ABSTRACT
Balanced progenitor activities are crucial for the development and maintenance of high turn-over organs such as the esophagus. However, the molecular mechanisms regulating these progenitor activities in the esophagus remain to be elucidated. Here, we demonstrated that Yap is required for the proliferation of esophageal progenitor cells (EPCs) in the developing murine esophagus. We found that Yap deficiency reduces EPC proliferation and stratification whereas persistent Yap activation increases cell proliferation and causes aberrant stratification of the developing esophagus. We further demonstrated that the role of YAP signaling is conserved in the developing human esophagus by utilizing 3D human pluripotent stem cell (hPSC)-derived esophageal organoid culture. Taken together, our studies combining loss/gain-of-function murine models and hPSC differentiation support a key role for YAP in the self-renewal of EPCs and stratification of the esophageal epithelium.
KEY WORDS: Esophagus, YAP, Progenitor cells, Human pluripotent stem cells, 3D organoids, Hippo pathway
Summary: YAP signaling regulates the conversion of simple columnar epithelium into stratified squamous cells in the developing mouse and human esophagus.
INTRODUCTION
The combination of human pluripotent stem cell (hPSC) differentiation and genetic mouse models has facilitated our understanding of disease mechanisms and human development. Previous studies employing this platform have demonstrated that signaling pathways such as WNT are crucial for the development of multiple endodermal organs, including the lung, thyroid, stomach, intestine and colon (Spence et al., 2011; Longmire et al., 2012; Mou et al., 2012; Huang et al., 2014; McCracken et al., 2014; Múnera et al., 2017). By contrast, we know very little about the molecular mechanisms regulating the esophagus, another endodermal organ lined by the stratified squamous epithelium. To begin to address this issue, we recently established an efficient protocol to directly differentiate hPSCs into p63+SOX2+ esophageal progenitor cells (EPCs), which resemble EPCs in the human fetal esophagus. These hPSC-derived EPCs are able to self-renew and differentiate into a stratified squamous epithelium in 3D organoid culture (Zhang et al., 2018). We used these EPCs combined with mouse genetic models to reveal that NOTCH and BMP signaling are important for the squamous differentiation of EPCs. Consistent with this, blocking NOTCH or BMP signaling with pharmacological inhibitors or genetic ablation leads to reduced differentiation of EPCs (Zhang et al., 2018).
During development of the mouse esophagus, EPCs are initially established from the dorsal foregut endoderm at around embryonic day (E) 10.5 (Jacobs et al., 2012; Que, 2015). These EPCs (p63+; also known as Trp63 in mouse and TP63 in human) form a single-layered epithelium in the nascent esophagus, and then they go through extensive proliferation and differentiation to generate a multi-layered epithelium composed of undifferentiated basal cells (p63+KRT5+) and differentiated suprabasal cells (KRT4+KRT13+) (Zhang et al., 2017). We have shown that NOTCH and BMP are essential for the squamous differentiation of progenitor cells in the developing esophagus. However, we know very little about the signaling pathway(s) controlling the proliferation of EPCs in both mouse and human esophagus. YAP (Yap1/YAP1) is a crucial regulator of proliferation in several endoderm-derived tissues, including the lung and intestine, which are lined by simple columnar epithelium (Cai et al., 2010; Zhao et al., 2014; Gregorieff et al., 2015; Yui et al., 2018). Treatment with the YAP inhibitor verteporfin, which blocks the interaction of YAP and its binding transcription factor TEAD, inhibits epithelial morphogenesis (Liu-Chittenden et al., 2012; Yui et al., 2018). Conversely, overexpression of YAP or deletion of the YAP suppressors Mst1 and Mst2 (Stk3) leads to the expansion of stem/progenitor cells in these organs (Camargo et al., 2007; Zhou et al., 2011). By contrast, it remains largely unknown how YAP regulates other endodermal organs, especially the esophagus where the epithelium consists of stratified and squamous cells.
We set out to address this issue by using Yap loss/gain-of-function mouse models and found that Yap is essential for the generation of the stratified squamous epithelium in the esophagus by regulating EPC proliferation. Additionally, YAP is important for the development of the human esophagus as YAP inhibition or knockdown reduces progenitor proliferation and stratification in 3D hPSC-derived esophageal organoids. These findings therefore support conserved roles for YAP in the development of both mouse and human esophagus.
RESULTS
Dynamic changes in Yap subcellular localization in the epithelium of mouse esophagus at early stages
We first sought to characterize Yap subcellular localization in the developing mouse esophagus. We immunostained wild-type (WT) esophageal sections for Yap and Sox2, an early marker of EPC fate, at key developmental stages of the esophagus. At E10.5, Yap displayed a nuclear subcellular localization in all EPCs (Fig. 1A). Strikingly, this localization pattern changed by E11.5, such that a clearly differential Yap subcellular localization pattern emerged: nuclear Yap was found solely in basally located EPCs, whereas suprabasal cells were characterized by cytoplasmic Yap (Fig. 1B). This differential Yap subcellular localization pattern remained unchanged throughout the subsequent developmental stages examined (Fig. 1C,D). Altogether, these observations demonstrate that Yap accumulates in the nucleus of basally located EPCs at early stages and basal cells at later stages, whereas suprabasal cells are characterized by cytoplasmic Yap.
Fig. 1.
Yap deletion examined at different developmental stages of the mouse esophagus. (A,B) Yap expression is incompletely ablated in the esophageal epithelium of Shh-Cre;Yaploxp/loxp (Yap KO) mutants at E10.5 and E11.5 (n=3). Note the complete loss of Yap in the ventral esophageal epithelium (arrows). (C,D) Yap expression is completely ablated in the esophageal epithelium of Shh-Cre;Yaploxp/loxp (Yap KO) mutants at E12.5 and E18.5. Note that expression of YAP is nuclear in EPCs at E10.5, and nuclear expression of Yap is localized to basally located EPCs at E11.5, E12.5 and E15.5. Apically oriented cells express cytoplasmic Yap starting at E11.5, E12.5 and E18.5. Note expression of both cytoplasmic and nuclear staining of YAP in wild type at E12.5 (arrows) and mostly nuclear in the basal cells (arrows) at E18.5 (n=3). Dotted line encircles the epithelium. d, dorsal; Epi, epithelium; es, esophagus; tr, trachea; v, ventral. Scale bars: 20 µm.
Yap is required for the proliferation of basal cells in the developing mouse esophagus
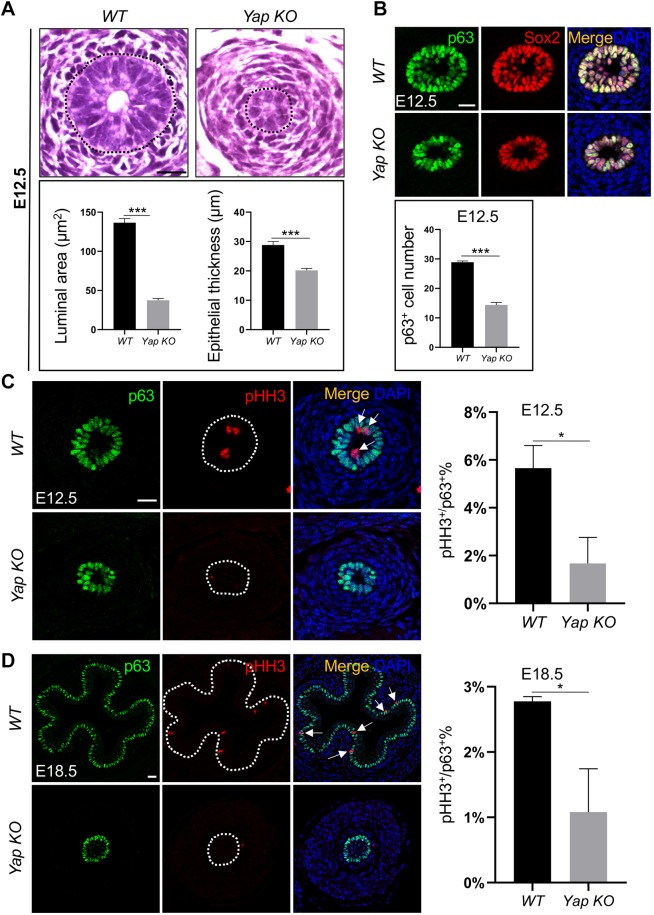
Nuclear Yap is typically associated with transcriptional programs of growth and proliferation (Mauviel et al., 2012; Varelas, 2014). We hypothesized that a Yap knockout (KO) at early stages of esophageal development inhibits basal cell proliferation and expansion. We deleted Yap using Shh-Cre, which is active in the nascent EPCs when the esophagus separates from the early foregut (Fig. 1A) (Rodriguez et al., 2010). Consistent with previous findings, Shh-Cre;Yaploxp/loxp (Yap KO) mutants died at birth as a result of respiratory failure (Mahoney et al., 2014). In Yap KO mutants, the trachea and esophagus separated normally (Fig. S1A). Of note, Shh expression in the esophagus is first induced in the ventral aspect of the esophageal epithelium at E10.5, and subsequently spreads to the dorsal aspect through E12.5 (Harris-Johnson et al., 2009). Consistent with this, we observed Yap deletion in the ventral aspect of the esophagus at E10.5 and E11.5 (Fig. 1A,B), and, by E12.5, continuing to E18.5, the entire esophageal epithelium was devoid of Yap protein, as assessed by immunofluorescence (IF) staining (Fig. 1C,D). EPC specification was unaffected by the loss of Yap, as they maintained expression of the anterior foregut marker Sox2 and basal cell marker p63 in Shh-Cre;Yaploxp/loxp mutants (Figs 1A and 2B). However, although the E12.5 esophageal epithelium became multilayered (28.8±1.2 µm thickness), Yap KO mutant esophageal epithelia were significantly thinner (20.2±0.7 µm, P<0.01; Figs 1C and 2A,B). The numbers of p63+ EPCs were also reduced at this stage in Yap KO mutants (14.4±0.9/section) compared with WT (28.9±0.4/section, P<0.01; Fig. 2B), suggesting that EPC proliferation was affected by Yap loss. Immunostaining for the proliferation marker phosphorylated histone H3 (pHH3) (5.6±1.0% in WT versus 1.7±1.1%, P<0.05 in Yap KO littermates; Fig. 2C), as well as in vivo 5-ethynyl-2′-deoxyuridine (EdU) labeling (41.8±2.9% in WT versus 30.0±3.2%, P<0.05 in Yap KO littermates; Fig. S1B), demonstrated a significant reduction in cell proliferation in Yap KO esophageal epithelia. Although basal cell proliferation appeared to be attenuated by E18.5 in WT conditions, this proliferation defect was still evident in E18.5 Yap KO samples (Fig. 2D, Fig. S1C). Examination of cleaved caspase-3 (Casp3) IF stain did not reveal evidence of apoptosis upon Yap deletion (Fig. S2A). Together, these results suggest that Yap positively regulates EPC proliferation during esophageal development.
Fig. 2.
Yap deletion affects the development of the mouse esophagus. (A) Deletion of Yap reduces the size of the esophageal lumen and thickness of the epithelium in Shh-Cre;Yaploxp/loxp (Yap KO) mutants at E12.5 (n=5). Black dotted line encircles the epithelium. (B) Expression of the progenitor markers p63 and Sox2 is maintained upon Yap deletion (n=3). Note the number of p63+ basal cells is significantly reduced at E12.5. (C,D) Yap deletion decreases the numbers of EPCs (p63+) and proliferating cells (pHH3+, arrows) at E12.5 (C) and E18.5 (D). White dotted line encircles the epithelium. Data represent mean+s.e.m. (n=5). *P<0.05, ***P<0.001 by unpaired, two-tailed Student's t-test. Scale bars: 20 µm.
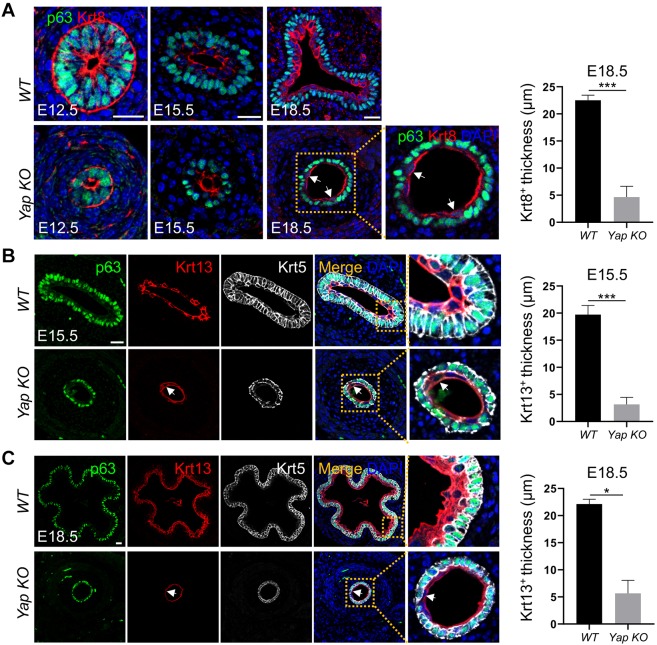
Conversion of the simple columnar esophageal epithelium into a stratified squamous epithelium involves the loss and gain of several keratin proteins. Krt8 is enriched in EPCs at E12.5, but as suprabasal cells mature they lose Krt8 expression and by E18.5 Krt8 is only expressed in the top layers of differentiated cells. Concomitantly, Krt4 and Krt13 are upregulated in the differentiating parabasal cells by E15.5, whereas the undifferentiated basal cells express Krt5 (Yu et al., 2005; Zhang et al., 2017).
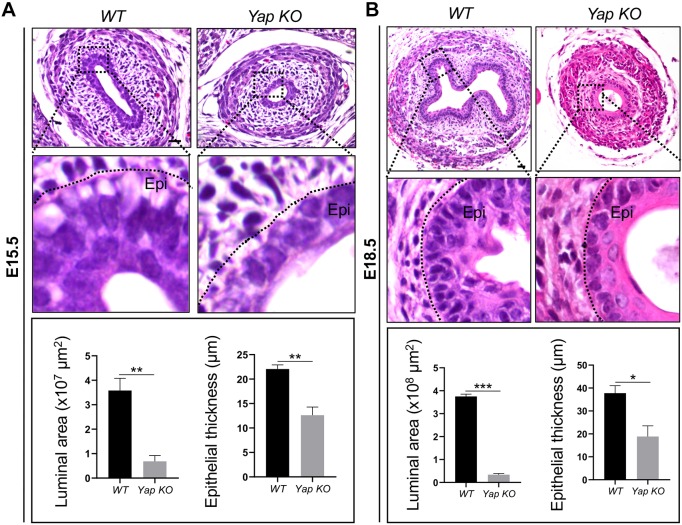
Yap deletion affected the stratification of the esophageal epithelium. This was noticeable at E15.5, but especially so at E18.5, at which stage the esophageal epithelium was 18.9±4.6 µm thick, compared with 37.8±3.2 µm thick in WT (P<0.05; Fig. 3A,B). In addition, the lumen lacked the characteristic papillae (Fig. 3B). Yap deletion did not affect the initial expression of Krt8 at E12.5 (Fig. 4A). Krt8-expressing suprabasal cells were detected at E15.5, and these cells co-expressed Krt13, though their number was decreased compared with WT littermates (Fig. 4A,B). By E18.5, the thickness of the Krt13+ suprabasal cell layer was reduced in the Yap KO mutants (Fig. 4C). This could be due to the lack of supply of basal cell-derived suprabasal cells. Notably, epithelial Yap deletion did not affect the differentiation of mesenchymal progenitors into muscle cells (Fig. S2B).
Fig. 3.
Yap deletion reduces the number of epithelial layers and lumen size. (A,B) Yap deletion results in a flat and simplified esophageal epithelium with a reduction in the size of the esophageal lumen and epithelial thickness at E15.5 and E18.5 (n=5 for each). Epi, epithelium. Black dotted line indicates the border between the epithelium and mesenchyme. Data represent mean+s.e.m. (n=5). *P<0.05, **P<0.01, ***P<0.001 by unpaired, two-tailed Student's t-test. Scale bars: 20 µm.
Fig. 4.
Yap deletion reduces squamous stratification in the developing mouse esophagus. (A) Yap deletion leads to a simplified epithelium with a reduced number of Krt8+ cells at different stages of esophageal development. Note a few Krt8+ cells (arrows) in E18.5 mutant esophagus and the thickness of Krt8+ cells is reduced (n=3). (B,C) Yap deletion reduces squamous differentiation and only a few cells on the top layer of the epithelium (arrows) express Krt13 at E15.5 (B) and E18.5 (C) (n=5). The thickness of Krt13+ cells is reduced in Yap KO mutants. Note the apical nonspecific staining of Krt8 and Krt13 in p63+ basal cells of E18.5 mutant esophagus. Also note the normal shedding keratin in the middle of the esophageal lumen and that the thickness of Krt13+ cells is reduced at E15.5 and E18.5. Data represent mean+s.e.m. (n=5). *P<0.05, ***P<0.001 by unpaired, two-tailed Student's t-test. Scale bars: 20 µm.
Ectopic nuclear Yap activation promotes esophageal hyperplasia
Given the dramatic hypoplastic phenotype upon Yap deletion, we next investigated whether constitutive nuclear accumulation of Yap in the esophageal epithelial cells generated an inverse phenotype. To accomplish this, we generated Shh-Cre;Yaploxp/loxp;R26Yap5SA mutants (Yap OE). This Rosa26-driven construct results in the constitutive expression of Yap5SA, a human Yap allele that was fused with a nuclear localization signal (NLS) and wherein five serines, normally phosphorylated by Lats1/2 to induce cytoplasmic sequestration of Yap, have been mutated into alanines, causing the constitutive accumulation of Yap in the nucleus to activate Yap transcriptional programs (Cotton et al., 2017). Thus endogenous Yap is replaced entirely by constitutively nuclear Yap in Shh-Cre;Yaploxp/loxp;R26Yap5SA compound mutants, allowing us to investigate the effect of nuclear accumulation of Yap in the absence of (endogenous) cytoplasmic Yap in EPCs. We also generated Shh-Cre;Yaploxp/+;R26Yap5SA, which retains one endogenous Yap allele and displayed a similar phenotype as the Shh-Cre;Yaploxp/loxp;R26Yap5SA esophageal epithelium. Thus, hereafter we only consider Shh-Cre;Yaploxp/loxp;R26Yap5SA compound mutants.
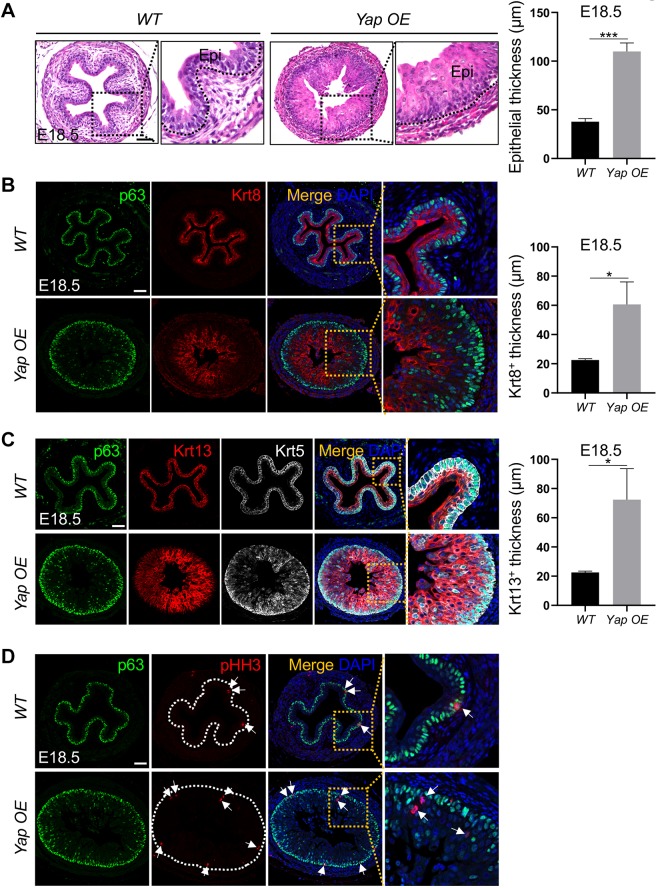
Yap overexpression caused a hyperplastic phenotype of the esophageal epithelium (Fig. 5A, Fig. S3A). By E18.5, the esophageal epithelium lacked its stereotypical stratified morphology, including papillae. Rather, the Yap OE epithelium was circular in shape and was significantly thicker (37.8±3.2 µm in WT versus 110.0±8.7 µm in Yap OE littermates, P<0.0001; Fig. 5A).
Fig. 5.
Ectopic Yap promotes the proliferation and stratification of esophageal epithelium. (A) Yap overexpression increases the thickness of the esophageal epithelium in Shh-Cre;Yaploxp/loxp;R26-Yap5SA (Yap OE) compound mutants (n=4). Black dotted line indicates the border between epithelium and mesenchyme. (B) Thickness of the suprabasal cell layers (Krt8+) is increased in the esophagus of E18.5 Yap OE mutants (n=3). (C) Thickness of the basal cell layers (p63+ Krt5+) and differentiated cell layers (Krt13+) is increased in the esophagus of E18.5 Yap OE mutants (n=5). (D) Yap overexpression increases the number of p63+ basal cells and pHH3+ proliferating epithelial cells (arrows) at E18.5 (n=6). Note the proliferating parabasal cells in mutants as shown in the magnified view. Epi, epithelium. White dotted line encircles the epithelium. Data represent mean+s.e.m. (n=5). *P<0.05, ***P<0.001 by unpaired, two-tailed Student's t-test. Scale bars: 50 µm.
Because Yap KO attenuated basal cell proliferation, we hypothesized that basal cell hyperplasia was responsible for the hyperplastic phenotype in Yap OE esophageal epithelium. Staining for markers of basal (p63 and Krt5) and suprabasal (Krt8 and Krt13) cells revealed that whereas p63+Krt5+ basal cells generally retained their basal position in the epithelium and mostly formed a single basal layer, the Krt8+Krt13+ suprabasal cell populations were dramatically expanded and had lost their characteristic stratified morphology. These suprabasal cells were interspersed by sporadic p63+Krt5+ basal cells (Fig. 5C), demonstrating disorganization of the esophageal epithelium. However, despite the Krt8+Krt13+ suprabasal hyperplastic phenotype, the majority of the suprabasal cells were negative for the proliferation marker pHH3 (Fig. 5D). This suggested that the hyperplastic phenotype was caused by hyperproliferative basal cells that produce excess Krt8+Krt13+ suprabasal cells. Altogether, these data corroborate the notion that Yap promotes esophageal basal cell proliferation during development.
Nuclear Yap regulation of Notch signaling is involved in the differentiation of esophageal basal cells into suprabasal cells
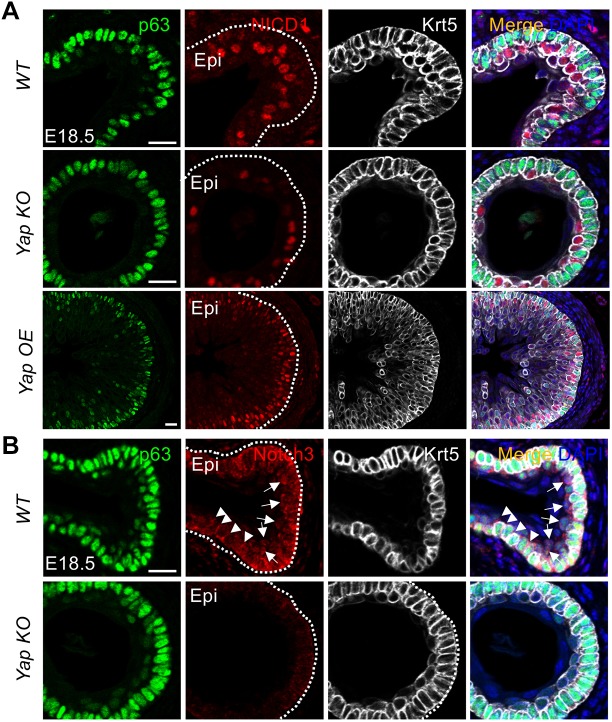
We next investigated potential mechanisms that might explain how Yap controls the squamous differentiation of esophageal basal cells. A previous study identified Notch signaling as an important pathway in the differentiation of EPCs (Zhang et al., 2018) and several studies have demonstrated that Yap directly activates Notch signaling by increasing the expression of Notch ligands and receptors (Tschaharganeh et al., 2013; Yimlamai et al., 2014). Thus, we investigated whether Yap modulates Notch signaling in esophageal basal cells by immunostaining. In E18.5 WT mice, esophageal epithelium suprabasal cells stained robustly for nuclear cleaved NICD1, a pattern corroborated by Notch3 staining, which is relatively enriched in the nuclei of suprabasal cells and in the plasma membrane of basal cells, indicative of Notch activation in suprabasal cells, but not basal cells (Fig. 6). Yap deletion leads to reduced nuclear NICD1 and Notch3 staining, whereas Yap OE esophageal epithelium displayed strong nuclear NICD1 staining in basal cell-adjacent suprabasal cells (parabasal cells) (Fig. 6A). In line with these findings, the mRNA levels of multiple ligands and receptors were decreased accompanied by reduced expression of the Notch signaling downstream target Hes1 (Fig. S3B). Together, these findings suggest that Yap crosstalks with Notch signaling during epithelial stratification in the developing esophagus.
Fig. 6.
Yap crosstalks with Notch during the differentiation of EPCs in the developing mouse esophagus. (A) Yap deletion and overexpression reduces and increases expression of cleaved NICD1, a Notch signaling effector, respectively. Note NICD1 expression is enriched in the nuclei of suprabasal cells at E18.5 (n=3 for each). (B) Yap deletion reduces expression of Notch3 in the developing mouse esophagus at E18.5 (n=3). Note that Notch3 is relatively enriched in the nuclei of parabasal cells (arrows), in contrast to relative enrichment in the plasma membrane of basal cells (arrowheads). Epi, epithelium. White dotted line indicates the basement membrane. Scale bars: 40 µm.
YAP plays a conserved role in hPSC-derived EPCs and YAP inhibition leads to reduced proliferation and stratification of 3D EPC organoids
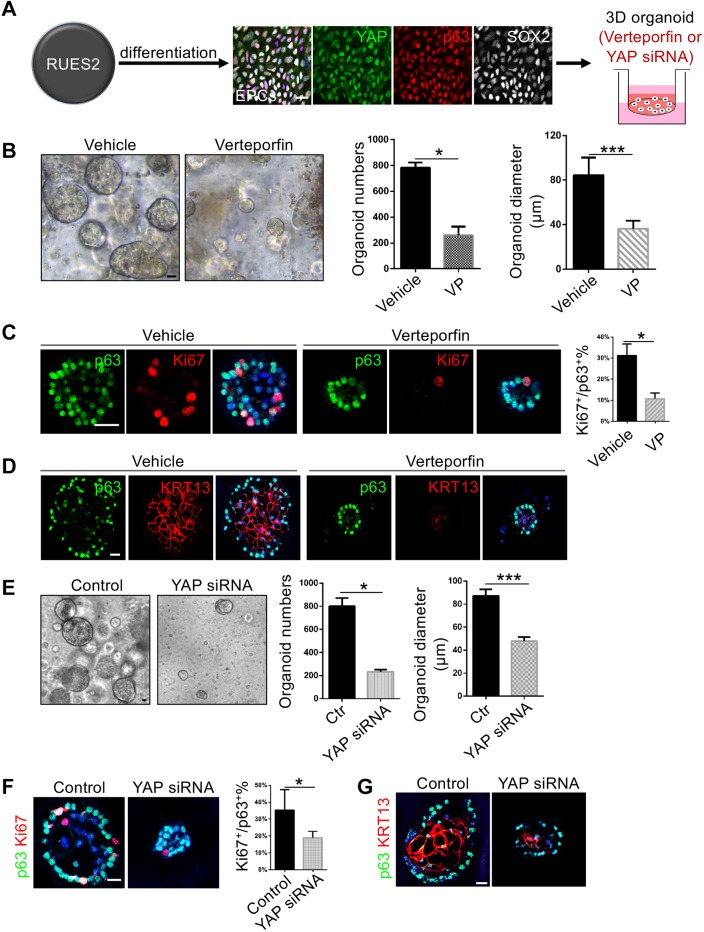
We next asked whether YAP is also required for the development of human esophagus. To begin to address this issue, we followed the protocol that we previously established to generate EPCs from hPSCs (Zhang et al., 2018). Here, we used the human embryonic stem cell line RUES2 (Fig. 7A). As previously described, we obtained a high differentiation efficiency with more than 80% cells being p63+ EPCs (data not shown) (Zhang et al., 2018). We then purified these EPCs with fluorescence-activated cell sorting (FACS) using antibodies against the cell surface markers EPCAM and ITGβ4. Importantly, we noticed that YAP is enriched in the nuclei of the purified p63+ EPCs (Fig. 7A). The expression of YAP was mainly localized in the nucleus and was higher in the peripheral cells of hPSC-derived esophageal organoids (Fig. S4A).
Fig. 7.
YAP is required for the growth of organoids established with hPSC-derived EPCs. (A) Schematic of the experimental protocol describing YAP inhibition and knockdown in organoids established with human embryonic stem cell RUES2-derived EPCs. Note YAP is expressed in p63+ SOX2+ EPCs. (B) YAP inhibition with verteporfin (VP) reduces the numbers and size of esophageal organoids (n=3). (C) YAP inhibition with VP reduces the proliferation (Ki67+) of EPCs (n=6). (D) YAP inhibition reduces the stratification of hPSC-derived esophageal organoids. (E) YAP knockdown by siRNA reduces the numbers and size of esophageal organoids (n=3). (F) YAP knockdown reduces the proliferation (Ki67+) of EPCs (n=5). (G) YAP knockdown reduces the stratification of hPSC-derived esophageal organoids. Data represent mean+s.e.m. *P<0.05, ***P<0.001 by unpaired, two-tailed Student's t-test. Scale bars: 20 μm.
YAP inhibition by verteporfin did not affect the efficiency of generating EPCs from the anterior foregut endodermal cells (Fig. S4B). We then treated the purified EPCs with verteporfin, and assessed whether blocking YAP affected EPCs in a 3D setting. Expression of YAP downstream targets was significantly reduced by verteporfin (Fig. S4C). Remarkably, verteporfin treatment reduced the number and size of 3D esophageal organoids (Fig. 7B). In line with these findings, the numbers of p63+Ki67+ cells were significantly reduced in individual organoids following drug treatment (Fig. 7C). Furthermore, YAP inhibition also reduced the stratification of the hPSC-derived-esophageal organoids as shown by expression of the basal cell marker p63 and suprabasal cell marker KRT13 (Fig. 7D). To confirm the role of YAP signaling in the growth of hPSC-esophageal organoids, we used siRNA to knock down YAP specifically. Consistently, YAP knockdown led to reduced expression of downstream targets (Fig. S4D). YAP knockdown also significantly reduced the numbers and sizes of organoids (Fig. 7E), accompanied by decreased proliferation and stratification of esophageal epithelium (Fig. 7F,G). Taken together, these results indicate that YAP plays conserved roles in regulation of the proliferation and squamous differentiation of hPSC-derived esophagus in a 3D setting.
DISCUSSION
Establishment of a stratified squamous epithelium in the esophagus requires coordinated proliferation and differentiation of EPCs. Here, we combined mouse genetic models and hPSC differentiation to demonstrate that Yap is primarily a crucial regulator of EPC proliferation, and that Yap, by controlling EPC proliferation, may indirectly impact subsequent epithelial stratification. This role appears to be evolutionarily conserved, as YAP inhibition or knockdown similarly leads to reduced number, size and stratification of hPSC-derived esophageal organoids.
We showed that basally located EPCs retain nuclear Yap throughout esophageal development. This agrees with previous findings in pulmonary and epidermal basal cells (Zhang et al., 2011; Mahoney et al., 2014; van Soldt et al., 2019). In these systems, the nuclear Yap is required for basal cell maintenance: Yap-deficient basal cells differentiate aberrantly, and constitutive nuclear Yap accumulation causes basal cell hyperplasia (Zhang et al., 2011). Surprisingly, we found that esophageal basal cells were not ablated upon Yap deletion. Thus, Yap seems to be required for the proliferation, but not for the initial specification and maintenance, of basal cells during esophageal development.
Yap is sequestered to the cytoplasm in differentiating basal cells, as was demonstrated in the developing and adult tracheal and epidermal epithelia. In these tissues, cytoplasmic Yap sequestration is a requirement for differentiation. Constitutive nuclear Yap accumulation inhibits the differentiation of airway epithelial progenitors in the developing lung as well as adult epidermal basal cells, but also causes ectopic expression of basal cell markers in adult tracheal epithelial cells (Zhang et al., 2011; Mahoney et al., 2014; Zhao et al., 2014; Elbediwy et al., 2016). Indeed, we found that esophageal suprabasal cells are also characterized by cytoplasmic Yap. However, overexpression of constitutive nuclear Yap did not hinder esophageal suprabasal cell differentiation. To the contrary, we observed aberrant expansion of the Krt8+Krt13+ suprabasal population despite ectopic nuclear Yap accumulation, presumably due to basal cell hyperproliferation and increased production of basal cell-derived suprabasal cells. Thus, our data are compatible with a model wherein Yap plays a role in the regulation of basal cell proliferation, but not maintenance of basal cell identity, as it does in epidermal or tracheal basal cells. Intriguingly, we were able to replicate these roles in hPSC-derived esophageal organoids, suggesting possible evolutionary conservation of these roles of Yap in esophageal development.
The mechanisms by which Yap exerts its regulatory role in the esophageal basal cells are an area for further study. One candidate of interest is Notch signaling, as generation of a stratified squamous epithelium involves the activity of Notch, and suppression of Notch signaling leads to reduced squamous differentiation (Zhang et al., 2018). Here, we provide evidence for Yap-Notch crosstalk during esophageal development, perhaps through inhibition of Notch activation in suprabasal cells.
Significantly, increased levels of both cytoplasmic and nuclear YAP have been found in dysplastic esophageal epithelium and esophageal malignancies (Lam-Himlin et al., 2006; Song et al., 2014 2015; Wang et al., 2018). YAP activation and nuclear localization is necessary for the anterior gradient homolog 2 (AGR2)-induced upregulation of the EGFR ligand amphiregulin in esophageal adenocarcinoma cell lines (Dong et al., 2011). In addition, the YAP-associated transcription factor p73 (TP73) is enriched in basal progenitor cells of the adult human esophagus (Strano et al., 2001; Matsha et al., 2007) and high levels of p73 are correlated with an increased risk of esophageal squamous cell carcinoma (ESCC) following human papillomavirus infection (Matsha et al., 2007). Our studies demonstrated that YAP is also enriched in EPCs of the developing esophagus. YAP inhibition leads to severely reduced proliferation of mouse and hPSC-derived EPCs. Conversely, overactivation of YAP leads to expanded EPCs, confirming that YAP is a crucial regulator of cell proliferation. We have previously shown that basal progenitor cells serve as the cell of origin for both ESCC and esophageal adenocarcinoma (Liu fet al., 2013; Jiang et al., 2017). It is possible that YAP acts as an oncogene to transform these EPCs at the very early stages of these malignancies.
In summary, we utilized mouse genetic models and hPSC differentiation to identify that Yap is a crucial regulator of esophageal epithelial morphogenesis. Inhibition of Yap by genetic ablation or verteporfin administration leads to reduced EPC proliferation. Conversely, ectopic nuclear accumulation of Yap promotes EPC proliferation and excess production of suprabasal cells, causing epithelial thickening. Our findings not only provide important insights into the mechanism regulating esophageal development in both mouse and human, but also offer the first genetic evidence that Yap is an important modulator of epithelial proliferation in the esophagus. Significantly, ESCCs with high levels of Yap have a low survival rate, and Yap can serve as an independent predictor for poor prognosis (Muramatsu et al., 2011). In light of these studies, our findings may have implications for the search for therapeutic targets against esophageal malignancy.
MATERIALS AND METHODS
Animal maintenance
Shh-Cre (Harfe et al., 2004), Yaploxp/loxp (Camargo et al., 2007) and R26Yap5SA (Cotton et al., 2017) mouse lines have been described previously. Mouse strains were maintained on a C57BL/6 and 129SvEv mixed background, were analyzed at 8-24 weeks of age, and both sexes were used. Tail DNA was used to genotype mice. Mice were maintained under pathogen-free conditions with a 12-h light/night cycle. Food and water were provided ad libitum and experimental procedures were conducted according to protocols approved by the Columbia University Institutional Animal Care and Use Committee (IACUC).
Differentiation and 3D organoid culture of hPSC-derived EPCs
The RUES2 human pluripotent stem cell line (Rockefeller University Embryonic Stem Cell Line 2, NIH approval number NIHhESC-09-0013) was kindly provided by the Mount Sinai Stem Cell Core facility and cultured as previously described (Zhang et al., 2018). This cell line has recently been authenticated and tested for contamination. To maintain RUES2 cells, CF-1 MEF (MTI-GlobalStem) mitotically arrested by irradiation was plated at a density of ∼25,000 cells/cm2. The next day, RUES2 cells were cultured on mouse embryonic fibroblasts in maintenance medium: 400 ml DMEM/F12 (Thermo Fisher Scientific), 100 ml KnockOut Serum Replacement (Thermo Fisher Scientific), 5 ml GlutaMAX (Thermo Fisher Scientific), 5 ml MEM non-essential amino acids solution (Thermo Fisher Scientific), 3.5 μl 2-mercaptoethanol (Sigma-Aldrich), 1 ml primocin (Thermo Fisher Scientific) and FGF2 (R&D Systems) at a final concentration of 20 ng/ml to make a total volume of ∼500 ml. Cells were kept in an incubator of 95% humidity, 95% air and 5% CO2 at 37°C. For passaging, cells were incubated with Accutase/EDTA (Innovative Cell Technologies) for 2 min and split at a ratio of 1:20. Human embryonic stem/induced pluripotent cell research was conducted under the approval of the Columbia University Human Embryonic and Human Embryonic Stem Cell Research Committee. Cells were cultured in serum-free differentiation (SFD) medium during differentiation induction. SFD medium was prepared as follows: 750 ml reconstituted IMDM (Thermo Fisher Scientific), 250 ml F-12 (Corning), 7.5 ml 7.5% Bovine Albumin Fraction V Solution (Thermo Fisher Scientific), 10 ml GlutaMAX (Thermo Fisher Scientific), 10 ml B27 (Thermo Fisher Scientific), 5 ml N2 (Thermo Fisher Scientific), 10 ml Penicillin/Streptomycin (Thermo Fisher Scientific), 50 μg/ml L-ascorbic acid (Sigma-Aldrich) and 0.04 μl/ml MTG (Sigma-Aldrich). Endoderm was induced with SFD medium supplemented with 10 μM Rock inhibitor Y-27632 (Tocris), 100 ng/ml Activin A, 2.5 ng FGF2 and 0.5 ng/ml BMP4 (R&D Systems) in 6-well Ultra-Low-Attachment plates (Corning) for 3 days. Anterior foregut progenitor cells were differentiated using SFD medium supplemented with 10 μM SB431542 (Tocris) and 100 ng/ml Noggin (R&D Systems) for 2 days. To induce esophageal differentiation, cells were further cultured with SFD medium supplemented with 100 ng/ml epidermal growth factor (EGF), 100 ng/ml Noggin and 10 μM SB431542 another 10 days and SFD medium supplemented 100 ng/ml EGF for 8 days. EGF was added to SFD medium to promote cell growth. The specification of EPCs was confirmed by the expression of p63 and SOX2. EPCs were re-plated and cultured for 5 days and purified with EPCAM and ITGβ4 surface markers. For 3D organoid culture 25, 000 ITGβ4+ EPCAM+ sorted EPCs in 75 μl medium were mixed with 75 μl Matrigel (Corning) in 24-well cell culture inserts (Falcon) and cultured for 2 weeks. The 3D organoid culture medium included SFD medium supplemented with 200 ng/ml EGF, 10 μM Y27632, 100 ng/ml noggin, 10 μM SB431542, 3 μM CHIR99021 and 20 ng/ml FGF2 (Zhang et al., 2018). To inhibit YAP signaling, 100 nM verteporfin was added into the medium and DMSO vehicle was used as control. For YAP knockdown, cells were transfected with YAP siRNA or control siRNA (Santa Cruz Biotechnology, sc-38637 and sc-37007, respectively) using Lipofectamine RNAiMAX (Thermo Fisher Scientific).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the RNeasy Plus Universal Mini Kit (Qiagen) and reverse transcribed using the SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific). All the amplifications were performed in the StepOnePlus Real-Time PCR System (Applied Biosystems) using SYBR Green Supermix (Bio-Rad). All experiments were performed at least in triplicate. Transcript levels of all genes were normalized to β-actin using 2(−ΔΔCT) method. The fold change of each gene was calculated as fold change of the indicated samples. qRT-PCR primer sequences are listed in Table S2.
Hematoxylin & Eosin staining, immunofluorescence, and microscopy imaging
For paraffin sections (8 μm), tissues were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, dehydrated with ethanol and Histoclear and embedded in paraffin. For cryosections (8 μm), tissues were fixed in 4% PFA in 1× PBS at 4°C overnight, infiltrated with 30% sucrose in 1× PBS overnight or until the tissue sinks, and embedded in OCT. Cells in culture were fixed in 4% PFA in 1× PBS at room temperature for 15 min. For Hematoxylin & Eosin (H&E) staining, tissues were stained with Hematoxylin solution for 5 min and rinsed with deionized water. Tissue was then stained with Eosin solution for 1 min and dehydrate with ethanol and mounted with Permount Mounting Medium (Fisher Scientific). To perform immunofluorescence staining, primary antibodies were incubated on the tissue at 4°C overnight and the next day further stained by secondary antibodies conjugated to Alexa Fluor 488, 568, Cy3 and 647 (Thermo Fisher Scientific, Jackson ImmunoResearch; Table S1) for 2 h. For EdU incorporation, pregnant mice were administered 1 mg (5 mg/ml stock solution in PBS) EdU solution (Thermo Fisher Scientific, C10340) by intraperitoneal injection 2 h prior to embryo harvest. EdU incorporation was assessed using Click-iT EdU Alexa Fluor 647 Imaging Kit (Thermo Fisher Scientific, C0340). Images were taken using a DMi8 fluorescence microscope (Leica Microsystems) and a Zeiss LSM700 confocal laser scanning microscope (Carl Zeiss). Brightfield images were acquired using a Nikon SMZ1500 inverted microscope (Nikon). Antibodies are listed in Table S1.
Quantification and statistical analysis
Data were analyzed using GraphPad Software Prism 6 and are presented as mean+s.e.m. Statistical significance was determined by two-tailed, unpaired Student's t-tests. For multiple groups, one-way ANOVA was used for comparisons followed by Bonferroni correction. At least three biological replicates were determined for each analysis. P-values of 0.05 or less were considered to be statistically significant.
Supplementary Material
Acknowledgements
We thank members of the Que and Cardoso laboratories for critically reading this manuscript and helpful inputs. We are also grateful to Lynna Tsai for her expertise in genotyping and immunostaining. We thank the Columbia Center for Translational Immunology (CCTI) Flow Cytometry Core at Columbia University Medical Center (supported in part by the Office of the Director, NIH under the award S10OD020056).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.D.B., Y.Z., B.J.v.S., A.K.R., S.S.A., W.V.C., J.Q.; Methodology: D.D.B., Y.Z., B.J.v.S., M.J., J.Q.; Validation: J.Q.; Formal analysis: D.D.B., B.J.v.S., S.S.; Investigation: D.D.B., Y.Z., B.J.v.S., M.J., S.S., H.N., J.Q.; Resources: H.N.; Writing - original draft: Y.Z., J.Q.; Writing - review & editing: D.D.B., Y.Z., A.K.R., W.V.C., J.Q.; Supervision: J.Q.; Project administration: J.Q.; Funding acquisition: J.Q.
Funding
This work in the Que lab was supported by the National Institutes of Health (DK113144, DK100342 and DK120650 to J.Q.), and the Price Family Foundation. D.D.B. was supported by the National Institutes of Health (T32-DK083256 and HL132996-02S1; Administrative Supplement, J.Q.), a training grant from the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) (U54AI117804), the NASPGHAN Foundation/NASPGHAN George Ferry Young Investigator Award (CEGIR is part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS and patient advocacy groups including APFED, CURED, and EFC), and gifts from the Phyllis and Ivan Seidenberg Family Fund for Children's Digestive Health. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.178855.supplemental
References
- Cai J., Zhang N., Zheng Y., de Wilde R. F., Maitra A. and Pan D. (2010). The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383-2388. 10.1101/gad.1978810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo F. D., Gokhale S., Johnnidis J. B., Fu D., Bell G. W., Jaenisch R. and Brummelkamp T. R. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054-2060. 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- Cotton J. L., Li Q., Ma L., Park J.-S., Wang J., Ou J., Zhu L. J., Ip Y. T., Johnson R. L. and Mao J. (2017). YAP/TAZ and hedgehog coordinate growth and patterning in gastrointestinal mesenchyme. Dev. Cell 43, 35-47.e4. 10.1016/j.devcel.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A., Gupta A., Pai R. K., Tun M. and Lowe A. W. (2011). The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J. Biol. Chem. 286, 18301-18310. 10.1074/jbc.M110.215707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A., Vincent-Mistiaen Z. I., Spencer-Dene B., Stone R. K., Boeing S., Wculek S. K., Cordero J., Tan E. H., Ridgway R., Brunton V. G. et al. (2016). Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 143, 1674-1687. 10.1242/dev.133728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M. R., Khomchuk Y. and Wrana J. L. (2015). Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715-718. 10.1038/nature15382 [DOI] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- Harris-Johnson K. S., Domyan E. T., Vezina C. M. and Sun X. (2009). beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287-16292. 10.1073/pnas.0902274106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. X. L., Islam M. N., O'Neill J., Hu Z., Yang Y.-G., Chen Y.-W., Mumau M., Green M. D., Vunjak-Novakovic G., Bhattacharya J. et al. (2014). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84-91. 10.1038/nbt.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I. J., Ku W.-Y. and Que J. (2012). Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev. Biol. 369, 54-64. 10.1016/j.ydbio.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Li H., Zhang Y., Yang Y., Lu R., Liu K., Lin S., Lan X., Wang H., Wu H. et al. (2017). Transitional basal cells at the squamous-columnar junction generate Barrett's oesophagus. Nature 550, 529-533. 10.1038/nature24269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam-Himlin D. M., Daniels J. A., Gayyed M. F., Dong J., Maitra A., Pan D., Montgomery E. A. and Anders R. A. (2006). The Hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Int. J. Gastrointest. Cancer 37, 103-109. 10.1007/s12029-007-0010-8 [DOI] [PubMed] [Google Scholar]
- Liu K., Jiang M., Lu Y., Chen H., Sun J., Wu S., Ku W.-Y., Nakagawa H., Kita Y., Natsugoe S. et al. (2013). Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell 12, 304-315. 10.1016/j.stem.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y., Huang B., Shim J. S., Chen Q., Lee S.-J., Anders R. A., Liu J. O. and Pan D. (2012). Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300-1305. 10.1101/gad.192856.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmire T. A., Ikonomou L., Hawkins F., Christodoulou C., Cao Y., Jean J. C., Kwok L. W., Mou H., Rajagopal J., Shen S. S. et al. (2012). Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398-411. 10.1016/j.stem.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney J. E., Mori M., Szymaniak A. D., Varelas X. and Cardoso W. V. (2014). The Hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137-150. 10.1016/j.devcel.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsha T., Donninger H., Erasmus R. T., Hendricks D., Stepien A. and Parker M. I. (2007). Expression of p53 and its homolog, p73, in HPV DNA positive oesophageal squamous cell carcinomas. Virology 369, 182-190. 10.1016/j.virol.2007.07.025 [DOI] [PubMed] [Google Scholar]
- Mauviel A., Nallet-Staub F. and Varelas X. (2012). Integrating developmental signals: a Hippo in the (path)way. Oncogene 31, 1743-1756. 10.1038/onc.2011.363 [DOI] [PubMed] [Google Scholar]
- McCracken K. W., Catá E. M., Crawford C. M., Sinagoga K. L., Schumacher M., Rockich B. E., Tsai Y.-H., Mayhew C. N., Spence J. R., Zavros Y. et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400-404. 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Zhao R., Sherwood R., Ahfeldt T., Lapey A., Wain J., Sicilian L., Izvolsky K., Lau F. H., Musunuru K. et al. (2012). Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10, 385-397. 10.1016/j.stem.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Múnera J. O., Sundaram N., Rankin S. A., Hill D., Watson C., Mahe M., Vallance J. E., Shroyer N. F., Sinagoga K. L., Zarzoso-Lacoste A. et al. (2017). Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell 21, 51-64.e6. 10.1016/j.stem.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T., Imoto I., Matsui T., Kozaki K.-I., Haruki S., Sudol M., Shimada Y., Tsuda H., Kawano T. and Inazawa J. (2011). YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 32, 389-398. 10.1093/carcin/bgq254 [DOI] [PubMed] [Google Scholar]
- Que J. (2015). The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus. Wiley Interdiscip. Rev. Dev. Biol. 4, 419-430. 10.1002/wdev.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P., Da Silva S., Oxburgh L., Wang F., Hogan B. L. M. and Que J. (2010). BMP signaling in the development of the mouse esophagus and forestomach. Development 137, 4171-4176. 10.1242/dev.056077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Ajani J. A., Honjo S., Maru D. M., Chen Q., Scott A. W., Heallen T. R., Xiao L., Hofstetter W. L., Weston B. et al. (2014). Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 74, 4170-4182. 10.1158/0008-5472.CAN-13-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Honjo S., Jin J., Chang S.-S., Scott A. W., Chen Q., Kalhor N., Correa A. M., Hofstetter W. L., Albarracin C. T. et al. (2015). The Hippo coactivator YAP1 Mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin. Cancer Res. 21, 2580-2590. 10.1158/1078-0432.CCR-14-2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M. et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105-109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S., Munarriz E., Rossi M., Castagnoli L., Shaul Y., Sacchi A., Oren M., Sudol M., Cesareni G. and Blandino G. (2001). Physical interaction with yes-associated protein enhances p73 transcriptional activity. J. Biol. Chem. 276, 15164-15173. 10.1074/jbc.M010484200 [DOI] [PubMed] [Google Scholar]
- Tschaharganeh D. F., Chen X., Latzko P., Malz M., Gaida M. M., Felix K., Ladu S., Singer S., Pinna F., Gretz N. et al. (2013). Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 144, 1530-1542.e12. 10.1053/j.gastro.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soldt B. J., Qian J., Li J., Tang N., Lu J. and Cardoso W. V. (2019). Yap and its subcellular localization have distinct compartment-specific roles in the developing lung. Development 146, dev175810 10.1242/dev.175810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X. (2014). The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614-1626. 10.1242/dev.102376 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang Z., Yu X., Huang X., Liu Z., Chai Y., Yang L., Wang Q., Li M., Zhao J. et al. (2018). Unbalanced YAP–SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene 38, 2042-2055. 10.1038/s41388-018-0476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D., Christodoulou C., Galli G. G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B. Z. and Camargo F. D. (2014). Hippo pathway activity influences liver cell fate. Cell 157, 1324-1338. 10.1016/j.cell.2014.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.-Y., Slack J. M. W. and Tosh D. (2005). Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev. Biol. 284, 157-170. 10.1016/j.ydbio.2005.04.042 [DOI] [PubMed] [Google Scholar]
- Yui S., Azzolin L., Maimets M., Pedersen M. T., Fordham R. P., Hansen S. L., Larsen H. L., Guiu J., Alves M. R. P., Rundsten C. F. et al. (2018). ‘YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration’. Cell Stem Cell 22, 35-49.e7. 10.1016/j.stem.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Pasolli H. A. and Fuchs E. (2011). Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 108, 2270-2275. 10.1073/pnas.1019603108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jiang M., Kim E., Lin S., Liu K., Lan X. and Que J. (2017). Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 66, 25-35. 10.1016/j.semcdb.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang Y., Jiang M., Huang S. X., Zhang W., Al Alam D., Danopoulos S., Mori M., Chen Y.-W., Balasubramanian R. et al. (2018). 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23, 516-529.e5. 10.1016/j.stem.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Fallon T. R., Saladi S. V., Pardo-Saganta A., Villoria J., Mou H., Vinarsky V., Gonzalez-Celeiro M., Nunna N., Hariri L. P. et al. (2014). Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 30, 151-165. 10.1016/j.devcel.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Zhang Y., Wu H., Barry E., Yin Y., Lawrence E., Dawson D., Willis J. E., Markowitz S. D., Camargo F. D. et al. (2011). Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 108, E1312-E1320. 10.1073/pnas.1110428108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.