ABSTRACT
As with all glial cells, the major role of retinal Müller glia (MG) is to provide essential neuronal support. However, the MG of some non-mammalian species have the additional ability to generate new retinal neurons capable of sight restoration. Unfortunately, mammalian MG do not possess this ability. However, if we could understand the reasons why, we may be able to devise strategies to confer regenerative potential. The recent discovery that the Hippo signaling pathway acts as an intrinsic block to mammalian MG proliferation, along with reports of adeno-associated virus (AAV)-based MG reprogramming and functional photoreceptor differentiation, may indicate a watershed moment in the field of mammalian retinal regeneration. However, as researchers delve deeper into the cellular and molecular mechanisms, and further refine MG reprogramming strategies, we should recall past misinterpretations of data in this field and proceed with caution. Here, we provide a summary of these emerging data and a discussion of technical concerns specific to AAV-mediated reprogramming experiments that must be addressed in order for the field to move forward.
KEY WORDS: Müller glia, Hippo signaling, Cellular reprogramming, Retinal regeneration
Summary: This Spotlight discusses strategies that can be used to unlock the endogenous regenerative potential of the mammalian retina, highlighting potential technical pitfalls and important considerations.
Introduction
Retinal degenerative diseases, as well as traumatic retinal injury, result in permanent loss of retinal neurons and thus sight, depriving many worldwide of one of our most valued senses. Owing to the clinical need for sight restoration, researchers are pursuing a variety of therapeutic strategies to delay or reverse retinal damage and neuronal death. Current approaches include delivery of trophic and anti-apoptotic factors, viral-mediated gene replacement therapy, transplantation of photoreceptors (rods and cones) and retinal pigment epithelium, optogenetic prosthesis, and bionic retinal implants (Cepko, 2012; Fine et al., 2015; Langhe and Pearson, 2019). However, it has become clear that a ‘one-size-fits-all’ approach to retinal repair is not likely to succeed, and treatments will have to be tailored to a variety of disease contexts, including stage of severity and affected cell type. Moreover, although progress has been made on all these fronts, this has not yet translated into a broad-spectrum clinical intervention to cure blindness.
An alternative strategy seeks to determine whether the mammalian retina contains endogenous, but dormant, regenerative potential that might be awakened to drive tissue self-repair. Precedent for endogenous retinal repair has been clearly established for other vertebrate species, such as the zebrafish, which undergoes retinal regeneration fueled by a population of cells known as Müller glia (MG) (Fausett and Goldman, 2006). MG are the major glial cell type in the retina. They are radially oriented, extend the entire thickness of the tissue, and exhibit elaborate lateral processes contacting neighboring neurons. This unique architecture provides exquisite sensitivity to changes in the retinal environment and allows MG to maintain retinal homeostasis and provide neural protection (Vecino et al., 2016). Although the homeostatic roles of zebrafish MG are similar to those performed by mammalian MG, zebrafish MG have the additional ability to enter the cell cycle in response to retinal damage and divide asymmetrically to self-renew and produce a single progenitor-like cell (Fig. 1). This multipotent daughter then clonally expands to generate a pool of cells capable of differentiation into new retinal neurons, including photoreceptors (Fausett and Goldman, 2006; Goldman, 2014). Unfortunately, mammalian MG have lost this ability, or it is actively suppressed.
Fig. 1.
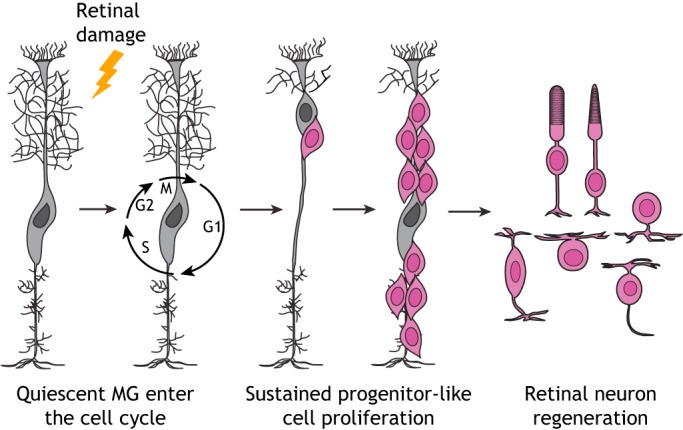
Zebrafish Müller glia-mediated retinal regeneration. In response to retinal damage, quiescent zebrafish Müller glia (MG) enter the cell cycle and divide asymmetrically to self-renew and produce a single progenitor-like cell (pink). This multipotent daughter then clonally expands to generate a pool of cells capable of differentiation into new retinal neurons including photoreceptors.
Recent studies from our labs, and the lab of Dr Muriel Perron, have identified the Hippo pathway as an essential molecular mechanism that blocks mammalian MG cell cycle entry and reprogramming to a progenitor-like cellular state (Hamon et al., 2019; Rueda et al., 2019). Here, we highlight these data and discuss the advantages of developing additional strategies to unlock the endogenous regenerative potential of the mammalian retina. We also discuss specific experimental pitfalls that should be considered and avoided as the field moves toward more translational experiments.
Extrinsic therapeutic strategies for mammalian retinal repair
The mammalian retina is not required for viability. It is also highly accessible for surgery, has a relatively simple anatomy, and benefits from straightforward functional and histological characterization. These advantages make it highly amenable to developing exogenous therapeutic approaches that, for several decades, have been the main thrust of studies aimed at treating retinal degenerative disease and injury. One of the more broadly applicable approaches is to deliver neurotrophic, neuroprotective, anti-apoptotic, anti-inflammatory and anti-angiogenic factors aimed at delaying or even halting the loss of diseased retinal cells (Fortuny and Flannery, 2018). Although these methods may eventually develop into an effective therapy, they do not directly treat the primary cause of retinal degeneration.
For a more direct treatment, adeno-associated viral (AAV) gene therapy holds tremendous promise. This new advancement is best illustrated by the FDA approval of the AAV-based therapy Luxturna in 2018. Luxturna is a therapy that involves AAV-mediated delivery of the RPE65 gene to the retinal pigment epithelium (Russell et al., 2017) replacing the biallelic mutant variants of RPE65 in patients with Leber's congenital amaurosis and retinitis pigmentosa (Trapani and Auricchio, 2018). Although extremely encouraging, AAV-mediated gene replacement therapy requires that a significant number of targetable, mutant photoreceptors are still present in the patient prior to treatment. Therefore, patients with significantly progressed disease are not likely to benefit. Furthermore, AAV gene replacement also requires that the causative mutation is known. Such an approach will not address more-complex cases, such as traumatic injury, or age-related macular degeneration and glaucoma, which have an unclear genetic etiology.
Another active area of research on retinal repair is cell replacement to treat mutated, damaged or lost photoreceptors. Over the last two decades, transplantation studies employing a wide variety of donor cell types, such as primary photoreceptors, retinal pigment epithelium (RPE) and retinal progenitor cells (RPCs), have been performed (Santos-Ferreira et al., 2016b). With the exception of RPE (Jin et al., 2019; Mandai et al., 2017), most cell types have shown little efficacy in terms of vision improvement in retinal degeneration mouse models, or clear signs of robust integration into the retina. In 2006, an apparent breakthrough occurred with the report that transplanted immature rod precursors exhibit efficient rod differentiation and synaptogenesis, host integration, and improvement of vision in mouse models of retinal degeneration (MacLaren et al., 2006). Over the following 10 years, much effort was spent expanding upon this initial finding (Barber et al., 2013; Pearson et al., 2012; Santos-Ferreira et al., 2015; Singh et al., 2013) and developing culture methods to derive large numbers of transplantable photoreceptor precursors from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Eiraku et al., 2011; Nakano et al., 2012). Retinal degeneration mutants injected with these ESC- and iPSC-derived photoreceptor precursors were also reported to exhibit improved retinal function along with donor photoreceptor integration (Assawachananont et al., 2014; Decembrini et al., 2014; Gonzalez-Cordero et al., 2013; Homma et al., 2013). With the development of human ESC- and iPSC-derived photoreceptor precursors, the field seemed poised for more translational experiments and clinical trials. However, in 2016, a red herring was exposed. Several independent reports emerged indicating that the vast majority of transplanted photoreceptor precursors actually do not integrate into the host retina. Rather, these donor cells participate in material exchange (likely RNA and/or protein) with host photoreceptors, including the donor cells' fluorescent protein label (Decembrini et al., 2017; Ortin-Martinez et al., 2017; Pearson et al., 2016; Santos-Ferreira et al., 2016a; Singh et al., 2016). Despite this apparent lack of donor photoreceptor integration, it is important to note that some functional improvement was reported for mouse retinal degeneration mutants. Therefore, donor cell material exchange may provide a therapeutic factor (or factors) that benefits the diseased host photoreceptors, and current research is focused on elucidating the mechanism underlying such material exchange.
Strategies for intrinsic mammalian retinal repair: MG-mediated retinal regeneration
Retinal cell transplantation was originally conceived as a potential therapy for end-stage retinal degenerative disease, when few photoreceptors are still present. However, the discovery of photoreceptor material exchange, without donor cell integration, has shifted this focus to something more akin to neurotropic/protective protein delivery or broad-spectrum gene therapy for diseased retinae that still contain significant numbers of photoreceptors. Thus, for cases of severe retinal degeneration or trauma, investigation into photoreceptor replacement from an intrinsic source, such as MG, is warranted.
As mentioned above, although zebrafish MG exhibit remarkable regenerative capacity, MG of the mammalian retina do not (Goldman, 2014). For several years now, studies of the cellular and molecular mechanisms driving zebrafish retinal regeneration have thus focused on identifying whether there are key factors present in the zebrafish, but not the mouse, retina that might confer regenerative potential. The most high-profile of these studies identified the proneural transcription factor Ascl1a as being essential for zebrafish MG-mediated retinal regeneration (Fausett et al., 2008). Ascl1 is not expressed in adult mouse MG, in either normal or damaged contexts. However, forced transgenic expression of Ascl1 in MG within the damaged retina of young mice results in the production of new retinal neurons, albeit those that are limited to bipolar and amacrine-like identities (Ueki et al., 2015). Although these results are intriguing, it is curious that this response to Ascl1 expression does not persist past 2 weeks of age (Ueki et al., 2015). A recent report (Jorstad et al., 2017) suggests this is due to limited chromatin accessibility within older MG. Here, the histone deacetylase inhibitor trichostatin-A was injected into Ascl1-overexpressing retinae and this was shown to prolong the production of new interneurons to adult stages (Jorstad et al., 2017). However, forced expression of Ascl1 did not result in significant proliferation of mouse MG, leading the authors to conclude that Ascl1 causes a direct trans-differentiation from MG to interneurons (Jorstad et al., 2017). Taken together, these studies suggest that although Acsl1 is able to promote mouse MG neuronal trans-differentiation, this process is limited in its efficiency and in the cell types produced. If we are to unlock the full regenerative potential of mammalian MG for retinal self-repair, it will be important to first define the endogenous molecular mechanisms restraining MG proliferation and reprogramming to a multipotent progenitor-like state.
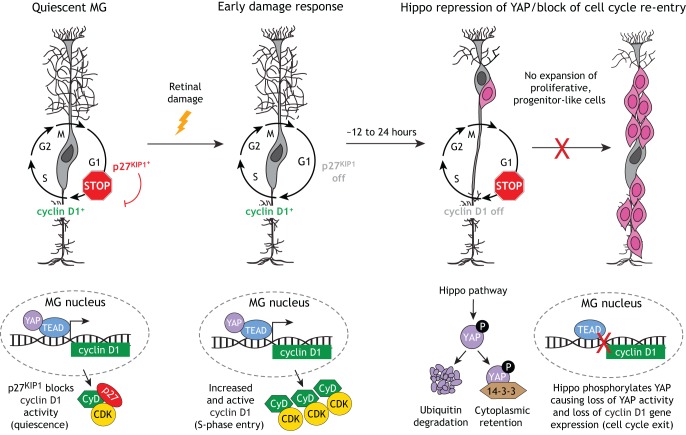
In a landmark study, quiescent adult mouse MG were shown to simultaneously express the S phase-promoting cyclin D3 protein and the cyclin kinase inhibitor p27KIP1 (also known as Cdkn1b) (Dyer and Cepko, 2000). p27KIP1 is known to inhibit the cyclin D/CDK complex to prevent S-phase entry. Co-expression of these proteins suggests that quiescent mouse MG are poised for cell cycle entry upon retinal damage. Indeed, 24 h after drug-induced retinal neuronal death, a small subset of MG enters S phase, coincident with loss of p27KIP1 expression while cyclin D3 expression persists (presumably in a de-repressed state). However, over an additional 24 h, cyclin D3 gene expression is turned off and proliferation stops. For almost two decades, the molecular mechanism suppressing sustained cyclin D3 expression and blocking MG proliferation remained unknown.
Recently, we and the lab of Dr Muriel Perron identified signaling by the Hippo pathway as an essential molecular block to sustained MG proliferation and reprogramming to a progenitor-like state (Hamon et al., 2019; Rueda et al., 2019) (Fig. 2). We determined that, within 12 h of retinal injury, mouse MG dramatically upregulate cyclin D1 gene expression (but not cyclin D3 as previously reported) coincident with S-phase entry of a subset of MG. By 48 h post-injury, cyclin D1 expression is repressed and MG do not re-enter the cell cycle (Rueda et al., 2019). Our data suggest that this rapid repression of cyclin D1 expression, and the cessation of MG proliferation, is due to Hippo pathway-mediated phosphorylation and repression of the TEAD transcriptional co-factor YAP (YAP1), which is a direct regulator of cyclin D1 transcription. We further showed that genetically bypassing this mechanism, either by Hippo loss of function or through transgenic expression of a phospho-deficient variant of YAP (YAP5SA), results in spontaneous and robust MG proliferation and reprogramming to a progenitor-like state (Rueda et al., 2019). In addition to these findings, the rationale for further investigation of the Hippo pathway as a molecular target for retinal regeneration strategies is supported by several studies in other tissues. Modulation of Hippo pathway activity has been shown to result in increased proliferation and plasticity within the heart, intestine and liver (Moya and Halder, 2016). As in the retina, this ability seems to be driven by deregulated activity of YAP (and its paralog TAZ), leading to an increase in the expression of YAP/TAZ-TEAD target genes that are essential for proliferation, self-renewal, tissue homeostasis and repair (Yu et al., 2015).
Fig. 2.
The Hippo pathway acts as an endogenous block to sustained mammalian MG proliferation and reprogramming. MG normally express low levels of the S phase-promoting protein cyclin D1 (CyD), which is a direct transcriptional target of YAP/TEAD. However, due to co-expression of the cyclin kinase inhibitor p27KIP1, cyclin D1 activity is repressed and MG are kept in a quiescent state. Upon retinal damage, p27KIP1 expression is lost coincident with upregulation of cyclin D1 expression and activity, which triggers MG to enter the S phase. However, over an additional 12-24 h, the MG exit the cell cycle and are prevented from expanding clonally into a proliferative, progenitor-like population. This block in MG cell cycle re-entry is due to Hippo pathway-mediated phosphorylation of YAP, which leads to YAP cytoplasmic retention or ubiquitin degradation. As a consequence, YAP is kept out of the nucleus and cyclin D1 is not expressed in MG.
Beyond the core Hippo pathway players, a variety of other pathways and molecules crosstalk with the Hippo pathway and may thus influence MG-mediated retinal regeneration (Ma et al., 2019; Moya and Halder, 2019). One pathway of particular importance is the Wnt pathway, which was previously shown to be required for efficient zebrafish retinal regeneration (Meyers et al., 2012; Ramachandran et al., 2011). Interestingly, recent reports suggest that β-catenin (Ctnnb1), the transcriptional effector of canonical Wnt signaling, plays a role in MG reprogramming in mice (Yao et al., 2016). When AAV is used to drive Ctnnb1 expression in adult mouse MG, these cells undergo spontaneous cell cycle entry in uninjured retinae in a fashion similar to, but not as robust as, YAP5SA transgenic MG (Rueda et al., 2019; Yao et al., 2016). Remarkably, when the same β-catenin-expressing cells are subsequently infected with AAV driving the rod-specifying factors Otx2, Crx and Nrl, they differentiate into rods capable of vision restoration in a mouse model of congenital blindness (Yao et al., 2018). Whether the Hippo and Wnt pathways crosstalk to block MG-mediated retinal regeneration remains to be experimentally determined. The interaction between the Hippo and Wnt pathways is complex and involves context-dependent, positive and negative interactions (Wang and Martin, 2017). However, considering the similarities between the effects of forced expression of YAP5SA and β-catenin on MG proliferation, it is likely that YAP and β-catenin cooperatively regulate a cohort of genes required for MG reprogramming (Yao et al., 2018). Consistent with this idea, we and others have reported that the YAP/TAZ/TEAD complex interacts with the TCF/LEF/β-catenin complex to activate a common gene regulatory network (Heallen et al., 2011).
Besides crosstalk with other signaling pathways, it is currently unclear which cue or cues are generated by retinal damage to then influence Hippo pathway-dependent YAP activity. Also, other than cyclin D1, we do not yet have a clear picture of the transcriptional targets directly downstream of YAP that drive MG proliferation and reprogramming. By answering these questions, we may be able to further refine MG reprogramming strategies. We also do not yet know the extent to which YAP5SA-reprogrammed MG resemble multipotent RPCs capable of generating all retinal cell types. Once induced, transgenic YAP5SA is driven by a ubiquitous promoter (Monroe et al., 2019). Therefore, most of the YAP5SA-reprogrammed MG are likely held in a progenitor-like state, making it difficult to assess whether these cells can differentiate into new retinal neurons. Thus, future efforts will be aimed at establishing reversible control over YAP5SA expression.
Targeting the Hippo pathway for retinal regenerative medicine: challenges and concerns
Now that the Hippo pathway has been identified as an endogenous, negative regulator of mammalian MG proliferation and cellular reprogramming, we have a new molecular entry point from which to further develop strategies to promote MG-mediated retinal regeneration. However, inducing the mammalian retina to undergo true regeneration to produce functional retinal neurons is unlikely to be accomplished by bypassing one signaling pathway. Studies of zebrafish retinal regeneration will likely continue to shed light on additional requirements, some of which will likely crosstalk with the Hippo pathway, that may then be applied to the mouse. Candidates for such factors include the PI3K-AKT-mTOR, JAK-STAT and MAPK-ERK pathways, as well as EGF signaling (Hamon et al., 2019; Rueda et al., 2019; Wan and Goldman, 2016). It will also be interesting to determine whether differential regulation of yap1 occurs in zebrafish MG compared to mice.
As researchers begin to delve deeper into YAP- and/or β-catenin-mediated mouse MG reprogramming (Hamon et al., 2019; Rueda et al., 2019; Yao et al., 2016, 2018), we recommend that it would be wise to remember the decade-long misinterpretation of retinal cell transplantations as a cautionary tale (Nickerson et al., 2018). Irrespective of whether the Hippo or Wnt pathways are being targeted, any strategy aimed at awakening the regenerative potential of resident MG comes with a specific set of practical matters and technical issues that we must address. Below, we highlight some of the most pressing concerns for the field going forward.
The need for precise and transient expression of MG reprogramming factors and directed neuron differentiation
MG serve a variety of essential roles to preserve retinal homeostasis, including the maintenance of retinal lamination (Vecino et al., 2016). Therefore, if the majority of MG in the retina are reprogrammed, the retina could lose homeostatic support, leading to further damage and degeneration. In fact, during zebrafish retinal regeneration, only a subset of MG normally enter the cell cycle and subsequently differentiate into new retinal neurons (Fausett and Goldman, 2006). Thus, any devised mammalian MG reprogramming strategy should take a cue from this system and ensure that only a limited number of MG are induced to proliferate. As a first step, we must precisely determine what percentage of the retina's MG population can proliferate before homeostasis is compromised and neurons are lost. Then, we must develop methods for rapid, but transient, bypass of the Hippo pathway or any other molecular target.
Once we have achieved temporally and spatially controlled reprogramming of MG to a progenitor-like state, there is no guarantee that these cells will spontaneously differentiate into the desired (missing) retinal cell types or do so in sufficient quantities. In this case, a two-step AAV reprogramming approach, similar to that previously reported (Yao et al., 2018), may be needed. As AAV is FDA-approved for retinal gene replacement, it is likely that this method will garner much attention as a possible delivery system for MG-reprogramming factors. Therefore, we next discuss specific considerations that should be addressed when designing AAV reprogramming experiments and interpreting the results.
AAV-mediated MG reprogramming: proceed with caution…and the proper controls
Previously, a variant of AAV6 called ShH10 was reported to have very high tropism for adult, rodent MG, but to also infect a subset of NeuN (Rbfox3)+ ganglion and amacrine cells (Byrne et al., 2013; Klimczak et al., 2009; Yao et al., 2016). To add an extra level of specificity to ShH10, a Gfap promoter was used to drive Ctnnb1 in MG, and it was shown that these MG go on to proliferate spontaneously (Yao et al., 2016, 2018). The authors reported that ShH10 Gfap promoter-driven expression is highly selective for MG but did not show the quantified data. However, this is an extremely important control for any MG-reprogramming experiment as any expression outside of MG could lead to a misinterpretation of results. Although there are many reports in the literature indicating Gfap promoter glial specificity, significant discrepancies also exist that indicate neuronal expression (Lee et al., 2008; Su et al., 2004). The use of any promoter in AAV gene delivery requires careful consideration of not only the cell of interest but also the experimental design. In the case of the Gfap promoter, attached sequences (such as GFP versus lacZ) can significantly affect cell specificity (Lee et al., 2008; Su et al., 2004). Thus, for any given AAV construct utilizing a specific promoter element, it is incumbent upon the researcher to design experiments that account for this potential pitfall.
Because no AAV serotype or promoter can be necessarily considered 100% specific to any cell type in any context (such as damage), we should embed multi-layered lineage-tracing techniques within AAV-mediated MG-reprogramming experiments. One obvious control is to implement Cre or Flp recombinase-based fate mapping. The ROSA26R-nTnG dual Cre reporter, for example, could allow the tracking of single cells. In this line, nuclear-localized tdTomato is expressed ubiquitously but cells then express a nuclear GFP upon Cre recombination (http://www.informatics.jax.org/allele/MGI:5504463). However, because it is currently unclear whether material exchange occurs between resident retinal cells, Cre or Flp fluorescent reporter data on its own should not be considered as a definitive readout of cell lineage (Boudreau-Pinsonneault and Cayouette, 2018). It is absolutely necessary to include labeling with 5-ethynyl-2′-deoxyuridine (EdU) or other thymidine analogs and Ki67 (Mki67) immunofluorescence in MG fate-mapping experiments. If one observes Cre reporter-labeled (GFP+), post-mitotic neurons that are also EdU+, but Ki67−, this would be a strong indication that they are derived from reprogrammed MG that proliferated, subsequently exited the cell, and re-differentiated into neurons. Alternatively, one may employ an EdU/5-bromo-2′-deoxyuridine (BrdU) pulse-chase experiment to assess cell cycle exit. Finally, an often-overlooked control is to perform Cre immunofluorescence to ensure that any Cre reporter labeling is not due to leaky AAV expression in neurons or material exchange of Cre from neighboring cells.
The reported success of two-step AAV-mediated MG reprogramming to functional rods (Yao et al., 2018) will likely lead to further investment in this strategy for the production of cones and ganglion cells. As we proceed with additional studies, there are several important technical questions that need to be answered. It was previously shown that when the Gfap promoter is used to drive Ctnnb1, MG enter a proliferative state (Yao et al., 2018). When these proliferative MG are subsequently infected with Gfap promoter-driven Otx2, Crx and Nrl, they were reported to then differentiate into functional rods. But what is the identity of the β-catenin-expressing MG prior to rod induction? Are they reprogrammed to an RPC-like state, which is now able to undergo neurogenesis? Questions such as these are extremely important and should be addressed through single cell mRNA sequencing (scRNA-seq) followed by comparisons with scRNA-seq data from endogenous RPCs. Nevertheless, even if β-catenin-expressing MG are indeed reprogrammed to an RPC-like state, it is curious that the Gfap promoter is reported to still be active in these cells (Yao et al., 2018). These data would seem to indicate that MG reprogrammed by β-catenin still retain some level of MG identity or that the Gfap promoter may not be entirely specific to MG. These data also contrast with our own immunofluorescence and scRNA-seq data indicating that YAP5SA-reprogrammed, proliferative MG lose MG identity, including a complete loss of Gfap expression (Rueda et al., 2019). Also, because re-infection of a retinal cell with an AAV of the same serotype is a primary feature of the two-step reprogramming strategy, the efficiency of this event should be quantified. Despite the known immune privilege of the mammalian retina, it is formally possible that MG may develop immunity to a specific AAV, making re-infection less likely. This potential technical limitation may be particularly true in the context of the damaged or diseased retina. To the best of our knowledge, these questions remain unanswered, but further exploring them will likely result in more effective and specific delivery of reprogramming factors.
Choosing the most appropriate models
Finally, the true test of successful MG-mediated retinal regeneration is to demonstrate functional recovery in a mouse model of retinal degeneration. Here, it is absolutely crucial to choose the correct disease scenario. Cell-based therapies, such as MG reprogramming, aim to replace photoreceptors lost to disease. Therefore, we should utilize mouse models of photoreceptor dystrophy. In mouse models such as the Gnat1/Gnat2 mutants, in which mutant rods do not exhibit significant degeneration, phenotypic improvement may be difficult to interpret (Calvert et al., 2000; Chang et al., 2006). Any ‘rescue effect’ attributed to genetic modification followed by reprogramming of MG might actually be due to material transfer, or non-specific AAV expression (gene therapy) in pre-existing mutant rods. These potential issues are precisely why carefully controlled cell lineage-tracing experiments must be employed. For example, if a thymidine analog such as EdU is used to label proliferative, reprogrammed MG, prior to cell cycle exit and re-differentiation into rods, the expected readout would be the appearance of EdU+ rods indicating bona fide MG-mediated retinal regeneration.
Concluding remarks
With the identification of the Hippo pathway, and possibly the Wnt pathway, as endogenous regulators of mammalian MG proliferation and reprogramming, the stage is set for a new and exciting era in the field of retinal regenerative medicine. Utilizing methods such as AAV to genetically manipulate these pathways may drive significant progress toward reprogramming strategies, resulting in bona fide MG-mediated retinal regeneration and, hence, functional recovery of vision. However, we must not lose sight of the complexities in interpreting retinal fate-mapping data, and we must employ the proper, multi-layered controls before concluding that a particular neuron is derived from a regenerative MG cell. As a final note, we would like to call for continued data and reagent sharing to aid in driving the field forward. Transcriptomic and epigenomic methods are now providing unprecedented resolution of cellular reprogramming. Therefore, it is essential that these data be made available through repositories such as the NCBI's Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo) as well as other user-friendly interfaces such as the St. Jude PeCan Data Portal (https://pecan.stjude.cloud/retinalnucleome). As more researchers employ AAV strategies to retinal reprogramming experiments, it is also essential that we provide complete transparency regarding construct design, promoter sequences and off-target effects, and make all plasmids available through repositories such as Addgene (https://www.addgene.org). Overall, we hope that these collaborative efforts will together pave the way towards robust and effective strategies to aid sight restoration.
Acknowledgements
We thank Benjamin Hall, and Drs Elda Rueda, Valerie Wallace, Phil Nickerson and Seth Blackshaw for their thoughtful critiques of our manuscript. We sincerely apologize for references that were omitted owing to space constraints.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health (NIH) [R01 EY024906 and R01 EY030448, to R.A.P.; R01 HL127717, R01 HL118761 and R01 HL130804 to J.F.M.], a Vivian L. Smith Foundation and MacDonald Research Fund award [16RDM001 J.F.M.], and a BrightFocus Foundation Macular Degeneration Research Grant [R.A.P.]. Deposited in PMC for release after 12 months.
References
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y. and Takahashi M. (2014). Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports 2, 662-674. 10.1016/j.stemcr.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. C., Hippert C., Duran Y., West E. L., Bainbridge J. W., Warre-Cornish K., Luhmann U. F., Lakowski J., Sowden J. C., Ali R. R. et al. (2013). Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 110, 354-359. 10.1073/pnas.1212677110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau-Pinsonneault C. and Cayouette M. (2018). Cell lineage tracing in the retina: could material transfer distort conclusions? Dev. Dyn. 247, 10-17. 10.1002/dvdy.24535 [DOI] [PubMed] [Google Scholar]
- Byrne L. C., Khalid F., Lee T., Zin E. A., Greenberg K. P., Visel M., Schaffer D. V. and Flannery J. G. (2013). AAV-mediated, optogenetic ablation of Muller Glia leads to structural and functional changes in the mouse retina. PLoS ONE 8, e76075 10.1371/journal.pone.0076075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert P. D., Krasnoperova N. V., Lyubarsky A. L., Isayama T., Nicolo M., Kosaras B., Wong G., Gannon K. S., Margolskee R. F., Sidman R. L. et al. (2000). Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl. Acad. Sci. USA 97, 13913-13918. 10.1073/pnas.250478897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L. (2012). Emerging gene therapies for retinal degenerations. J. Neurosci. 32, 6415-6420. 10.1523/JNEUROSCI.0295-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B., Dacey M. S., Hawes N. L., Hitchcock P. F., Milam A. H., Atmaca-Sonmez P., Nusinowitz S. and Heckenlively J. R. (2006). Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest. Ophthalmol. Vis. Sci. 47, 5017-5021. 10.1167/iovs.05-1468 [DOI] [PubMed] [Google Scholar]
- Decembrini S., Koch U., Radtke F., Moulin A. and Arsenijevic Y. (2014). Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports 2, 853-865. 10.1016/j.stemcr.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S., Martin C., Sennlaub F., Chemtob S., Biel M., Samardzija M., Moulin A., Behar-Cohen F. and Arsenijevic Y. (2017). Cone genesis tracing by the Chrnb4-EGFP mouse line: evidences of cellular material fusion after cone precursor transplantation. Mol. Ther. 25, 634-653. 10.1016/j.ymthe.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M. A. and Cepko C. L. (2000). Control of Muller glial cell proliferation and activation following retinal injury. Nat. Neurosci. 3, 873-880. 10.1038/78774 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T. and Sasai Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Fausett B. V. and Goldman D. (2006). A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 26, 6303-6313. 10.1523/JNEUROSCI.0332-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett B. V., Gumerson J. D. and Goldman D. (2008). The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 28, 1109-1117. 10.1523/JNEUROSCI.4853-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine I., Cepko C. L. and Landy M. S. (2015). Vision research special issue: sight restoration: prosthetics, optogenetics and gene therapy. Vision Res. 111, 115-123. 10.1016/j.visres.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Fortuny C. and Flannery J. G. (2018). Mutation-independent gene therapies for rod-cone dystrophies. Adv. Exp. Med. Biol. 1074, 75-81. 10.1007/978-3-319-75402-4_10 [DOI] [PubMed] [Google Scholar]
- Goldman D. (2014). Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 15, 431-442. 10.1038/nrn3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cordero A., West E. L., Pearson R. A., Duran Y., Carvalho L. S., Chu C. J., Naeem A., Blackford S. J. I., Georgiadis A., Lakowski J. et al. (2013). Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 31, 741-747. 10.1038/nbt.2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon A., Garcia-Garcia D., Ail D., Bitard J., Chesneau A., Dalkara D., Locker M., Roger J. E. and Perron M. (2019). Linking YAP to muller glia quiescence exit in the degenerative retina. Cell Rep 27, 1712-1725.e1716. 10.1016/j.celrep.2019.04.045 [DOI] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L. and Martin J. F. (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458-461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K., Okamoto S., Mandai M., Gotoh N., Rajasimha H. K., Chang Y.-S., Chen S., Li W., Cogliati T., Swaroop A. et al. (2013). Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors. Stem Cells 31, 1149-1159. 10.1002/stem.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.-B., Gao M.-L., Deng W.-L., Wu K.-C., Sugita S., Mandai M. and Takahashi M. (2019). Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 69, 38-56. 10.1016/j.preteyeres.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Jorstad N. L., Wilken M. S., Grimes W. N., Wohl S. G., VandenBosch L. S., Yoshimatsu T., Wong R. O., Rieke F. and Reh T. A. (2017). Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 548, 103-107. 10.1038/nature23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak R. R., Koerber J. T., Dalkara D., Flannery J. G. and Schaffer D. V. (2009). A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PLoS ONE 4, e7467 10.1371/journal.pone.0007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhe R. and Pearson R. A. (2019). Rebuilding the retina: prospects for muller glial-mediated self-repair. Curr. Eye Res. 10.1080/02713683.2019.1669665 [DOI] [PubMed] [Google Scholar]
- Lee Y., Messing A., Su M. and Brenner M. (2008). GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481-493. 10.1002/glia.20622 [DOI] [PubMed] [Google Scholar]
- Ma S., Meng Z., Chen R. and Guan K.-L. (2019). The hippo pathway: biology and pathophysiology. Annu. Rev. Biochem. 88, 577-604. 10.1146/annurev-biochem-013118-111829 [DOI] [PubMed] [Google Scholar]
- MacLaren R. E., Pearson R. A., MacNeil A., Douglas R. H., Salt T. E., Akimoto M., Swaroop A., Sowden J. C. and Ali R. R. (2006). Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203-207. 10.1038/nature05161 [DOI] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. et al. (2017). Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038-1046. 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Meyers J. R., Hu L., Moses A., Kaboli K., Papandrea A. and Raymond P. A. (2012). beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 7, 30 10.1186/1749-8104-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. O., Hill M. C., Morikawa Y., Leach J. P., Heallen T., Cao S., Krijger P. H. L., de Laat W., Wehrens X. H. T., Rodney G. G. et al. (2019). YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in Vivo. Dev. Cell 48, 765-779.e767. 10.1016/j.devcel.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya I. M. and Halder G. (2016). The Hippo pathway in cellular reprogramming and regeneration of different organs. Curr. Opin. Cell Biol. 43, 62-68. 10.1016/j.ceb.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Moya I. M. and Halder G. (2019). Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211-226. 10.1038/s41580-018-0086-y [DOI] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M. and Sasai Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771-785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nickerson P. E. B., Ortin-Martinez A. and Wallace V. A. (2018). Material exchange in photoreceptor transplantation: updating our understanding of donor/host communication and the future of cell engraftment science. Front. Neural Circuits 12, 17 10.3389/fncir.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A., Tsai E. L. S., Nickerson P. E., Bergeret M., Lu Y., Smiley S., Comanita L. and Wallace V. A. (2017). A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells 35, 932-939. 10.1002/stem.2552 [DOI] [PubMed] [Google Scholar]
- Pearson R. A., Barber A. C., Rizzi M., Hippert C., Xue T., West E. L., Duran Y., Smith A. J., Chuang J. Z., Azam S. A. et al. (2012). Restoration of vision after transplantation of photoreceptors. Nature 485, 99-103. 10.1038/nature10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. A., Gonzalez-Cordero A., West E. L., Ribeiro J. R., Aghaizu N., Goh D., Sampson R. D., Georgiadis A., Waldron P. V., Duran Y. et al. (2016). Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 7, 13029 10.1038/ncomms13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Zhao X.-F. and Goldman D. (2011). Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc. Natl. Acad. Sci. USA 108, 15858-15863. 10.1073/pnas.1107220108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda E. M., Hall B. M., Hill M. C., Swinton P. G., Tong X., Martin J. F. and Poché R. A. (2019). The hippo pathway blocks mammalian retinal muller glial cell reprogramming. Cell Rep. 27, 1637-1649.e1636. 10.1016/j.celrep.2019.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S., Bennett J., Wellman J. A., Chung D. C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. et al. (2017). Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849-860. 10.1016/S0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T., Postel K., Stutzki H., Kurth T., Zeck G. and Ader M. (2015). Daylight vision repair by cell transplantation. Stem Cells 33, 79-90. 10.1002/stem.1824 [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J. and Ader M. (2016a). Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat. Commun. 7, 13028 10.1038/ncomms13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T. F., Borsch O. and Ader M. (2016b). Rebuilding the missing Part-A review on photoreceptor transplantation. Front. Syst. Neurosci. 10, 105 10.3389/fnsys.2016.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. S., Charbel Issa P., Butler R., Martin C., Lipinski D. M., Sekaran S., Barnard A. R. and MacLaren R. E. (2013). Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 110, 1101-1106. 10.1073/pnas.1119416110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. S., Balmer J., Barnard A. R., Aslam S. A., Moralli D., Green C. M., Barnea-Cramer A., Duncan I. and MacLaren R. E. (2016). Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 7, 13537 10.1038/ncomms13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Hu H., Lee Y., d'Azzo A., Messing A. and Brenner M. (2004). Expression specificity of GFAP transgenes. Neurochem. Res. 29, 2075-2093. 10.1007/s11064-004-6881-1 [DOI] [PubMed] [Google Scholar]
- Trapani I. and Auricchio A. (2018). Seeing the light after 25 years of retinal gene therapy. Trends Mol. Med. 24, 669-681. 10.1016/j.molmed.2018.06.006 [DOI] [PubMed] [Google Scholar]
- Ueki Y., Wilken M. S., Cox K. E., Chipman L., Jorstad N., Sternhagen K., Simic M., Ullom K., Nakafuku M. and Reh T. A. (2015). Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 112, 13717-13722. 10.1073/pnas.1510595112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecino E., Rodriguez F. D., Ruzafa N., Pereiro X. and Sharma S. C. (2016). Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 51, 1-40. 10.1016/j.preteyeres.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Wan J. and Goldman D. (2016). Retina regeneration in zebrafish. Curr. Opin. Genet. Dev. 40, 41-47. 10.1016/j.gde.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. and Martin J. F. (2017). Hippo pathway: an emerging regulator of craniofacial and dental development. J. Dent. Res. 96, 1229-1237. 10.1177/0022034517719886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K., Qiu S., Tian L., Snider W. D., Flannery J. G., Schaffer D. V. and Chen B. (2016). Wnt regulates proliferation and neurogenic potential of muller glial cells via a Lin28/let-7 miRNA-dependent pathway in adult mammalian retinas. Cell Rep. 17, 165-178. 10.1016/j.celrep.2016.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K., Qiu S., Wang Y. V., Park S. J. H., Mohns E. J., Mehta B., Liu X., Chang B., Zenisek D., Crair M. C. et al. (2018). Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 560, 484-488. 10.1038/s41586-018-0425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.-X., Zhao B. and Guan K.-L. (2015). Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811-828. 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]