Abstract
Although arsenic trioxide (arsenite, AsIII) has shown a remarkable efficacy in the treatment of acute promyelocytic leukemia patients, multidrug resistance is still a major concern for its clinical use. Multidrug resistance-associated protein 4 (MRP4), which belongs to the ATP-binding cassette (ABC) superfamily of transporters, is localized to the basolateral membrane of hepatocytes and the apical membrane of renal proximal tubule cells. Due to its characteristic localization, MRP4 is proposed as a candidate in the elimination of arsenic and may contribute to resistance to AsIII. To test this hypothesis, stable HEK293 cells overexpressing MRP4 or MRP2 were used to establish the role of these two transporters in AsIII resistance. The IC50 values of AsIII in MRP4 cells were approximately 6-fold higher than those in MRP2 cells, supporting an important role for MRP4 in resistance to AsIII. The capacity of MRP4 to confer resistance to AsIII was further confirmed by a dramatic decrease in the IC50 values with the addition of MK571, an MRP4 inhibitor, and cyclosporine A, a well-known broad-spectrum inhibitor of ABC transporters. Surprisingly, the sensitivity of the MRP2 cells to AsIII was similar to that of the parent cells, although insufficient formation of glutathione and/or Se conjugated arsenic compounds in the MRP2 cells might limit transport. Given that MRP4 is a major contributor to arsenic resistance in vitro, further investigation into the correlation between MRP4 expression and treatment outcome of leukemia patients treated with arsenic-based regimens is warranted.
Keywords: arsenite, cytotoxicity, multidrug resistance-associated protein 4, MRP2, multidrug resistance
Introduction
Arsenic and arsenic-containing compounds are widely distributed in the environment and exist in organic and inorganic forms. Although a well-known poison, arsenic has been used medicinally for over 2,000 years (1). In particular, administration of arsenic trioxide (arsenite, AsIII), an arsenic derivative, has demonstrated a remarkable efficacy in the treatment of relapsed and refractory acute promyelocytic leukemia (APL) (1–4). Detailed pharmacokinetic studies of AsIII in APL patients have been carried out to optimize treatment (3,5–8). Both inorganic arsenic and methylated arsenic metabolites accumulate in red blood cells during repeated administration of AsIII to APL patients (7). Arsenic metabolites are also detected in cerebrospinal fluid (6) at concentrations of arsenic necessary for induction of differentiation (4,9). Recent data from our laboratory demonstrated that the profiles of arsenic species in peripheral blood (PB) plasma were very similar to those of bone marrow (BM) plasma, suggesting that the profiles of PB plasma could be predictive biomarkers for the treatment outcome of APL patients (8). These findings on the pharmacokinetics of AsIII in APL patients provide new insight into clinical applications of AsIII, and may contribute to designing better therapeutic protocols (1).
Multidrug resistance is a major concern for the clinical use of anticancer drugs. ATP-binding cassette (ABC) transporters contribute to drug resistance via ATP-dependent drug efflux (1,10). Multidrug resistance-associated proteins 1 and 2 (MRP1/2), and multidrug resistance protein 1 (MDR1; also known as P-glycoprotein, P-gp) have been implicated in the efflux of arsenic, and may contribute to resistance to arsenic therapy (1,11,12). Furthermore, we recently demonstrated the MRP2 and aquaporin-9 (AQP9), a member of the aquaporin superfamily, involvement in arsenic uptake (1,13–15), contributing to the differential sensitivity of primary human-derived normal cells to arsenite (14,16–18). Although these previous findings provide fundamental knowledge for understanding the cellular handling and elimination pathways of arsenic in cancer cells as well as normal cells, more studies are required to provide detailed information on the efficacy and safety of arsenic for clinical use.
MRP4 transports antiviral agents such as the nucleoside/nucleotide analogs azidothymidine (AZT), adefovir [9-(2-phosphonylmethoxyethyl) adenine or PMEA] and tenofovir (TFV) (19–21), and anticancer drugs such as camptothecins and methotrexate (22–24). Of note, recent studies have demonstrated that hematopoietic progenitor cell differentiation affects expression and function of MRP4, and that MRP4 has a relevant role in tumor growth and apoptosis and in the eradication of leukemic stem cells, suggesting MRP4 as a new potential therapeutic target for acute myeloid leukemia (25,26). It is noteworthy that MRP4 is localized to the basolateral membrane of hepatocytes and the apical membrane of renal proximal tubule cells, distinguishing itself from other members of the MRP family (21). Considering the localization of MRP4, biomethylation of arsenic primarily in liver and elimination of arsenic by the kidney (1), MRP4 is an ideal candidate for the elimination of arsenic and may contribute to resistance to AsIII. Only recently have studies been reported regarding the role of MRP4 in arsenic resistance (27,28). In the present study, stable human embryonic kidney epithelial (HEK)293 cells overexpressing MRP4 and MRP2 were created and used to investigate the cytotoxicity of AsIII against both MRP overexpressing cells and reference cells transfected with an empty vector, in order to clarify whether MRP4 cells have the capacity to confer drug resistance to AsIII.
Materials and methods
Reagents
Sodium arsenite (AsIII) was purchased from Tri Chemical Laboratories (Yamanashi, Japan). Cyclosporin A (CsA), a broad-spectrum inhibitor of ABC transporters, was kindly provided by Professor Toshihiko Hirano, Department of Clinical Pharmacology, School of Pharmacy, Tokyo University of Pharmacy and Life Sciences. 3-[[3-[2-(7-chloroquinolin-2-yl) vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid (MK571), an inhibitor of MRP4, was purchased from Merck (Darmstadt, Germany).
Construction of stable HEK293 cell lines expressing human MRP2 or MRP4 cDNA
Human ABCC2 or ABCC4 cDNA was subcloned into the pcDNA5/FRT vector (Invitrogen, Carlsbad, CA, USA) as described previously (19,20). Briefly, HEK293 Flp-In cells were seeded at 5×105 cells/well in 6-well plates in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) without antibiotics. After 24 h of cultivation, 0.4 µg MRP2 or MRP4 plasmid was transfected into HEK293 Flp-In cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Empty pcDNA/FRT vector was transfected as a negative control (empty vector cells). DMEM fresh media were added at 6 h post-transfection. The selection of stable cell clones expressing MRP2 or MRP4 reference plasmid was started in 75 µg/ml hygromycin (Invitrogen) the following day. Media were changed every 2–3 days, and selection of stable transfectants generally took 10–14 days. HEK293 cells stably expressing MRP2 reference (MRP2 cells) or MRP4 reference (MRP4 cells) and empty vector cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml of penicillin and 100 µg/ml of streptomycin at 37°C in a humidified atmosphere (5% CO2 in air).
MRP2 protein isolation and western blot analysis
Proteins were isolated using PARIS™ kit (Ambion Life Technologies, Foster City, CA, USA) according to the manufacturer's protocol. Proteins (30 µg/lane) were separated on 4–15% Tris-HCl Criterion gels (Bio-Rad, Hercules, CA, USA) by SDS-PAGE at 80 V and then transferred onto nitrocellulose membranes at 250 mA for 2 h. Membranes were blocked in 2% skim milk for 1 h at room temperature and incubated overnight at 4°C with anti-MRP2 antibody (M2-III-6) at 1:250 dilution (Thermo Fisher Scientific, Rockford, IL, USA) or anti-GAPDH antibody (ab9483) at 1:100,000 dilution (Abcam, Cambridge, MA, USA). The following day, membranes were washed three times with phosphate-buffered saline (PBS) and then incubated with IRdye 800CW goat anti-mouse fluorescent secondary antibody (Li-COR, Lincoln, NE, USA) at 1:10,000 dilution. Membranes were washed with PBS and visualized on an odyssey® Sa Infrared Imaging system (Li-COR).
Evaluation of MRP2 membrane expression by immunocytochemistry
Empty vector and MRP2 reference cells were seeded at 2.5×104/chamber on 4-chamber slides 24 h prior to staining. The following day cells were washed with cold PBS two times and permeabilized with cold acetone for 10 min on ice. An equal volume of 8% paraformaldehyde was added directly to the cells and incubated for 10 min at room temperature. Cells were washed with PBS two times and blocked with 2 mg/ml BSA solution for 30 min at room temperature. Primary anti-MRP2 (M2-III-6) antibody was added to BSA solution to the final dilution of 1:20. After three washes with PBS, the slides were incubated with a fluorescently conjugated secondary antibody (Alexa Fluor® 488; Thermo Fisher Scientific) for 1 h at room temperature protected from light. DAPI (Thermo Fisher Scientific) stain at 1:3,000 dilution was added 20 min before the end of incubation. After three washes with PBS, images were mounted and then captured using a Retiga CCD-cooled camera and associated QCapture Pro software (QImaging, Surrey, BC, Canada).
Functional assays of MRP2 cells
Evaluation of MRP2 transport activity was performed using a carboxyfluorescein (CF) fluorescent dye retention assay. CF was applied to cells in its di-acetate (CFDA) form (Sigma-Aldrich, St. Louis, MO, USA) which is non fluorescent and highly lipophilic; it freely enters the cells through passive diffusion. Once inside the cell the acetate groups of CFDA are cleaved by esterases yielding fluorescent CF, which has low permeability characteristics and is a substrate for ABC efflux transporters, including MRP2 (29). Briefly, empty vector and MRP2 overexpressing cells were trypsinized, washed with warm PBS and re-suspended at a cell density of 1×106 cells/ml in fresh DMEM without FBS. Cells were then incubated for 30 min at 37°C with 10 µM of CFDA. Following accumulation, cells were pelleted, washed twice with warm PBS, re-suspended in DMEM media supplemented with 10% FBS and allowed to efflux for 30 min at 37°C. Following efflux, cells were pelleted, washed twice with ice-cold PBS and then re-suspended in ice-cold PBS supplemented with 10% FBS for analysis. Retention of CF was determined by measuring fluorescence using flow cytometry performed on a BD FACSCalibur (BD Biosciences, Mississauga, ON, Canada). Briefly, 10,000 cell events were collected for each sample. Cells were co-stained with propidium iodide (PI; Thermo Fisher Scientific) to exclude non-viable cells from further analysis. CF fluorescence was measured in FL-1 channel (excitation wavelength 488 nm and emission wavelength 530 nm), and PI fluorescence was measured in FL-3 channel (excitation wavelength 488 nm and emission wavelength 600 nm). Each experiment was repeated four times. Data were normalized to empty vector transfected cells and a t-test was used on normalized data to test for differences in MRP2 overexpressing cells and reference cells.
Functional assays of MRP4 cells
Transport assays for MRP4 were carried out using two reported MRP4 substrates [adenine-8-3H-tenofovir disoproxil (TFV) (3.8 Ci/mmol, 98.1% purity); 3H-9-(2-phosphonylmethoxyethyl)-adenine (PMEA)] (Moravek Biochemicals, Brea, CA, USA) as described previously (19,20,30). Briefly, MRP4 and empty vector cells were seeded at 2.5×105 cells/well in triplicate in poly-D-lysine-coated 24-well plates (BD Biosciences, San Jose, CA, USA). After preincubation for 24 h, cells were incubated with 1 µM TFV or 100 nM PMEA in glucose-free DMEM supplemented with 10 µM NaN3 and 10 µM 2-deoxy-D-glucose, respectively, for 2 h at 37°C. After accumulation, cells were washed with ice-cold PBS and supplemented with complete DMEM. Supernatant fractions were collected at 0, 30 and 90 min, and cells were washed and lysed with 800 µl/well of an aqueous solution of 10% sodium dodecyl sulfate and 1 N NaOH. Supernatants were added to Ecolite scintillation fluid (MP Biomedicals, Santa Ana, CA, USA) and extracellular amounts of TFV and PMEA were analyzed by scintillation counting. The remaining cell lysate was used to determine the protein concentration with a BCA™ Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA); TFV and PMEA levels were normalized to protein concentrations.
Cell viability assay
The cytotoxicity of AsIII to MRP4, MRP2 and empty vector cells was investigated by XTT dye-reduction assays according to the method previously described with slight modifications (14,31). Briefly, the cells were seeded in 96-well plates (Iwaki, Tokyo, Japan) at a density of 5×103 cells/well in 0.1 ml complete DMEM and cultivated for 24 h. Cultures in triplicate were treated with various concentrations of AsIII in the presence or absence of transporter inhibitors at the concentrations indicated. Cells were pre-incubated with transporter inhibitors at the indicated concentrations for 30 min. After treatment with AsIII for an additional 48 h, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; Sigma, MD, USA) and phenazine methosulfate (Wako Pure Chemical Industries, Osaka, Japan) were added into each well at final concentrations of 0.2 mg/ml and 1 mM, respectively. After incubation at 37°C for 4 h, the plates were mixed, and the absorbance at 450 nm was measured with a microplate reader (Safire, Tecan, Switzerland). The relative cell viability was expressed as the ratio of the absorbance of each treatment group against those of the corresponding untreated control group. Data are shown as means ± SD from more than five independent experiments. The IC50 values of AsIII for all three cell types were calculated using GraphPad Prism® 5 software.
Statistical analysis
Data were analyzed using Student's t-test and ANOVA with a Dunnett's post test method. A p-value <0.05 was considered as statistically significant.
Results
Confirmation of MRP2 expression and transporter activity in the MRP2 stable cell line
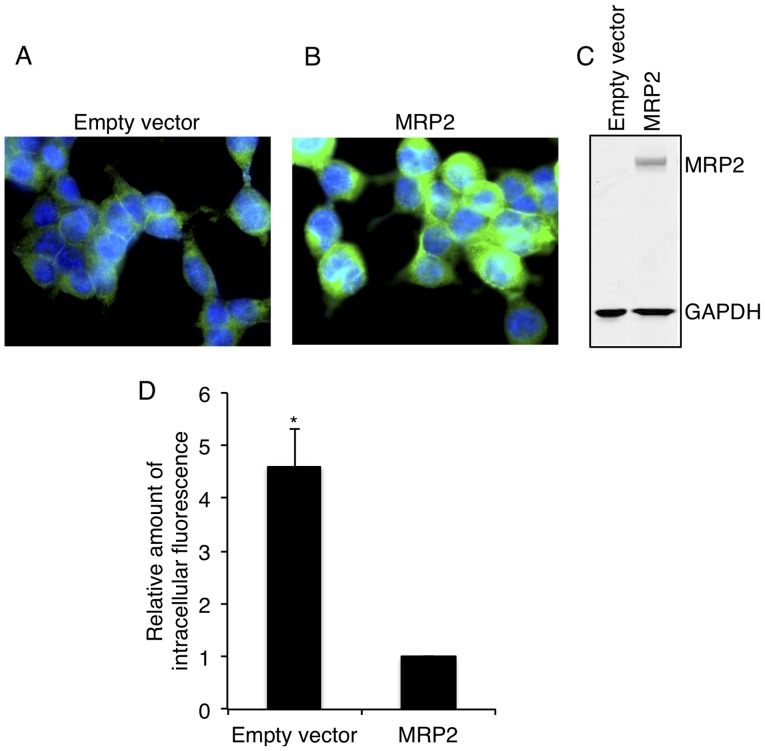
Protein expression of MRP2 was confirmed using immunocytochemistry and western blotting. As shown in Fig. 1, MRP2 cells had significantly higher levels of membrane (Fig. 1A and B) and total (Fig. 1C) MRP2 expression compared to empty vector transfected cells. Functional activity of MRP2 was confirmed in MRP2 reference cells using a CF retention assay. As shown in Fig. 1D, retention of CF in MRP2 overexpressing cells was 4.5-fold lower than that in empty vector cells, indirectly confirming high efflux of fluorescent dye from MRP2 overexpressing cells and, therefore, providing strong evidence for MRP2 functional activity.
Figure 1.
MRP2 expression and transporter activity in MRP2 stable cell line. Immunocytochemistry (A and B) and western blotting (C) for the expression of MRP2 protein, and CF fluorescent dye retention assay (D) for MRP2 activity were carried out as described under Materials and methods. Results are shown as the means ± SD from four independent experiments. For each experiment, data were normalized to empty vector cells. Significant differences were observed between MRP2 and empty vector cells (*P<0.05).
Confirmation of transporter activity in the MRP4 stable cell line
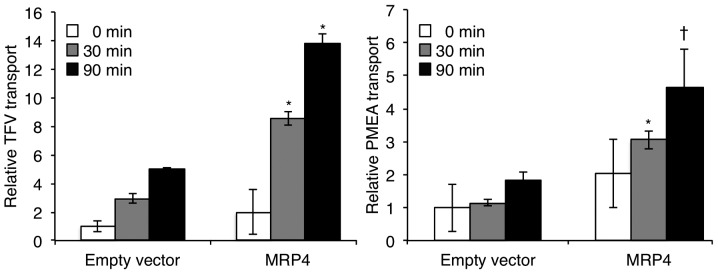
Accumulation of TFV or PMEA in the supernatant fractions of each cell type was assessed for confirmation of MRP4 function. As shown in Fig. 2, the accumulation of TFV or PMEA in the supernatant of MRP4 cells increased with time, and was much higher than that of empty vector cells. Compared to empty vector cells, an approximately 3-fold and 2.7-fold increase in the efflux of TFV and PMEA, respectively, was observed in the MRP4 cells at the 30 and 90 min time-points (Fig. 2), indicating functional MRP4.
Figure 2.
Transporter activity in MRP4 stable cell line. Transport assays for MRP4 activity were carried out using two reported MRP4 substrates (TFV and PMEA) as described under Materials and methods. Data are shown as the means ± SD from three independent experiments. For each experiment, data were normalized to empty vector cells. Significant differences were observed between MRP4 and empty vector cells at each time-point (*P<0.01, ✝P<0.05).
AsIII-induced cytotoxicity in MRP4 and MRP2 cells
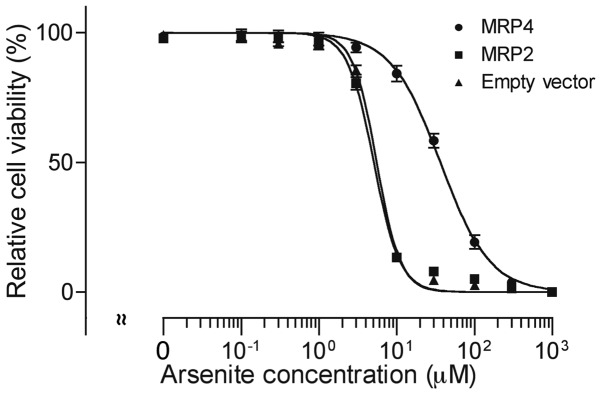
After treatment with 0, 0.1, 0.3, 1, 3, 10, 30, 100, 300 and 1,000 µM of AsIII for 48 h, cell viability was investigated by XTT assay. A significant dose-dependent decrease in cell viability was observed in all three cell types (Fig. 3). AsIII exhibited much lower cytotoxicity in MRP4 cells in comparison with MRP2 and empty vector cells. The IC50 values of AsIII were 36.6±8.1, 5.1±0.3 and 5.5±0.3 µM in MRP4, MRP2 and empty vector cells (MRP4 vs. empty vector, P<0.01; MRP2 vs. empty vector, P>0.05), respectively.
Figure 3.
AsIII-induced cytotoxicity in MRP4 and MRP2 overexpressing cells. After treatment with 0-1,000 µM of AsIII for 48 h, cell viability was quantified with an XTT assay as described in Materials and methods. Data are shown as means ± SD from more than five independent experiments. The IC50 value for AsIII in MRP4 cells was significantly greater than the corresponding value in empty vector cells (P<0.01).
Effect of MK571 on AsIII-induced cytotoxicity in MRP4 and empty vector cells
Both MRP4 and empty vector cells were exposed to various concentrations of AsIII (0–1,000 µM) in the presence or absence of 10 or 25 µM MK571 for 48 h, followed by the assessment of cell viability. The IC50 value of AsIII in MRP4 cells decreased significantly from 28.2±3.3 to 11.2±3.3 and 6.3±1.0 µM by the addition of 10 and 25 µM MK571 (MRP4 without MK571 vs. MRP4 with 10 or 25 µM MK571, P<0.01), respectively (Fig. 4A). Interestingly, the addition of MK571 also slightly enhanced AsIII-induced cytotoxicity in empty vector cells, with the IC50 value of AsIII decreasing from 4.8±0.8 to 3.3±0.4 and 2.5±0.2 µM by the addition of 10 and 25 µM MK571 (empty vector cells without MK571 vs. empty vector cells with 10 or 25 µM MK571, P<0.05), respectively (Fig. 4B).
Figure 4.
Effect of MK571 on AsIII-induced cytotoxicity in MRP4 overexpressing cells. MRP4 (A) and empty (B) vector cells were exposed to various concentrations of AsIII (0–1,000 µM) in the presence or absence of 10 or 25 µM MK571 for 48 h, followed by the assessment of cell viability. Data are shown as means ± SD from more than five independent experiments. The IC50 value for AsIII in MRP4 and empty vector cells decreased significantly in the presence of MK571 (P<0.01 for MRP4 and P<0.05 for empty vector).
Effect of CsA on AsIII-induced cytotoxicity in MRP4 and empty vector cells
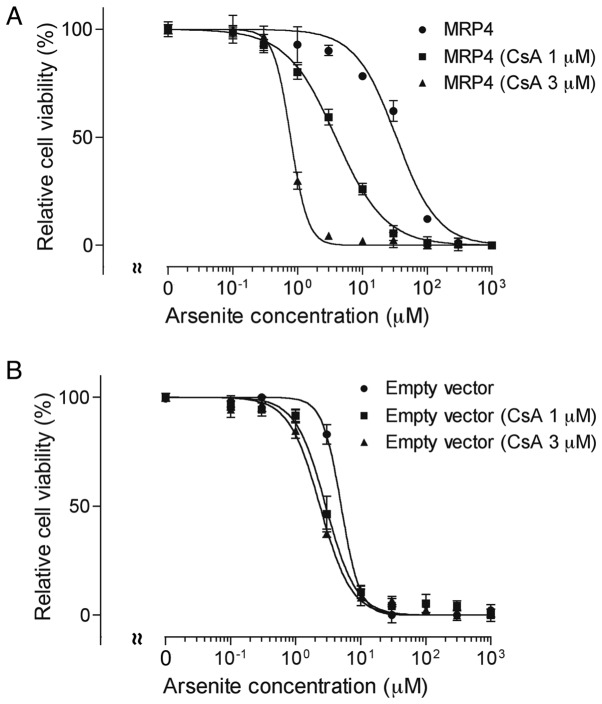
Both MRP4 and empty vector cells were exposed to various concentrations of AsIII (0–1,000 µM) in the presence or absence of 1 or 3 µM CsA for 48 h, followed by the assessment of cell viability. In comparison to MK571, CsA, a broad-spectrum inhibitor of ABC transporters, more potently inhibited MRP4 and increased AsIII-induced cytotoxicity (Fig. 5A). The IC50 value of AsIII in MRP4 cells decreased ≥90% after the addition of 1 and 3 µM CsA, respectively (Fig. 5A). There was some increase in AsIII cytotoxicity as well in empty vector cells (5.3±0.2 vs 3.1±0.6 and 2.3±0.2 µM) (empty vector cells without CsA vs. empty vector cells with 1 or 3 µM CsA, P<0.05) (Fig. 5B).
Figure 5.
Effect of CsA on AsIII-induced cytotoxicity in MRP4 overexpressing cells. MRP4 (A) and empty vector (B) cells were exposed to various concentrations of AsIII (0–1,000 µM) in the presence or absence of 1 or 3 µM CsA for 48 h, followed by the assessment of cell viability. Data are shown as means ± SD from more than five independent experiments. The IC50 value for AsIII in MRP4 and empty vector cells decreased significantly in the presence of CsA (P<0.01 for MRP4 and P<0.05 for empty vector).
Discussion
MRP2 and MRP4 overexpressing cells were used in the current study to evaluate the contribution of these ABC efflux transporters to AsIII cytotoxicity. A 6-fold difference in IC50 values of AsIII between these cells and a significant reduction of the IC50 value in MRP4 cells provide strong support that MRP4 is a major mediator of AsIII efflux from cells and plays a major role in AsIII resistance. MRP2 has been demonstrated to be involved in the efflux of arsenic, conferring resistance to AsIII in other experimental systems (12,32,33). Wild-type and MRP2-deficient Wistar (TR−) rats have been used to show that MRP2 is responsible for the biliary excretion of arsenic triglutathione [As(GS)3] and monomethyl arsenic diglutathione [CH3As(GS)2] (33). These in vivo findings are supported by cellular transport assays demonstrating that As(GS)3 is also a substrate for human MRP2 (12). A recent in vitro study using MRP2-enriched membrane vesicles demonstrated that MRP2 transports selenobis(S-glutathionyl) arsiniumion [((GS)2AsSe)−] (32). These previous findings suggest that glutathione and/or the essential trace element selenium (Se) are required for the excretion and detoxification of arsenic. In contrast, using MRP2 overexpressing cells in the present study, there was no evidence that MRP2 plays a critical role in AsIII transport and cytotoxicity. These conflicting results might reflect low levels of glutathione and/or Se conjugation in MRP2 overexpressing HEK293 cells, although more detailed analysis of the molecular events such as the amount of glutathione and its conjugated arsenic compounds is required to confirm this hypothesis.
Inhibition studies further support the role of MRP4 to confer resistance to AsIII. MK571, an MRP4 inhibitor (21), was used to demonstrate that MRP4 confers resistance to a series of camptothecin analogs, including irinotecan and SN-38 (23,24). Consistent with these previous studies, the addition of MK571 significantly potentiated AsIII-triggered cytotoxicity in the MRP4 cells in a dose-dependent manner. CsA, a well-known broad-spectrum inhibitor of ABC transporters, increased AsIII-triggered cytotoxicity to a greater degree than MK571, supporting a role for multiple ABC transporters in AsIII cytotoxicity. Collectively, our experimental results implicate MRP4 in resistance to AsIII. Considering that multidrug resistance-reversing activity of CsA has been reported in phase II studies with myeloma and acute leukemia, increased recognition of drug-drug interactions is necessary for optimal treatment of patients with arsenic-based regimens.
Our results are not completely in agreement with other studies of arsenic resistance. Increased resistance was not detected in MRP4-transfected NIH3T3 cells after exposure to sodium meta-arsenite for 72 h (28). Furthermore, a recent report demonstrated that after treatment with increasing concentrations of inorganic and methylated species of arsenic for 72 h, MRP4-transfected HEK293 cells conferred resistance to arsenate and methylated species of arsenic except for arsenite (27). Plausible explanations for the differences between our findings and previous reports are differences in cell lines, arsenite reagent, treatment durations and stable versus transient overexpression systems.
MRP4 has been proposed to contribute to arsenic elimination due to its characteristic localization in both the basolateral membrane of hepatocytes and the apical membrane of renal proximal tubule cells (21,27). Based on transport studies using MRP4-enriched membrane vesicles, the diglutathione conjugate of MMAIII, monomethylarsenic diglutathione [MMA(GS)2], and DMAV are transported by MRP4 (27). It is noteworthy that MRP4 has been reported to regulate intracellular cyclic adenosine monophosphate (cAMP) levels, an endogenous substrate identified for MRP4 (21), in AML cell lines and contribute to cell proliferation and differentiation (34). Moreover, Copsel et al recently reported that MRP4 blockade strongly reduced tumor growth by inducing cell cycle arrest and apoptosis in U937 xenografted mice, and further demonstrated that increased cAMP levels and MRP4 inhibition resulted in leukemic stem cell differentiation (25). Of note, a rapid increase in intracellular cAMP has been linked to all-trans retinoic acid (ATRA)-induced differentiation in the human APL cell line NB4 and in fresh APL cells (35). More importantly, cAMP facilitates the degradation of AsIII-mediated fusion protein promyelocytic leukemia (PML)-retinoic acid receptor α (RARα), a fusion gene generated by the t(15;17) translocation in APL and though to play a central role in the initiation of leukemogenesis (1,4,36). These previous findings raised the possibility that MRP4 would be a novel promising target in APL therapy. The expression of MRP4 increased in CD34+ cells differentiated toward megakaryocytes with thrombopoietin, and a similar increase was also observed in a megakaryoblastic cell line (M-07e) derived from a patient with megakaryoblastic leukemia, when differentiated toward megakaryocytes (26). However, the expression of MRP4 decreased in CD34+ cells differentiated toward monocytes with G-CSF, suggesting a relevant role of MRP4 in hematopoietic progenitor cell differentiation (26). Therefore, considering the expression status of MRPs, including MRP4, is important for providing meaningful clinical benefits for patients with different types of hematological disorders.
It is noteworthy that the MRP4 gene is highly polymorphic, and numerous nonsynonymous single-nucleotide polymorphisms (SNPs) have been identified (21). Functional studies have shown that although there was no evidence for a complete loss of function allele, two variants (G187W and G487E) show a significantly reduced function compared to reference MRP4 as evidenced by higher intracellular accumulation of ATZ and PMEA, two antiviral substrates for MRP4 (19). Although no disease has so far been directly linked to altered MRP4 activity, evaluating the functional effects of high frequency variants on the disposition of arsenic has important implications for hematologic malignancy patients treated with arsenic-based regimens.
In conclusion, our results demonstrated the capacity of MRP4 to confer resistance to AsIII as evidenced by cell survival assays when treated with AsIII in the presence or absence of its two differential inhibitors. Given that MRP4 is widely distributed in the body, and plays a pivotal role in the drug concentrations achieved clinically, monitoring its expression levels may have important implications for predicting not only clinical efficacy but also side effects of arsenite and its metabolites. Obviously, clinical data are required to support the role of MRP4 in drug disposition and efficacy. Based on our findings and previous studies showing that MRP4 could be a major contributor to arsenic resistance, further investigation into the correlation between the expression level of MRP4 and treatment outcome of leukemia patients treated with arsenic-based regimens is warranted.
References
- 1.Yuan B, Yoshino Y, Kaise T, Toyoda H. Application of arsenic trioxide therapy for patients with leukemia. In: Sun H, editor. Biological Chemistry of Arsenic, Antimony and Bismuth. John Wiley and Sons, Ltd; Chichester: 2011. pp. 263–292. [Google Scholar]
- 2.Cohen MH, Hirschfeld S, Flamm Honig S, Ibrahim A, Johnson JR, O'Leary JJ, White RM, Williams GA, Pazdur R. Drug approval summaries: Arsenic trioxide, tamoxifen citrate, anastrazole, paclitaxel, bexarotene. Oncologist. 2001;6:4–11. doi: 10.1634/theoncologist.6-1-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 4.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 5.Fujisawa S, Ohno R, Shigeno K, Sahara N, Nakamura S, Naito K, Kobayashi M, Shinjo K, Takeshita A, Suzuki Y, et al. Pharmacokinetics of arsenic species in Japanese patients with relapsed or refractory acute promyelocytic leukemia treated with arsenic trioxide. Cancer Chemother Pharmacol. 2007;59:485–493. doi: 10.1007/s00280-006-0288-4. [DOI] [PubMed] [Google Scholar]
- 6.Kiguchi T, Yoshino Y, Yuan B, Yoshizawa S, Kitahara T, Akahane D, Gotoh M, Kaise T, Toyoda H, Ohyashiki K. Speciation of arsenic trioxide penetrates into cerebrospinal fluid in patients with acute promyelocytic leukemia. Leuk Res. 2010;34:403–405. doi: 10.1016/j.leukres.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Yoshino Y, Yuan B, Miyashita SI, Iriyama N, Horikoshi A, Shikino O, Toyoda H, Kaise T. Speciation of arsenic trioxide metabolites in blood cells and plasma of a patient with acute promyelocytic leukemia. Anal Bioanal Chem. 2009;393:689–697. doi: 10.1007/s00216-008-2487-9. [DOI] [PubMed] [Google Scholar]
- 8.Iriyama N, Yoshino Y, Yuan B, Horikoshi A, Hirabayashi Y, Hatta Y, Toyoda H, Takeuchi J. Speciation of arsenic trioxide metabolites in peripheral blood and bone marrow from an acute promyelocytic leukemia patient. J Hematol Oncol. 2012;5:1. doi: 10.1186/1756-8722-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 10.Morjani H, Madoulet C. Immunosuppressors as multidrug resistance reversal agents. Methods Mol Biol. 2010;596:433–446. doi: 10.1007/978-1-60761-416-6_19. [DOI] [PubMed] [Google Scholar]
- 11.Lee TC, Ho IC, Lu WJ, Huang JD. Enhanced expression of multidrug resistance-associated protein 2 and reduced expression of aquaglyceroporin 3 in an arsenic-resistant human cell line. J Biol Chem. 2006;281:18401–18407. doi: 10.1074/jbc.M601266200. [DOI] [PubMed] [Google Scholar]
- 12.Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J Biol Chem. 2004;279:32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharjee H, Carbrey J, Rosen BP, Mukhopadhyay R. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem Biophys Res Commun. 2004;322:836–841. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino Y, Yuan B, Kaise T, Takeichi M, Tanaka S, Hirano T, Kroetz DL, Toyoda H. Contribution of aquaporin 9 and multidrug resistance-associated protein 2 to differential sensitivity to arsenite between primary cultured chorion and amnion cells prepared from human fetal membranes. Toxicol Appl Pharmacol. 2011;257:198–208. doi: 10.1016/j.taap.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iriyama N, Yuan B, Yoshino Y, Hatta Y, Horikoshi A, Aizawa S, Takeuchi J, Toyoda H. Aquaporin 9, a promising predictor for the cytocidal effects of arsenic trioxide in acute promyelocytic leukemia cell lines and primary blasts. Oncol Rep. 2013;29:2362–2368. doi: 10.3892/or.2013.2388. [DOI] [PubMed] [Google Scholar]
- 16.Yuan B, Ohyama K, Bessho T, Toyoda H. Contribution of inducible nitric oxide synthase and cyclooxygenase-2 to apoptosis induction in smooth chorion trophoblast cells of human fetal membrane tissues. Biochem Biophys Res Commun. 2006;341:822–827. doi: 10.1016/j.bbrc.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Yuan B, Ohyama K, Bessho T, Uchide N, Toyoda H. Imbalance between ROS production and elimination results in apoptosis induction in primary smooth chorion trophoblast cells prepared from human fetal membrane tissues. Life Sci. 2008;82:623–630. doi: 10.1016/j.lfs.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Yuan B, Ohyama K, Takeichi M, Toyoda H. Direct contribution of inducible nitric oxide synthase expression to apoptosis induction in primary smooth chorion trophoblast cells of human fetal membrane tissues. Int J Biochem Cell Biol. 2009;41:1062–1069. doi: 10.1016/j.biocel.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Ferrin TE, et al. The human multidrug resistance protein 4 (MRP4, ABCC4): Functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325:859–868. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly L, Fukushima H, Karchin R, Gow JM, Chinn LW, Pieper U, Segal MR, Kroetz DL, Sali A. Functional hot spots in human ATP-binding cassette transporter nucleotide binding domains. Protein Sci. 2010;19:2110–2121. doi: 10.1002/pro.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62:3144–3150. [PubMed] [Google Scholar]
- 23.Tian Q, Zhang J, Chan SY, Tan TM, Duan W, Huang M, Zhu YZ, Chan E, Yu Q, Nie YQ, et al. Topotecan is a substrate for multidrug resistance associated protein 4. Curr Drug Metab. 2006;7:105–118. doi: 10.2174/138920006774832550. [DOI] [PubMed] [Google Scholar]
- 24.Tian Q, Zhang J, Tan TM, Chan E, Duan W, Chan SY, Boelsterli UA, Ho PC, Yang H, Bian JS, et al. Human multidrug resistance associated protein 4 confers resistance to camptothecins. Pharm Res. 2005;22:1837–1853. doi: 10.1007/s11095-005-7595-z. [DOI] [PubMed] [Google Scholar]
- 25.Copsel S, Bruzzone A, May M, Beyrath J, Wargon V, Cany J, Russel FG, Shayo C, Davio C. Multidrug resistance protein 4/ATP binding cassette transporter 4: A new potential therapeutic target for acute myeloid leukemia. Oncotarget. 2014;5:9308–9321. doi: 10.18632/oncotarget.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oevermann L, Scheitz J, Starke K, Köck K, Kiefer T, Dölken G, Niessen J, Greinacher A, Siegmund W, Zygmunt M, et al. Hematopoietic stem cell differentiation affects expression and function of MRP4 (ABCC4), a transport protein for signaling molecules and drugs. Int J Cancer. 2009;124:2303–2311. doi: 10.1002/ijc.24207. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee M, Carew MW, Roggenbeck BA, Whitlock BD, Naranmandura H, Le XC, Leslie EM. A novel pathway for arsenic elimination: Human multidrug resistance protein 4 (MRP4/ABCC4) mediates cellular export of dimethylarsinic acid (DMAV) and the diglutathione conjugate of monomethylarsonous acid (MMAIII) Mol Pharmacol. 2014;86:168–179. doi: 10.1124/mol.113.091314. [DOI] [PubMed] [Google Scholar]
- 28.Lee K, Klein-Szanto AJ, Kruh GD. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst. 2000;92:1934–1940. doi: 10.1093/jnci/92.23.1934. [DOI] [PubMed] [Google Scholar]
- 29.Lee G, Piquette-Miller M. Influence of IL-6 on MDR and MRP-mediated multidrug resistance in human hepatoma cells. Can J Physiol Pharmacol. 2001;79:876–884. doi: 10.1139/y01-071. [DOI] [PubMed] [Google Scholar]
- 30.Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi H, Yuan B, Yuhara E, Imai M, Furutani R, Fukushima S, Hazama S, Hirobe C, Ohyama K, Takagi N, et al. Involvement of histone H3 phosphorylation via the activation of p38 MAPK pathway and intracellular redox status in cytotoxicity of HL-60 cells induced by Vitex agnus-castus fruit extract. Int J Oncol. 2014;45:843–852. doi: 10.3892/ijo.2014.2454. [DOI] [PubMed] [Google Scholar]
- 32.Carew MW, Leslie EM. Selenium-dependent and -independent transport of arsenic by the human multidrug resistance protein 2 (MRP2/ABCC2): Implications for the mutual detoxification of arsenic and selenium. Carcinogenesis. 2010;31:1450–1455. doi: 10.1093/carcin/bgq125. [DOI] [PubMed] [Google Scholar]
- 33.Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW. The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem. 2000;275:33404–33408. doi: 10.1074/jbc.M007030200. [DOI] [PubMed] [Google Scholar]
- 34.Copsel S, Garcia C, Diez F, Vermeulem M, Baldi A, Bianciotti LG, Russel FG, Shayo C, Davio C. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. 2011;286:6979–6988. doi: 10.1074/jbc.M110.166868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Tao J, Zhu Q, Jia PM, Dou AX, Li X, Cheng F, Waxman S, Chen GQ, Chen SJ, et al. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18:285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Q, Zhang JW, Zhu HQ, Shen YL, Flexor M, Jia PM, Yu Y, Cai X, Waxman S, Lanotte M, et al. Synergic effects of arsenic trioxide and cAMP during acute promyelocytic leukemia cell maturation subtends a novel signaling cross-talk. Blood. 2002;99:1014–1022. [PubMed] [Google Scholar]