Abstract
No pharmacotherapies are approved for the treatment of cocaine use disorder (CUD). Galantamine, a cholinesterase inhibitor, has shown initial promise for cocaine use reduction in methadone-maintained individuals with CUD and cognitive improvement in abstinent individuals with past CUD. However, galantamine has not previously tested in individuals with current CUD and no comorbid opioid use disorder or methadone maintenance. The goal of this 13-week, double-blind, placebo-controlled, randomized controlled trial was to test the efficacy of galantamine (8mg or 16mg/day; extended release (ER)) for reducing cocaine use and improving cognitive function in individuals with cocaine use disorder (CUD). Ninety-three treatment-seeking cocaine users were randomized to placebo (n=32), 8 mg/day galantamine (n=31) or 16 mg/day galantamine (n=30). The medication was well-tolerated with minimal reports of side-effects. However, there were no significant treatment group differences in cocaine use outcomes (as measured by self-report or urines). The 16mg galantamine group had a greater improvement in working memory capacity (Backwards Digit Span), but there were no other significant treatment group differences on key cognitive outcomes. These findings did not provide support for the efficacy of galantamine as a treatment for cocaine use in this sample of individuals with CUD.
Keywords: Cocaine use disorder (CUD), galantamine, clinical trial, cholinesterase inhibitor, cognition
1. INTRODUCTION
Cocaine use disorder (CUD) remains an important public health problem in the United States with significant costs to the individual and society (John & Wu, 2017), yet there are no FDA-approved pharmacotherapies for the treatment of CUD. The brain acetylcholine (Ach) system may be a promising target for the treatment of CUD (Mehmet Sofuoglu & Mooney, 2009). Among medications targeting Ach, acetylcholinesterase inhibitors have shown promise in reducing cocaine use in preclinical and clinical studies. Cholinesterase inhibitors increase synaptic Ach levels by blocking its breakdown, and among those galantamine is also an allosteric modulator of nicotinic receptors (Giacobini, 2004; Schilstrom, Ivanov, Wiker, & Svensson, 2007). In preclinical studies, administration of cholinesterase inhibitors reduces self-administration of cocaine in primates and rodents (Kenneth Grasing, Yang, & He, 2011; Liu et al., 2012; Wilson & Schuster, 1973), and reduces liking/motivation for cocaine as indicated by diminished cocaine-induced conditioned place preference (Hikida, Kitabatake, Pastan, & Nakanishi, 2003). In addition, clinical trials have shown effects of galantamine on reducing cocaine use. In a small pilot study, individuals with CUD who were also stabilized on methadone for opioid use disorder were randomized to galantamine (8mg until week 4 then increased to 16mg; N=14) or placebo (N=14)(M. Sofuoglu & Carroll, 2011). The group randomized to galantamine showed a trend towards better cocaine use outcomes (reduced cocaine use as measured by self-reported days of use and urine toxicology)(M. Sofuoglu & Carroll, 2011). In a separate larger 12-week clinical trial (N=120), also in individuals with CUD who were also stabilized on methadone for opioid use disorder, the group randomized to galantamine (8mg) showed significantly better cocaine use outcomes (reduced cocaine use as measured by self-reported days of use and trend towards lower urine toxicology) than the placebo group (Carroll, Nich, DeVito, Shi, & Sofuoglu, 2018).
While there are several non-exclusive potential mechanisms through which cholinesterase inhibitors, such as galantamine, may be hypothesized to influence CUD (e.g., ACh influences sleep, nociception, mood, stress response and reward (K. Grasing, 2016; Sarter, Lustig, Howe, Gritton, & Berry, 2014)), a key potential mechanism is through galantamine’s cognitive enhancing capabilities. Galantamine is one of several cholinesterase inhibitors used as a cognitive enhancer in the treatment of Alzheimer’s disease. Cognitive enhancement has been proposed as potential target for substance use disorders, including CUD (M. Sofuoglu, 2010; M. Sofuoglu, DeVito, Waters, & Carroll, 2013, 2016). ACh is implicated in cognitive processes with relevance to addiction. For example, ACh in the prefrontal cortex mediates attentional processes (Sarter et al., 2014). Furthermore, the ratio of dopamine to ACh in the nucleus accumbens may affect reward and aversion spectrum (i.e., greater dopamine relative to ACh levels may facilitate reward, diminished dopamine relative to ACh levels may facilitate aversive states) (Hoebel, Avena, & Rada, 2007). However, findings are mixed on whether galantamine improves cognitive function in individuals with cocaine use disorders. A randomized placebo-controlled 10-day trial of galantamine (8mg/day extended release (ER)) assessed the effects of galantamine on cognitive function in abstinent individuals who met criteria for past cocaine dependence (N=34). The group randomized to galantamine displayed greater improvements in attention (as measured by signal detection (A’), response latency and accuracy measures on the Rapid Visual Information Processing (RVP) task) relative to the group randomized to placebo (M. Sofuoglu, Waters, Poling, & Carroll, 2011). However, a 12-week clinical trial (N=120), in individuals with current CUD who were also stabilized on methadone for opioid use disorder, found no significant benefit of galantamine (8mg) over placebo on cognitive function (including the RVP task), despite better cocaine use outcomes in the galantamine group (Carroll et al., 2018).
In the current trial, we tested the efficacy of galantamine in reducing cocaine use and improving cognitive function in individuals with current CUD and who do not have co-morbid opioid use disorder and are not receiving methadone. We hypothesized that galantamine treatment (8 or 16mg/day) would be more effective than placebo in a) reducing cocaine use and b) improving attention function during the trial. If these initial hypotheses were supported, we also hypothesized that galantamine’s efficacy in reducing cocaine use would be mediated by galantamine’s effects on improving attention (as assessed with the RVP task).
2. METHODS
2.1. Participants
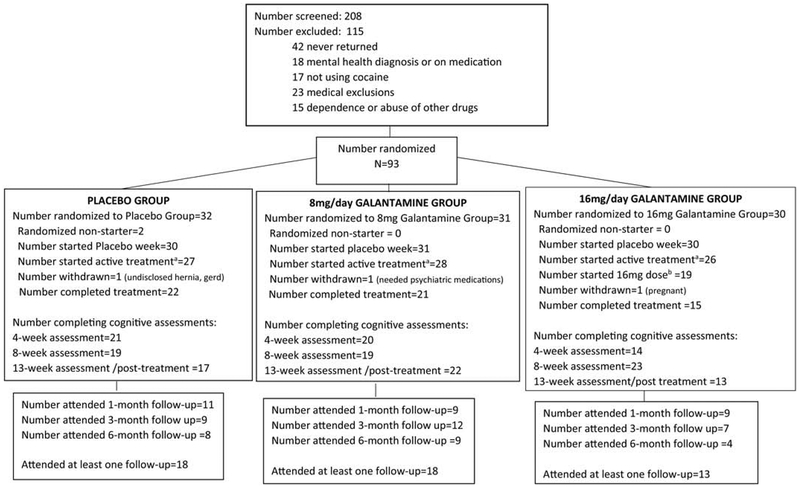
Ninety-three treatment-seeking cocaine users between the ages of 18 to 55 were recruited from the greater New Haven, CT area between July 2011 and October 2015 (for Consort Diagram see Fig 1). To be included in the study, participants had to meet the DSM-IV criteria for current cocaine dependence as determined by a study physician and confirmed with the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 1996), report using cocaine at least once per week in the prior 30 days, and provide a cocaine-positive drug urine test at screening. Women had to test negative for pregnancy, use effective birth control methods, and take monthly urine pregnancy tests participation. Participants had to be fluent in English, have a 6th grade or higher reading level, be willing to be randomized to treatment, and commit to 13 weeks of treatment. Potential participants were excluded if they met DSM-IV criteria for substance use disorders other than cocaine or tobacco, or lifetime schizophrenia or bipolar disorder, or have a depressive or anxiety disorder with current use of a prescribed psychotropic medication that cannot be discontinued; if they had significant medical conditions, including asthma or chronic obstructive lung disease, history or current gastrointestinal ulcer, hepatic or renal deficit and cardiac rhythm disturbances, screening liver function test (AST or ALT) greater than 3 times normal, known allergy or adverse reaction to galantamine or any other medical conditions that the study physician deems contraindicated for galantamine treatment; or current use of other medications including drugs that slow heart rate (e.g., beta-blockers). Eligibility criteria were determined through medical evaluation which included blood work, electrocardiogram (ECG), urine analysis, urine toxicology, medical and psychiatric evaluation.
Figure 1. Consort Diagram.
a“Started active treatment” refers to the number who attended at least one session after the completion of the week 0 placebo lead-in week and received pills (i.e., placebo group received placebo; 8 or 16mg galantamine groups received 8mg galantamine).
b“Started 16mg dose” refers to the number of individuals from the group randomized to 16mg galantamine who attended at least one session after the completion of the titration period (week 4) and received their target dose (16mg galantamine).
The study protocol was approved by the VA Connecticut Human Studies Subcommittee and the Yale University Human Investigations Committee and was registered at clinicaltrials.gov (NCT 01531153).
2.2. Procedures/Interventions
2.2.1. Overview of Treatment Conditions:
This study was a double-blind, placebo-controlled, randomized outpatient clinical trial in which participants were randomized to one of three medication treatment groups: placebo, 8 mg/day galantamine or 16 mg/day galantamine. All participants received Contingency Management (CM) to reinforce medication adherence, and all were offered weekly individual drug counseling. Urn randomization was used to balance treatment groups for gender, severity of cocaine use (1–10, 11–20, or 21–30 days of cocaine use in month prior to treatment)), and smoking status (no use, 1–10, or ≥11 average cigarettes per day at baseline). These variables were chosen for the urn because gender and severity of cocaine use have been shown to predict treatment responses in cocaine users (Poling, Kosten, & Sofuoglu, 2007), and smoking status is relevant, given galantamine’s actions on nicotinic receptors and its potential efficacy for smoking cessation (Ashare et al., 2016; Diehl et al., 2006; MacLean, Waters, Brede, & Sofuoglu, 2018).
2.2.2. Visits:
Subjects were asked to attend clinic visits two to three times per week during the treatment trial. At clinic visits, subjects submitted a urine sample and breathalyzer, filled in self reports on side effects (SAFTEE (Levine & Schooler, 1986)) and substance use (Timeline Follow-back method), had their heart rate and blood pressure measured, and received the study medication and CM payments. ECG was collected at week 6 and study termination. Follow-up visits were scheduled for 1, 3 and 6-months post-treatment. A cognitive assessment (detailed below in section 2.3.1) was administered at baseline, treatment weeks 4, 8, and 13, and follow-up visits.
2.2.3. Galantamine and Placebo Schedule:
The medication schedule was designed to initiate treatment without delay, while also enabling the gradual dose titration recommended for galantamine. Therefore, during week 0 of treatment, all participants were initiated with a daily placebo pill. This week allowed participants to start the clinic visit routine and behavioral therapy (CM) to encourage compliance prior to initiation of the galantamine and was single-blind (researchers were aware that all participants were receiving a one week placebo lead-in). In week 1 of treatment, the group randomized to placebo remained on placebo, while the groups randomized to 8 or 16mg of galantamine were both started on 8mg of galantamine. Galantamine was the extended release (ER) formulation and was taken once per day. Subjects had take-home medication for non-clinic days. With once daily dosing, steady-state plasma levels are expected to be reached within one week (Robinson & Plosker, 2006). For those assigned to 16 mg/day, the dose of galantamine was increased to 16 mg at the end of treatment week 4. Treatment groups remained on their full dosage through week 12 (end of study), at which point the study medication was discontinued.
2.2.4. Contingency Management (CM):
CM to reinforce pill compliance. For all subjects, pills (galantamine or placebo) were dispensed at each clinic visit. For each pill-dispensing instance that the subject complied with, they earned a minimum payment with amounts escalating per consecutive pill session attended. If participants failed to attend a scheduled pill dispensing session, the amount earned reset to the minimum for the subsequent dispensing session, and again escalated for each consecutive dispensing session attended (Peirce et al., 2006; Petry et al., 2005). The variable (Table 2) which reflects pill compliance, as reinforced by CM, is “Percent of Clinic/Medication Dispensing Visits Attended (of Expected)”. In addition to CM payments, participants received reimbursement gift cards or bus tokens for participating in the trial, to defer transportation costs.
Table 2.
Treatment Retention and Cocaine Use Outcomes
| Treatment Retention and Outcome Variables | Galantamine | Placebo | Total Sample | Statistics: 3 group (8mg, 16mg, placebo) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8mg | 16mg | ||||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | F | df | p | |
| A. Treatment/Protocol Adherence | |||||||||||||||
| Days in Treatment Protocol (week 1 to 12)a | 60.1 | 34.1 | 31 | 45.9 | 37.3 | 30 | 58.1 | 34.2 | 32 | 54.8 | 35.4 | 93 | 1.46 | 2,90 | 0.24 |
| Days in Treatment Protocol (week 4 to 12)b | 38.2 | 23.5 | 31 | 27.6 | 26.1 | 30 | 36.3 | 24.0 | 32 | 34.2 | 24.7 | 93 | 1.62 | 2,90 | 0.20 |
| Days of Data Reported on Substance Use Calendar (TLFB) (week 4 to 12)b | 47.2 | 15.7 | 25 | 34.9 | 25.1 | 21 | 41.9 | 21.1 | 26 | 41.7 | 21 | 72 | 2 | 2,69 | 0.14 |
| % Urines Submitted (of Expected) (week 4 to 12)b | 85.8 | 21.4 | 24 | 77.9 | 22 | 17 | 84.4 | 21.6 | 24 | 83.2 | 21.5 | 65 | 0.71 | 2,62 | 0.50 |
| % Clinic/Medication Dispensing Visits Attended (of Expected) (week 4 to 12)b, c | 71.9 | 44.3 | 30 | 48.2 | 48 | 29 | 68.8 | 43.2 | 31 | 63.2 | 45.9 | 90 | 2.38 | 2,87 | 0.10 |
| B. Cocaine Use Outcomes | |||||||||||||||
| % Negative Cocaine Urines (week 4 to 12)b | 31.0 | 36.8 | 23 | 22.9 | 31.1 | 17 | 28.1 | 36.6 | 24 | 27.8 | 34.9 | 64 | 0.25 | 2,62 | 0.78 |
| % Days Cocaine Abstinence (self-report) (week 4 to 12)b | 27.9 | 25.5 | 22 | 29 | 21.3 | 15 | 29.3 | 27.7 | 24 | 28.7 | 25.1 | 61 | 0.02 | 2,59 | 0.98 |
| Longest Consecutive Days Cocaine Abstinence (self-report) (week 4 to 12)b | 13.9 | 14.7 | 25 | 8.2 | 10.1 | 21 | 13.4 | 15.2 | 26 | 12.1 | 13.8 | 72 | 1.16 | 2,69 | 0.32 |
Week 1 to 12 encapsulates the full galantamine treatment period, including titration. Week 0, where all groups received placebo, is not included.
Week 4 to 12 is restricted to the galantamine treatment period following titration, therefore the group randomized to 16mg galantamine were receiving 16mg galantamine/day during this time.
The pills (galantamine or placebo) were dispensed at each clinic/medication dispensing visit: participants were observed (by a member of the research team) ingesting that day’s pill(s), then were given take-home pills to cover them until the next scheduled clinic visit. CM payments were based on adherence with this visit protocol.
2.2.5. Individual Drug Counseling:
Regardless of treatment assignment, all participants were offered weekly one-hour individual psychotherapy sessions. Counselors held a Licensed Alcohol and Drug Counselor or Master’s degree or above and were supervised in their treatment delivery by a licensed psychologist. Like other study staff, the counselor was blinded to participants’ medication treatment condition.
2.3. Assessments/Outcomes:
The main outcome measures were cocaine use and cognitive function.
2.3.1. Substance Use Outcomes:
Substance use outcomes were measured with biomarkers and self-report. Urine samples, collected at clinic visits during the 13-week treatment and at follow-ups, were tested for cocaine metabolite (benzoylecgonine), and other drugs of abuse (opioids, benzodiazepines, cannabis, amphetamines). Self-reported day-to-day use of cocaine and other substances (alcohol, tobacco, heroin, other opioids, stimulants, sedatives and cannabis) was collected weekly using the Timeline Follow Back method.
Cocaine use outcomes include: percent cocaine negative urines, and two measures of self-reported abstinence during the treatment period (percent days of cocaine abstinence, longest duration of continuous abstinence). Primary cocaine outcomes were calculated from week 4 (once dose titration was complete and placebo, 8mg and 16mg galantamine groups were receiving their assigned treatments) through the end of the treatment period, but then secondarily also repeated for the treatment period including the titration period but excluding the placebo lead-in week (i.e., including week 1–12, excluding week 0.
Secondary substance use study outcomes included severity of cocaine abstinence symptoms and nicotine dependence. The Cocaine Selective Severity Assessment (CSSA) is a clinician-administered instrument that measures early cocaine abstinence symptomatology by rating 18 signs and symptoms associated with early cocaine abstinence based on a scale of 0 (no symptoms) to 7 (maximum score) (Kampman et al., 1998). The CSSA was administered by the counselor during individual drug counseling sessions at baseline, monthly during treatment and at follow-up. The Fagerstrom Test for Nicotine Dependence (FTND) is a self-report measure that assesses the degree of nicotine dependence (Fagerstrom, 1978), and was collected at baseline, at the end of treatment, and at follow-up.
2.3.1. Cognitive Outcomes:
Cognitive function was assessed with three computerized tasks and a paper/pencil task.
CANTAB Rapid Visual Information Processing (RVP) task assessed sustained attention. In this task, digits appear rapidly one at a time on the computer screen and the participant is asked to press a button when they see the target sequences (e.g., ‘3’ followed by a ‘5’ followed by a ‘7’, with no other numbers in between) and withhold responding for non-targets. The main outcome measure for RVP is A’, a measure of target sensitivity, where higher scores (range 0–1) indicate greater ability to detect and respond to the target sequence and avoid responding to non-target sequences. Secondary RVP measures include B”, a measure of response bias, where scores (range −1 to +1) closer to −1 indicate a bias towards under-responding (to targets) and scores closer to +1 indicate a bias towards over-responding (to non-targets), and false alarms (commission errors to non-targets).
CANTAB Stop Signal Task (SST) assesses the ability to inhibit a prepotent response and consists of trials with arrows on left or right of the screen and the subject presses the corresponding button depending on the direction of the arrow (‘Go’ trials). On a subset of trials (‘Stop’ trials), the arrow is followed by an auditory ‘stop signal’. The delay between the presentation of the arrow and the sound of the beep (stop-signal delay) is variable therefore unpredictable. The main outcome is Stop Signal Reaction Time (SSRT), a measure of response inhibition, and represents the estimated length of time between the go stimulus and the stop stimulus at which the subject is able to successfully inhibit their response on approximately 50% of the trials (range 0 to 1500 ms; lower scores reflect better response inhibition). Secondary outcomes for SST were median and standard deviation of reaction time on correct ‘go’ trials.
Drug Stroop:
The computerized ‘Drug Stroop’ task (DeVito, Kiluk, Nich, Mouratidis, & Carroll, 2018; DL Reeves, Schlege, & Gilliland, 1991; D. Reeves, Winter, Kane, Elsmore, & Bleiberg, 2002) consists of a practice and a task condition. During the practice, letters (non-words) were displayed in different colored fonts and participants were asked to press quickly and accurately on buttons corresponding with each color fonts, to teach the color-button pairings. During the task, participants used the same color-button pairings to press for words that were either cocaine-related ‘drug’ (e.g., cocaine) or ‘neutral’ (e.g., chair) words. The Drug Stroop effect is the response time to correct trials for the ‘drug’ relative to ‘neutral’ trials and serves as a measure of attentional bias to drug-related stimuli and cognitive control (i.e., more drug-stimuli-related slowing showing more attentional bias and less cognitive control).
Digit Span:
Participants were read lists of numerical digits of increasing length and asked to repeat aloud the digits either in the order they were read (Forward Digit Span; a measure of memory capacity) or in the reverse order (Backward Digit Span; a measure of working memory). Key outcomes include the number of correct spans and the longest correct span, calculated separately for Forward and Backward conditions (Weschler 2008). Digit Span has been shown to be sensitive to treatment with galantamine and other cholinesterase inhibitors (Buchanan et al., 2008).
2.3.1. Treatment/Protocol Adherence Measures:
Measures assessing treatment/protocol adherence (see Table 2) include the a) time between initiation and either completion or drop out from the protocol (i.e., Days in Treatment Protocol); b) the number of protocol days for which substance use calendar data was provided (Days of Data Reported on Substance Use Calendar (TLFB)); percent of scheduled urine specimens provided at clinic visits (i.e., % Urines Submitted (of Expected)); pill adherence, as reinforced by CM, namely by attendance at visits during which that day’s pill administration was observed and pills were dispensed for take-home doses (i.e., % Clinic/Medication Dispensing Visits Attended (of Expected)).
2.4. Statistical Analyses
Data analyses were conducted on the full randomized (intent-to-treat) sample. The primary treatment group comparison included three-group comparison (8mg, 16mg, placebo) to enable assessment of differential dose effects, wherein pairwise comparisons between groups were assessed in the event of significant finding in these three-group analyses. As a secondary approach, reported in the Supplemental Materials (Supplemental Tables 1–3), analyses were repeated with a two-group comparison: galantamine (8 and 16mg groups combined) versus placebo groups, to assess overall medication effects. For the substance use outcomes that were averaged over the treatment period (e.g., % negative urines) the primary approach to focus on the treatment weeks after full dose titration was achieved (i.e., treatment week 4) through the end of treatment (i.e., treatment week 12). In addition, group analyses of the summary measures from the entire treatment period (which includes the placebo run-in week when all groups were on placebo and the titration weeks when the 16mg galantamine group was still on 8mg galantamine) were run to check for consistency (data not shown).
Comparability of treatment groups on baseline characteristics (demographic, substance use, mood, cognitive function) and measures of treatment engagement and retention was evaluated using chi-square tests for categorical measures and ANOVA for continuous measures. Survival analyses were run to evaluate time to drop out by treatment condition.
ANOVAs were used to assess treatment group differences in the primary cocaine use outcomes (percentage of cocaine positive urines during the trial, self-reported percentage of days without cocaine use during study participation), other drug and alcohol use measure and adverse effects. Repeated measures ANOVAs assessed changes in cognitive function from baseline (week 0) to end of treatment (week 12), with treatment group as a between-subject factor.
Multilevel longitudinal models were run to assess change across treatment time by treatment condition. Separate models examined whether galantamine was associated with a reduction in the probability of obtaining a cocaine negative urine result or change in key cognitive indicators, by treatment condition.
3. RESULTS
3.1. Baseline Characteristics, Treatment Adherence and Safety
3.1.1. Baseline Characteristics:
The baseline subject characteristics are presented in Table 1. Treatment groups were generally well-matched on baseline measures, with one exception: the placebo group was slightly older on average than the 8 or 16mg galantamine groups (Table 1).
Table 1.
Baseline and demographics by treatment condition
| 47 | 6. | 3 | 43 | 8. | 3 | 48 | 7. | 3 | 46. | 7. | 9 | 3. | 2, | 0. | |
| Age | .4 | 8 | 1 | .3 | 3 | 0 | .1 | 2 | 2 | 3 | 6 | 3 | 59 | 90 | 03 |
| 86 | 9. | 3 | 85 | 2 | 85 | 10 | 3 | 86. | 10 | 9 | 0. | 2, | 0. | ||
| Shipley Estimated IQ | .7 | 9 | 1 | .8 | 10 | 8 | .4 | .8 | 2 | 0 | .1 | 1 | 14 | 88 | 87 |
| Days Use in Past 28 Days | |||||||||||||||
| 6. | 9. | 3 | 7. | 7. | 2 | 8. | 3 | 8. | 8 | 0. | 2, | 0. | |||
| Alcohol | 9 | 3 | 1 | 9 | 4 | 8 | 9 | 9 | 0 | 7.9 | 6 | 9 | 4 | 86 | 68 |
| 18 | 7. | 3 | 16 | 7. | 2 | 18 | 7. | 3 | 17. | 7. | 9 | 0. | 2, | 0. | |
| Cocaine | .5 | 6 | 1 | .8 | 6 | 9 | .3 | 6 | 0 | 9 | 5 | 0 | 46 | 87 | 63 |
| 3. | 7. | 3 | 0. | 2. | 2 | 1. | 2. | 3 | 8 | 2. | 2, | 0. | |||
| Marijuana | 4 | 5 | 1 | 61 | 7 | 8 | 4 | 6 | 0 | 1.8 | 5 | 9 | 54 | 86 | 09 |
| 0. | 2. | 3 | 1. | 5. | 2 | 0. | 1. | 3 | 3. | 8 | 0. | 2, | 0. | ||
| Benzodiazepine | 6 | 6 | 1 | 4 | 2 | 8 | 2 | 0 | 0 | 0.7 | 3 | 9 | 88 | 86 | 42 |
| 3 | 2 | 3 | 9 | 2, | |||||||||||
| Heroin | 0 | 0 | 1 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 87 | ||
| 19 | 12 | 3 | 17 | 12 | 2 | 21 | 10 | 3 | 19. | 11 | 9 | 0. | 2, | 0. | |
| Nicotine | .3 | .1 | 1 | .7 | .4 | 9 | .7 | .8 | 0 | 57 | .8 | 0 | 86 | 87 | 43 |
| Barratt Impulsivity Scale (BIS-11) | |||||||||||||||
| 26 | 5. | 2 | 27 | 6. | 2 | 7. | 2 | 26. | 6. | 7 | 0. | 2, | 0. | ||
| Non-planning impulsivity | .5 | 1 | 8 | .7 | 3 | 5 | 26 | 2 | 3 | 7 | 2 | 6 | 52 | 73 | 6 |
| 23 | 4. | 2 | 4. | 2 | 23 | 4. | 2 | 24. | 4. | 7 | 0. | 2, | 0. | ||
| Motor impulsivity | .9 | 2 | 8 | 25 | 5 | 5 | .8 | 6 | 5 | 2 | 4 | 8 | 56 | 75 | 57 |
| 14 | 3. | 2 | 17 | 2 | 16 | 5. | 2 | 16 | 4. | 7 | 2, | 0. | |||
| Cognitive impulsivity | .9 | 6 | 7 | .7 | 4 | 6 | .9 | 3 | 5 | 5 | 4 | 8 | 3 | 75 | 06 |
| 4. | 2. | 2 | 3. | 2. | 1 | 3. | 2. | 2 | 2. | 6 | 0. | 2, | 0. | ||
| Nicotine dependence: FTND | 1 | 3 | 2 | 4 | 5 | 9 | 2 | 4 | 2 | 3.6 | 4 | 3 | 9 | 60 | 41 |
| 47 | 22 | 3 | 50 | 20 | 2 | 49 | 23 | 2 | 49. | 22 | 8 | 0. | 2, | 0. | |
| Cocaine Withdrawal: CSSA | .7 | .8 | 1 | .2 | .4 | 3 | .7 | .3 | 7 | 1 | .1 | 1 | 1 | 78 | 91 |
Abbreviation: FTND=Fagerstrom Test of Nicotine Dependence, CSSA=Cocaine Selective Severity Assessment
In order to be eligible for the study, participants had to have a cocaine positive urine prior to starting. There were zero positive urines for heroin, oxycodone, amphetamines, or methamphetamines.
3.1.2. Treatment Retention and Adherence:
Of the 93 subjects randomized to a treatment condition, 81 (87%) initiated treatment (i.e., completed the placebo lead-in week and began the titration period (placebo group receiving placebo; 8 and 16mg groups receiving 8mg galantamine), and 58 completed treatment (62% of the randomized sample, 71.6% of the sample that initiated treatment) (for Consort Diagram see Fig. 1). Treatment groups did not significantly differ in terms of treatment retention (i.e., mean days in treatment) or treatment/protocol adherence (e.g., days of substance use calendar collected, percent of urine specimens submitted out of expected during the protocol) (Table 2A, Supplemental Table 2). There was no significant difference in the time to drop out by treatment group (for details, see Supplemental Fig. 1).
3.1.3. Treatment Safety:
The medication was largely well-tolerated. When restricting to weeks 4–12 (when treatment groups were receiving their assigned treatments), more instances of ‘insomnia’ were reported for the 16mg galantamine group (n=4; 8mg galantamine n=0; placebo n=1; x2=6.72, df=2, p=0.04); while placebo reported more instances of heartburn (n=2) and abdominal pain (n=2) than the galantamine group (8 or 16mg combined; n=0 heartburn, n=0 abdominal pain; x2=3.88; p=0.05). No other symptoms differed across groups during weeks 4–12 and there were no main effects of group (8mg, 16mg, placebo) on reported adverse effects across the full duration of the trial (including titration period) (data not shown). One subject was withdrawn from each group for medical reasons, but these were all deemed to be unrelated to the medication (Fig 1).
3.2. Substance Use Outcomes During Treatment
There were no significant differences between treatment groups (8, 16mg, placebo) in primary cocaine use outcomes (% cocaine negative urines, % days self-reported cocaine abstinence, or longest duration of cocaine abstinence) from week 4 (when titration complete and groups receiving their assigned medication conditions) through the end of treatment (Table 2B). These findings remained consistent when considering or the entire treatment period (including the titration period) (data not shown). Furthermore, the generalized linear model (GLM) analysis of cocaine-negative urines from weeks 4 through 12 found no significant change in the probability of submitting a cocaine negative urine over time (Wald Chi-Square=0.03, df=1, p=0.86), and no significant differences by treatment condition (Wald Chi-Square=2.44, df=2, p=0.29), nor interactions of treatment condition and time (Wald Chi-Square=2.27, df=2, p=0.32) (Supplemental Figure 2). HLM analyses found cocaine withdrawal severity (CSSA) reduced over the treatment period (week: F1, 76.6=9.37, p=0.003) but did not differ by treatment group (group: F2, 121.1=0.005, p=0.995; week*group: F2, 75.9=0.41, p=0.67).
Rates of other substance use during treatment weeks 4–12 did not significantly differ by treatment groups (F≤ 0.58; p≥0.57) and remained low during this time period (percent days self-reported abstinence: heroin (99.9%), other opioids (99.2%), marijuana (92.6%), speed (99.8%), alcohol (79.4%), and cigarettes (41.3%)).
3.3. Cognitive Outcomes
ANOVAs assessing end of treatment (week 12) cognitive performance relative to pre-treatment baseline found improved short term memory capacity (Longest Digit Span Forward) and working memory capacity (Longest Digit Span Backwards (LDSB)) and time by treatment group interaction reflecting a greater improvement in working memory (LDSB) in those receiving 16mg galantamine relative to 8mg or placebo (Table 3). There were no effects of time or treatment group on the primary outcome measure of RVP A’, a measure of attention. However, a time by treatment group interaction on RVP B” indicated that all groups at baseline showed a bias towards over-responding (to non-targets), and the 16mg galantamine group showed a greater reduction in the over-responding bias (i.e., consistent with less motor impulsivity) by end of treatment (Table 3). Correct response times improved (were faster) at end of treatment relative to baseline in both the drug and neutral trials of the Drug Stroop task but there was no significant change in the Drug Stroop Effect and no time by treatment group effects on task performance. There were no time or treatment group effects on the stop signal task, a measure of response inhibition (Table 3). Similarly, HLM analyses of primary cognitive outcomes, including baseline and all three within-treatment cognitive timepoints (weeks 4, 8, 12), also demonstrated improved memory performance across repeated measures in treatment (Longest Digit Span Forward, week: F1, 177.0=16.6, p<0.001), however it did not differ by treatment group (group: F2, 121.6=0.61, p=0.55; week*group: F2, 176.7=0.97, p=0.38) and the other key cognitive outcomes (as listed in Table 3A) did not show significant effects of week group, or group*week (data not shown).
Table 3.
Cognitive Measures
| Galantamine | Placebo | Total | Statistics: 3 Group (8, 16mg, placebo) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task Variable (Cognitive Domain) | Timepoint | 8 mg | 16 mg | Time | Group | Groupx Time | ||||||||||
| mean | sd | n | mean | sd | n | mean | sd | n | mean | sd | n | F, p | F, p | F, p | ||
| A. Primary Cognitive Outcomes | ||||||||||||||||
| RVP A’ (Attention/Signal Detection) | Baseline (Wk 0) | 0.86 | 0.06 | 17 | 0.86 | 0.05 | 12 | 0.85 | 0.08 | 15 | 0.86 | 0.06 | 44 | .41, .53 | .38, .69 | 1.31, .28 |
| End of Treatment (Wk 12) | 0.88 | 0.07 | 17 | 0.84 | 0.12 | 12 | 0.87 | 0.08 | 15 | 0.87 | 0.09 | 44 | ||||
| Longest Digit Span Forward (Memory Capacity) | Baseline (Wk 0) | 5.9 | 1.7 | 22 | 5.7 | 1.3 | 13 | 6.4 | 2.3 | 17 | 6 | 1.8 | 52 | 17.18, .00 | 1.43, .25 | .48, .62 |
| End of Treatment (Wk 12) | 6.5 | 1.4 | 22 | 6.8 | 1 | 13 | 7.4 | 1.2 | 17 | 6.9 | 1.3 | 52 | ||||
| Longest Digit Span Backwards (Working Memory) | Baseline (Wk 0) | 4.2 | 1.7 | 22 | 3.2 | 0.9 | 13 | 4 | 1.7 | 17 | 3.9 | 1.6 | 52 | 4.9, .03 | .58, .56 | 5.89, .005 |
| End of Treatment (Wk 12) | 4.2 | 1.2 | 22 | 4.3 | 1.4 | 13 | 3.9 | 1.6 | 17 | 4.1 | 1.4 | 52 | ||||
| Stop Signal Response Time (SSRT) (Response Inhibition) | Baseline (Wk 0) | 215.3 | 89.9 | 17 | 277.2 | 142.6 | 12 | 244.1 | 111.4 | 15 | 242 | 113.5 | 44 | .01, .92 | .67, .52 | .22, .81 |
| End of Treatment (Wk 12) | 241.9 | 140.2 | 17 | 267.9 | 196.6 | 12 | 234.8 | 129.2 | 15 | 246.6 | 151.1 | 44 | ||||
| Drug Stroop Effect (Attentional Bias) | Baseline (Wk 0) | −2.25 | 13.5 | 16 | 3.1 | 5.5 | 3 | 1.5 | 4.3 | 15 | 0.6 | 9.1 | 44 | .13, .72 | .42, .66 | 2.11, .13 |
| End of Treatment (Wk 12) | 2.3 | 6.4 | 16 | 0.22 | 2.9 | 13 | 1.4 | 3.9 | 15 | 1.4 | 4.8 | 44 | ||||
| B. Secondary Cognitive Outcomes | ||||||||||||||||
| Digit Span: Forward Total Score (Memory Capacity) | Baseline (Wk 0) | 9.6 | 3 | 22 | 9.9 | 2.3 | 13 | 10.7 | 2.1 | 19 | 10.1 | 2.6 | 54 | 6.23, .02 | .78, .47 | .27, .77 |
| End of Treatment (Wk 12) | 10.5 | 2.6 | 22 | 10.7 | 1.8 | 13 | 11.2 | 3.1 | 19 | 10.8 | 2.6 | 54 | ||||
| Digit Span: Backwards Total Score (Working Memory) | Baseline (Wk 0) | 8.3 | 3.1 | 22 | 7.2 | 1.5 | 13 | 7.6 | 1.9 | 19 | 7.8 | 2.4 | 54 | .79, .38 | .82, .44 | 2.03, .14 |
| End of Treatment (Wk 12) | 8.3 | 2.1 | 22 | 8.5 | 2.7 | 13 | 7.3 | 2.7 | 19 | 8 | 2.5 | 54 | ||||
| RVP: B” (Response Bias) | Baseline (Wk 0) | 0.77 | 0.42 | 17 | 0.91 | 0.13 | 12 | 0.77 | 0.22 | 15 | 0.81 | 0.3 | 44 | 2.13, .15 | .20, .82 | 4.44, .02 |
| End of Treatment (Wk 12) | 0.81 | 0.16 | 17 | 0.57 | 0.56 | 12 | 0.81 | 0.19 | 15 | 0.74 | 0.34 | 44 | ||||
| RVP: False Alarm (Motor Impulsivity) | Baseline (Wk 0) | 0.04 | 0.08 | 17 | 0.01 | 0.02 | 12 | 0.03 | 0.04 | 15 | 0.03 | 0.05 | 44 | .03, .87 | .18, .84 | 1.93, .16 |
| End of Treatment (Wk 12) | 0.03 | 0.03 | 17 | 0.03 | 0.04 | 12 | 0.02 | 0.02 | 15 | 0.03 | 0.03 | 44 | ||||
| SST: Median Correct Go RT (Response Speed) | Baseline (Wk 0) | 706.3 | 260 | 17 | 619.7 | 285.7 | 12 | 680 | 158.3 | 15 | 673.7 | 235.4 | 44 | .87, .36 | .02, .99 | 1.51, .23 |
| End of Treatment (Wk 12) | 608.6 | 201.6 | 17 | 669.7 | 323.5 | 12 | 631.5 | 150.6 | 15 | 633.1 | 223.3 | 44 | ||||
| SST: SD Correct Go RT (Attention) | Baseline (Wk 0) | 276.5 | 138.5 | 17 | 302.3 | 174.6 | 12 | 363.4 | 214.4 | 15 | 313.1 | 177 | 44 | 3.36, .07 | 1.05, .36 | .15, .86 |
| End of Treatment (Wk 12) | 337.8 | 262.9 | 17 | 410.6 | 276.1 | 12 | 492.6 | 530.5 | 15 | 410.4 | 375.9 | 44 | ||||
| Drug Stroop: Mean RT for Correct Neutral Trials | Baseline (Wk 0) | 791.8 | 189.4 | 16 | 808.2 | 112.7 | 13 | 854.5 | 142.2 | 15 | 818 | 153 | 44 | 4.64, .04 | .78, .47 | .52, .60 |
| End of Treatment (Wk 12) | 772.0 | 186 | 16 | 750 | 138.2 | 13 | 828.7 | 180.1 | 15 | 784.8 | 170.5 | 44 | ||||
| Drug Stroop: Mean RT for Correct Drug Trials | Baseline (Wk 0) | 789.5 | 191 | 16 | 811.2 | 112.6 | 13 | 856 | 140.8 | 15 | 818.6 | 153.4 | 44 | 4.48, .04 | .79, .46 | .71, .50 |
| End of Treatment (Wk 12) | 774.3 | 184.8 | 16 | 750.3 | 139.1 | 13 | 830.1 | 179.9 | 15 | 786.2 | 170.2 | 44 | ||||
Abbreviations: RVP= Rapid Visual Information Processing Task; SST= Stop Signal Task; RT= Response Time; Wk= Week Bold indicates statistical significance (p<0.05); bold italics indicate a trend significance (p<0.10).
Analyses did not consider whether improvement on a measure of attention (RVP A’) moderated improvements in cocaine use outcomes. These analyses were not pursued due to the lack of significant treatment or group effects on cocaine use or on the hypothesized cognitive moderator (attention and signal detection as measured by RVP A’).
4.0. Discussion
4.1. Summary of Findings:
In this placebo-controlled randomized clinical trial of galantamine (8 or 16mg) in individuals with CUD, the hypotheses were largely unsupported. Treatment groups did not differ in terms of cocaine use outcomes (urine or self-report) during treatment. There was some indication of cognitive enhancing effects of the higher dose of galantamine (16mg) improving working memory and reducing a bias towards over-responding on a continuous performance type task. However, the cognitive enhancing effects were modest and limited to a subset of the measures assessed. The medication was well-tolerated, with limited reports of adverse effects and no medication-related treatment withdrawals. Taken together, these findings do not provide strong support for the efficacy of galantamine to treat CUD in this subgroup of current cocaine users with no co-morbid illicit substance use disorders.
4.2. Cocaine Use Outcomes: Consistency with prior findings
The current study built off prior findings which had shown efficacy of galantamine in reducing cocaine use (Carroll et al., 2018; M. Sofuoglu & Carroll, 2011) and improving cognitive function, including attention (M. Sofuoglu et al., 2011). The inconsistencies between the current findings and prior positive reports may relate to important differences in the clinical populations targeted within each study. Two prior studies, an RCT (N=120; (Carroll et al., 2018) and a small pilot (M. Sofuoglu & Carroll, 2011), found galantamine reduced cocaine use significantly more than placebo. While the treatment regime was similar (e.g., in the RCT: 8mg galantamine, extended-release formulation, offer of platform behavioral treatment (counseling sessions), 12-week treatment protocol), both of the prior studies included individuals who were stabilized on methadone throughout the trial, as a treatment for their co-morbid opioid use disorder. In fact, galantamine not only reduced cocaine use, but also significantly reduced opioid use in the RCT sample (Carroll, DeVito, Yip, Nich, & Sofuoglu, in press (2019)). In contrast, in the current study, in which cocaine use was not affected by galantamine, not only was co-morbid substance use disorder (other than tobacco) an exclusion criteria, opioid use was extremely low in this sample, other drug and alcohol use rates were also low in this sample, and rates of other drug use did not change differentially by treatment condition. This raises the possibility that galantamine’s efficacy in reducing cocaine use may be either restricted to CUD with co-morbid opioid use disorders who are receiving methadone, or may be indirectly related to galantamine’s efficacy in reducing opioid use in this co-morbid population. Additional research into galantamine’s efficacy in reducing opioid use in individuals with and without co-morbid CUD is warranted.
4.3. Cognitive Outcomes: Consistency with prior findings
The cognitive findings were mixed. While the primary cognitive outcome (i.e., attention, as measured by RVP A’) did not show the hypothesized cognitive enhancing effect of galantamine, other cognitive measures- including working memory (digit span backwards) did show modest cognitive enhancing effects from the higher dose (16mg) of galantamine. One prior placebo-controlled trial in currently abstinent individuals with past CUD (and no current other substance use disorders except tobacco) had shown cognitive enhancing effects of galantamine (8mg/day) on several RVP measures, including RVP A’ (i.e., signal detection), but did not include a measure of working memory (M. Sofuoglu et al., 2011). In contrast, the RCT in methadone-maintained current CUD did not show any significant cognitive enhancing effects of the lower dose of galantamine (8mg)- including on working memory or RVP measures, despite improved cocaine use outcomes with galantamine (Carroll et al., 2018). Given the possibility for intermittent cocaine use and withdrawal to impact cognitive function, galantamine may have differential cognitive enhancing effects in those with current CUD versus those who are in prolonged abstinence with past CUD. Although they were modest, the effects of galantamine on working memory at the higher galantamine dose (16mg) in the current study with active cocaine users, and improvement in attention with a lower dose (8mg) in a past study in abstinent CUD suggest that there may be a role for galantamine as a cognitive enhancer in some CUD subgroups. Furthermore, this pattern of findings could be consistent with higher galantamine doses showing greater cognitive enhancing efficacy (relative to lower doses) in more clinically severe individuals (e.g., current cocaine users), while lower doses may be sufficient in less clinically severe groups (e.g., individuals with CUD who are currently maintaining abstinence); a pattern seen with galantamine’s efficacy in individuals with mild versus moderate Alzheimer’s disease (Aronson, Van Baelen, Kavanagh, & Schwalen, 2009). If so, the lack of cognitive enhancing effects of the 8mg dose in the prior study with a clinically severe sample (i.e., active cocaine users with co-morbid opioid use disorder) may have reflected an insufficient dose to improve cognitive outcomes. As galantamine’s effects on cognition and cocaine use outcomes appears dissociable, that also raises the possibility that the optimal galantamine dose for each clinical sample may also differ based on the primary outcome target: cognitive improvement or reduced cocaine use. Importantly, taken together, these current and prior findings suggest that cognitive enhancement with galantamine is neither necessary nor sufficient for galantamine to improve cocaine use outcomes, and is therefore unlikely to be the key factor moderating galantamine’s ability to reduce cocaine use. However, galantamine’s cognitive enhancing effects may have value as an adjunct treatment in the context of other cognitive or behavioral treatments. For example, working memory training may improve cocaine use outcomes(Schulte et al., 2018), so future research may consider whether cognitive enhancement with galantamine may facilitate working memory improvements above working memory training alone, to further improve cocaine use outcomes.
4.4. Conclusions
Findings did not support galantamine as a treatment for reducing cocaine use in the subgroup of individuals with current CUD who do not have co-morbid opioid use disorder and who are not receiving methadone. Galantamine may have modest efficacy as a cognitive enhancer in this subgroup of individuals with CUD.
Supplementary Material
Highlights.
No pharmacotherapies are approved for use in cocaine use disorder (CUD)
13-week randomized controlled trial of galantamine outpatients with CUD
Galantamine (8 or 16mg/day) was not superior to placebo for cocaine use outcomes
Galantamine at 16mg/day was associated with modest cognitive improvements
Though well-tolerated, galantamine did not demonstrate efficacy as CUD treatment
ACKNOWLEDGEMENTS:
We thank Stacy Minnix, Ellen Mitchell, Lance Barnes, Christopher Cryan for their contributions to data collection and data management.
FUNDING: New England Mental Illness Research Education and Clinical Center (MIRECC), R01 DA029577, P50-DA09241 and R21/33 DA041661. DISCLOSURES: Nothing to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: Nothing to declare
References
- Aronson S, Van Baelen B, Kavanagh S, & Schwalen S (2009). Optimal dosing of galantamine in patients with mild or moderate Alzheimer’s disease: post Hoc analysis of a randomized, double-blind, placebo-controlled trial. Drugs Aging, 26(3), 231–239. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, & Schmidt HD (2016). Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry, 6, e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Conley RR, Dickinson D, Ball MP, Feldman S, Gold JM, & McMahon RP (2008). Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry, 165(1), 82–89. [DOI] [PubMed] [Google Scholar]
- Carroll KM, DeVito EE, Yip SW, Nich C, & Sofuoglu M (in press (2019)). Double blind placebo-controlled trial of galantamine for methadone-maintained individuals with cocaine use disorder: Secondary analysis of effects on illicit opioid use. The American Journal on Addictions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, DeVito EE, Shi JM, & Sofuoglu M (2018). Galantamine and Computerized Cognitive Behavioral Therapy for Cocaine Dependence: A Randomized Clinical Trial. J Clin Psychiatry, 79(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Kiluk BD, Nich C, Mouratidis M, & Carroll KM (2018). Drug Stroop: Mechanisms of response to computerized cognitive behavioral therapy for cocaine dependence in a randomized clinical trial. Drug Alcohol Depend, 183, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Nakovics H, C. B, Smolka MN, Batra A, & Mann K (2006). Galantamine reduces smoking in alcohol-dependent patients: A randomized, placebo controlled trial. International Journal of Clinical Pharmacology & Therapeutics, 44(12), 614–622. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav, 3(3–4), 235–241. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1996). Structured clinical interview for DSM-IV Axis I disorders patient edition. [DOI] [PubMed]
- Giacobini E (2004). Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol Res, 50(4), 433–440. [DOI] [PubMed] [Google Scholar]
- Grasing K (2016). A threshold model for opposing actions of acetylcholine on reward behavior: Molecular mechanisms and implications for treatment of substance abuse disorders. Behav Brain Res, 312, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasing K, Yang Y, & He S (2011). Reversible and persistent decreases in cocaine self-administration after cholinesterase inhibition: different effects of donepezil and rivastigmine. Behavioural pharmacology, 22(1), 58–70. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, & Nakanishi S (2003). Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A, 100(10), 6169–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, & Rada P (2007). Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol, 7(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, & Wu LT (2017). Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend, 180, 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, & Epperson LE (1998). Reliability and validity of the cocaine selective severity assessment [In Process Citation]. Addict Behav, 23(4), 449–461. [DOI] [PubMed] [Google Scholar]
- Levine J, & Schooler N (1986). SAFTEE: A technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin, 343–381. [PubMed] [Google Scholar]
- Liu H, Lai M, Zhou X, Zhu H, Liu Y, Sun A, … Zhou W (2012). Galantamine attenuates the heroin seeking behaviors induced by cues after prolonged withdrawal in rats. Neuropharmacology, 62(8), 2515–2521. [DOI] [PubMed] [Google Scholar]
- MacLean RR, Waters AJ, Brede E, & Sofuoglu M (2018). Effects of galantamine on smoking behavior and cognitive performance in treatment-seeking smokers prior to a quit attempt. Hum Psychopharmacol, 33(4), e2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, … Li R (2006). Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry, 63(2), 201–208. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, … Li R (2005). Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry, 62(10), 1148–1156. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, & Sofuoglu M (2007). Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse, 33(2), 191–206. [DOI] [PubMed] [Google Scholar]
- Reeves D, Schlege R, & Gilliland K (1991). The UTCPAB and the NATO AGARD STRES Battery: Results from standardization studies. Medical Defense Biosciences Review. [Google Scholar]
- Reeves D, Winter K, Kane R, Elsmore T, & Bleiberg J (2002). ANAM 2001’s User’s Manual. National Cognitive Recovery Foundation. [Google Scholar]
- Robinson DM, & Plosker GL (2006). Galantamine extended release. CNS drugs, 20(8), 673–681; discussion 682–673. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, & Berry AS (2014). Deterministic functions of cortical acetylcholine. European Journal of Neuroscience, 39(11), 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Ivanov VB, Wiker C, & Svensson TH (2007). Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology, 32(1), 43–53. [DOI] [PubMed] [Google Scholar]
- Schulte MHJ, Wiers RW, Boendermaker WJ, Goudriaan AE, van den Brink W, van Deursen DS, … Waters AJ (2018). The effect of N-acetylcysteine and working memory training on cocaine use, craving and inhibition in regular cocaine users: correspondence of lab assessments and Ecological Momentary Assessment. Addict Behav, 79, 24–31. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M (2010). Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction, 105(1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, & Carroll KM (2011). Effects of galantamine on cocaine use in chronic cocaine users. Am J Addict, 20(3), 302–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, & Carroll KM (2013). Cognitive enhancement as a treatment for drug addictions. Neuropharmacology, 64, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, & Carroll KM (2016). Cognitive Function as a Transdiagnostic Treatment Target in Stimulant Use Disorders. J Dual Diagn, 12(1), 90–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, & Mooney M (2009). Cholinergic functioning in stimulant addiction. CNS drugs, 23(11), 939–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Poling J, & Carroll KM (2011). Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol, 19(1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, & Schuster CR (1973). Cholinergic influence on intravenous cocaine self-administration by rhesus monkeys. Pharmacology Biochemistry and Behavior, 1(6), 643–649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.