lengths starting at 16 – 21 6/7 weeks, then every 2 weeks until 22 6/7 weeks unless the cervical length was observed to be 25–29 mm, after which the scan frequency was increased to weekly. After informed consent, women who developed a cervical length < 25mm at 16 –22 6/7 weeks were randomized to receive a cerclage or not.
All women undergoing serial ultrasound evaluations and with complete data were considered. BMI, determined by subject measurements at the time of enrollment to the study, was calculated in the usual fashion: a subject’s weight divided by the square of her height (kg/m2). BMI is defined by the World Health Organization (WHO)14 as follows: normal 18.5 to < 25, overweight 25 to < 30, and obese ≥ 30 kg/m2. BMI was evaluated both as a categorical variable (based on the WHO categories) and a continuous variable.
Chi-square and Student t-tests were used to evaluate demographic characteristics between randomized and non-randomized women, as well as between the two randomization groups. Fisher’s exact test and Wilcoxon rank-sum test were used where appropriate. Frequencies (percentages), means (standard deviations), and median (interdecile range), are presented.
Linear regression analysis was used to examine the relationship between BMI and both the shortest observed cervical length and gestational age at delivery. Logistic regression was used to examine the relationship between BMI and preterm birth < 35 weeks. Multivariable regression models examined the effects of BMI on gestational age at delivery and on preterm birth < 35 weeks while controlling for gestational age of earliest prior preterm birth, cervical length at randomization, cerclage randomization group, and the interaction between BMI and randomization group. We selected a two-sided alpha level of < 0.05 to represent statistical significance. SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
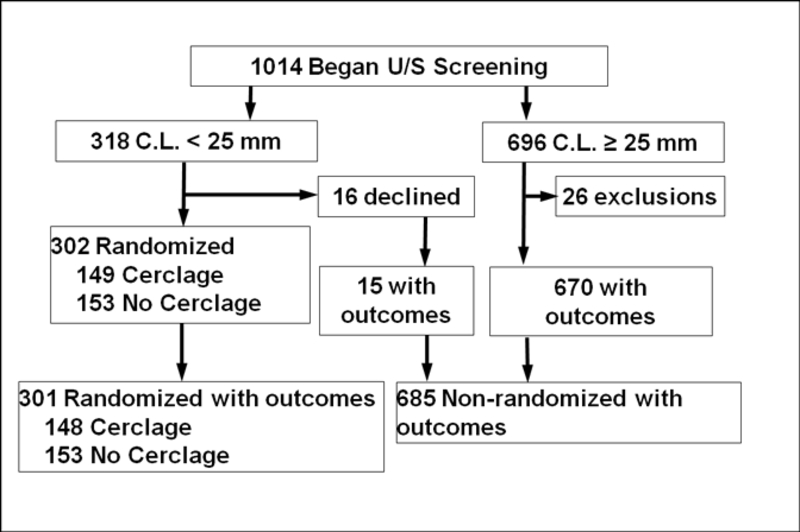
Of 986 women with prior SPTB who were screened with transvaginal ultrasound cervical lengths between 16 and 22 6/7 weeks and had delivery information available, 318 had a cervical length < 25mm, of which 301 agreed to randomization (Figure 1). Of these, 148 were randomized to receive a cerclage and 153 were randomized to not receive a cerclage. The demographic and sonographic characteristics for the randomized subjects compared to those who were not randomized can be found in Table 1, and the characteristics for the randomized subjects who did or did not receive a cerclage can be found in Table 2. The majority (57%) of the randomized women were African American.
Figure 1:
Flowchart of Subjects
Table 1.
Baseline characteristics for all subjects
| Randomized (n=301) | Non-randomized (n = 685) | P value | |
|---|---|---|---|
| Maternal age (y) | 26.4 ± 5.3 | 26.7 ± 5.3 | 0.43 |
| Number of prior births (n) | 2 (1, 4)† | 2 (1, 4)† | 0.12†† |
| Body Mass Index (kg/m2) at enrollment | 29.6 ± 7.7 | 28.8 ± 7.0 | 0.14 |
| Body Mass Index category (n) | |||
| Normal (< 25 kg/m2) | 93 (31) | 229 (33) | 0.51 |
| Overweight (25–30 kg/m2) | 99 (33) | 201 (29) | |
| Obese (> 30 kg/m2) | 109 (36) | 255 (37) | |
| Race/ethnicity | |||
| Black (non-Hispanic) | 173 (57) | 224 (33) | <0.01 |
| White (non-Hispanic) | 53 (18) | 131 (19) | |
| Hispanic | 44 (15) | 208 (31) | |
| Asian | 1 (0.3) | 11 (2) | |
| Other | 30 (10) | 111 (16) | |
| Gestational age of earliest preterm birth (wks) | 24.4 ± 4.8 | 26.6 ± 4.5 | <0.01 |
| Gestational age of most recent birth (wks) | 26.8 ± 6.6 | 30.4 ± 6.5 | <0.01 |
| Gestational age at enrollment (wks) | 17.4 ± 1.3 | 17.8 ± 1.4 | <0.01 |
Data presented as n (%), mean (range), or mean ± 1 SD.
Race and ethnic group are self-reported
Median and interdecile range
Wilcoxon rank-sum test
Table 2.
Baseline characteristics for 301 subjects randomly assigned to cerclage or to no-cerclage groups
| Cerclage (n = 148) | No-cerclage (n = 153) | P value | |
|---|---|---|---|
| Maternal age (y) | 26.4 ± 5.5 | 26.6 ± 5.1 | 0.75 |
| Number of prior births (n) | 2 (1,4)† | 2 (1,4)† | 0.66†† |
| Body Mass Index (kg/m2) at enrollment | 29.2 ± 7.8 | 29.9 ± 7.5 | 0.44 |
| Body Mass Index category (n) | 0.40 | ||
| Normal (< 25 kg/m2) | 51 (34) | 42 (27) | |
| Overweight (25–30 kg/m2) | 45 (30) | 54(35) | |
| Obese (> 30 kg/m2) | 52 (35) | 57 (37) | |
| Race/ethnicity | 0.36 | ||
| Black (non-Hispanic) | 80 (54) | 93 (61) | |
| White (non-Hispanic) | 25 (6.9) | 28 (18) | |
| Hispanic | 27 (8.2) | 17 (11) | |
| Asian | 1 (0.7) | 0 (0) | |
| Other | 15 (0.1) | 15 (9.8) | |
| Gestational age of earliest preterm birth (wks) | 24.2 ± 4.8 | 24.5 ± 4.7 | 0.58 |
| Gestational age of most recent birth (wks) | 26.4 ± 6.7 | 27.1 ± 6.5 | 0.37 |
| Gestational age at randomization (wks) | 19.4 ± 1.9 | 19.5 ± 2.0 | 0.56 |
| Cervical length at randomization (mm) | 18.6 ± 6.3 | 19.5 ± 5.3 | 0.21 |
| Gestational age at enrollment (wks) | 17.4 ± 1.2 | 17.4 ± 1.4 | 0.95 |
Data presented as n (%), mean (range), or mean ± 1 SD.
Race and ethnic group are self-reported
Median and interdecile range
Wilcoxon rank-sum test
For the entire cohort of 986 women, the mean BMI at enrollment was 29.0 ± 7.2 kg/m2 and the mean gestational age at delivery was 36.2 ± 4.8 weeks. When considering BMI as a continuous variable, it was not associated with shortest observed cervical length (p=0.68), gestational age at birth (p=0.12), or preterm birth < 35 weeks (p=0.68; 95% CI: 0.98–1.03). When considering BMI as a 3-level categorical variable, based on the WHO weight classes, similar non-significant conclusions were reached: shortest observed cervical length (p=0.86), gestational age at birth (p=0.20), or preterm birth < 35 weeks (p=0.69).
When the analysis was limited to the 301 randomized patients with cervical lengths < 25 mm, there was no significant relationship between BMI and shortest observed cervical length (p=0.18) or preterm birth <35 weeks (p=0.27; 95% CI: 0.99–1.05). However, a significant interaction between BMI and cerclage group was noted (p=0.03); therefore, the analysis was stratified by randomization group. For women assigned to cerclage, the mean BMI at enrollment was 29.2 ± 7.8 kg/m2 and the mean gestational age at delivery was 35.0 ± 5.5 weeks. In this group, BMI was not found to be a risk factor in regards to gestational age at birth (p=0.67).
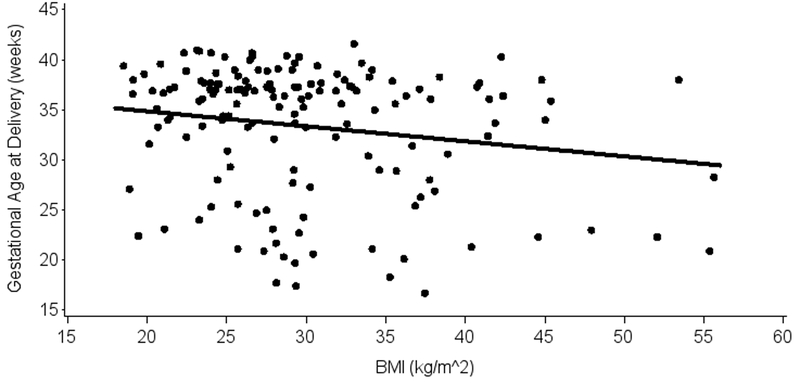
In the women randomized to not receive a cerclage, the mean BMI at enrollment was 29.9 ± 7.5 kg/m2 and the mean gestational age at delivery was 33.5 ± 6.4 weeks. Using multivariable linear regression controlled for shortest cervical length in these women, BMI was significantly associated with gestational age at delivery, with an estimated slope of −0.14 weeks per kg/m2 (p=0.03, 95% CI: (−0.26)-(−0.02)). This observed effect is driven primarily by women with extremely large BMI, as demonstrated in Figure 2, and is nullified with the exclusion of women with BMI > 47 (n=5). For the randomized women, no significant effects of BMI were observed when categorized into WHO weight classes.
Figure 2:
Relationship of Body Mass Index and Gestational Age at Delivery in Women Randomized to Not Receive a Cerclage
DISCUSSION
The results from our secondary analysis suggest that BMI alone does not appear to play a role in preterm birth in a population of women at high risk for recurrent preterm birth.
One of the strengths of our study is the focused patient population. The population evaluated in this study was at high risk for recurrent preterm birth secondary to their history of a preterm birth and their shortened cervices. As well, the majority of these subjects were at high risk for preterm birth because of their ethnicity (African American). Prior studies evaluating the relationship of BMI and preterm birth have examined generally low-risk, heterogeneous populations, where the relationship of BMI may be obscured by various confounding factors. However, in this study, we analyzed a very high-risk population, homogeneous for at least two strong preterm birth risk factors, and, therefore, the relationship between preterm birth and BMI may be more readily observed. The importance of the study population is highlighted in the following two studies: Baeten et al15 reported greater rates of preterm delivery for obese women in a cohort of women identified from Washington State’s birth certificate records and Cnattingius et al16 reported a lower rate of SPTB in a Swedish population based cohort. Both of these studies evaluated all births in a particular geographic location without reference to known preterm birth risk factors, a common theme in previously published studies of BMI’s relationship with SPTB.6–11 McDonald’s meta-analysis of 84 studies also evaluated low risk populations, which is reflected in their conflicting results of increased risk of SPTB before 33 weeks gestation and no difference in risk of preterm birth prior to 37 weeks gestation between normal weight, overweight, and obese women.6 In narrowing our population to such a high risk group as we did, we decreased the generalizability of the results, but were able to more closely evaluate one of the factors which might play a role in this particular group’s high risk status.
An expected weakness of this study is that this is a secondary analysis and therefore the parent study was not designed for this particular endpoint. With the 301 subjects who were randomized, the power to evaluate a 0.20 correlation or higher between BMI and gestational age at delivery was > 90%. The relatively low frequencies of BMI at the upper strata likely translated into low numbers of preterm births in general. Our analysis is also based on BMI at the time of enrollment, and we did not analyze the relationships or associations of preconception BMI or gestational weight gain in this investigation. Another weakness of this study is the inability to specifically analyze the concomitant use of 17-hydroxyprogesterone caproate. Similar to the parent trial, while our particular analyses showed no effect when progesterone was incorporated into the models, the effect of progesterone in this study could only be analyzed on a “patient intention to use” basis, rather than on actual use or preparation used. Results of the secondary analysis regarding the use of progesterone have been published elsewhere.17
The results of this study highlight the need for further research in this area of obstetrics, including evaluation of BMI changes in women with subsequent preterm births. Biologic plausibility would suggest the heightened inflammatory response described in obese women (in adipose tissue, placenta, vascular endothelium, and circulating plasma) as an etiology for shortening of their cervices18; however, this would not explain why a cerclage might improve the gestational age at delivery in these women, as suggested by the fact that no significant interaction in the group who received cerclages was noted. Consistent evidence supports the utility of cervical length measurements as one of the best predictors of spontaneous preterm birth.19 Our results, together with those of the parent trial, may suggest that this high risk population, regardless of BMI, would benefit from the placement of cerclage if pathologic cervical shortening is demonstrated.
ACKNOWLEDGEMENT
We wish to acknowledge other members of the Vaginal Ultrasound Trial Consortium: Susan Ramin, MD; Mark Tomlinson, MD; Eric Knudtson, MD; Robert Egerman, MD; Richard Silver, MD; Helen How, MD; Mike Gordon, MD.
Funding
The Eunice Kennedy Shriver National Institute of Child Health and Development provided funding via grant U01 HD039939.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA 2010; 303: 235–241. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2008 Natl Vital Stat Rep; 58 (16). Hyattsville, MD: National Center for Health Statistics. Released April 6, 2010. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol 2007;110:405–415. [DOI] [PubMed] [Google Scholar]
- 5.McManemy J, Cooke E, Amon E, Leet T. Recurrent risk for preterm delivery. Am J Obstet Gynecol 2007; 196:576.e1–576e.7. [DOI] [PubMed] [Google Scholar]
- 6.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ 2010; 341:c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torloni MR, Betran AP, Daher S, Widmer M, Dolan SM, Menon R, Bergel E, Allen T, Merialdi M. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. Journal of Maternal-Fetal and Neonatal Medicine 2009; 22: 957–970. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Y, Cahill AG, Macones GA, Zhu F, Odibo AO. The Association between Prepregnancy Maternal Body Mass Index and Preterm Delivery. Am J Perinat 2010; 27: 293–298. [DOI] [PubMed] [Google Scholar]
- 9.Flick AA, Brookfield KF, de la Torre L, Tudela CM, Duthely L, Gonzalez-Quintero VH. Excessive weight gain among obese women and pregnancy outcomes. Am J Perinat 2010; 27: 333–338. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenberg HM, Iams JD, Goldenberg RL, Newman RB, Weiner SJ, Sibai BM, Caritis SN, Miodovnik M, Dombrowski MP. Maternal obesity, uterine activity, and the risk of spontaneous preterm birth. Obstet Gynecol 2009; 113: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology 2010; 21: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obesity in pregnancy. ACOG Committee Opinion No 315. American College of Obstetricians and Gynecologists. Obstet Gynecol 2005; 106: 671–5. [DOI] [PubMed] [Google Scholar]
- 13.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, Egerman RS, Wing DA, Tomlinson M, Silver R, Ramin SM, Guzman ER, Gordon M, How HY, Knudtson EJ, Szychowski JM, Cliver S, Hauth JC. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 2009; 201: 375.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Global Database on Body Mass Index. http://apps.who.int/bmi. World Health Organization, 2011.
- 15.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001; 91: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 1998; 338: 147–52. [DOI] [PubMed] [Google Scholar]
- 17.Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, Sheffield JS, Perez-Delboy A, Wing DA, Guzman ER. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol 2010; 202: 351.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010;140: 373–385. [DOI] [PubMed] [Google Scholar]
- 19.Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol 2003; 46: 947–62. [DOI] [PubMed] [Google Scholar]