i. Summary
A biobank is an important nexus between clinical and research aspects of pathology. The collection and storage of high quality surgical samples is essential for diagnosis post-surgery, and can also be used to create vaccines, identify therapeutic targets or establish eligibility of cancer patients in a clinical trial. Therefore, personnel handling surgical tissues should follow standard operating procedures (SOP) to maximize efficiency and preserve tissue quality. This chapter is intended to familiarize novice biobank personnel with the issues associated with different steps of surgical tissue collection including patient consent, sample collection, tissue storage, quality control and distribution.
Keywords: Surgical, Tissue, Biospecimen, Biobank, Biorepository, Procurement, Nucleic acids, Pathology
1. Introduction
Surgical tissues collected from routine surgeries are primarily used for intraoperative diagnosis. Additionally, tissues collected are banked and stored for future access on behalf of patients. Since surgical tissues are of great interest for both patients and clinical researchers, every effort must be made to collect tissue appropriately. Adherence to well thought out protocols regarding collection, identification, distribution, and storage of surgical tissues plays a significant role in tissue procurement. In the last decade, National Cancer Institute (NCI) and International Society for Biological and Environmental Repositories (ISBER), besides other domestic and international organizations, have published standard practice guidelines detailing methods to preserve high quality biospecimens (1–3).
Tissue samples may include normal tissue, malignant or benign tumor tissue, or other diseased tissue. Most human biospecimens are collected and stored in pathology departments which are the legal custodians of patients’ biological samples in the U.S (4). Permission to procure samples for clinical testing is covered in consents signed prior to surgery. Research on patient biospecimens typically requires pre-approval of the research project by an Institutional Review Board (IRB) and an informed consent separate from the surgical consent signed by the patients. If it is not feasible to obtain a research consent prior to surgery, excess tissue may be collected, frozen and stored in the pathology department, but is not released to research laboratories. The reason for this precautionary collection without a consent is that, rather than being discarded, the stored tissue may be used for diagnostic testing if the original samples are inadequate. Secondly, the patient may still be interested in participating in research but was unable to sign a consent, due to coming in emergently or for other logistical reasons. In the past, patients have been disappointed to find out that they were unable to enter clinical trials for rare or serious diagnosis secondary to extra tissue samples not being collected due to lack of a research specific consent prior to surgery. Once the consent is obtained later, the stored tissue is released for research or clinical trials. In some circumstances, regulations may permit the release of unconsented excess de-identified surgical tissue to researchers (5). The biobank technician should strive to understand the regulations pertinent in their jurisdiction. Typically, the biobank director is familiar with the regulations and can help clarify the biobank’s collection policies. Your institution’s IRB office can also be helpful in providing guidance in the matter.
For our brain tumor program, technicians go directly to the operating room to collect the tumor tissue once they are telephoned or paged by the operating room staff that the surgical tissues are ready for procurement. Our technicians then take the specimens to the pathologist for triaging. Alternatively, operating room staff may bring the specimen to the pathology departments’ grossing room. The pathologists then evaluate patient tissue with a frozen section study. If the diagnosis is clear cut, the pathologist is typically free to release larger quantities of tissue for clinical trials/research. If the diagnosis is unclear, the pathologist may retain more tissue for diagnosis. Sometimes no frozen section is requested by the surgeons and the pathologist will decide about tissue release based on the known clinical history. For example, resection of a metastatic tumor in patient with known widespread metastatic disease may not warrant a frozen section. In all cases, it is imperative that the pathologist or designee (e.g. pathology resident or fellow) determines that there is adequate tissue for diagnosis prior to release of excess tissue to the biobank. Under no circumstances should a biobank technician unilaterally bank a biospecimen without approval and release from the pathologist or his/her designee. Ideally, the pathology triage area should be in close proximity to the operating rooms to minimize time to stabilization.
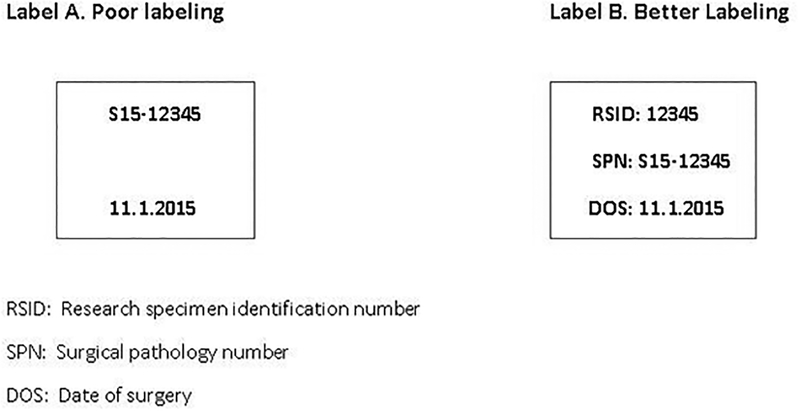
Surgical tissues have unique biological characteristics. The challenge for biobanks is to preserve the quality of original biospecimens for future research use. For this reason, understanding the different conditions that may affect tissue quality of biospecimens is essential (6). Rapid stabilization by freezing or fixation in formalin is important (see Note 1). When not feasible, the biospecimen should be kept on wet ice or in a refrigerator till it can be stabilized. Accurate and careful labeling of biospecimens is an important step in tissue procurement (7) (Fig.1). For example, mislabeling may result in flawed experimental data through incorrect linkages to patient clinical data. Furthermore, in the event that a patient chooses to withdraw his or her specimens from a study, the biobank needs to identify the appropriate specimens to comply (see Note 2). In many instances, histologic quality control should be performed before distribution to ensure that the specific disbursement sample contains sufficient lesional tissue of interest. A pathologist evaluates the histologic section of the biospecimen to be disbursed.
Figure 1.
Proper labeling technique
2. Materials
2.1. Personal Protective equipment
Treat all biospecimens as potentially infectious. All personnel who handle surgical specimens should wear proper personal protective clothing.
Goggles or safety glasses
Face mask
Gloves
Lab coats
Face shields
Gowns
Hair nets
Shoe covers
2.2. Procurement supplies
Some of these supplies might be carried by the biobank technician and others pre-positioned in the pathology grossing or triage area near the operating rooms. Maintenance of these supplies should be carried out periodically (see Note 3).
Collection container (e.g. a hand carried cooler that can hold containers for dry ice and wet ice as well as all other needed supplies)
Distribution and protocol sheets (detailing amounts and types of tissue to be disbursed to the different requesting research laboratories as well as special protocols e.g. special media requirements)
Patient’s information (medical record number, Name, Gender, Date of birth, Surgery date may be provided to the biobank if a specific protocol is requested)
Chemical fixative-usually formalin (Table 1)
Dry ice
Wet ice
Ruler to measure sample in 3 dimensions
Cryovials (1.8 ml)
Scalpels and forceps (sterile as appropriate)
Culture media, stabilizing solutions (for RNA, DNA)
Thermos with isopentane/dry ice (available in operating room area)
Digital balance for weighing samples (available in operating room area)
Tongs for holding cryovials to freeze
Specimen jars/Sterile specimen jars
Permanent marker for writing on specimen containers
Pens for writing on paperwork
Digital camera to photograph gross specimen
Table 1:
Chemical Fixatives
| Chemical fixative | Fixation time (Time in solution) | Mechanism | Comment |
|---|---|---|---|
| Crosslinking fixatives – Formaldehyde (Often used as 10% neutral buffered formalin) | 12–24 hours | Creating covalent chemical bonds between proteins in tissue, tend to preserve the secondary structure of proteins and may protect significant amounts of tertiary structure as well | Most commonly used fixative in histology, good tissue penetration and good for IHC techniques |
| Crosslinking fixative - Glutaraldehyde | 1 hour | Same as above | More extensive cross linking than formaldehyde interfering with with, gives best overall cytoplasmic and nuclear detail for electron microscopy |
| Precipitating or denaturing fixatives - alcohols | 1–6 hours | Reducing the solubility of protein molecules or by disrupting the hydrophobic interactions that give many proteins their tertiary structure | Examples: Ethanol and methanol: most common precipitating fixatives, fixation frozen sections and smears, rarely used alone for fixing blocks unless studying nucleic acids. Methacarn (Methanol-Carnoy) |
| Mercury (B-5 fixative) | 4–8 hours | Unknown mechanism that increases staining brightness and give excellent nuclear detail | Fast but penetrates poorly and produce tissue shrinkage. Favored for hematopoietic and reticuloendothelial tissues. Change to 70% ethanol after the 4–8 hours. |
| Methacarn (Methanol-Carnoy) | 1–4 hours | Quick fixation and can be stored in fixative for several weeks without apparent harm to tissue morphology or antigenicity. Good RNA quality. |
2.3. Supplies for subdividing frozen biospecimens for disbursement
At the time of disbursement, frozen sample cryovials are retrieved from freezers. From the retrieved sample, a portion or aliquot needs to be cut off to provide the requested quantity. We take an even smaller sample from that aliquot to perform histologic quality assurance before releasing the sample.
Razor blades
Parafilm
Forceps and scalpels
Petri dish
Dry ice
Styrofoam box for dry ice
Sample information (location of samples in freezer)
Centrifuge tubes
Cryoprotective embedding medium (OCT: Optimal Cutting Temperature compound)
Acetone (or other fixative)
Cryotome
Microtome
Biohazard hood
Fume hood
2.4. General Processing and Storage Equipment
Computers and related equipment
Printer
Digital balance (0–70 gram)
Freezers (−20°C, −80°C, −150°C)
Refrigerator (0–4°C)
Cryostat for frozen section procedure (temperature at interval of −18°C to −22°C)
Oven for deparaffinizing slides
Fume hood for Hematoxylin and eosin staining
Biohazard hood
Drawer cabinet (histocassette storage)
3. Methods
3.1. Collection and Storage of Surgical Tissues
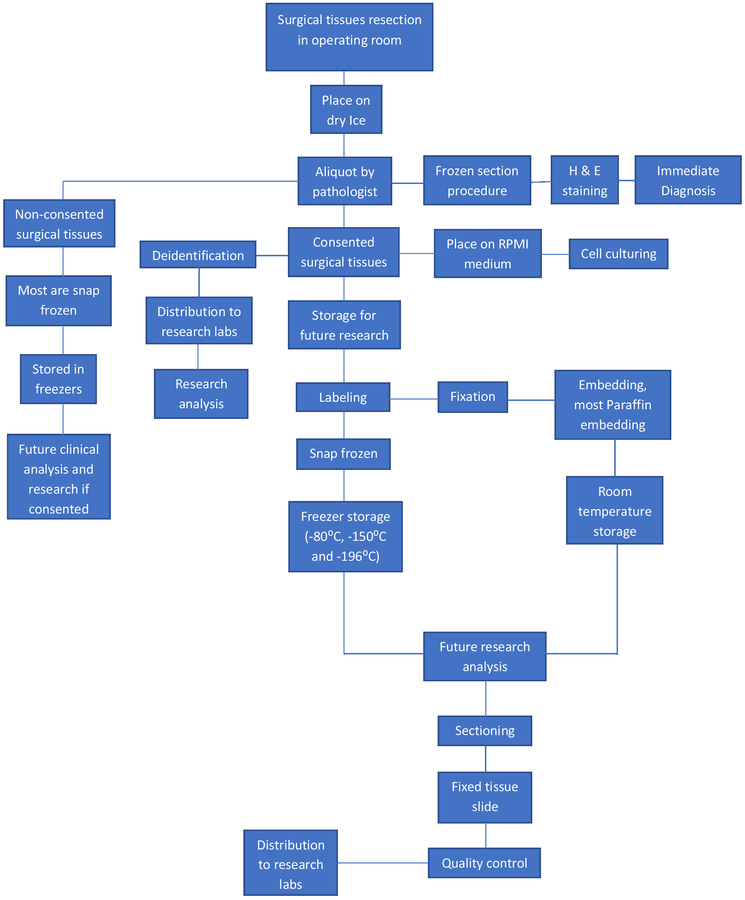
Collection of the tissue samples requires coordination with operating room and research staff. Typically, samples will be saved (frozen and in formalin). Keeping samples cold on wet ice or in the refrigerator till they can be expeditiously stabilized by freezing or formalin fixation is an important principle to remember. Samples may also be placed in culture media or other specialized media for preserving nucleic acids as requested by researchers (Fig. 2).
Figure 2.
Surgical procurement workflow
Ensure that the distribution and protocol sheets and sufficient collection supplies are in the cooler on the day of surgery.
Check the Operating Room (OR) schedule in the morning for the relevant patient cases.
Check the consent status of patients if not already known.
Obtain wet ice once paged by OR staff for a case.
Put on appropriate surgery wear (booties, hat, mask etc.).
Go to patient’s designated OR.
Obtain specimen jars from OR staff.
Check that provided patient paperwork corresponds with the specimen jar labeling.
Document the time that the specimen is collected.
Label the container if not already labeled.
Put the containers on wet ice.
Take samples back to surgical pathology.
Put wet ice container in a refrigerator while waiting for pathologist availability and confirmation of adequate tissue for diagnosis.
Ask pathologist or pathologist assistant to assist in providing lesional tissue and/or control normal tissues.
If a pathologist performed the frozen section analysis, ask for the diagnosis and record the information. The diagnosis may dictate the appropriate protocol to follow.
After a sample is provided, photograph with ruler if part of the biobank standard operating procedure. Upload to the biobank information system after tissue processing is completed.
Weigh the sample in a sterile container; tare the scale with the empty container. Sterility is important for samples that may be cultured.
Cut the sample to the desired size with a sterile scalpel on a sterile surface (for our brain tumor program, we often cut 3–5 mm pieces).
Place each piece in a labeled cryovial or specimen jar (see Note 2).
Put representative tissue into a container with culture media or other specialized media if required by your protocol or by a special request. Maintain the media on wet ice or at room temperature depending on the requirements of the protocol.
Drop cryovials in the isopentane/dry ice mix for 5 min (Snap freezing).
Put cryovials inside Styrofoam box with dry ice.
Put a representative tissue sample in formalin.
Document the time when stabilization (freezing, formalin fixation etc.) occurs.
Take samples back to the biobank and call or page the relevant research laboratories to come and get their fresh or frozen tissue.
When the research laboratories retrieve their samples as appropriate for their IRB-approved protocol, have them sign a form with the quantity and type of biospecimen provided to them. Enter the data in the biobank information system.
Place frozen cryovials into freezer boxes and into a freezer.
Document each cryovial’s location (freezer box number, freezer shelf location, freezer number, and room number) in the biobank information system.
Specimens in formalin should be cut so as to fit into histocassettes (see Note 4). Thickness should be about 0.2 cm and the width and length should be such that the specimen fits easily in the cassette (see Note 5).
Label the cassettes with a pencil or cassette printer prior to adding the tissue.
The tissue specimens should be left to fix in formalin for 12–18 hrs and then submitted to a pathology laboratory for tissue processing and embedding into a paraffin block.
After the paraffin blocks are returned to the biobank, paraffin sections can be cut for histologic quality assurance.
The paraffin blocks and slides can be stored in block and slide cabinets (see Note 6). The locations should be entered into the Biobank Information Management System.
3.2. Histologic Quality Assurance of Surgical Tissues
It is important to understand that, while tissues may be collected from an organ that is ostensibly normal, inflamed, cancerous or otherwise diseased, the tissue sample often is heterogeneous. There may be varying proportions of normal tissue, diseased tissue, inflammation, hemorrhage, and necrosis. In addition, there is heterogeneity within normal tissues as well as diseased tissue. For example, the white matter of the brain differs from the cortical grey matter or the renal cortex differs from the renal medulla. A malignancy may have low grade and high-grade areas. You may have aliquoted the sample into multiple cryovials or containers and each aliquot may have differing proportions of the desired lesional or normal tissue. Hence, histologic evaluation of the samples is an important step to perform on the sample to be released to the researcher.
3.2.1. Frozen tissue histology
Chip a 0.2–0.3 cm fragment off the aliquot of frozen tissue that has been requested for disbursement.
Place the small fragment in a cryoprotective embedding medium (OCT) and freeze in an isopentane/dry ice solution.
Label super-frost slides.
Section tissue in a cryostat at 5 microns (4–6 microns is typical).
Pick up sections on super-frost slides labeled as to the specimen identity.
Fix slides in acetone for 15 minutes, at −20°C.
Perform H&E (Hematoxylin & Eosin) staining.
Provide to a pathologist to review.
Document the pathologist’s findings in the biobank information system.
If the specimen does not meet criteria sufficient for use, prepare another aliquot from the frozen tissue specimen (either from a different area of the frozen tissue or from a different cryovial of the same specimen).
Provide the histologic quality assurance data on the biospecimen to the researcher or end-user. For example, we provide the estimated percentage of tumor on the slides. Typically, the end-user lets us know their minimum criteria which is dependent on the downstream assay.
3.2.2. Formalin fixed paraffin embedded tissue block histology
Preparation of paraffin tissue sections for histology includes using a microtome (see Note 7) to cut sections from a previously prepared FFPE tissue block (see Note 8). Sections of 5 microns can be cut on a microtome for light microscopy (see Note 9). Again, the pathologist should review the H&E stained section and the findings should be recorded in the biobank information system and provided to the end-user as appropriate.
4. Notes
A number of factors impact each step of tissue procurement and change suitability of a particular sample for a research project. In vivo factors include co-existing medical conditions, treatments (e.g. radiation, medications, alternative medical therapies, and anesthesia) and warm ischemia. Some ex vivo factors include warm or cold ischemia, transport temperature, and time to stabilization. Ischemia can affect a variety of cell properties include gene expression and protein phosphorylation profile. Surgical tissues should be placed on wet ice as soon as possible. The advantage of this cooling is minimizing ischemic and other changes till the samples can be stabilized by freezing or formalin fixation.
There must be at least 2 identifiers on every container. Typically, our biobank team receives samples with patient name, medical record number, specimen designation (e.g. right temporal lobe tumor), and frozen section diagnosis. Dates are always desirable as part of labeling, but they must be specified as to what they reflect. Blocks will be labeled with patient’s identification number (7).
A variety of instruments or equipment is used to support the process of biospecimen procurement. All instruments and equipment should be properly operated, maintained, serviced, and monitored to ensure that malfunctions of these instruments and equipment do not adversely affect the service requests or the safety of the laboratory personnel. The procedures and schedules for instrument maintenance should be as frequent as specified by the manufacturer. The laboratory director periodically evaluates the results of instrument maintenance and function for all devices. The evaluations are documented on the Equipment Maintenance forms. Freezer and refrigerator temperature should be checked daily. If temperature is within the acceptable range it will be recorded by date but if outside of acceptable range, it should be rechecked in 15 minutes to see if the unit recovers to within the parameters. If the unit fails to recover to normal ranges, management will be notified. Thermometers must pass quality assurance yearly using a NIST-certified thermometer. Document results and any actions taken on the Mechanical Refrigeration Unit Daily and Monthly.
Sectioning tissue can be dangerous and carry biohazard risk. Personnel sectioning tissue should receive adequate training in operating sectioning equipment and using safety precautions.
Different section thicknesses are appropriate for various usages: 4–5 microns for immunostaining and 70–100 microns for nucleic acid extraction samples. For the latter, tissue scraped from multiple slides is also feasible.
Paraffin-embedded specimens are stored under conditions that protect them from light, moisture and heat. The paraffin blocks are typically stored at room temperature.
Microtomes are very heavy pieces of equipment. They must be situated in a secure position, on a firm surface, not too near to the edge of the bench. Microtomes should be kept as clean as possible and be left in a safe condition for service personnel
Extended storage of unstained FFPE slides should be avoided as this may result in the loss of antigenicity for immunohistochemistry. Antigenicity loss is variable depending on the antigen/antibody. It is preferable to cut fresh slides from the paraffin block. However, it may be unavoidable. Storage in a refrigerator may extend the shelf life for immunohistochemistry.
For light microscopy, paraffin wax is most frequently used. Paraffin wax does not provide a sufficiently hard matrix for cutting very thin sections for electron microscopy. Instead, resins are used. Epoxy resins are the most commonly employed embedding media, but acrylic resins are also used, particularly where immunohistochemistry is required.
Physical damage via breakage, fire, and flooding are risks to biospecimens. Loss of electrical power can lead to loss of frozen tissue biospecimens. Consider diverse storage locations or storage at room temperature (8).
Acknowledgement
This work was supported in part by NIH:NCI P50-CA211015, NIH:NIMH U24 MH100929, the Art of the Brain Foundation, and the Henry E. Singleton Brain Cancer Research Program.
References
- 1.Tuck Melissa K., Chan Daniel W., Chia David, et al. (2009) Standard Operating Procedures for Serum and Plasma Collection: Early Detection Research Network Consensus Statement Standard Operating Procedure Integration Working Group. J Proteome Res 8(1):113–117. Doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shabikhani Maryam, Lucey Gregory M., Wei Bowen, et al. (2014) The procurement, storage, and quality assurance of frozen blood and tissue biospeciments in pathology, biorepository, and biobank setting. Clin Biochem 47(0): 258–266. doi: 10.1016/j.clinbiochem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ISBER (2012) Best practices for repositories collection, storage, retrieval, and distribution of biological materials for research international society for biological and environmental repositories. Biopreserve Biobank 10(2):79–161. doi: 10.1089/bio.2012.1022. [DOI] [PubMed] [Google Scholar]
- 4.Harty-Golder B (2004) Retention and ownership of blocks. MLO Med Lab Obs 36:37. [PubMed] [Google Scholar]
- 5.Hakimian R, Taube S, Bledsoe M, et al. (2004) National Cancer Institute Cancer Diagnosis Program: 50- State Survey of Laws Regarding the Collection, Storage, and Use of Human Tissue Specimens and Associated Data for Research. Bethesda, MD: National Institutes of Health; NIH publication No: 05–5628 [Google Scholar]
- 6.Yong William H., Dry Sarah M., and Shabikhani Maryam (2014) A Practical Approach to Clinical and Research Biobanking In: Day Christina E. (ed.) Histopathology, Methods and Protocols, Los Angeles, CA, USA: [DOI] [PubMed] [Google Scholar]
- 7.Kay Andrew B, Estrada Daniel K, Mareninov Sergey, et al. (2011) Considerations for uniform and accurate biospecimen labeling in a biorepository and research environment. J Clin Pathol 64: 634–636. Doi: 10.1136/jcp.2010.080655 [DOI] [PubMed] [Google Scholar]
- 8.Lou Jerry J, Mirsadraei Leili, Sanchez Desiree E, et al. (2014) A review of room temperature storage of biospecimen tissue and nucleic acids for anatomic pathology laboratories and biorepositories. Clin Biochem 47(0): 267–273. Doi: 10.1016/j.clinbiochem.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]