Abstract
Objective: To explore the prevalence of micronutrient deficiencies in patients with diabetic foot ulcers and correlate this with foot disease severity and other clinical factors.
Approach: Prospective cohort study of diabetic patients with foot ulcers seen in multidisciplinary foot clinics across Adelaide or admitted to the Vascular Surgery Unit at the Royal Adelaide Hospital between February 2017 and September 2018. A total of 131 patients were included in the study. Plasma serum levels of vitamins A, C, D, and E, copper, zinc, and ferritin were measured. Demographic and clinical data, including BMI, smoking status, duration of diabetes, HbA1c, and WIfI score, were obtained.
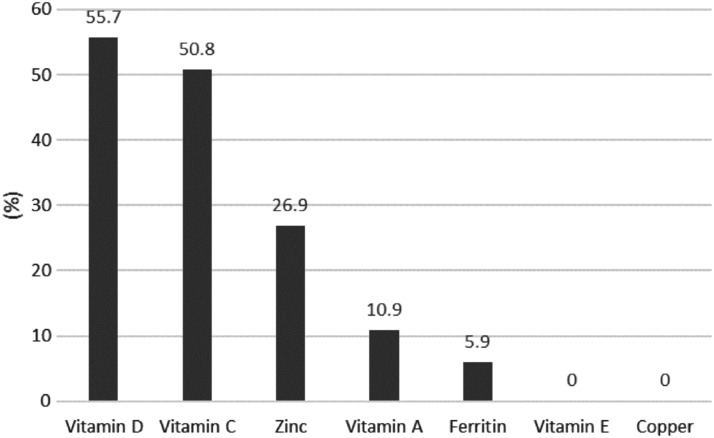
Results: The most prevalent nutritional deficiency found was vitamin D affecting 55.7% of patients. Suboptimal levels of vitamin C affected 73% of patients, comprising marginal levels in 22.2% and deficient levels in 50.8%. Zinc deficiency, vitamin A deficiency, and low ferritin levels were present in 26.9%, 10.9%, and 5.9% of patients, respectively. There was no correlation between BMI, grip strength, duration of diabetes, HbA1c, or smoking status with micronutrient deficiency. Increased severity of diabetic foot disease was associated with lower vitamin C levels (p = 0.02).
Innovation: This study has demonstrated that the deficiency of micronutrients, especially vitamin D, vitamin C, zinc, and vitamin A, is common in diabetic patients with foot ulcers.
Conclusions: The prevalence of micronutrient deficiency is high in a diabetic population with foot ulcers/wounds. Special concerns regarding the high prevalence of vitamin C and zinc deficiency, given their roles in wound healing. Although further research needs to be performed to determine the clinical implications of our findings, micronutrient deficiency should be considered in diabetic patients with foot wounds.
Keywords: chronic wounds, diabetes, nutrition
Guilherme Pena, MD.

Introduction
Diabetic foot complications carry a substantial physical, psychological, and financial burden for the patients and community. People who suffer from diabetes have a lifetime risk of nearly 25% of developing a foot ulcer, and more than 50% of patient ulcers will develop infection.1 A history of foot ulcer is significantly associated with negative outcomes. Approximately 85% of all amputations in diabetic patients are preceded by foot ulceration, which subsequently deteriorates to foot infection or gangrene.2
Diabetic foot complications are recognized as the most common cause of nontraumatic lower limb amputation internationally. Worldwide it is estimated that every 20 s a lower limb amputation is performed as a consequence of diabetes.3 The challenge to heal a diabetic foot ulcer is compounded by the high rate of reulceration once healed. Approximately 40% of patients have recurrence of an ulcer within 1 year after healing, almost 60% within 3 years, and 65% within 5 years.3
Multifactorial efforts should be made to give affected patients the best chance to heal foot ulcers. Traditionally, this has taken the form of local wound care, debridement, offloading, attention to infection, and revascularization if required. However, there are many other patient-related factors that are frequently overlooked that may influence wound healing (Table 1). One of the factors most commonly neglected is patient nutrition.
Table 1.
Factors considered to affect wound healing
| Poor or impaired perfusion |
| Infection |
| Smoking and alcoholism |
| Aging |
| Chronic diseases (e.g., diabetes, chronic kidney disease, AIDS) |
| Malnutrition |
| Medication (e.g., glucocorticoid steroids, nonsteroidal anti-inflammatory drugs, chemotherapy) |
| Obesity |
| Edema |
| Presence of foreign body |
| Venous insufficiency |
Clinical Problem Addressed
Wound healing is a complex, dynamic, and interactive process involving soluble mediators, blood cells, extracellular matrix, and parenchymal cells. Wound healing has three phases that overlap in time; inflammation, tissue formation, and tissue remodeling.4 The relationship between nutrition and wound healing has been recognized for centuries. It is widely known that macronutrient malnutrition, especially protein, adversely affects wound healing. Equally important are micronutrients, which are critical components of cellular metabolism. A number of vitamins and minerals play a significant role in the immune system and wound healing, particularly important are vitamin C, vitamin A, and zinc.5 Despite their roles, they are not routinely measured or monitored in clinical practice.
The primary goal of this study was to assess the prevalence of vitamin and micronutrient deficiency in diabetic patients with foot ulcers seen either in an outpatient setting within multidisciplinary foot clinics, or inpatients admitted to our vascular service. The secondary goal was to correlate micronutrient levels with disease severity and other clinical factors, namely duration of diabetes, HbA1c levels, grip strength, and smoking status.
Materials and Methods
This study is part of a major project assessing factors influencing outcomes in patients with diabetic foot disease. Ethics has been obtained from the Central Adelaide Local Health Network Ethics Committee, and written consent was obtained from all participants. Subjects consisted of patients seen at Multidisciplinary Foot Clinics at The Queen Elizabeth Hospital and Lyell McEwin Hospital, or admitted under the Vascular Surgery service at the Royal Adelaide Hospital, all within the Adelaide metro area of South Australia. Eligibility criteria included being diabetic, age ≥18 years, able to have follow-ups in Adelaide, and presence of foot ulcer(s).
A total of 131 patients were recruited for the study between February 2017 and September 2018. Plasma levels of vitamins A, C, D, and E; and copper, zinc, and ferritin were measured at recruitment. All the samples were venous blood taken by a phlebologist in the hospital or at an outpatient pathology collection center. Specimens were handled and transported as per collection guide and all processed by SA Pathology. Table 2 shows reference levels used for analysis. Demographic information and clinical data were prospectively obtained during patient assessment, including age, gender, weight, height, BMI, and smoking status. In addition, grip strength and WIfI score were also recorded. Grip strength is a measurement of muscle function as an indicator of functional as well as nutritional status.6 The WIfI is a validated classification that stratifies patients with threatened lower extremity, including patients with diabetic foot ulcers, based on three major factors that impact amputation risk and clinical management: Wound, Ischemia, and foot Infection.7
Table 2.
Reference levels of micronutrients
| Vitamin A | <0.7 μmol/L | Deficient |
| ≥0.7 μmol/L | Nondeficient | |
| Vitamin C | <11.4 μmol/L | Deficient |
| 11.4–22.7 μmol/L | Marginal | |
| >22.7 μmol/L | Adequate | |
| Vitamin D | <60 nmol/L | Deficient |
| ≥60 nmol/L | Nondeficient | |
| Vitamin E | <12 μmol/L | Deficient |
| ≥12 μmol/L | Nondeficient | |
| Zinc | <9 μmol/L | Deficient |
| ≥9 μmol/L | Nondeficient | |
| Copper | <10 μmol/L | Deficient |
| ≥10 μmol/L | Nondeficient | |
| Ferritin | <30 μg/L | Deficient |
| ≥30 μg/L | Nondeficienta |
Ferritin is an acute-phase reactant and significantly higher cutoff levels for ferritin are used to define iron deficiency accompanied by inflammation.
The association between the nutrients most commonly cited as important for wound healing, namely vitamin C, vitamin A, and zinc, and smoking status, grip strength, duration of diabetes (since diagnosis), HbA1c levels, and burden of diabetic foot disease assessed by WIfI, was assessed.
Statistics
Continuous measures are summarized as means with standard deviations and medians with interquartile range. Categorical measures are presented as counts and percentages. Associations between nutrient deficiencies and continuous predictors were determined using the Kruskal–Wallis test (vitamin C deficiency) or Wilcoxon test (remaining nutrients) as appropriate. The associations between nutrient deficiency and categorical predictors were assessed using Pearson's chi-square or Fisher's exact test as appropriate. There was no formal statistical assessment for vitamin E or copper deficiencies as all patients were in the normal range for these nutrients.
Results
One hundred thirty-one patients were enrolled in the study. The characteristics of the participants are shown in Table 3. Figure 1 summarizes the prevalence of micronutrient deficiencies. The elements most frequently found to be deficient, in descending order, were vitamin D, vitamin C, zinc, ferritin, and vitamin A. None of the patients had low levels of vitamin E or copper.
Table 3.
Summary of participant characteristics
| Variable | Level | Number | Percent |
|---|---|---|---|
| Gender | Female | 27 | 20.6 |
| Male | 104 | 79.4 | |
| Smoking status | Current | 31 | 23.7 |
| Exsmoker | 62 | 47.3 | |
| Never | 38 | 29 | |
| WIfI category | 1 | 25 | 19.1 |
| 2 | 32 | 24.4 | |
| 3 | 33 | 25.2 | |
| 4 | 41 | 31.3 |
| Variable | Mean | SD |
|---|---|---|
| Age (years) | 66.3 | 13.1 |
| Duration of diabetes (years) | 16.4 | 10.7 |
| HbA1c (%) | 8.8 | 4.4 |
| BMI | 29.4 | 6.1 |
| Grip strength (kg) | 29.1 | 9.6 |
Figure 1.
Percentage of participants with vitamin and mineral deficiencies.
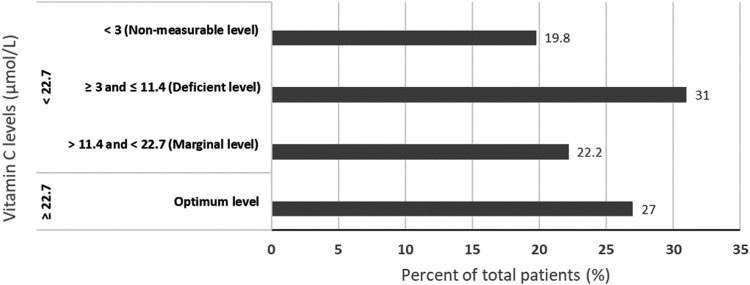
Twenty-seven percent of patients had normal levels of vitamin C. The remainder had suboptimum levels with just over half of all the patients having low or no measurable plasma levels of this vitamin (Fig. 2).
Figure 2.
Vitamin C levels.
There was no association between the duration of diabetes, HbA1c levels, grip strength, smoking habits, and BMI and levels of vitamin A, C, and zinc. Patients with a higher burden of foot disease as assessed by the WIfI score had lower levels of vitamin C (p = 0.02) and higher ferritin level (p = 0.004). Lower grip strength and smoking habit were associated with lower vitamin D levels (p = 0.02 and 0.01, respectively) (Table 4).
Table 4.
Association between micronutrients and clinical parameters
| Smoking Status (p Value)a | Grip Strength (p Value) | Duration of Diabetes (p Value) | HbA1c (p Value) | WIfI Scoreb(p Value) | |
|---|---|---|---|---|---|
| Vitamin C | 0.13 | 0.77 | 0.21 | 0.70 | 0.02 |
| Vitamin A | 1.0 | 0.39 | 0.46 | 0.82 | 0.06 |
| Zinc | 0.30 | 0.87 | 0.98 | 0.41 | 0.05 |
| Ferritin | 0.34 | 0.63 | 0.78 | 0.47 | 0.004 |
| Vitamin D | 0.02 | 0.01 | 0.21 | 0.19 | 0.73 |
Bold values are statistically significant.
There was no formal statistical assessment for vitamin E or copper deficiencies as all patients were in the normal range for these nutrients.
Assessed as current smoker or nonsmoker.
Fisher's exact test (Pearson's chi-square test otherwise).
Discussion
Vitamins and minerals are required in small amounts, yet they are critical to the cellular metabolism, including the wound healing process. This study has demonstrated that micronutrient deficiencies are very common in diabetic patients with foot ulcers/wound.
Vitamin D deficiency was the most common deficiency detected, and its prevalence is consistent with previous reports.8 This was expected as vitamin D deficiency is recognized as a global public health problem, usually related to sunscreen use and sun avoidance behaviors.9 Diabetic patients with foot ulcers may be at particular risk of vitamin D deficiency due to reduced level of physical activity, which is often considered a surrogate for the amount of time spent outdoors and therefore sun exposure. Vitamin D is well recognized for its role in the bone homeostasis. However, vitamin D signaling has also many extraskeletal effects. These include regulation of cell proliferation, immune and muscle function, skin differentiation, and reproduction, as well as vascular and metabolic properties.10 Despite the abundance of preclinical data regarding vitamin D and skin cell interaction, there is no good-quality evidence to support a substantial role in wound healing. The association between reduced levels of vitamin D and smoking habits is consistent with previous reports, however, the mechanism of this association is unclear.11,12 Vitamin D deficiency was associated with lower grip strength. This is can be explained by the fact that lower muscular strength is one of the aspects of sarcopenia and frailty that commonly accompany aging. It is well known that geriatric patients are at higher risk of vitamin D deficiency due to a lower sunshine exposure and a reduced capacity of the older skin to synthesize vitamin D under the influence of UV light.13
The prevalence of deficiencies in vitamin C, zinc, and vitamin A was higher than anticipated, and concerning, given the pivotal roles these nutrients play in wound healing. Iron status was assessed by ferritin levels, which is a marker of iron stores in the body. Six percent of the patients had ferritin less than 30 μg/L and were considered deficient. However, the prevalence of iron deficiency is likely higher than identified by our study. Ferritin is an acute-phase reactant, and a significantly higher cutoff level for ferritin is used to define iron deficiency accompanied by inflammation.14 This explains why there was a positive correlation between ferritin levels and WIfI score, for which one of the components is the presence and severity of infection. None of the study patients had biochemical deficiency of copper or vitamin E. Deficiency of copper and vitamin E in adults is extremely rare and generally related to physiological abnormalities such as the malabsorption syndrome. It is not anticipated that deficiency of these elements will be a consequence of simple reduced intake.15,16
Vitamin C (ascorbic acid) is an important water-soluble vitamin, essential for collagen, carnitine, and neurotransmitter biosynthesis.17 Vitamin C is a cofactor for prolyl and lysyl hydroxylase, two essential enzymes in the collagen biosynthesis pathway, and as such ensures the maintenance of normal mature collagen networks in humans. Hydroxyproline serves to stabilize the triple helix, and hydroxylysine is necessary for the formation of the intermolecular crosslinks in collagen, which is critical to the biological functions of this protein.18 This failure of collagen synthesis in deficiency of vitamin C leads to the manifestations characteristic of scurvy.17,18 An interesting experiment that validates the role of vitamin C in skin hemostasis was conducted in 1939 by John Crandon, a second-year surgical resident at Boston City Hospital. Crandon commenced a vitamin C-free diet and published a detailed description of the dramatic changes he experienced during this period. Notably, at about 6 months into the experiment, his appendectomy scar from years ago began to disintegrate. In addition, a back incision, performed as part of the experiment, failed to heal and a biopsy demonstrated lack of “intercellular substance.” Following the administration of IV vitamin C, the wounds rapidly healed.19
Only 27% of patients in the present study had optimum levels of vitamin C, 22% had marginal levels, and 51% were deficient. Approximately 1 in 5 patients had nonmeasurable levels. These findings were surprising, given this vitamin is found widely in fruits and vegetables. Although low plasma ascorbic acid levels do not necessarily indicate scurvy, serum levels have a linear relationship with vitamin C intake and clinical cases of scurvy always have low or no measurable plasma ascorbic acid.20
Many common conditions are thought to result in pro-oxidant states that contribute to low vitamin C levels, including cigarette smoking, diabetes, and acute illnesses.21,22 In this study, there was no correlation between vitamin C levels and smoking history, diabetic control (measured by HbA1c), or duration of diabetes. Patients with more advanced foot disease burden assessed by WIfI classification had lower vitamin C levels. This could be related to the effect of inflammation and acute illness on plasma vitamin C levels or could be related to lower vitamin intake in the group with more advanced disease. It is known that diabetic foot disease has a profound impact on patients' quality of life. Diabetic foot ulceration is associated with restricted mobility, social isolation, and reduced self-esteem.23 It can be assumed that patients with more severe disease burden assessed by the WIfI score would experience more drastic impact on mobility and social life and therefore would be less likely to have a diet rich in fresh fruit and vegetables.
It is well documented that patients with scurvy present with weakening of connective tissues and poor wound healing.17,18 However, there are no definitive data showing that increasing the vitamin C concentration directly enhances its biochemical or molecular function in human tissues, or that higher vitamin C levels confer a wound-healing benefit in patients without scurvy. There is lack of evidence from well-designed studies to support the theory that vitamin C supplementation above the recommended daily allowances improves wound healing. However, it does make sense to treat patients with reduced levels, especially in the presence of chronic ulcers or large wounds.
Zinc is the second-most abundant trace element in the human body after iron24 and its main sources are animal products and seafood. It is an essential trace element crucial for the function of more than 300 enzymes and it is important for cellular processes such as cell division and apoptosis.25 Zinc plays an important role in wound healing as it serves as a cofactor in numerous transcription factors and enzyme systems, including zinc-dependent matrix metalloproteinases. Matrix metalloproteinases are a group of calcium-dependent zinc-containing enzymes that are involved in the degradation of extracellular matrix (ECM). Metalloproteinases and their inhibitors are essential for the regulation of ECM degradation and deposition during wound repair.26 In this study, 27% of diabetic patients with foot ulcers had low levels of this mineral. There was no correlation of zinc levels with diabetic duration and control, grip strength, or smoking status. The patients with more advanced foot disease tended to have lower zinc levels and this correlation nearly reached statistical significance (p = 0.05). It is important to note that serum zinc concentrations may not fully reflect the physiological zinc status in an individual and factors such as inflammation may affect plasma levels.
The role of oral supplementation of zinc in wound healing is controversial. A frequently cited randomized controlled trial (RCT) from 1967 demonstrated decreased wound healing time by 43% in patients with pilonidal sinus wounds receiving oral zinc sulfate supplements.27 Serum zinc concentrations in participants in this study were not measured. A more recent RCT with patients with diabetic foot ulcers demonstrated a statistically significant improvement in wound healing following 12 weeks of supplementation of zinc.28 However, both studies had small sample size and did not select patients based on zinc parameters.
Vitamin A is an essential, dietary, fat-soluble vitamin that has multiple functions, including an important role in wound healing. It is involved in epithelial differentiation and proliferation, stimulation of angiogenesis, collagen synthesis, and fibroplasia. Vitamin A also has a unique ability to reverse the inhibitory effects of glucocorticosteroids on wound healing.29 Vitamin A is available in the human diet in two forms: preformed vitamin A, found in food from animal sources, and provitamin A carotenoids, such as beta-carotene present in fruits and vegetables. Most of the vitamin A in the body is stored in the liver, and the plasma contains only ∼1% of the total body reserve. Levels can be reduced in the setting of inflammation and liver disease. Vitamin A deficiency is a public health problem in low-income countries, especially in Africa and Southeast Asia, where it is the leading cause of preventable blindness. Although vitamin A deficiency is considered rare in developed countries, 11% of the diabetic patients with foot ulcers included in this study had low retinol levels (<0.7 μmol/L) and one patient had severe deficiency (<0.35 μmol/L).
The reason for low vitamin levels in the studied patients is likely multifactorial, with poor intake playing a major role. To ensure adequate micronutrient intake, a diverse diet is required. Micronutrient-rich foods include fruits, vegetables, meat, dairy, seafood, nuts, and seeds. Maintaining a rich and diverse diet may be particularly difficult for low-income households as it may be less affordable than a more energy-dense diet. It is known that diabetic ulcers are associated with reduced quality of life, decrease mobility, and social isolation.23 This potentially impairs the ability of affected patients to go shopping frequently for fresh food. This is particularly important for vitamin C, which is found in many fruits and vegetables. Vitamin C levels in food depend on transport, storage, and cooking practices.30 In addition, there may be insufficient education about nutrition, poor cooking skills, and a heathy diet rich in vegetables and fruits may also be perceived as not palatable or boring.
This study's primary aim was to assess the prevalence of micronutrient deficiency in diabetic patients with foot ulcers. We acknowledge that there are many potential confounders that may influence the micronutrient levels and would be ideal to have a detailed nutritional history from the patients, including history of malabsorption syndromes, dietary intake, and supplement use. It would be also interesting to compare the results with control groups of individuals without diabetes and a group of diabetic patients without history of foot ulcers. The sample size of this study was large, and the selection criteria were broad to include a whole heterogeneity of diabetic population with foot ulcers. Although further research needs to be performed to determine the clinical implications of our findings, vitamin and mineral deficiency should be considered in all diabetic patients with foot wounds. Foot ulcers are a common and challenging complication of diabetes and constitute a substantial burden for these patients. The management should be based on intensive multimodality therapy aiming to achieve wound healing, reduce risk of reulceration, and an improved quality of life.
Innovation
This study has demonstrated that the prevalence of micronutrient deficiency, especially vitamin D, vitamin C, zinc, and vitamin A, is high in diabetic patients with foot ulcers. Currently, there is no good-quality evidence to support micronutrient supplementation to improve wound healing and this study did not assess clinical correlation with outcomes. However, in light of the physiological role of some micronutrients, especially vitamins C and A, and zinc, the complexity of the diabetic foot disease, and the high prevalence of micronutrient deficiency found in this study, we suggest assessing the levels of these vitamins and minerals in patients with diabetic foot ulcers and considering supplementary treatment if deficiency is found.
Key Findings
Suboptimal levels of vitamin C affected 73% of diabetic patients with foot ulcers, comprising marginal levels in 22.2% and deficient levels in 50.8%.
Zinc deficiency was found in ∼27% of the patients.
Vitamin A deficiency was present in ∼11% of the patients.
Although further research needs to be performed to determine the clinical implications of our findings, micronutrient deficiency should be considered in diabetic patients with foot wounds.
Acknowledgment and Funding Sources
The authors thank Ruth Battersby for assisting with data management.
Abbreviations and Acronyms
- ECM
extracellular matrix
- RCT
randomized controlled trial
Author Disclosure and Ghostwriting
The authors declare no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Guilherme Pena, MD, is a vascular surgery trainee with special interest in diabetic foot. Currently undertaking a higher degree by research in the topic “diabetic foot” at the University of Adelaide. Beatrice Kuang, MBBS, is a vascular surgery service registrar. Prue Cowled, PhD, BSc (Hons), is the principal medical scientist for the Department of Surgery at the University of Adelaide. Stuart Howell, PhD, is a senior statistician at the University of Adelaide. Joseph Dawson, MBBS, ChM, MD, MRCS, FRCS, FRACS, is a consultant vascular surgeon at the Royal Adelaide Hospital. Ross Philpot, MBBS, FRACP, is an infectious diseases specialist at the Queen Elizabeth Hospital with special interest in diabetic foot and nutrition. Robert Fitridge, MBBS, MS, FRACS, is Professor of vascular surgery at the University of Adelaide and Head of the Multidisciplinary Diabetic Foot Service at The Queen Elizabeth Hospital and Lyell McEwin Health Service, which he cofounded in the mid-1990s. He is a member of the Baker IDI/NHMRC working group, which developed national guidelines (“Prevention, Identification, and Management of foot complications in diabetes”) for the management of the diabetic foot and member of the International Working Group on the Diabetic Foot.
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 2. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev 2000;16 Suppl 1:S75–S83 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 [DOI] [PubMed] [Google Scholar]
- 4. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 5. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norman K, Stobaus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011;30:135–142 [DOI] [PubMed] [Google Scholar]
- 7. Mills JL Sr., Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–234.e1–e2 [DOI] [PubMed] [Google Scholar]
- 8. Daly RM, Gagnon C, Lu ZX, et al. Prevalence of vitamin D deficiency and its determinants in Australian adults aged 25 years and older: a national, population-based study. Clin Endocrinol (Oxf) 2012;77:26–35 [DOI] [PubMed] [Google Scholar]
- 9. van Schoor N, Lips P. Worldwide vitamin D status. In: Bouillon R. Vitamin D: Classical and Novel Actions. New York, NY: Elsevier, 2011: 671–680 [DOI] [PubMed] [Google Scholar]
- 10. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extra-skeletal actions of vitamin D: current evidence and outstanding questions 2018. [Epub ahead of print] DOI: 10.1210/er.2018-00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brot C, Jørgensen NR, Sørensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr 1999;53:920. [DOI] [PubMed] [Google Scholar]
- 12. Krall EA, Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res 1999;14:215–220 [DOI] [PubMed] [Google Scholar]
- 13. Visser M, Lips P, Deeg DJH. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 2003;88:5766–5772 [DOI] [PubMed] [Google Scholar]
- 14. Camaschella C. Iron-deficiency anemia. N Engl J Med 2015;372:1832–1843 [DOI] [PubMed] [Google Scholar]
- 15. Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol 2008;80:523–531 [DOI] [PubMed] [Google Scholar]
- 16. Institute of Medicine Panel on Dietary AaR, Compounds. Vitamin E. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): National Academies Press (US), 2000 [PubMed] [Google Scholar]
- 17. Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2003;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyera N, Galey I, Bernard BA. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int J Cosmet Sci 1998;20:151–158 [DOI] [PubMed] [Google Scholar]
- 19. Crandon JH, Lund CC, Dill DB. Experimental human scurvy. N Engl J Med 1940;223:353–369 [Google Scholar]
- 20. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66–74 [DOI] [PubMed] [Google Scholar]
- 21. Schectman G, Byrd JC, Hoffmann R. Ascorbic acid requirements for smokers: analysis of a population survey. Am J Clin Nutr 1991;53:1466–1470 [DOI] [PubMed] [Google Scholar]
- 22. Sinclair AJ, Taylor PB, Lunec J, Girling AJ, Barnett AH. Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet Med 1994;11:893–898 [DOI] [PubMed] [Google Scholar]
- 23. Kinmond K, McGee P, Gough S, Ashford R. ‘Loss of self’: a psychosocial study of the quality of life of adults with diabetic foot ulceration. J Tissue Viability 2003;13:6–8, 10, 2 passim [DOI] [PubMed] [Google Scholar]
- 24. Scrimshaw NS, Young VR. The requirements of human nutrition. Sci Am 1976;235:50–64 [DOI] [PubMed] [Google Scholar]
- 25. Jansen J, Karges W, Rink L. Zinc and diabetes—clinical links and molecular mechanisms. J Nutr Biochem 2009;20:399–417 [DOI] [PubMed] [Google Scholar]
- 26. Caley MP, Martins VL, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 2015;4:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pories WJ, Henzel JH, Rob CG, Strain WH. Acceleration of wound healing in man with zinc sulphate given by mouth. Lancet 1967;1:121–124 [DOI] [PubMed] [Google Scholar]
- 28. Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen 2017;25:512–520 [DOI] [PubMed] [Google Scholar]
- 29. Wicke C, Halliday B, Allen D, et al. Effects of steroids and retinoids on wound healing. Arch Surg 2000;135:1265–1270 [DOI] [PubMed] [Google Scholar]
- 30. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA 1999;281:1415–1423 [DOI] [PubMed] [Google Scholar]