Abstract
Background
Long-term exposure to hypertonic and high glucose in peritoneal dialysis fluid can result in peritoneal fibrosis. Spleen tyrosine kinase (SYK) has a role in inflammation and fibrosis. This study aimed to investigate the role of SYK in an in vivo rat model of peritoneal fibrosis and in rat peritoneal mesothelial cells (PMCs) in vitro and to investigate the underlying mechanisms.
Material/Methods
Sprague-Dawley rats (N=24) were randomized into the sham control group (N=6); the peritoneal fibrosis group (N=6) treated with intraperitoneal chlorhexidine digluconate; the SYK inhibitor group (N=6), treated with chlorhexidine digluconate and fostamatinib; and the TGF-β inhibitor group (N=6), treated with chlorhexidine digluconate and LY2109761. The rat model underwent daily intraperitoneal injection with 0.5 ml of 0.1% chlorhexidine digluconate. Rat peritoneal mesothelial cells (PMCs) were cultured in vitro in high glucose. SYK expression was measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot. Enzyme-linked immunosorbent assay (ELISA) and qRT-PCR measured inflammatory mediators. Transforming growth factor-β1 (TGF-β1) and Smad3 were detected by Western blot. Short hairpin RNA (shRNA) was used to target the SYK gene.
Results
SYK was upregulated in the rat model of peritoneal fibrosis and was induced rat PMCs cultured in high glucose. Knockdown of SYK and inhibition of TGF-β1 significantly reduced fibrosis and inflammation. Findings in the in vivo rat model confirmed that SYK mediated peritoneal fibrosis by regulating TGF-β1/Smad3 signaling.
Conclusions
In a rat model and in rat PMCs, expression of SYK increased peritoneal fibrosis through activation of the TGF-β1/Smad3 signaling pathway.
MeSH Keywords: Nephrology, Peritoneal Dialysis, Peritoneal Fibrosis
Background
Peritoneal dialysis is widely used for the treatment of diseases that include chronic kidney failure [1] and peritonitis [2]. Peritoneal dialysis uses peritoneum as a semi-permeable membrane and can remove metabolites, toxic substances, and correct water and electrolyte imbalance [3,4]. However, the development of peritoneal fibrosis is currently a major challenge for long-term peritoneal dialysis [3,5]. Peritoneal fibrosis can result in loss of the mesothelial cell layer of the peritoneum, thickening of the sub-mesothelial layer, and vascular angiogenesis [6]. Previously published studies have shown that several factors, including hypoxia, disorders of the endocrine system, and the release of inflammatory mediators were associated with the development of peritoneal fibrosis [7]. However, the molecular mechanisms underlying the pathogenesis of peritoneal fibrosis remain unclear.
Spleen tyrosine kinase (SYK), a cytosolic non-receptor tyrosine kinase, is widely expressed in red blood cells, platelets, vascular endothelial cells, fibroblasts, bronchial epithelial cells, hepatocytes, and osteoclasts [7,8]. SYK is involved in intracellular signaling and transmission of activation signals of membrane receptors to downstream effector molecules [9,10]. The immune and inflammatory responses associated with SYK expression are involved in the pathogenesis of allergic diseases, some types of cancer, and infectious diseases [11]. The findings from a recently published study identified SYK is a potential therapeutic target to control liver fibrosis [12]. However, the role of SYK and the underlying molecular mechanisms in the pathogenesis of peritoneal fibrosis remain unclear.
Previously published studies have shown that cytokines are involved in the development of peritoneal fibrosis, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β, and monocyte chemoattractant protein-1 (MCP-1) [13–15]. The transforming growth factor-β (TGF-β) signaling pathway includes a large family of multifunctional cytokines that regulate cell growth, proliferation, differentiation, migration, and cell apoptosis [16]. The TGF-β/Smad3 signaling pathway is an important pathway that has a role in the development of liver fibrosis [17]. SYK has been shown to coordinate with TGF-β in erythropoiesis [18]. However, whether SYK can modulate TGF-β/Smad3 signaling in peritoneal fibrosis remains to be investigated.
Therefore, this study aimed to investigate the role of SYK in an in vivo rat model of peritoneal fibrosis and rat peritoneal mesothelial cells (PMCs) in vitro and to investigate the underlying mechanisms.
Material and Methods
Animals and treatment
Adult male Sprague–Dawley (SD) rats (N=24) between 8–10 weeks old, weighing 205–300 g were purchased from Hunan SJA Laboratory Animal Co. Ltd. (Hunan, China). The rats were maintained in with a 12-hourly light and dark cycle and a controlled temperature (23–25°C), and had free access to food and water. This study was approved by the Institutional Animal Care Committee at The First Affiliated Hospital of Hunan Normal University.
After four days of regular feeding, the rats were randomized into four groups: the sham control group (N=6); the peritoneal fibrosis group treated with intraperitoneal chlorhexidine digluconate (N=6); the SYK inhibitor group treated with chlorhexidine digluconate and fostamatinib (N=6); and the TGF-β inhibitor group treated with chlorhexidine digluconate and LY2109761 (N=6). Rats in the model of peritoneal fibrosis underwent intraperitoneal injection with 0.5 ml 0.1% chlorhexidine digluconate (Sigma-Aldrich, St. Louis, MO, USA) daily for three weeks. Control rats were injected with an equal volume of 0.9% saline daily for three weeks. The SYK inhibitor, fostamatinib (40 mg/kg), was given by gavage twice daily at the same time when the rats were treated with chlorhexidine digluconate. The TGF-β inhibitor, LY2109761 (50 mg/kg), was given by gavage twice daily at the same time when the rats were treated with chlorhexidine digluconate. All rats were euthanized after 21 days of treatment, at which time, peritoneal tissues sampled from all rats.
Histology and immunohistochemistry
Rat peritoneal tissue was fixed in 10% formalin, embedded in paraffin wax, sectioned onto glass slides and stained histochemically with hematoxylin and eosin (H&E) and Masson’s trichrome for collagen. Tissue sections were viewed and analyzed by light microscopy.
Immunohistochemistry was performed on the tissue section of rat peritoneal tissue by incubating the dewaxed tissue sections overnight at 4°C in the primary antibody to SYK (Abcam, Cambridge, MA, USA). After washing, the tissue sections were incubated in the secondary antibody at 37°C for 45 min. The intensity of immunostaining (IHC score) was calculated visually as the sum of the staining intensity and area of tissue staining, as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining); 0 (none), 1 (<1/100), 2 (1/100 to 1/10), 3 (1/10 to 1/3), 4 (1/3 to 2/3), and 5 (>2/3) [12].
Cell culture and cell transfection
Rat peritoneal mesothelial cells (PMCs) were isolated using standard trypsin/EDTA method, as previously described [19]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 100 μg/mL penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C with 5% CO2. The cells were divided into two groups, the high glucose (HG) group, in which cells were treated with 126 mmol/L glucose and were cultured in 10% FBS-DMEM medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) for 3 days, and the control group, in which cells were treated with a normal concentration of glucose (5.6 mmol/L) in 10% FBS-DMEM medium for 3 days.
Cells were transfected with short hairpin RNA (shRNA) for SYK (sh-SYK) or included a negative control (NC) (5 nM) (Shanghai GeneChem Co. Ltd., Shanghai, China), and with pcDNA3.1-SYK or blank vector (5 nM) (Shanghai GeneChem Co. Ltd., Shanghai, China) using Lipofectamine 2000 (GenePharma Co., Shanghai, China), according to the manufacturer’s instructions. After 48 h of transfection, the transfected cells were harvested and analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). The TGF-β inhibitor, LY2109761 (0.5 mg/mL), was used to suppress TGF-β signaling [20,21]. The SYK inhibitor, fostamatinib (5 μmol/L) was used to inhibit SYK [22].
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to measure inflammatory cytokines in serum or cell supernatants. After the rats were euthanized, blood samples (2 ml) were collected in tubes without EDTA to obtain the serum. The supernatant was obtained after centrifugation at 1800 x g for 10 min at room temperature. Serum levels of TNF-α, IL-6, IL-1β, and MCP-1 were measured using commercial ELISA kits, including a rat TNF-α ELISA Kit (ab46070) (Abcam, Cambridge, MA, USA), a rat IL-6 ELISA Kit (ab234570) (Abcam, Cambridge, MA, USA), a rat IL-1β ELISA Kit (ab100767) (Abcam, Cambridge, MA, USA), and a rat MCP-1 ELISA Kit (ab219045) (Abcam, Cambridge, MA, USA), according to manufacturer’s instruction.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Tiangen Biotech, Beijing, China). The PrimeScript™ One Step qRT-PCR kit (Takara, Dalian, China) was used to convert RNA to cDNA. The PCR reactions were performed using an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primer sequences were as follows:
SYK, forward: 5′-CATGTCAAGGATAAGAACATCATAGA-3′;
SYK, reverse: 5′-AGTTCACCACGTCAIAGTAGTAAATT-3′;
TNF-α, forward: 5′-GTGATCGGTCCCAACAAGGA-3′;
TNF-α, reverse: 5′-TTTGCTACGACGTGGGCTAC-3′;
IL-1β, forward: 5′-GGACAGAACATAAGCCAACA-3′;
IL-1β, reverse: 5′-TCAGAGGCAGGGAGGGA-3′;
IL-6, forward: 5′-AGCCACTGCCTTCCCTACTTC-3′;
IL-6, reverse: 5′-GGTCCTTAGCCCACTCCTTCTG-3′;
MCP-1 Forward: 5′-ACTTGACCCATAAATCTGA-3′;
MCP-1 reverse: 5′-TGGAAGGGAATAGTGTAAT-3′;
GAPDH, forward: 5′-CCACAGTCCATGCCATCAC-3′;
GAPDH, eeverse: 5′-GCTTCACCACCTTCTTGATG-3′.
Relative expression levels were calculated by the 2−ΔΔCt method. GAPDH was used as an internal control.
Western blot
Cell lysates were extracted using RIPA buffer (CW Biotech Co. Ltd., Beijing, China). The BCA protein assay kit (CW Biotech Co. Ltd., Beijing, China) was performed to measure the protein concentration. Equal amounts of proteins were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to polyvinylidene fluoride (PVDF) membranes and incubated with primary antibodies, including anti-SYK antibody (ab40781) (Abcam, Cambridge, MA, USA), anti-collagen I antibody (ab34710) (Abcam, Cambridge, MA, USA), anti-vimentin antibody (ab8978) (Abcam, Cambridge, MA, USA), anti-VEGF-A antibody (ab46154) (Abcam, Cambridge, MA, USA), anti-E-cadherin antibody (ab1416) (Abcam, Cambridge, MA, USA), anti-α-SMA antibody, (ab32575) (Abcam, Cambridge, MA, USA), anti-TGF-β1 antibody (ab64715) (Abcam, Cambridge, MA, USA), anti-p-Smad3 antibody (ab52903) (Abcam, Cambridge, MA, USA), and anti-Smad3 antibody (ab40854) (Abcam, Cambridge, MA, USA). The membranes were then incubated in the secondary horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1/1000) (ab6721) (Abcam, Cambridge, MA, USA) or anti-mouse immunoglobulin G secondary antibody (1/1000) (ab6785) (Abcam, Cambridge, MA, USA). The results were analyzed using the enhanced chemiluminescence (ECL) Western blot substrate (Pierce Biotechnology, Shanghai, China). GAPDH was used as an internal control.
Statistical analysis
Data were expressed as the mean±standard deviation (SD). Comparison between two groups was performed using Student’s t-test, and comparison between three or more groups using one-way analysis of variance (ANOVA) followed by Tukey’s test. Pearson’s correlation analysis was used. A P-value of <0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS version 22.0 software (IBM, Chicago, IL, USA).
Results
Upregulation of SYK expression in peritoneal fibrosis
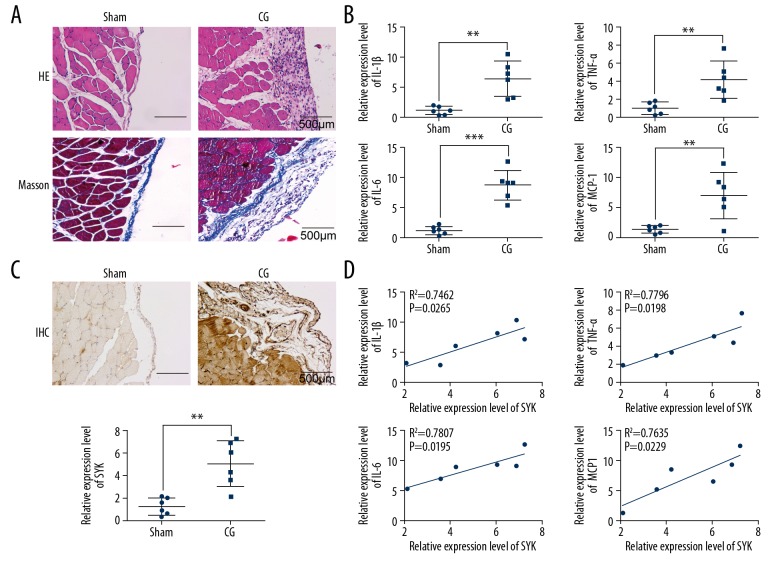
The rat model of peritoneal fibrosis was established to investigate the role of SYK in peritoneal fibrosis. Histology of the peritoneal tissue in the rat model group treated with intraperitoneal chlorhexidine digluconate showed significant hyperplasia and fibrosis compared with the sham control group (Figure 1A). Also, immunohistochemistry and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) showed that the expression of SYK was significantly upregulated in the peritoneal tissues in the rat model group compared with the sham control group (Figure 1B). In the rat model group treated with intraperitoneal chlorhexidine digluconate, the expression of inflammatory cytokines interleukin-1β (IL-1β), TNF-α, IL-6, and MCP-1 were significantly increased compared with the sham control group (Figure 1C). The expression of SYK was significantly correlated with the expression of IL-1β, TNF-α, IL-6, and MCP-1 (Figure 1D). These data indicated both SYK and inflammatory cytokines were upregulated in the rat model of peritoneal fibrosis treated with intraperitoneal chlorhexidine digluconate.
Figure 1.
Upregulation of SYK expression in peritoneal fibrosis. (A) Photomicrograph of the histology of changes in peritoneal tissue in the rat model of peritoneal fibrosis compared with the control. There is mesothelial cell hyperplasia and peritoneal thickening in the chlorhexidine digluconate-induced peritoneal fibrosis model group compared with the normal control group. Scale bar, 500 μm. (B) Immunohistochemistry and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) were used to detect the expression of SYK. Scale bar, 500 μm. ** P<0.01 vs. sham. (C) The expression of inflammatory cytokines, IL-1β, TNF-α, IL-6, and MCP-1 in rat serum were detected. ** P<0.01 and *** P<0.001 vs. sham. (D) The expression of SYK was significantly correlated with the levels of various inflammatory cytokines. All experiments were independently conducted in triplicate. CG – chlorhexidine digluconate.
High glucose induced peritoneal mesothelial cell fibrosis and inflammation
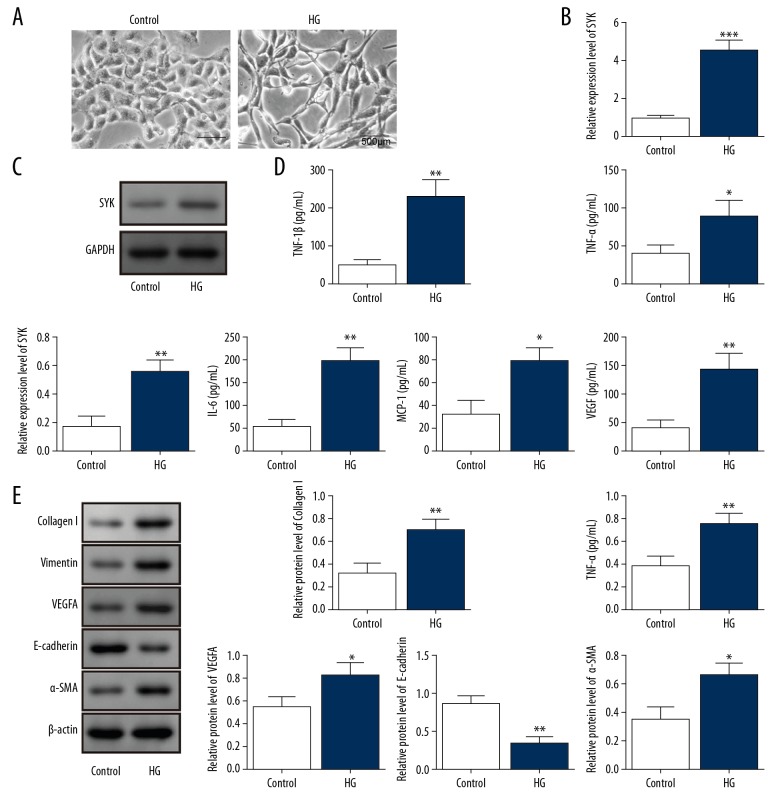
Rat peritoneal mesothelial cells (PMCs) were stimulated by high glucose for the in vitro model of peritoneal fibrosis. The morphology of the peritoneal mesothelial cells in the high glucose group showed a fibroblast-like spindle cell morphology when compared with the untreated control group (Figure 2A). Also, mRNA and protein levels of SYK were significantly increased in the high glucose group (Figure 2B, 2C). In the high glucose group, the levels of vascular endothelial growth factor (VEGF) and the inflammatory cytokines IL-1β, TNF-α, IL-6, and MCP-1 were significantly higher than the control group (Figure 2D). The expression of biomarkers of fibrosis, including α-SMA, collagen I, vimentin and VEGF-A were also significantly upregulated, while the expression of E-cadherin was significantly inhibited in the high glucose group (Figure 2E), indicating the transformation of PMCs. These findings supported that high expression of SYK was increased in the in vitro model of peritoneal fibrosis.
Figure 2.
High glucose induced peritoneal mesothelial cell (PMC) fibrosis and inflammation. (A) Microscopy shows that the rat peritoneal mesothelial cell (PMC) morphology in cells cultured in high levels of glucose have a fibroblast-like morphology compared with the control group. Scale bar, 500 μm. (B, C) SYK mRNA and protein levels were measured using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot analysis. ** P<0.01 and *** P<0.001 vs. control. (D) Levels of inflammatory cytokines IL-1β, TNF-α, IL-6, and MCP-1 in the supernatants were measured using enzyme-linked immunosorbent assay (ELISA). * P<0.05 and ** P<0.01 vs. control. (E) Expressions of α-SMA, collagen I, vimentin, VEGF-A, and E-cadherin were determined using Western blot. * P<0.05 and ** P<0.01 vs. control. All experiments were independently conducted in triplicate. HG – high glucose.
Knockdown of SYK reduced the expression of mediators of fibrosis and inflammation in PMCs in the high glucose group
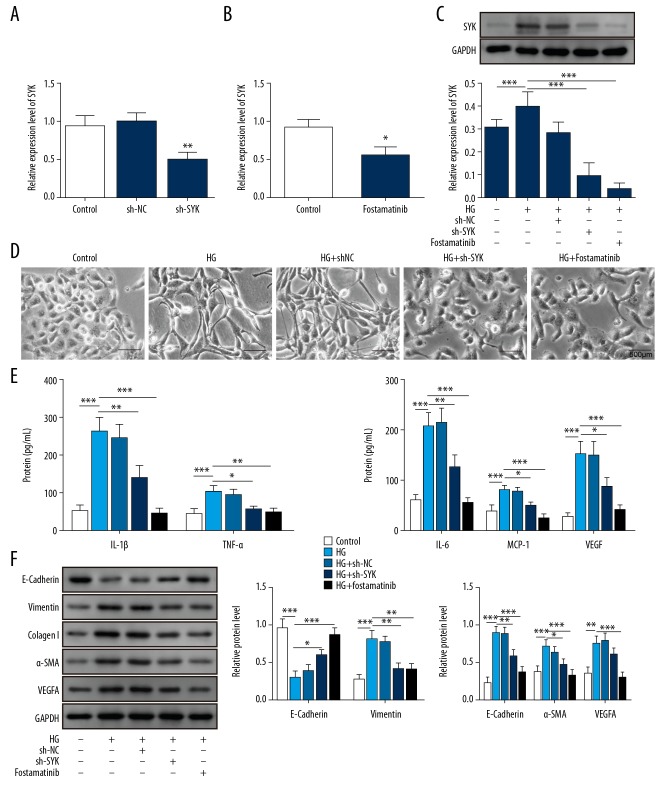
Short hairpin RNA (shRNA) that specifically targeted SYK and the use of its inhibitor, fostamatinib, were used to knock down SYK expression. As shown in Figure 3A and 3B, mRNA expression and protein expression levels of SYK were significantly down-regulated after transfection with sh-SYK or treatment with fostamatinib, supporting the successful knockdown of SYK (Figure 3C). When SYK expression was inhibited, fibril formation of rat PMCs was significantly reduced, and the cell morphology appeared to be normal in the SYK knockdown cells (Figure 3D). The expression of inflammatory and fibrosis-associated proteins was significantly inhibited in cells transfected with sh-SYK or treated with fostamatinib (Figure 3E, 3F). These findings supported that SYK was involved in fibrosis and inflammation associated with high glucose levels in rat PMCs.
Figure 3.
Knockdown of SYK reduced peritoneal mesothelial cell (PMC) fibrosis and inflammation in conditions of high glucose. (A, B) After knockdown of SYK and pretreating the rat PMCs with the SYK inhibitor, SYK expression was significantly reduced. ** P<0.01 vs. shNC. * P<0.05 vs. control. (C) High glucose treatment promoted upregulation of SYK, but the effect was significantly reversed after knockdown of SYK or pretreatment with SYK inhibitor. (D) High glucose treatment induced the transformation of peritoneal mesothelial cells to fibroblast-like cells, which was blocked by knockdown of SYK or pretreatment with the SYK inhibitor. Scale bar, 500 μm. (E) High glucose treatment induced the release of inflammatory cytokines, and the release of these factors was significantly reduced after knockdown of SYK or pretreating PMCs with its inhibitor. ** P<0.01 and *** P<0.001 vs. control. ** P<0.01 and *** P<0.001 vs. HG. * P<0.05 and ** P<0.01 vs. HG+shNC. (F) High glucose treatment promoted the upregulated expression of α-SMA, collagen I, vimentin, and VEGF-A, while the expression of E-cadherin was significantly inhibited. However, this effect was reversed after knockdown of SYK or pretreatment with its inhibitor. All experiments were independently conducted in triplicate. HG – high glucose.
SYK mediated peritoneal fibrosis by activating TGF-β1/Smad3 signaling in rat PMCs in vitro
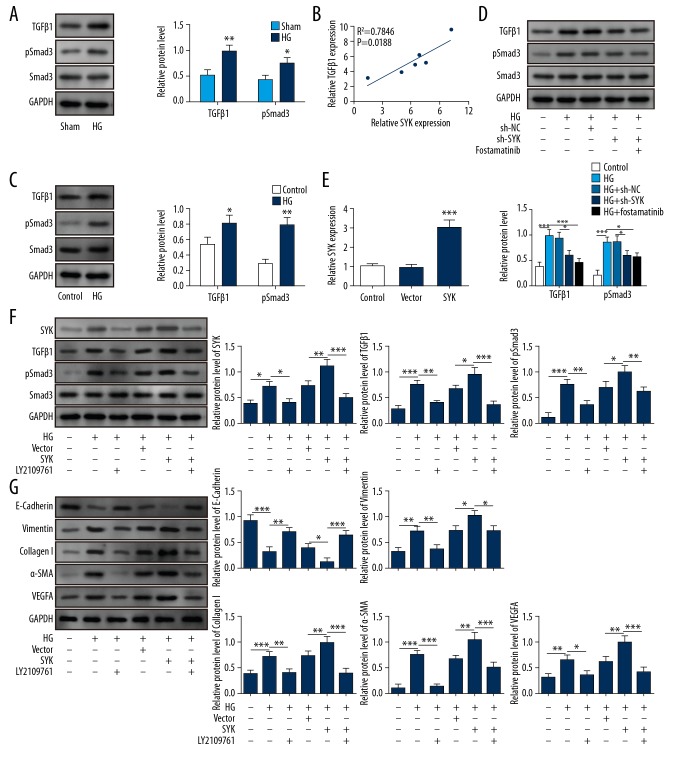
To clarify the underlying mechanisms for SYK in the pathogenesis of peritoneal fibrosis, we further investigated the relationship between SYK and the TGF-β1/Smad3 signaling pathway in rat PMCs cultured in high levels of glucose. As shown in Figure 4A, the activation of the TGF-β1/Smad3 signaling pathway was observed in rat peritoneal tissues of chlorhexidine digluconate-induced model of peritoneal fibrosis. SYK and TGF-β1 levels in all rat peritoneal tissues were increased (Figure 4B). Also, in rat PMCs cultured in high levels of glucose, both TGF-β1 and p-Smad3 was significantly increased (Figure 4C). However, when SYK was inhibited, the expression of TGF-β1 and p-Smad3 were down-regulated (Figure 4D). Overexpression of SYK significantly increased the expression of SYK, TGF-β1, and p-Smad3, as well as fibrosis-related proteins collagen I, vimentin, VEGF-A, E-cadherin, and α-SMA (Figure 4E–4G). However, inhibition of TGF-β1 by LY2109761 reversed the effects of SYK overexpression. These findings supported that SYK might modulate the pathogenesis of peritoneal fibrosis through regulation of TGF-β1/Smad3 signaling pathway.
Figure 4.
SYK mediated peritoneal mesothelial cell fibrosis by activating TGFb1/Smad3 signaling. (A) Western blot was performed to assess the expression of TGFb1/Smad3 signaling. * P<0.05 and ** P<0.01 vs. sham. (B) Pearson correlation between TGFb1 and SYK in the peritoneal fibrosis model. (C) The expression of TGF-β1/Smad3 signaling of high glucose-induced rat peritoneal mesothelial cells (PMCs) in the presence or absence of short hairpin RNA (shRNA) for SYK or its inhibitor was determined using Western blot. * P<0.05 and ** P<0.01 vs. the control. (D) High glucose (HG) induced activation of TGF-β1/Smad3 signaling. This effect was reversed after knockdown of SYK or pretreatment with its inhibitor. *** P<0.001 vs. the control. * P<0.05 and *** P<0.001 vs. HG. * P<0.05 vs. HG+shNC. (E) Overexpression of SYK shown by quantitative reverse transcription-polymerase chain reaction (qRT-PCR). *** P<0.001 vs. vector. (F) After pretreatment with the TGF-β signaling inhibitor, phosphorylation of SYK and activation of TGF-β1/Smad3 signaling were significantly inhibited. After pretreatment with the TGF-β signaling inhibitor, high glucose induction and SYK overexpression treatment also showed similar effects. * P<0.05 and *** P<0.001 vs. control. * P<0.05 and ** P<0.01 vs. HG. * P<0.05 and ** P<0.01 vs. HG+vector. ** P<0.01 and *** P<0.001 vs. HG+SYK. (G) Pretreatment with the TGF-β signaling inhibitor significantly inhibited the upregulation of α-SMA, collagen I, vimentin, and VEGF-A, and promoted the expression of E-cadherin. This effect was partially increased after high glucose induction and SYK overexpression. ** P<0.01 and *** P<0.001 vs. control. * P<0.05, ** P<0.01 and *** P<0.001 vs. HG. * P<0.05 and ** P<0.01 vs. HG+vector. * P<0.05 and *** P<0.001 vs. HG+SYK. All experiments were independently conducted in triplicate. HG – high glucose.
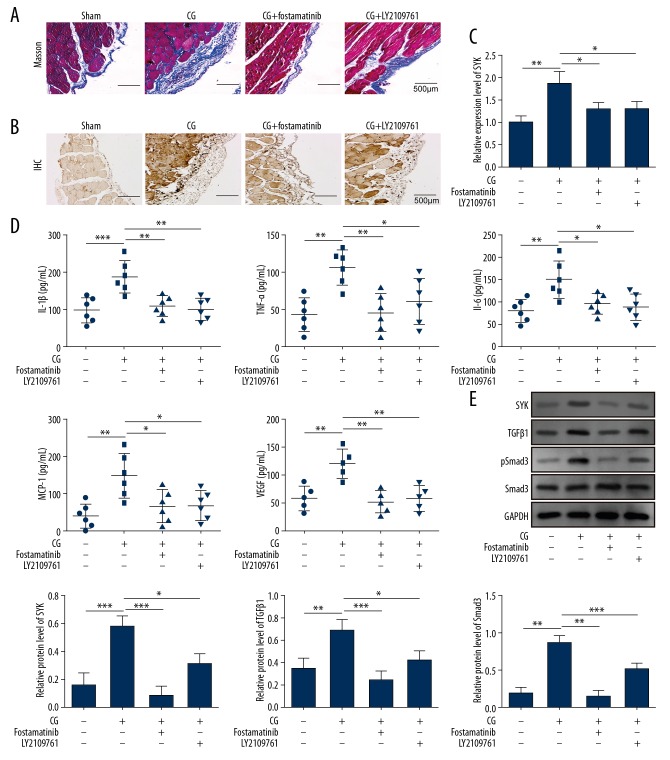
SYK mediated peritoneal fibrosis by regulating TGF-β1/Smad3 signaling in the rat model in vivo
The findings from the in vivo rat model supported that the role of SYK regulated by TGF-β1/Smad3 signaling. In the chlorhexidine digluconate-induced rat model, when SYK was inhibited by fostamatinib, or TGF-β1 was suppressed by LY2109761, Masson’s trichrome staining showed reduced collagen production in the rat peritoneal tissue (Figure 5A). SYK mRNA expression was also significantly reduced (Figure 5B, 5C). Down-regulation of SYK was associated with significantly reduced levels of inflammatory mediators (Figure 5D). Western blot showed the inhibition of TGF-β1 or SYK significantly reduced the expression of TGF-β1 and p-Smad3 in the chlorhexidine digluconate-induced rat model (Figure 5E). These results supported that SYK could regulate the development of peritoneal fibrosis by TGF-β1/Smad3 signaling in vivo in the rat model of peritoneal fibrosis.
Figure 5.
SYK mediated peritoneal fibrosis by regulating the TGF-β1/Smad3 signaling. Data show the changes after the use of the SYK inhibitor or the TGF-β signaling inhibitor. (A) Abnormal hyperplasia of the peritoneal tissue is reduced. Scale bar, 500 μm. (B) The expression of SYK in peritoneal tissues is significantly reduced. Scale bar, 500 μm. (C) Upregulation of SYK is inhibited, as in Figure A. ** P<0.01 vs. sham. * P<0.05 vs. chlorhexidine digluconate. (D) Significant inhibition of the expression of inflammatory cytokines. ** P<0.01 and *** P<0.001 vs. sham. * P<0.05 and ** P<0.01 vs. chlorhexidine digluconate. (E) Phosphorylation of SYK is inhibited and also blocks the activation of TGF-β signaling. ** P<0.01 and *** P<0.001 vs. sham. * P<0.05, ** P<0.01 and *** P<0.001 vs. chlorhexidine digluconate. All experiments were independently conducted in triplicate. CG – chlorhexidine digluconate.
Discussion
Despite the widespread use of peritoneal dialysis, patients may experience complications that include changes in the peritoneum, loss of peritoneal mesothelial cells, and epithelial-mesenchymal transition (EMT), which may impair peritoneal ultrafiltration and results in peritoneal inflammation and fibrosis [6]. SYK is a non-receptor tyrosine kinase (NRTK) that transmits the activation signal of membrane receptors to downstream effector molecules. There have been few previous studies on the effects of SYK in the development of peritoneal fibrosis. The findings from this study showed that SYK expression was increased in peritoneal fibrosis, and inhibition of SYK reduced the severity of fibrosis and inflammation through regulating TGF-β/Smad3 signaling in both in vivo and in vitro models.
SYK plays an important role in the immune and inflammatory responses and is involved in neoplasia, including in human hepatocellular carcinoma [11,23,24]. The role of SYK in fibrosis has previously been reported. Chen et al. showed that inhibition of SYK suppressed renal fibrosis through its anti-inflammatory effects and by down-regulation of the MAPK-p38 pathway [25]. Qu et al. showed that SYK was upregulated in liver fibrosis and suggested its role as a potential therapeutic target in the control of fibrosis [12]. In the present study, SYK was found to be upregulated in peritoneal fibrosis, and the inhibition of SYK could suppress fibrosis in rat peritoneal tissues.
Previously published studies have shown that TGF-β and its downstream regulatory signals are associated with myocardial fibrosis and other fibrotic diseases [26,27]. Akhmetshina et al. showed that TGF-β could mediate fibrosis through activation of Wnt signaling [28]. In a recent study, inhibition of TGF-β1 signaling was shown to result in suppression of lung fibrosis [29]. TGF-β1 has also been reported to induce peritoneal fibrosis through the activation of the Smad signaling pathway [30]. The findings from this study showed that TGF-β/Smad3 signaling was activated in the rat models of peritoneal fibrosis, and the inhibition of TGF-β significantly suppressed fibrosis of the peritoneal tissues and cells.
Previously published studies have shown that TGF-β signaling is closely associated with the activity of SYK, and the kinase activity of SYK is essential for the activation of some signaling receptor downstream effector molecules [25]. Upregulation and phosphorylation of SYK are important pathways for activation of the TGF-β1 signaling pathway [31]. The SYK inhibitor R406 has been shown to down-regulate inflammation through regulation of TGF-β1 signaling in an in vitro model of Pseudomonas aeruginosa infection [32]. Bin et al. also showed that the inhibition of SYK resulted in suppression of TGF-β1 signaling and resulted in the inhibition of epithelial-mesenchymal transition (EMT) in corneal epithelial cells [33]. Inhibition of TGF-β1 by LY2109761 has previously been shown to suppress the expression of SYK, indicating that the interaction between SYK and TGF-β1 was bidirectional [33]. In the present study, the activation of SYK was shown to increase the progression of peritoneal fibrosis through activation of the TGF-β1/Smad3 signaling pathway, and inhibition of TGF-β1 also resulted in down-regulation of SYK. These findings are supported by previous studies. However, further in vivo studies are needed to provide insights into the relationship between SYK and TGF-β1 signaling.
Conclusions
This study aimed to investigate the role of SYK in an in vivo rat model of peritoneal fibrosis and in rat peritoneal mesothelial cells (PMCs) in vitro and to investigate the underlying mechanisms. The findings showed that the expression of SYK increased peritoneal fibrosis through activation of the TGF-β1/Smad3 signaling pathway and inhibition of SYK activity reduced inflammation and peritoneal fibrosis. The findings from this preliminary study should be supported by further studies to determine whether SYK and the TGF-β1/Smad3 signaling pathway have potential roles as novel therapeutic targets to control peritoneal fibrosis.
Footnotes
Source of support: This study was supported by the grants from the Hunan Provincial Peoples’ Hospital ‘Renshu’ Research Fund, Peoples’ Republic of China (No. RS201810)
Conflict of interest
None.
References
- 1.Yu X, Yang X. Peritoneal dialysis in China: Meeting the challenge of chronic kidney failure. Am J Kidney Dis. 2015;65(1):147–51. doi: 10.1053/j.ajkd.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Sam R, Sahani M, Ulozas E, et al. Utility of a peritoneal dialysis leukocyte test strip in the diagnosis of peritonitis. Artif Organs. 2015;26(6):546–48. doi: 10.1046/j.1525-1594.2002.06886_2.x. [DOI] [PubMed] [Google Scholar]
- 3.Oh KH, Margetts PJ. Cytokines and growth factors involved in peritoneal fibrosis of peritoneal dialysis patients. Int J Artif Organs. 2005;28(2):129–34. doi: 10.1177/039139880502800208. [DOI] [PubMed] [Google Scholar]
- 4.Alnatour M, Thompson D. Peritoneal dialysis. Semin Intervent Radiol. 2016;33(01):3–5. doi: 10.1055/s-0036-1571804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raby AC, Gonzálezmateo GT, Williams A, et al. Targeting Toll-like receptors with soluble Toll-like receptor 2 prevents peritoneal dialysis solution-induced fibrosis. Kidney Int. 2018;94(2):346–62. doi: 10.1016/j.kint.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Chang JH, Yoon SJ, Han SH, et al. The impact of dialysis modality on arterial stiffness in patients with end-stage renal disease. Ren Fail. 2010;32(8):947–53. doi: 10.3109/0886022X.2010.502607. [DOI] [PubMed] [Google Scholar]
- 7.Hung KY, Liu SY, Yang TC, et al. High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxid Med Cell Longev. 2014;2014(3) doi: 10.1155/2014/642793. 642793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitai M, Fukuda N, Ueno T, et al. Effects of a spleen tyrosine kinase inhibitor on progression of the lupus nephritis in mice. J Pharmacolog Sci. 2017;134(1):29. doi: 10.1016/j.jphs.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Pan G, Qiao X, Sun H, et al. Activated spleen tyrosine kinase promotes malignant progression of oral squamous cell carcinoma via mTOR/S6 signaling pathway in an ERK1/2-independent manner. Oncotarget. 2017;8(48):83900–912. doi: 10.18632/oncotarget.19911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabeling C, Herbert J, Hocke AC, et al. Spleen tyrosine kinase inhibition blocks airway constriction and protects from Th2-induced airway inflammation and remodeling. Allergy. 2017;72(7):1061–72. doi: 10.1111/all.13101. [DOI] [PubMed] [Google Scholar]
- 11.Attila M, Jürgen R, Tybulewicz VLJ. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu YQ, Zhong S, Meng N, et al. NLRP3 inflammasome activation results in liver inflammation and fibrosis in mice infected with Schistosoma japonicum in a Syk-dependent manner. Sci Rep. 2017;7(1):8120. doi: 10.1038/s41598-017-08689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh KH, Margetts PJ. Cytokines and growth factors involved in peritoneal fibrosis of peritoneal dialysis patients. Int J Artif Organs. 2005;28(2):129–34. doi: 10.1177/039139880502800208. [DOI] [PubMed] [Google Scholar]
- 14.Subeq YM, Ke CY, Lin NT, et al. Valsartan decreases TGF-β1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine. 2011;53(2):223–30. doi: 10.1016/j.cyto.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Yokoi H, Kasahara M, Mori K, et al. Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int. 2012;81(2):160–69. doi: 10.1038/ki.2011.305. [DOI] [PubMed] [Google Scholar]
- 16.Hu B, Mao Z, Jiang X, et al. Role of TGF-beta1/Smad3-mediated fibrosis in drug resistance mechanism of prolactinoma. Brain Res. 2018;1698:204–12. doi: 10.1016/j.brainres.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64(3):157–67. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HC, Huang DY, Wu MS, et al. Spleen tyrosine kinase mediates the actions of EPO and GM-CSF and coordinates with TGF-β in erythropoiesis. Biochim Biophys Acta. 2017;1864(4):687–96. doi: 10.1016/j.bbamcr.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Tamura M, Osajima A, Nakayamada S, et al. High glucose levels inhibit focal adhesion kinase-mediated wound healing of rat peritoneal mesothelial cells. Kidney Int. 2003;63(2):722–31. doi: 10.1046/j.1523-1755.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Shan N, Zhao C, et al. LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells. Int J Clin Exp Pathol. 2015;8(5):4923–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Kleber S, Röhrich M, et al. Blockade of TGF-β Signaling by the TGFβR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71(23):7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 22.Bernard S, Danglade D, Gardano L, et al. Inhibitors of BCR signalling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int J Cancer. 2015;136(12):2761–74. doi: 10.1002/ijc.29326. [DOI] [PubMed] [Google Scholar]
- 23.Ma FY, Blease K, Nikolic-Paterson DJ. A role for spleen tyrosine kinase in renal fibrosis in the mouse obstructed kidney. Life Sci. 2016;146:192–200. doi: 10.1016/j.lfs.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Pamuk ON, Can G, Ayvaz S, et al. Spleen tyrosine kinase (Syk) inhibitor fostamatinib limits tissue damage and fibrosis in a bleomycin-induced scleroderma mouse model. Clin Exp Rheumatol. 2015;33(4 Suppl 91):S15–22. [PubMed] [Google Scholar]
- 25.Chen KH, Hsu HH, Yang HY, et al. Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway. Int J Biochem Cell Biol. 2016;74:135–44. doi: 10.1016/j.biocel.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Rao F, Liu F, et al. Involvement of Smad3 pathway in atrial fibrosis induced by elevated hydrostatic pressure. J Cell Physiol. 2018;233(6):4981–89. doi: 10.1002/jcp.26337. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Zhang Y, Mao H, et al. A crosstalk between the Smad and JNK signaling in the TGF-beta-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells. PLoS One. 2012;7(2):e32009. doi: 10.1371/journal.pone.0032009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Kim TJ, Peng DH, et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J Clin Invest. 2017;127(10):3675. doi: 10.1172/JCI94624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan WJ, Yu X, Huang XR, et al. Opposing roles for Smad2 and Smad3 in peritoneal fibrosis in vivo and in vitro. Am J Pathol. 2014;184(8):2275–84. doi: 10.1016/j.ajpath.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zou N, Wang J, et al. TGF-beta1/Smad3 signaling pathway mediates T-2 toxin-induced decrease of type II collagen in cultured rat chondrocytes. Toxins. 2017;9(11) doi: 10.3390/toxins9110359. pii: E359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhazmi A, Choi J, Ulanova M. Syk inhibitor R406 down-regulates inflammation in an in vitro model of Pseudomonas aeruginosa infection. Can J Physiol Pharmacol. 2018;96(2):182–90. doi: 10.1139/cjpp-2017-0307. [DOI] [PubMed] [Google Scholar]
- 33.Ga Bin P, Daejin K, Yeong Seok K, et al. The Epstein-Barr virus causes epithelial-mesenchymal transition in human corneal epithelial cells via Syk/src and Akt/Erk signaling pathways. Invest Ophthalmol Vis Sci. 2014;55(3):1770–79. doi: 10.1167/iovs.13-12988. [DOI] [PubMed] [Google Scholar]