ABSTRACT
Low-density lipoprotein (LDL) deposition, aggregation and retention in the endothelial sub-intima are critical initiating events during atherosclerosis. Macrophages digest aggregated LDL (agLDL) through a process called exophagy. High-density lipoprotein (HDL) plays an atheroprotective role, but studies attempting to exploit it therapeutically have been unsuccessful, highlighting gaps in our current understanding of HDL function. Here, we characterized the role of HDL during exophagy of agLDL. We find that atherosclerotic plaque macrophages contact agLDL and form an extracellular digestive compartment similar to that observed in vitro. During macrophage catabolism of agLDL in vitro, levels of free cholesterol in the agLDL are increased. HDL can extract free cholesterol directly from this agLDL and inhibit macrophage foam cell formation. Cholesterol-balanced hydroxypropyl-β-cyclodextrin similarly reduced macrophage cholesterol uptake and foam cell formation. Finally, we show that HDL can directly extract free cholesterol, but not cholesterol esters, from agLDL in the absence of cells. Together, these results suggest that the actions of HDL can directly extract free cholesterol from agLDL during catabolism, and provide a new context in which to view the complex relationship between HDL and atherosclerosis.
KEY WORDS: Atherosclerosis, Cholesterol, Foam cell, HDL, LDL, Macrophage
Summary: High-density lipoprotein specifically and dose dependently extracts free cholesterol from aggregated LDL in the context of atherosclerosis, adding new complexity to the role of high-density lipoprotein in foam cell formation.
INTRODUCTION
During the initiating stages of atherosclerosis, low-density lipoproteins (LDLs) percolate through the endothelium, where they bind to the sub-endothelial intima and become aggregated, oxidatively modified and retained (Tabas, 1999; Borén et al., 2000). Macrophages encountering such aggregated LDL (agLDL) form an extracellular compartment, stabilized by actin polymerization, to which lysosomal enzymes are secreted to degrade it (Tamminen et al., 1999; Grosheva et al., 2009; Haka et al., 2009). We have termed this process digestive exophagy and called the compartment formed the lysosomal synapse (LS). We have extensively characterized the LS, and a recent study found that the susceptibility of LDL to aggregate was predictive of future cardiovascular deaths, independently of other risk factors (Ruuth et al., 2018). In response to agLDL, macrophages take up free cholesterol (FC), and the FC is esterified and stored in lipid droplets. These macrophages become foam cells and die, leaving behind cholesterol and cell debris that become the necrotic core of a plaque. Like LDL, high-density lipoproteins (HDL) can bind to and transport cholesterol in the body. In addition to ABCA1- and ABCG1-mediated cholesterol efflux from cells, HDL can also accept FC from the plasma membrane of cells, and transfer occurs through aqueous diffusion (Karlin et al., 1987; Johnson et al., 1991; Lewis and Rader, 2005). Cholesterol-binding moieties such as hydroxypropyl-β-cyclodextrin (HPCD) also have the ability to remove FC from the plasma membrane of cells. Elevated HDL is associated with anti-atherogenic effects and better cardiovascular health (Lewis and Rader, 2005). Several studies have found that increasing HDL levels by using inhibitors of cholesterol ester transfer protein (CETP) failed to decrease frequency of cardiovascular events, but a study of anacetrabib, a CETP inhibitor, found somewhat lower incidence of major coronary events in patients undergoing intensive statin treatment (Group et al., 2017). These mixed results highlight significant gaps in our understanding of HDL function. Our data suggest that HDL (and HPCD) can extract FC from agLDL in a dose-dependent manner, and this likely contributes to the complex function of HDL during atherosclerosis.
RESULTS AND DISCUSSION
Macrophages use the LS to degrade agLDL in a mouse model of atherosclerosis
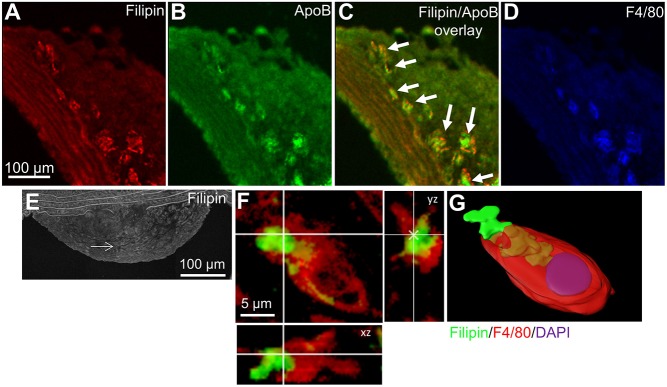
To characterize the in vivo interactions of macrophages with agLDL, we examined lesions in Apoe−/− mice. We observed extensive colocalization of FC and LDL in aortic lesions in regions containing macrophages (Fig. 1A–D). Closer examination of this revealed that plaque macrophages make an extensive invaginated structure with FC-enriched agLDL (Fig. 1E–G) that is very similar to our observations in cell culture (Singh et al., 2016). We also observed plaque macrophages forming a compartment using F-actin to interact with agLDL enriched with FC (Singh et al., 2019) (Fig. S1A–C). These data suggest that atherosclerotic plaque macrophages use a compartment similar to the LS to degrade agLDL.
Fig. 1.
Atherosclerotic plaque macrophages contact agLDL that is highly enriched in FC. (A–D) Aortic sections from hyperlipidemic Apoe−/− mice containing atherosclerotic plaque were stained for FC using filipin (red) (A), for LDL using anti-ApoB antibody (green) and for macrophages using anti-F4/80 antibody (blue) (D). An overlay of filipin and ApoB staining (C) is also shown. (E–G) Aortic sections from hyperlipidemic Apoe−/− mice containing atherosclerotic plaque (E) were stained for free cholesterol using filipin (green) and for macrophages using anti-F4/80 antibody (red). Arrows indicate the location of the macrophage shown in F and G. (F) A single slice from a confocal z-stack showing a macrophage forming a LS around free cholesterol-enriched agLDL and showing orthogonal xz and yz planes. (G) 3D reconstruction of the macrophage shown in F. The agLDL (green and tan) is contained in a surface connected compartment.
HDL can reduce cholesterol uptake during exophagy and lipid accumulation
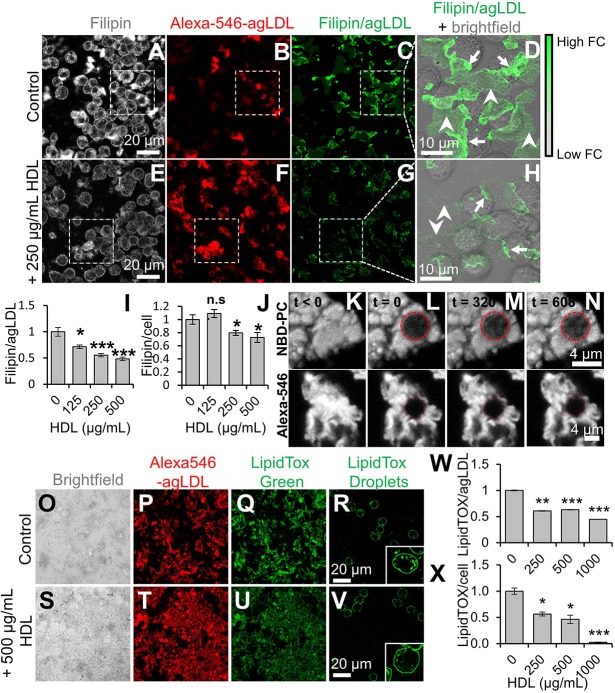
Macrophage catabolism of agLDL generates FC that can be labeled with filipin (Grosheva et al., 2009). We observed this process with and without the addition of HDL. In the absence of HDL, J774 macrophages efficiently catabolized agLDL and generated FC (Fig. 2A,B). We generated a filipin and agLDL image to highlight regions enriched in FC associated with agLDL at contact sites with macrophages (Fig. 2C,D, arrows). In the presence of 250 μg/ml HDL (Fig. 2E–H), FC enrichment was still observed at macrophage contact sites with agLDL, but it was reduced (Fig. 2H, arrows). FC in agLDL at sites away from macrophage contact areas was also reduced (Fig. 2H, arrowheads). Quantification revealed that HDL decreased FC in agLDL in a dose-dependent manner, with a 50% decrease at 500 µg/ml HDL (Fig. 2I). There was also a reduction in cell-associated filipin labeling in the presence of HDL (Fig. 2J). The LS can become acidified and hold a proton gradient, so it would be inaccessible to large molecules such as HDL (Singh et al., 2016). We hypothesized that FC might diffuse out of the LS and into regions of agLDL not in contact with macrophages, which would be accessible to HDL and that HDL might then extract. To analyze lipid and particle diffusion within agLDL, we incorporated NBD-PC (which incorporates into lipids) or Alexa-Fluor-546 (which labels ApoB protein within LDL) into agLDL and performed fluorescence recovery after photobleaching (FRAP). Immediately after photobleaching, the circular bleach region appeared to be dark compared to pre-bleaching (Fig. 2K–L). We observed this same region after 320 s (Fig. 2M) and 608 s (Fig. 2N) after photobleaching. Gradually, the NBD-PC signal was recovered in the photobleached area, showing that a lipid was mobile within agLDL. By contrast, the Alexa-Fluor-546 signal was not recovered, showing that individual LDL particles within agLDL are not mobile. Therefore, it seems likely that FC observed at sites not in contact with macrophages comes from diffusion of FC within an agLDL particle.
Fig. 2.
HDL reduces macrophage FC uptake from agLDL and foam cell formation. (A–H) J774 macrophages were incubated with cross-linked Alexa546–agLDL for 4 h in absence (A–C) or presence (E–G) of 250 µg/ml HDL. Cells were fixed, FC stained with filipin, and cells analyzed by confocal microscopy. Filipin and agLDL images (C,G) show FC intensity relative to agLDL fluorescence. (D,H) Filipin and agLDL from areas highlighted by the white dashes boxes in C and G overlaid on corresponding bright-field images. Arrows show agLDL in contact with cells, where FC is enriched due to catabolism of agLDL. Arrowheads show agLDL not directly in contact with cells. Images taken as described in A–H for cells in the presence of the indicated amount of HDL were used to quantify (I) the ratio of FC to agLDL and (J) the amount of free cholesterol relative to that in control cells (set at 1) for at least 10 fields containing >100 cells. (K–N) agLDL was labeled with NBD-PC and (K) observed prior to photobleaching. (L) agLDL was photobleached at t=0 in the area indicated by the red dashed circle. Fluorescence recovery after photobleaching within the photobleached area was observed after (M) 320 s and (N) 608 s. (O–V) J774 macrophages were incubated with Alexa546–agLDL stabilized by a collagen matrix for 24 h at 37°C in the presence of 0, 250, 500 or 1000 µg/ml HDL. Cells were fixed, and cholesteryl esters were stained with LipidTox Green and analyzed by confocal microscopy. Shown are bright-field, Alexa546–agLDL, total LipidTox Green and cell-associated LipidTox Green images of cells incubated with 0 (O–R) and 500 (S–V) µg/ml HDL. Cell-associated LipidTox Green images were obtained after background subtraction of the agLDL-associated LipidTox Green signal. Insets show a representative cell from the same field. Images were used to quantify agLDL-associated LipidTox fluorescence (W) and cell-associated LipidTox fluorescence (X) for at least 10 fields containing >100 cells relative to that in control cells (set at 1). Data were compiled from at least three independent experiments. Error bars show s.e.m. *P≤0.05; **P≤0.01; ***P≤0.001; n.s. not statistically significant.
To analyze the effect of HDL on lipid accumulation, we treated J774 macrophages with agLDL in the absence or presence of HDL and quantified LipidTOX staining of agLDL and lipid droplets in cells. In absence of HDL, macrophages accumulated cholesteryl ester (CE) robustly (Fig. 2O–R); upon incubation with 500 μg/ml HDL, this was reduced (Fig. 2S–V). HDL treatment reduced the LipidTOX signal from agLDL by ∼50% at the highest dose of 1000 µg/ml (Fig. 2W). HDL also reduced CE accumulation in macrophages, with a 99% reduction at 1000 µg/ml (Fig. 2X). Thus, HDL removal of FC from agLDL can significantly reduce foam cell formation. This result might suggest that HDL could extract CE from agLDL, but another explanation seems more likely. Removal of FC from the outer shell of the agLDL would provide easier access of lysosomal acid lipase to the CE in the core of the lipoproteins, increasing the rate of CE hydrolysis.
HPCD reduces cholesterol uptake during exophagy and subsequent lipid accumulation
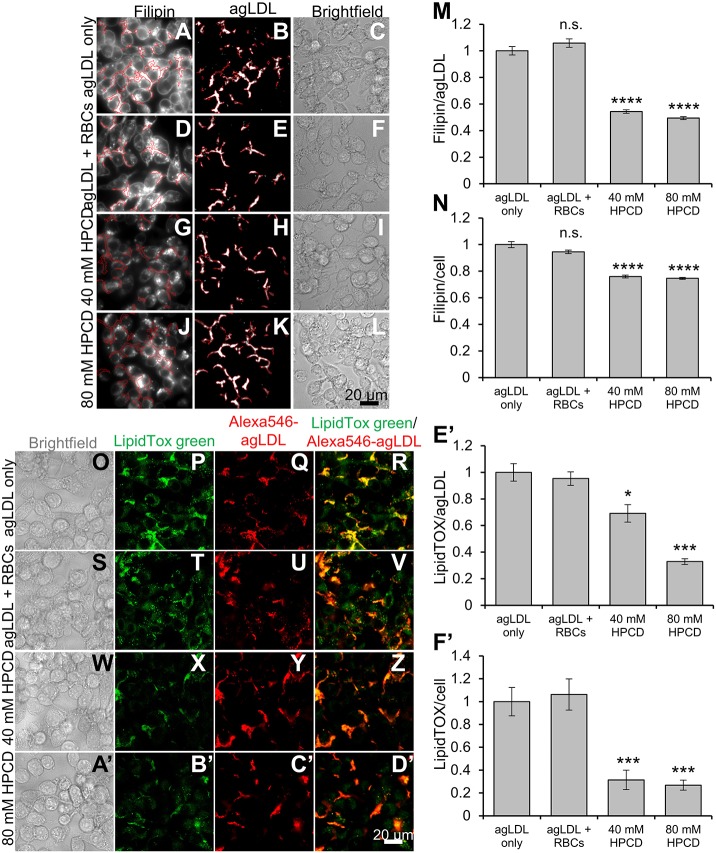
A recent study reported that the cholesterol chelator HPCD can reduce plaque development during atherosclerosis (Zimmer et al., 2016). We investigated whether HPCD could extract FC from agLDL. To do this, we cholesterol balanced HPCD by equilibration with sheep red blood cells as described previously (Iaea et al., 2017). This cholesterol-balanced HPCD does not increase or decrease cholesterol levels in cells under normal conditions because it is pre-equilibrated with cells. However, if cells have a higher than normal cholesterol (i.e. a higher chemical potential of cholesterol in their membranes), cholesterol-balanced HPCD will remove the excess cholesterol. Macrophages were treated with agLDL and then either had no further treatment or were treated with media preincubated with RBCs with or without cholesterol-balanced HPCD. In conditions lacking HPCD, cells catabolized agLDL efficiently, and FC was observed in agLDL and cells (Fig. 3A–C,D–F). Upon addition of 40 mM (Fig. 3G–I) or 80 mM cholesterol-balanced HPCD (Fig. 3J–L), FC in agLDL was reduced by ∼50% (Fig. 3M). This suggested that HPCD can extract FC from agLDL. We also observed a ∼25% reduction of FC within cells under conditions containing HPCD (Fig. 3N). These results suggest that HPCD can extract FC and reduce cholesterol uptake by macrophages.
Fig. 3.
Cholesterol-balanced HPCD reduces FC uptake from untethered agLDL and foam cell formation. HPCD was pre-incubated with RBCs to cholesterol balance HPCD. J774 macrophages were incubated with Alexa546–agLDL for 1 h and then subsequently for 3 h with (A–C) no further treatment, (D–F) RBCs alone, (G–I) RBCs and 40 mM HPCD or (J–L) RBCs and 80 mM HPCD. The cells were fixed and FC stained with filipin and analyzed by confocal microscopy. Confocal images were used to quantify the average filipin intensity in (M) agLDL and in (N) cells, and (M) agLDL size for at least 10 fields containing >100 cells relative to that in control cells (agLGL only, set at 1). (O–F′) HPCD was pre-incubated with RBCs to cholesterol balance HPCD. J774 macrophages were incubated with Alexa546–agLDL for 1 h and then subsequently for 3 h with (O–R) no further treatment, (S–V) RBCs alone, (W–Z) RBCs and 40 mM HPCD or (A′–D′) RBCs and 80 mM HPCD. The cells were fixed and cholesteryl esters stained with LipidTox Green and analyzed by confocal microscopy. Confocal images were used to quantify LipidTox Green intensity in (E′) agLDL and in (F′) cells for at least 10 fields containing >100 cells relative to that in control cells (agLGL only, set at 1). Data were compiled from at least three independent experiments. Error bars show s.e.m. *P≤0.05, ***P≤0.001 ****P≤0.0001; n.s. not statistically significant.
Since cholesterol-balanced HPCD, like HDL, could extract FC, we hypothesized that it too might have the ability to reduce lipid accumulation and foam cell formation. To test this, macrophages were treated with agLDL and then either had no further treatment or were treated with media preincubated with RBCs with or without cholesterol-balanced HPCD. We then assessed CE accumulation through LipidTOX staining. In both agLDL-only and agLDL plus RBC conditions, macrophages accumulated CE (Fig. 3O–V). Addition of 40 mM or 80 mM HPCD effectively reduced CE accumulation in macrophages (Fig. 3W–D′). Quantification of LipidTOX signal with agLDL revealed that 40 mM HPCD reduced agLDL-associated LipidTOX staining to 65% of control, and 80 mM reduced LipidTOX staining to ∼30% of control (Fig. 3E′). We suggest that removal of FC might accelerate hydrolysis of CE and explain the difference in agLDL-associated LipidTOX staining. Quantification of LipidTox labeling in cells showed that both 40 mM or 80 mM HPCD reduced LipidTOX staining in cells to ∼33% of control conditions (Fig. 3F′). This result suggests that HPCD can reduce cholesterol uptake and CE accumulation from agLDL.
HDL extracts FC from agLDL in a cell-free environment and during agLDL catabolism
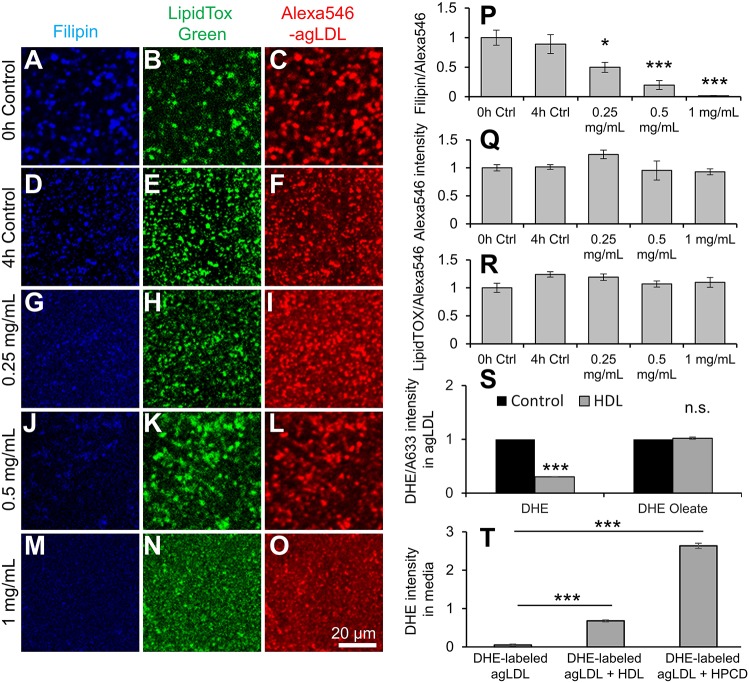
HDL mediates reverse cholesterol transport from cells in an ABCA1- and ABCG1-dependent manner (Lewis and Rader, 2005). However, this method of cholesterol efflux is too slow to explain HDL reduction of FC in agLDL and cells within 4 h (Fig. 2). Furthermore, we have shown above that extraction of FC using cholesterol-balanced HPCD, which functions independently of ABCA1 and ABCG1 can reduce FC accumulation and foam cell formation. To test whether the function of HDL in reducing FC in agLDL occurs directly, we incubated Alexa-Fluor-546–agLDL (Alexa546–agLDL) with HDL over 4 h, prior to staining FC using filipin and CE using LipidTOX Green. We observed in untreated controls at 0 and 4 h that Alexa546–agLDL contained FC and CE (Fig. 4A–C,D–F). Treatment with increasing amounts of HDL reduced FC but not CE or the amount of agLDL present (Fig. 4G–O). Quantification of filipin staining relative to the amount of Alexa546–agLDL revealed that HDL was able to dose dependently decrease the amount of FC in agLDL in the absence of cells (Fig. 4P). Under these conditions, the total amount of agLDL (Fig. 4Q) and the CE content in agLDL (Fig. 4R) were unchanged. These results show that HDL can extract FC from agLDL directly in the absence of cells. We confirmed these results by fluorescence spectroscopy. Alexa-Fluor-633–agLDL (Alexa633–agLDL) was reconstituted with dehydroergosterol (DHE; to mimic FC) or DHE-oleate (to mimic CE) prior to incubation with HDL for 4 h. HDL was able to reduce DHE but not DHE-oleate fluorescence in Alexa633-agLDL relative to the Alexa633-agLDL amount (Fig. 4S). This suggests that HDL can extract FC but not CE directly from agLDL. If HDL and cholesterol-balanced HPCD reduce foam cell formation by extracting FC from agLDL, we should be able to observe FC in the medium of cells treated with agLDL. We labeled agLDL with DHE (to mimic FC), and then treated J774 macrophages for 4 h in the presence or absence of HDL or cholesterol-balanced HPCD and assayed DHE fluorescence in the medium. We previously showed that most of the FC from agLDL catabolism goes into the cell and that ∼2.5% FC per hour is released into the medium (Haka et al., 2009). Consistent with this, we find some release of DHE into the medium in the absence of added sterol acceptors (Fig. 4T). In the presence of 500 μg/ml HDL or 40 mM cholesterol-balanced HPCD, we observed a 10-fold or 50-fold increase in DHE fluorescence in the medium, respectively (Fig. 4T). These results show that both HDL and cholesterol-balanced HPCD can promote FC extraction and sequestration into the medium during agLDL degradation.
Fig. 4.
HDL can extract freFC directly from agLDL in absence of cells and during agLDL catabolism. (A–O) Alexa546–agLDL in a collagen matrix was left untreated for (A–C) 0 or (D–F) 4 h, or (G–O) treated with the given concentrations of HDL for 4 h prior to staining of FC or CE using filipin or LipidTOX Green, and analysis by confocal microscopy. Images were used to quantify (P) filipin, (Q) Alexa546–agLDL and (R) LipidTOX and Alexa546 intensity for at least 10 fields containing >500 agLDL aggregates relative to that in control cells (set at 1). (S) Alexa633–AgLDL was reconstituted with DHE or DHE-oleate and then incubated with or without 500 µg/ml HDL for 4 h. AgLDL was pelleted by centrifugation, resuspended and measured by fluorescence spectroscopy. (T) J774 macrophages were incubated for 4 h with DHE-labeled agLDL or DHE-labeled agLDL plus 500 μg/ml HDL or 40 mM cholesterol-balanced HPCD. The medium was collected, agLDL removed by centrifugation and DHE fluorescence measured by fluorescence spectroscopy. Data compiled from at least three independent experiments. Error bars show s.e.m. *P≤0.05; ***P≤0.001; n.s. not statistically significant.
While higher HDL levels correlate with better cardiovascular health (Williams and Feldman, 2011), therapies aiming to increase HDL levels in the body have generally failed to reduce the incidence of cardiac events (Group et al., 2017; Nicholls et al., 2018). This suggests that we do not fully understand the role of HDL in heart disease. Here, we investigated the role of HDL in the macrophage catabolism of agLDL that leads to foam cell formation. We find that HDL extracts FC but not CE from agLDL, and this occurs directly and even in the absence of cells. This reduction in FC reduces CE accumulation and foam cell formation in macrophages. These results highlight a previously uncharacterized role for HDL and extend our current knowledge of HDL function in macrophage foam cell formation.
A recent study showed that administration of HPCD can reduce atherosclerosis in mice (Zimmer et al., 2016). Here we show that cholesterol-balanced HPCD can extract FC from agLDL and reduce FC and CE accumulation in macrophages. Interestingly, HPCD has been shown to reduce cholesterol in atherosclerotic plaques, and its effect on atherosclerosis was ABCA1- and ABCG1-independent (Zimmer et al., 2016). Therefore, the effect observed in this study might be partially responsible for HPCD-mediated reduction in atherosclerosis. HPCD has other effects and it was recently found to inhibit cholesterol crystal-induced inflammation (Bakke et al., 2017). These studies suggest that hydroxypropyl-β-cyclodextrin might be a potential therapy for atherosclerosis. Further studies will exploit these differences to probe the function of both HDL and HPCD during atherosclerosis.
MATERIALS AND METHODS
Cells and cell culture
J774a.1 macrophages (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 units/ml penicillin and 50 µg/ml streptomycin in a humidified atmosphere (5% CO2) at 37°C and used at low passage numbers. Cells were confirmed to be contamination free.
Reagents
Alexa-Fluor-546, Alexa-Fluor-633, LipidTOX Green, Alexa-Fluor-488–phalloidin and BS(PEG)5 were purchased from Invitrogen (Carlsbad, CA). C6-NBD-PC was purchased from Avanti Polar Lipids (Birmingham, AL). HPCD, cholesterol, filipin and PureCol EZ gel solution were purchased from Sigma-Aldrich (St Louis, MO). Sheep red blood cells were purchased from Innovative Research (Novi, MI).
Lipoproteins and labeling
Human LDL was prepared from donor plasma as described previously (Havel et al., 1955). Human HDL was purified using density-gradient ultracentrifugation of donor plasma (Havel et al., 1955), but instead of LDL, crude HDL was collected from the high-density fraction. The density of the solution was increased from 1.019 g/ml to 1.21 g/ml by addition of 0.273219 g/ml of sodium bromide (Sigma) and samples centrifuged for 48 h at 259,000 g. Purified HDL was collected from the top layer and stored at −80°C in aliquots until needed. LDL was labeled using succinimidyl esters of Alexa546 (Invitrogen) and C6-NBD-PC. LDL was aggregated by vigorous vortexing for 30 s (Buton et al., 1999). LDL was reconstituted with DHE or DHE-oleate as described previously (Wüstner et al., 2005).
Hyperlipidemic Apoe−/− mice
Female Apoe−/− mice (cat #002052) were obtained from Jackson Laboratories and placed on a high-fat diet (21% milk fat, 0.15% cholesterol; Harlan Teklad) for 24 weeks. Mice were euthanized, perfused with PBS and aortas taken for sectioning. All animal research was performed according to the guidelines of the Weill Cornell Medicine IACUC.
Aortic fixation and sectioning
Aortas were fixed overnight in 3% (w/v) paraformaldehyde at 4°C. Fixed aortas were placed in a solution of 30% sucrose in PBS and stored at 4°C overnight. Aortas were then gently agitated in embedding medium [1:2 ratio of 30% sucrose in PBS in optimal cutting temperature (OCT) medium] and then frozen in the same medium using 2-methylbutane and liquid nitrogen. Samples were then cut into 8 μm sections using a Cryostat, mounted onto glass slides and stored at −80°C.
Immunohistochemistry
For free cholesterol visualization, samples were stained with 50 μg/ml of filipin in PBS for 1 h at room temperature. For antibody labeling of sections, blocking was performed by incubation with 5% FBS for 1 h at room temperature. LDL was stained using anti-ApoB antibody (Abcam; Ab20737) at 1:100 dilution overnight at 4°C, followed by Alexa546-conjugated anti-rabbit-IgG secondary antibody at 1:250 dilution for 4 h at room temperature. For F4/80 labeling, sections were stained with primary antibody (Abcam; Ab6640) at 1:500 dilution overnight at 4°C, followed by Alexa633-anti-rat-IgG secondary antibody at 1:500 dilution for 4 h at room temperature. F-actin was stained using 0.02 U/ml Alexa-Fluor-488–phalloidin for 1 h at room temperature. All antibody labeling was carried out in PBS containing 2% FBS. Samples were washed with PBS and coverslips attached using Vectashield mounting medium prior to imaging.
FC/CE staining using filipin and LipidTox
Cells were fixed with 3% paraformaldehyde for 20 min at room temperature and stained with filipin (50 µg/ml in PBS) for 45 min and LipidTox (1:1000 in PBS) for 20 min at room temperature. Cells were washed repeatedly with PBS and analyzed by confocal microscopy.
Cholesterol balancing HPCD
Prior to use, HPCD was cholesterol balanced by incubation with 109 sheep red blood cells as described previously (Iaea et al., 2017). Subsequently, the red blood cells were removed by centrifugation (26,800 g for 10 min), prior to addition of supernatant to cells.
Fluorescence recovery after photobleaching
AgLDL was labeled with NBD-PC. After plating onto glass coverslips, agLDL was allowed to settle prior to imaging. A prebleach image was acquired before bleaching. A circular portion of NBD-PC in agLDL was photobleached for 30 s by repeatedly scanning a circular region for 30s at full power of the 488 nm laser so that the fluorescence was measurably reduced in the region. Subsequently, images were taken at given increments.
Dehydroergosterol reconstitution and fluorescence spectroscopy
Alexa633–agLDL was labeled with dehydroergosterol (DHE) using a 2 min pulse, and then chased for 30 min. Then agLDL was incubated with or without HDL for 4 h at 37°C. AgLDL was pelleted by centrifugation at 10,000 g for 10 min, resuspended in PBS and fluorescence analyzed using a SpectraMax M2 fluorometer (MDS Analytical Technologies, Sunnyvale, CA). The integrated DHE signal from agLDL was normalized to the integrated Alexa633–agLDL signal (600–750 nm). For DHE measurements in the media, Alexa546–agLDL was labeled with DHE for 30 min and then washed in Phenol Red-free DMEM prior to use. After 4 h treatment, the medium was collected, centrifuged at 10,000 g for 10 min remove agLDL and fluorescence analyzed using a SpectraMax M2 fluorometer using excitation at 324 nm and emission at 397 nm with a filter cutoff of 325 nm.
Confocal microscopy
For imaging, cells were plated on poly-D-lysine-coated glass coverslips attached beneath a 7 mm hole in a 35 mm tissue culture dish. Images were acquired with a Zeiss LSM510 or LSM880 laser-scanning confocal microscope using 40× Air, 0.8 NA or 40× oil, 1.3 NA objectives, respectively. For actin measurements, z-stacks were obtained with a step size of 0.98 μm. All data were analyzed with MetaMorph image analysis software (Molecular Devices, Dowingtown, PA) or MATLAB software (Mathworks, Natick, MA).
Image quantification
All image quantification was performed using MetaMorph or MATLAB software. For assessment of neutral lipid content, images were thresholded to exclude any fluorescence not associated with lipid droplets. Then the integrated LipidTOX Green fluorescence per field was quantified and divided by the number of cells in the field. FC and CE in agLDL were quantified as follows. A binary mask was generated by applying an inclusive threshold to the Alexa546–agLDL signal. This mask was then applied to the filipin or LipidTOX Green signal to only include FC or CE in agLDL. FC or CE signal colocalized with agLDL was quantified and divided by agLDL area to give FC or CE/agLDL. FC and CE in cells were quantified by applying an exclusive threshold to Alexa546-agLDL signal and applying it to the filipin or LipidTOX green signal to exclude FC or CE signal in agLDL. The remaining signal was divided by the number of cells in the field to give FC or CE/cell.
Statistics
For pairwise comparisons, a Student's two-tailed t-test was performed. For comparisons of more than two groups, one-way ANOVA followed by Bonferroni correction was performed. All statistical comparisons were performed using Excel software.
Supplementary Material
Acknowledgements
The authors thank Leona Cohen-Gould at the EM & Histology Core and Optical Microscopy Core at Weill Cornell Medical College for her help and support. We thank Dr J. David Warren (Department of Biochemistry, Weill Cornell Medical College) for supplying DHE-oleate.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.K.S., F.R.M.; Methodology: R.K.S., F.W.L., A.S.H.; Formal analysis: R.K.S., F.W.L.; Writing - original draft: R.K.S.; Writing - review & editing: R.K.S., F.R.M.; Project administration: F.R.M.; Funding acquisition: F.R.M., R.K.S., F.W.L.
Funding
The project described was supported by National Institutes of Health (grant R01-HL093324). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.K.S. is an American Heart Association Stanley Stahl Postdoctoral Fellow (ID: 15POST22990022). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.237271.supplemental
References
- Bakke S. S., Aune M. H., Niyonzima N., Pilely K., Ryan L., Skjelland M., Garred P., Aukrust P., Halvorsen B., Latz E. et al. (2017). Cyclodextrin reduces cholesterol crystal-induced inflammation by modulating complement activation. J. Immunol. 199, 2910-2920. 10.4049/jimmunol.1700302 [DOI] [PubMed] [Google Scholar]
- Borén J., Gustafsson M., Skålén K., Flood C. and Innerarity T. L. (2000). Role of extracellular retention of low density lipoproteins in atherosclerosis. Curr. Opin. Lipidol. 11, 451-456. 10.1097/00041433-200010000-00002 [DOI] [PubMed] [Google Scholar]
- Buton X., Mamdouh Z., Ghosh R., Du H., Kuriakose G., Beatini N., Grabowski G. A., Maxfield F. R. and Tabas I. (1999). Unique cellular events occurring during the initial interaction of macrophages with matrix-retained or methylated aggregated low density lipoprotein (LDL). J. Biol. Chem. 274, 32112-32121. 10.1074/jbc.274.45.32112 [DOI] [PubMed] [Google Scholar]
- Grosheva I., Haka A. S., Qin C., Pierini L. M. and Maxfield F. R. (2009). Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler. Thromb. Vasc. Biol. 29, 1615-1621. 10.1161/ATVBAHA.109.191882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group H. T. R. C., Bowman L., Hopewell J. C., Chen F., Wallendszus K., Stevens W., Collins R., Wiviott S. D., Cannon C. P., Braunwald E. et al. (2017). Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl. J. Med. 377, 1217-1227. 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- Haka A. S., Grosheva I., Chiang E., Buxbaum A. R., Baird B. A., Pierini L. M. and Maxfield F. R. (2009). Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell 20, 4932-4940. 10.1091/mbc.e09-07-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Eder H. A. and Bragdon J. H. (1955). The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345-1353. 10.1172/JCI103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaea D. B., Mao S., Lund F. W. and Maxfield F. R. (2017). Role of STARD4 in sterol transport between the endocytic recycling compartment and the plasma membrane. Mol. Biol. Cell 28, 1111-1122. 10.1091/mbc.e16-07-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., Mahlberg F. H., Rothblat G. H. and Phillips M. C. (1991). Cholesterol transport between cells and high-density lipoproteins. Biochim. Biophys. Acta 1085, 273-298. 10.1016/0005-2760(91)90132-2 [DOI] [PubMed] [Google Scholar]
- Karlin J. B., Johnson W. J., Benedict C. R., Chacko G. K., Phillips M. C. and Rothblat G. H. (1987). Cholesterol flux between cells and high density lipoprotein. Lack of relationship to specific binding of the lipoprotein to the cell surface. J. Biol. Chem. 262, 12557-12564. [PubMed] [Google Scholar]
- Lewis G. F. and Rader D. J. (2005). New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 96, 1221-1232. 10.1161/01.RES.0000170946.56981.5c [DOI] [PubMed] [Google Scholar]
- Nicholls S. J., Andrews J., Kastelein J. J. P., Merkely B., Nissen S. E., Ray K. K., Schwartz G. G., Worthley S. G., Keyserling C., Dasseux J.-L. et al. (2018). Effect of serial infusions of CER-001, a pre-beta high-density lipoprotein mimetic, on coronary atherosclerosis in patients following acute coronary syndromes in the CER-001 atherosclerosis regression acute coronary syndrome trial: a randomized clinical trial. JAMA Cardiol. 3, 815-822. 10.1001/jamacardio.2018.2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuth M., Nguyen S. D., Vihervaara T., Hilvo M., Laajala T. D., Kondadi P. K., Gistera A., Lähteenmäki H., Kittilä T., Huusko J. et al. (2018). Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur. Heart J. 39, 2562-2573. 10.1093/eurheartj/ehy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K., Barbosa-Lorenzi V. C., Lund F. W., Grosheva I., Maxfield F. R. and Haka A. S. (2016). Degradation of aggregated LDL occurs in complex extracellular sub-compartments of the lysosomal synapse. J. Cell Sci. 129, 1072-1082. 10.1242/jcs.181743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K., Haka A. S., Bhardwaj P., Zha X. and Maxfield F. R. (2019). Dynamic actin reorganization and Vav/Cdc42-dependent actin polymerization promote macrophage aggregated LDL uptake and catabolism. Arterioscler. Thromb. Vasc. Biol. 39, 137-149. 10.1161/ATVBAHA.118.312087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. (1999). Nonoxidative modifications of lipoproteins in atherogenesis. Annu. Rev. Nutr. 19, 123-139. 10.1146/annurev.nutr.19.1.123 [DOI] [PubMed] [Google Scholar]
- Tamminen M., Mottino G., Qiao J. H., Breslow J. L. and Frank J. S. (1999). Ultrastructure of early lipid accumulation in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19, 847-853. 10.1161/01.ATV.19.4.847 [DOI] [PubMed] [Google Scholar]
- Williams P. T. and Feldman D. E. (2011). Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman's Livermore Cohort. Atherosclerosis 214, 196-202. 10.1016/j.atherosclerosis.2010.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüstner D., Mondal M., Tabas I. and Maxfield F. R. (2005). Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic 6, 396-412. 10.1111/j.1600-0854.2005.00285.x [DOI] [PubMed] [Google Scholar]
- Zimmer S., Grebe A., Bakke S. S., Bode N., Halvorsen B., Ulas T., Skjelland M., De Nardo D., Labzin L. I., Kerksiek A. et al. (2016). Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8, 333ra350 10.1126/scitranslmed.aad6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.