Abstract
Although pre-exposure prophylaxis (PrEP) has been approved for primary HIV prevention for individuals aged 18 years or older since 2012, PrEP utilization has been suboptimal. To understand trends in PrEP provision from the health care providers' perspective, we systematically assessed each specific stage along the PrEP implementation cascade (i.e., awareness, willingness, consultation, and prescription) among health care professionals (HCPs) in the United States. Between June and December 2018, we conducted a systematic review of published studies on this topic. A total of 36 eligible studies were identified and included in the analyses. Random-effect models were employed to examine the pooled prevalence of each key stage along the cascade. Time trend and subgroup analyses were conducted. A thematic analysis was used to identify barriers and facilitators along the PrEP cascade. In this study, a total of 18,265 HCPs representing diverse demographics were included. The pooled prevalence of PrEP awareness was 68% [95% confidence interval (CI) = 55–80%], willingness to prescribe PrEP was 66% (95% CI = 54–77%), PrEP consultation was 37% (95% CI = 25–51%), and prescription provision was 24% (95% CI = 17–32%). Subgroup analyses revealed that PrEP provision among HCPs was lowest in the south, but has been improving annually nationwide. Infectious disease specialists [odds ratio (OR) = 4.06, 95% CI = 3.12–5.28; compared with primary care providers] and advanced practice registered nurses/physician assistants (OR = 1.51, 95% CI = 1.09–2.09; compared with physicians) had higher odds of prescribing PrEP. Barriers and facilitators regarding optimal PrEP implementation were embedded within individual, dyadic, social, and structural levels. This meta-analysis has comprehensively examined the trend and pattern of PrEP implementation among HCPs. To achieve optimal implementation of the PrEP cascade in the United States, tailored training and programs need to be provided to HCPs.
Keywords: systematic review, meta-analysis, PrEP implementation cascade, health care professionals, United States
Introduction
An estimated 1.1 million people in the United States are living with HIV, with 38,739 new HIV diagnoses in 2017.1 In 2017, the national rate of new diagnoses was 11.8 per 100,000 though regional differences exist: the south having the highest incidence (16.1), followed by the northeast (10.6), west (9.4), and midwest (7.4).2 The most heavily affected subgroups include men who have sex with men (MSM) of all races and ethnicities with young black MSM shouldering a disproportionate burden, followed by black and Latino heterosexuals.1,2 HIV continues to burden these subgroups despite intensive behavioral and biomedical prevention efforts (e.g., HIV testing and condom promotion).1,3
To curb the HIV epidemic, the US Food and Drug Administration (FDA) approved the use of daily oral pre-exposure prophylaxis (PrEP, brand name Truvada®) for primary HIV prevention in 2012 for “at-risk individuals aged 18 years or older,” and expand this indication to adolescents in 2018, based upon sufficient evidence of efficacy and safety from multiple clinical trials.4–9 In 2014, the Centers for Disease Control and Prevention (CDC) released clinical practice guidelines for health care providers to facilitate in prescribing PrEP to individuals with indications for its use,10 and the US Preventive Services Task Force (USPSTF) issued a final grade A recommendation statement endorsing provision of PrEP to persons at high risk of HIV acquisition.11,12 Based upon these recommendations, before prescribing PrEP, clinicians should confirm HIV-negative serostatus, assess kidney function, and test for hepatitis B and C, sexually transmitted infections, and pregnancy (when pertinent).11
Although health care professionals (HCPs) have made tremendous efforts, the uptake and provision of PrEP among people at risk of HIV acquisition has been slow.13 Although there has been a 73% overall increase in PrEP uptake since 2012, of a large number of persons who were at substantial risk of HIV infection in 2016 [1,232,000, 95% confidence interval (CI) = 661,000–1,803,000],13,14 only a small proportion (6.4%) were prescribed the medication. Even with this small proportion of PrEP users, significant disparities are observed across different geographical regions and at-risk groups. For instance, the rate of PrEP uptake in the northeast (47.4/100,000) was approximately two times higher than that in the south (22.6/100,000), west (28.1/100,000), and midwest (23.5/100,000), respectively.15 There were 14 times more male PrEP users than female users in 2016,15 and PrEP users are predominantly white.16 Further, PrEP use remains underutilized among various at-risk groups, including MSM (3–6%),17–19 black and Hispanic women (2%),20 injecting drug users (IDUs) (<2%),21 and sex workers (no reported data) in the United States.21–23 In addition, significant racial/ethnic disparities have been observed among PrEP users: blacks account for 44% of new HIV infections, only 10% of those on PrEP in 2015 were black.12,20 Thus, the provision of PrEP for people at risk of HIV acquisition and concomitant health disparities has become a top priority and significant research gap.
Nunn et al. proposed a “PrEP implementation cascade” model that suggests that progression along stages of the cascade must involve interaction and engagement among patients, HCPs, and other critical stakeholders in the system.24 Most available studies describing barriers to PrEP use, however, primarily focus on patient-level factors ranging from individual (e.g., ethnicity/race and age),25,26 dyadic (e.g., mistrust with HCPs),27 social (e.g., HIV stigma),28–30 or structural factors (e.g., insurance coverage,)31–33 with a smaller number of studies examining the role of health providers34–36 and other key stakeholders (e.g., pharmacists and community workers)34,35,37–42 as well as barriers embedded within PrEP implementation (e.g., prescription logistics and location of PrEP clinics).31–33
Studies focusing on HCPs either quantitatively examine factors that may influence PrEP prescription including sociodemographic variables (e.g., gender and race/ethnicity), practice characteristics (e.g., years of practice, expertise, and discussion of sexual history with patients),43–45 cognitive variables (e.g., attitudes and beliefs),36 and structural factors (e.g., available trainings and cost)36 among HCPs in different settings (e.g., primary care settings and HIV clinics),46–48 or qualitatively examined their concerns (e.g., patients' risk compensation behaviors and medication side effects) and solutions (e.g., tailored training) regarding PrEP provision.29,41,49 Further, several previous reviews have synthesized findings to describe the critical role of HCPs in PrEP implementation.34,35,46,47,50 For instance, a series of reviews conducted by Krakower and Mayer have comprehensively described different types of providers along the PrEP implementation cascade, in addition to proposed practical strategies for promoting optimal PrEP usage.34,35,50 However, lack of a systematic quantitative assessment of the provider PrEP implementation cascade may serve as a limitation for interpreting findings from these and other reviews. Besides, the “purview paradox” [i.e., neither infectious disease (ID) specialists nor primary care providers (PCPs) believe that PrEP care falls within their practice] has been consistently mentioned as a key barrier to PrEP implementation,29,51,52 but no studies have assessed this phenomenon quantitatively. Further, Pinto et al. identified multi-level challenges among HIV service providers and individuals at risk for infection (e.g., cognitive barriers and PrEP stigma) along the PrEP implementation cascade.29 However, most existing reviews segment health providers (e.g., physicians) from other HCPs (e.g., pharmacists) who also play a key role in PrEP implementation.34,35,46,47,50
To date, no studies have systematically examined PrEP implementation among HCPs in the United States as well as corresponding barriers and facilitators using statistically rigorous analytical strategies, such as meta-analysis. To fill this gap, the goal of this study is to identify and synthesize existing data along the PrEP implementation cascade among HCPs in the United States with three specific aims: (1) to quantitatively estimate the proportion of HCPs at each specific stage (e.g., awareness, willingness to prescribe, patient consultation, and actual prescription) along the PrEP cascade and quantitatively evaluate the “purview paradox” phenomena among HCPs, (2) to identify barriers and facilitators associated with PrEP implementation reported by each individual study, and (3) to make pragmatic recommendations for future programs aiming to promote PrEP implementation within the current health system in the United States.
Methods
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (Supplementary Table S1).53–56 For the first aim, studies were included if they reported provider-level data on PrEP care implementation in the United States. For the second aim, we extracted identified barriers and facilitators along the PrEP implementation cascade from included studies. Where multiple articles reported a single cohort, only the article with the most comprehensive data was included. We registered this review in the International Prospective Register of Systemic Reviews (Registration No. CRD42019122876).
Search strategy and study selection
Between June and December 2018, we conducted a comprehensive literature search from multiple databases including PubMed/MEDLINE, Web of Science, PsycINFO, EMBASE, and Google Scholar, with the following key words HIV and/or AIDS; health professionals and/or health providers; and PrEP care cascade or implementation (Supplementary Table S2). We also conducted a thorough search of conference proceedings, as well as references from reviews and articles that met our inclusion criteria. Two reviewers (C.Z. and Y.L.) independently reviewed articles identified in the initial search and disagreement was resolved by discussion (inter-rater reliability >95%). We also contacted one study author to clarify data information, but no response was obtained.
Published articles were included if they (1) presented results on PrEP implementation cascade for a sample including at least one type of HCPs and specialty [e.g., pharmacists, advanced nurse practitioners, and physician assistants (PAs), or physicians in specialties including HIV/ID, obstetrics-gynecology (OBGYNs), family medicine, internal medicine, PCPs] in the United States; (2) used quantitative (e.g., randomized control trials and cross-sectional/cohort studies) or qualitative (e.g., focus group and in-depth interviews) or mixed-method study designs; (3) reported quantitative measures (proportions) for any stages of the PrEP implementation (e.g., PrEP awareness, acceptance/willingness to prescribe PrEP, consultation on PrEP use, and PrEP prescription); or provided sufficient information to calculate pooled estimates; and (4) were peer reviewed and published in English, and could be searched from indexed databases or published sources. We excluded articles if they were (1) descriptive studies (e.g., case studies or case reports) or studies without quantitative measurements; (2) studies that only report data at patient- or institution- or state level; (3) reviews/meta-analyses; and (4) theoretical/modeling studies without original data.
Data extraction
Statistical analyses
Aim 1: synthesized pooled proportions
Prevalence of PrEP awareness (proportion of those who had ever heard of PrEP), willingness to prescribe PrEP (proportion of those who self-reported willingness to prescribe PrEP), PrEP consultation (proportions of those who ever provided PrEP-related consultation to patients), and PrEP prescription (proportion of those who have prescribed PrEP), were the key estimates in the current analyses. We calculated these PrEP implementation estimates by selected provider demographic characteristics, specialties, and professions, for studies in which these data were available. We also calculated the odds ratio (OR) for each PrEP cascade outcome by selected provider characteristics. For example, using the raw data we calculated the ratio of the odds of prescribing PrEP among advanced practice registered nurses (APRNs; number of APRNs who prescribed PrEP/the number of APRNs who did not prescribe PrEP) to the odds of prescribing PrEP among physicians (number of physicians who prescribed PrEP/the number of physicians who did not prescribe PrEP).
We employed the DerSimonian–Laird random-effects model to weight and pool the individual estimates,57 as all included studies were conducted among different populations across heterogeneous settings.58 Unlike the fixed effects model that assumed that all studies shared identical true effect sizes, the random-effects model was designed to capture variances of estimates across studies.58,59 In addition to the overall synthesis, we also conducted subgroup analyses to examine the pooled estimates by location of data collection, study design, and years of when data were collected.
In the analyses, we assessed differences (ORs) in implementing each stage of PrEP cascade across specialties (e.g., ID vs. PCPs) and provider types (e.g., APRNs vs. physicians). In addition, we calculated the pooled proportions for each specific stage along the PrEP cascade using the information provided by each study, with time trend analyses performed to assess whether the significant variance was observed across years. Further, a series of bivariate random-effects meta-regression analyses using aggregate-level data were performed to assess factors that may be associated with each specific stage of the PrEP implementation cascade as well as to explain heterogeneity of included studies better.60 In addition, sensitivity analyses were employed to examine the stability of the pooled estimates by evaluating whether the overall pooled estimates were sensitive to the exclusion of any individual studies (e.g., study with extreme weights or sample sizes). The I2-statistic and its corresponding 95% CIs describe heterogeneity, with higher percentages indicating higher heterogeneity.58 Publication bias was assessed by funnel plots (asymmetry indicating existing publication biases) and Egger's test (testing the asymmetry statistically).61 We performed all statistical and meta-analysis using STATA®15 (College Station, TX).
Aim 2: barriers and facilitators associated with PrEP care implementation
We extracted factors that were associated with stages of the PrEP cascade that were either reported by HCPs from qualitative studies or identified by analytical models from quantitative studies. Two reviewers (C.Z. and Y.L.) independently coded data from included studies based upon pre-established themes (i.e., barriers and facilitators regarding PrEP provision at individual, dyadic, and structural levels). The inter-rater reliability was >95%, and disagreement was solved by discussion. We further employed a thematic analysis to synthesize data to identify common themes in findings, and a narrative review of both quantitative and qualitative studies was presented.
Results
Search results
The initial search using keywords yielded 300 results. After initial screening by reading titles, 178 were retained for further assessment. An additional 38 articles were identified by hand searching reference lists, resulting in a total of 216 records. These records were assessed by reading abstracts, which yielded 94 references reviewed for full text. Of these, 50 articles were excluded for various reasons: 8 review articles, 15 PrEP care model articles, 7 conceptual articles, 8 reporting patient-level data, 4 reporting state/institution-level data, and 8 no relevant data, resulting in a total of 44 articles that met all inclusion criteria. Among all included publications, four pairs of articles reported the same data,36,62–68 and we retained only one from each pair with the most comprehensive information. Therefore, a total of 36 studies were retained that reported data regarding PrEP care implementation among HCPs including 26 quantitative studies,36,62–65,68–92 10 qualitative studies,49,51,65–67,93–99 and 1 study using mixed methods93 (Supplementary Fig. S1).
Study characteristics
Detailed study characteristics (i.e., authors, publication year, location, time of the survey, recruitment, study design, and key measurements) are reported in Supplementary Tables S3 and S4. Of the 36 included studies, 26 were published since 2015; however, two-thirds of the data were collected before 2015. The majority of quantitative studies employed cross-sectional designs. One study employed a longitudinal open cohort design to assess the PrEP implementation cascade across several years (e.g., 2009–2015),87 and two studies employed pre-post study designs to assess the effectiveness of a PrEP training intervention,60 and PrEP awareness before and after the publications of the PrEP efficacy results among physicians.63,64 For qualitative studies, three employed focus groups49,94,98 and the remainder used in-depth interviews for data collection.
The majority of studies employed convenience sampling strategies, with only a few using purposive sampling,36,51,62,93,97 snowball sampling,96,98 and probability-based sampling.91 Most data in quantitative studies were collected online (e.g., e-mails survey or web-based data collection), with three studies using in-person data collection (e.g., distributed paper-based questionnaires during professional conferences/workshops)73,84,86 and one study using a mail-out survey.89
Aim 1: pooled estimate of each stage of PrEP implementation cascade
A total of 18,265 US-based HCPs with various sociodemographic and backgrounds were identified and included for the Aim 1 analysis. Specifically, 51% (95% CI = 43–58%) of the included participants were female, 69% (95% CI = 66–72%) were white, 28% (95% CI = 17–39%) were APRNs/PAs (as we cannot separate APRNs from PAs among several included studies,13,85,89,91,93 we combined APRNs with PAs as one category), 39% (95% CI = 25–52%) were PCPs, 31% (95% CI = 17–48%) were trained in ID, and 4% (95% CI = 0–10%) were OBGYNs. Among all studies, two studies collected data from a total of 476 pharmacists.86,90
In the meta-analyses, we calculated the pooled proportion of each specific stage along the PrEP cascade using all included HCPs as the denominator. At baseline, the pooled prevalence of being aware of PrEP was 68% (95% CI = 55–80%), willingness to prescribe PrEP of the total participants was 66% (95% CI = 54–77%), provided a PrEP consultation was 37% (95% CI = 25–51%), and ever prescribed PrEP was 24% (95% CI = 17–32%). The bivariate meta-regression revealed that OBGYNs were less likely to be aware of PrEP, but no other statistically significant associations were identified between key characteristics and PrEP care implementation among included participants (Table 1 and Supplementary Fig. S2).
Table 1.
Meta-Analysis for Pre-Exposure Prophylaxis (PrEP) Implementation Cascade Among Health Care Professionals (N = 36)
| Baseline | DF | Heterogeneity | I2 statistics and its 95% CI | Publication bias | Postinterventions | DF | Heterogeneity | I2 statistics and its 95% CI | Publication bias | |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Age | 44.61 (41.15 to 48.07) | 6 | <0.0001 | 96.80% (95.09% to 97.88%) | Y | 42.11 (40.85 to 43.38) | 2 | 0.556 | 0.00% (0% to 94.27%) | N |
| Female (%) | 0.51 (0.43 to 0.58)a | 30 | <0.0001 | 97.98% (97.62% to 98.29%) | Y | — | — | — | — | — |
| White (%) | 0.69 (0.66 to 0.72)a | 24 | <0.0001 | 91.49% (86.87% to 93.60%) | Y | — | — | — | — | — |
| ID specialist (%) | 0.31 (0.17 to 0.48)a | 29 | <0.0001 | 99.46% (99.39% to 99.51%) | Y | — | — | — | — | — |
| Nursing (%)b | 0.28 (0.17 to 0.39)a | 17 | <0.0001 | 99.10% (98.93% to 99.23%) | Y | — | — | — | — | — |
| OBGYN (%) | 0.05 (0.01 to 0.11)a | 5 | <0.0001 | 96.33% (94.06% to 97.73%) | Y | — | — | — | — | — |
| Family medicine (%) | 0.42 (0.30 to 0.56)a | 18 | <0.0001 | 99.41% (99.32% to 99.48%) | Y | — | — | — | — | — |
| PrEP implementation cascade | ||||||||||
| PrEP awareness | 0.68 (0.55 to 0.80)a | 10 | <0.0001 | 98.65% (98.27% to 98.95%) | Y | 0.88 (0.74 to 0.97)c | 2 | p < 0.0001 | 85.97% (59.20% to 95.17%) | Y |
| Age | −0.02 (−0.06 to 0.03)d | — | — | — | — | — | — | — | — | |
| Female (%) | 0.36 (−0.97 to 1.69)d | — | — | — | — | — | — | — | — | |
| White (%) | −0.07 (−1.72 to 1.57)d | — | — | — | — | — | — | — | — | |
| ID specialist (%) | −0.03 (−0.50 to 0.44)d | — | — | — | — | — | — | — | — | — |
| Nursing (%) | −0.25 (−1.48 to 0.98)d | — | — | — | — | — | — | — | — | — |
| OBGYN (%) | −1.86 (−3.19 to −0.53)d,* | — | — | — | — | — | — | — | — | — |
| Family medicine (%) | −0.58 (−1.17 to −0.02)d | — | — | — | — | — | — | — | — | — |
| Rx willingness | 0.66 (0.54 to 0.77)a | 18 | <0.0001 | 98.06% (97.61% to 98.42%) | Y | 1.00 (0.95 to 1.00) | 0 | — | — | — |
| Age | −0.0 (−0.07 to 0.01)d | — | — | — | — | — | — | — | — | |
| Female (%) | −0.68 (−1.70 to 0.34)d | — | — | — | — | — | — | — | — | |
| White (%) | 0.15 (−1.22 to 1.52)d | — | — | — | — | — | — | — | — | |
| ID specialist (%) | −0.14 (−0.68 to 0.39)d | — | — | — | — | — | — | — | — | — |
| Nursing (%) | −0.48 (−1.26 to 0.29)d | — | — | — | — | — | — | — | — | — |
| OBGYN (%) | — | — | — | — | — | — | — | — | — | — |
| Family medicine (%) | 0.35 (−0.56 to 1.27)d | — | — | — | — | — | — | — | — | — |
| PrEP consulting | 0.37 (0.25 to 0.51)a | 4 | <0.0001 | 97.33% (95.66% to 98.35%) | Y | — | — | — | — | — |
| Age | −0.31 (−0.76 to 0.13)d | — | — | — | — | — | — | — | — | — |
| Female (%) | −0.53 (−2.24, 1.18)d | — | — | — | — | — | — | — | — | — |
| White (%) | −0.44 (−2.52 to 1.64)d | — | — | — | — | — | — | — | — | — |
| ID specialist (%) | 0.23 (−0.47 to 0.93)d | — | — | — | — | — | — | — | — | — |
| Nursing (%) | −0.31 (−1.87 to 1.25)d | — | — | — | — | — | — | — | — | — |
| OBGYN (%) | −0.42 (−3.62 to 2.79)d | — | — | — | — | — | — | — | — | — |
| Family medicine (%) | −1.54 (−4.83 to 1.76)d | — | — | — | — | — | — | — | — | — |
| PrEP prescription | 0.24 (0.17 to 0.32)a | 27 | <0.0001 | 98.85% (98.67% to 99.00%) | Y | 0.08 (0.00–0.26)a | 3 | p < 0.0001 | 93.86% (87.45% to 97.00%) | Y |
| Age | −0.02 (−0.05 to 0.02)d | — | — | — | — | — | — | — | — | — |
| Female (%) | −0.23 (−0.81 to 0.36)d | — | — | — | — | — | — | — | — | — |
| White (%) | −0.72 (−1.70 to 0.26)d | — | — | — | — | — | — | — | — | — |
| ID specialist (%) | −0.01 (−0.38 to 0.36)d | — | — | — | — | — | — | — | — | — |
| Nursing (%) | −0.40 (−1.06 to 0.26)d | — | — | — | — | — | — | — | — | — |
| OBGYN (%) | −0.42 (−1.65 to 0.81)d | — | — | — | — | — | — | — | — | — |
| Family medicine (%) | −0.28 (−0.67 to 0.13)d | — | — | — | — | — | — | — | — | — |
Proportions are based upon meta-analysis using metaprop command.
Nursing cohort includes registered nurse, nurse practitioners, and physician assistants.
Based upon a single data point.
β-coefficient and its corresponding 95% CI, from bivariate regression using metareg command.
p < 0.05.
95% CI, 95% confidence interval; DF, degree of freedom; ID, infectious disease; OBGYNs, obstetrics-gynecology; PrEP, pre-exposure prophylaxis.
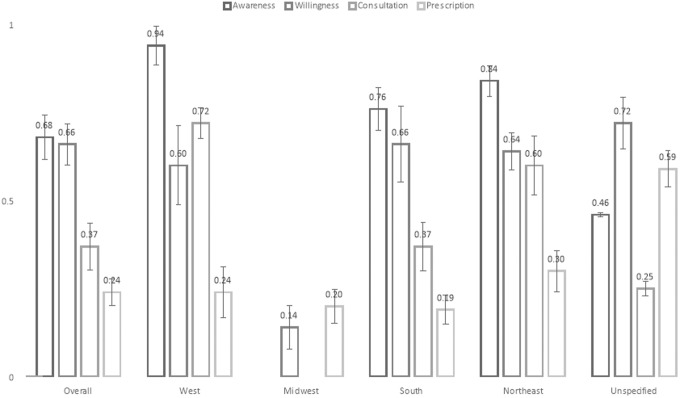
Subgroup analyses by geographic location revealed that PrEP care was implemented least optimally in the southern United States, which had the lowest prevalence of provider consultation (37%) and prescription (19%) compared with other regions. In contrast, HCPs in the west reported the highest proportions of PrEP awareness (94%) and consultation (72%). In the northeast, participants reported the highest proportion of provider prescriptions (30%), which was 20% higher than the overall proportion (Table 2 and Fig. 1).
Table 2.
Subgroup Analyses Based Upon Location, Survey Time, and Types of Health Care Professionals (Baseline, N = 36)
| |
Location of the survey |
Study design |
|||||
|---|---|---|---|---|---|---|---|
| West | Midwest | Southern | Northeast | Unspecifieda | Quantitative (n = 26) | Qualitative (n = 10) | |
| Demographics | |||||||
| Age | 43.00 (37.40–48.60) | — | 40.80 (34.89–46.71) | 43.18 (41.93–44.43) | 45.53 (43.89–47.17) | 45.97 (41.82–50.10) | 43.80 (41.62–45.99) |
| DF | 0 | — | 2 | 3 | 3 | 4 | 2 |
| Female (%) | 0.53 (0.39–0.66) | — | 0.63 (0.48–0.76) | 0.48 (0.36–0.60) | 0.56 (0.46–0.67) | 0.56 (0.48–0.63) | 0.31 (0.10–0.56) |
| DF | 4 | — | 6 | 12 | 13 | 23 | 7 |
| White (%) | 0.65 (0.58–0.72) | — | 0.76 (0.69–0.82) | 0.68 (0.57–0.77) | 0.68 (0.63–0.73) | 0.69 (0.65–0.73) | 0.65 (0.53–0.77) |
| DF | 1 | — | 3 | 6 | 10 | 21 | 3 |
| ID specialist (%) | 0.26 (0.06–0.54) | — | 0.13 (0.04–.25) | 0.37 (0.25–0.50) | 0.45 (0.19–0.72) | 0.27 (0.12–0.45) | 0.49 (0.26–0.72) |
| DF | 6 | — | 9 | 13 | 12 | 21 | 8 |
| Nursing (%) | 0.11 (0.06–0.19) | — | 0.48 (0.43–0.54) | 0.20 (0.14–0.26) | 0.33 (0.16–0.52) | 0.20 (0.11–0.32) | 0.09 (0.00–0.25) |
| DF | 0 | — | 1 | 4 | 5 | 13 | 5 |
| OBGYN (%) | — | — | 0.00 (0.00–0.02) | 0.02 (0.01–0.04) | 0.10 (0.04–0.18) | 0.03 (0.00–0.11) | 0.03 (0.00–0.08) |
| DF | — | — | 0 | 1 | 2 | 5 | 1 |
| Family medicine/PCP (%) | 0.32 (0.16–0.53) | — | 0.26 (0.22–0.31) | 0.10 (0.03–0.21) | 0.50 (0.34–0.65) | 0.43 (0.28–0.59) | 0.23 (0.07–0.43) |
| DF | 0 | — | 1 | 4 | 10 | 15 | 4 |
| PrEP care implementation | |||||||
| Awareness % | 0.94 (0.89–0.98) | — | 0.76 (0.69–0.83) | 0.84 (0.74–0.91) | 0.46 (0.45–0.47) | 0.80 (0.65–0.92) | 0.48 (0.06–0.92) |
| DF | 2 | — | 3 | 6 | 1 | 8 | 2 |
| Willingness % | 0.60 (0.37, 0.81) | 0.14 (0.02–0.26) | 0.66 (0.44–0.86) | 0.64 (0.53–0.74) | 0.72 (0.55–0.84) | 0.59 (0.45–0.73) | 0.89 (0.73–0.99) |
| DF | 6 | 0 | 10 | 12 | 7 | 14 | 3 |
| Consultant % | 0.72 (0.63–0.80) | — | 0.37 (0.24–0.51) | 0.60 (0.43–0.76) | 0.59 (0.55–0.63) | 0.44 (0.27–0.63) | — |
| DF | 1 | — | 4 | 4 | 0 | 5 | — |
| Prescription % | 0.24 (0.11–0.39) | 0.20 (0.10–0.29) | 0.19 (0.12–0.28) | 0.30 (0.19–0.42) | 0.25 (0.16–0.36) | 0.24 (0.16–0.32) | 0.31 (0.10–0.56) |
| DF | 6 | 0 | 9 | 15 | 14 | 20 | 7 |
Cannot identify specific locations where the data were collected.
PCP, primary care provider.
FIG. 1.
PrEP care implementation among health care professionals by different locations in the United States. Midwest has very limited number of studies. Standard errors are presented to illustrate the variance across different variables. PrEP, pre-exposure prophylaxis.
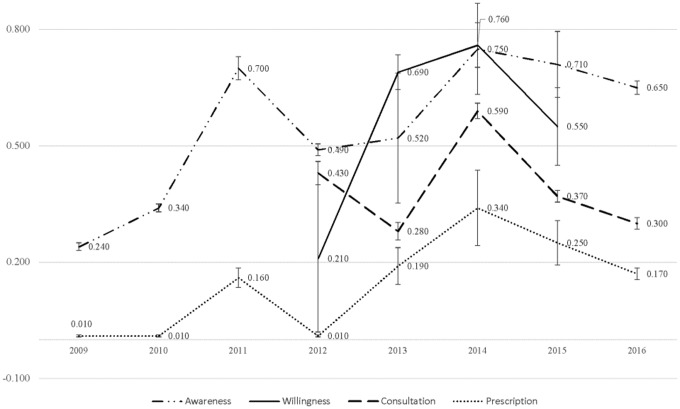
Although PrEP implementation among HCPs differed across regions, it improved over time nationally, especially for PrEP prescription (p-trend <0.05). Provider willingness to prescribe PrEP and PrEP consultation also increased over time (Fig. 2). We further examined PrEP implementation between 2013 and 2015, the most recent years after FDA approval, and found that PrEP was better implemented than that when considering all available data across years between 2009 and 2016 (Supplementary Table S5).
FIG. 2.
PrEP care implementation among health care professionals in the United States (by year). A series of trend tests using “ntrend” command have been used. The trend tests showed PrEP awareness across years is insignificant (z = 0.29; p = 0.771); PrEP willingness across year is insignificant (z = 1.85, p = 0.065); PrEP consultation across year is insignificant (z = 1.20, p = 0.229); PrEP prescription across year is significant (z = 2.96, p = 0.003). Standard errors are presented to illustrate the variance across different variables.
We further assessed PrEP implementation among studies with different designs and found HCPs in quantitative studies reported higher awareness and consultation, but lower prevalence of willingness and prescription than those who participated in qualitative studies (Table 2).
We also examined the “purview paradox” quantitatively. Our analysis showed that compared with PCPs, IDs had higher odds of being aware of PrEP (OR = 6.11, 95% CI = 3.56–10.48), willingness to prescribe PrEP (OR = 3.06, 95% CI = 2.27–4.11), and prescribing PrEP (OR = 4.06, 95% CI = 3.12–5.28). When we examined this phenomenon across different years, we found that PrEP awareness, willingness, and prescription increased incrementally over time among IDs (Supplementary Fig. S3). Our analysis also revealed that the odds of PrEP awareness were lower among APRNs/PAs than among physicians (OR = 0.61, 95% CI = 0.45–0.83); however, once awareness was established, the odds of prescribing PrEP were 1.51 (95% CI = 1.09–2.09) times higher among APRNs/PAs than among physicians, whereas the odds of APRNs/PAs willingness to prescribe were similar (OR = 1.00, 95% CI = 0.98–1.02) to that of physicians (Supplementary Fig. S4a, b). Further, when we examined the cascade over time, we found that the PrEP implementation increased among APRNs/PAs (Supplementary Fig. S5).
Heterogeneity, publication biases, and outlier assessment were all systematically assessed. High between-study heterogeneity was detected across studies evaluating PrEP awareness (I2 = 98.65%, 95% CI = 98.27–98.95%), willingness (I2 = 98.06%, 95% CI = 97.61–98.42%), consultation (I2 = 97.33%, 95% CI = 95.66–98.35%), and prescription (I2 = 98.85%, 95% CI = 98.67–99.00%) (Table 1). Asymmetries were also evident in funnel plots across all stages along the PrEP implementation cascade, but Egger's test only suggested significant publication biases for studies assessing willingness and prescription (Supplementary Fig. S6). Besides, meta-regression revealed that most outcomes were stable across different practice specialties except for OBGYN (Table 1). Sensitivity analyses, including or excluding studies with extreme weights, showed no significant differences. However, if pharmacists were excluded from the analysis of PrEP consultation, the pooled proportion of consultation became higher among other types of HCPs (p = 0.41, 95% CI = 0.28–0.56 when excluding pharmacists vs. p = 0.37, 95% CI = 0.25–0.51 when including pharmacists).
Aim 2: identified barriers and facilitators associated with PrEP care implementation
A narrative review of barriers and facilitators along the PrEP implementation cascade is presented for each study included in our analysis. After a thematic analysis, multi-level barriers were identified: (1) drug level: provider concerns about efficacy, safety, side effects, and drug resistance, (2) patient level: lack of requests for PrEP and low adherence, (3) provider level: lack of awareness/knowledge/skills, lack of training, workload management, and concerns for potential risk compensation (e.g., reduced condom use after initiating PrEP), and (4) structural level: cost/insurance coverage, lack of PrEP care models, no guidance for specific groups including adolescents, IDUs, and HIV serodiscordant couples. Almost all studies mentioned cost/insurance coverage and safety/efficacy issues as major concerns among HCPs. In contrast, PCPs considered better PrEP knowledge, skills, and experience, having patients who were MSM, serodiscordant couples or who requested PrEP use, and availability of evidence and guidance as to the most promising facilitators of PrEP implementation (Table 3).
Table 3.
Barriers and Facilitators Along Pre-Exposure Prophylaxis Care Implementation Identified from All Included Studies (n = 36)
| First author (year) | Location | Time of survey | Sample size | Barriers of prescription | Facilitators of willingness |
|---|---|---|---|---|---|
| Adams (2016),S1 quantitative | Online | June 2014 | N = 260 | Concerns: efficacy of PrEP, adherence, and medical cost; patients who are high-risk heterosexual and IDUs | Patients who are MSM with HIV+ partners |
| Arnold (2012),S26 qualitative | SF/Oakland/LA | 2011 | N = 22 | Little consensus on the target population for PrEP; current model of care is not well suited; lack of capacity of prescription among health providers; monitoring patients | Providers have beliefs in public health benefits of PrEP |
| Bacon (2017),S2 quantitative | San Francisco | May 2014 | N = 99 | Concerns: side effects (75%), resistance (60%), adherence (58%), drug cost (53%), risk compensation (41%), workload (19%) | |
| Blackstock (2016),S38 quantitative | Nationwide | April to May 2015 | N = 266 | Safety and risk compensation, lack of PrEP knowledge | Practice in settings with more HIV+ patients; better HIV knowledge; perceive PrEP extremely safe |
| Blaylock (2018),S3 quantitative | Online | 2015–2017 | N = 1,599 | Medication adverse effects, compliance, and a need for more clear evidence | 68% (n = 1082) endorsed provision of PrEP in the military service |
| Blumenthal (2015),S4 quantitative | New Yew and California | 2014 | N = 233 | Higher HIV knowledge score and from NY region and practice at private settings | |
| Calabrese (2014),S5 quantitative | Northeastern United States | January 2013 | N = 102 | Patients who are black as they may increase risk compensation after using PrEP | Female gender, positive feelings toward black (vs. white), and lower predicted patient risk compensation |
| Calabrese (2016/2017),S27,S28 qualitative | Nationwide | September 2014 to February 2015 | N = 18 | Cost/insurance; implementation logistics; eligibility determination should be shared with patients; adherence concerns; side effects; risk compensation; others (lack of PrEP awareness, medical mistrust, absence of existing ties to the medical system, and structural hurdles) | Patient-centered approach while making decision for PrEP use/relevant training on sexual history taking and sexual minority competence |
| Castel (2015),S6 quantitative | Miami, FL, and Washington, DC | March 2012–2013 | N = 142 | Less likelihood of intending to prescribe to patients with a history of missing medical visits or history of medication nonadherence | PrEP knowledge and experience |
| Clement (2017),S39 quantitative | North Carolina | Baseline: October 2015; post-test: September 2016 | Baseline = 115, follow-up = 79 | Baseline versus follow-up (PrEP prescribers): cost/insurance (58% vs. 25%); patients' compliance (37% vs. 21%); need additional training and lack of necessary knowledge for patient monitoring (32% vs. 15%); baseline versus follow-up (PrEP nonprescribers): lack of knowledge (60% vs. 45%); lack of comfort (42% vs. 37%); no PrEP candidates (42% vs. 37%) | |
| Collier (2017),S29 qualitative | New York City | 2014 | N = 21 | Frequency of and stigma associated with medical visits; burden of pill taking for multiple health concerns | PrEP is being covered by health insurance plan; a good contingency plan for condom failures; PrEP can reduce HIV incidence |
| Doblecki-lewis (2016),S30 qualitative | Southeastern Florida | October to December 2014 | N = 22 | Perceived limitations due to cost/insurance, patient adherence, and immigration status of those who would potentially benefit from the intervention were widely reported; low PrEP demand from patients and low PrEP awareness among patients; risk compensation; lack of risk assessment during clinical visits | A coordinated treatment support system that is inclusive of all individuals at risk for HIV infection regardless of legal status or ability to pay |
| Edelman (2017),S7 quantitative | Online | April to May 2015 | N = 250 | Compared with PCPs delivering care to more HIV-infected clinic patients, PCPs delivering care to fewer HIV-infected patients were more likely to report low willingness to prescribe PrEP to PWID | |
| Finocchario-Kessler (2016),S31 qualitative | Atlanta, Baltimore, Houston, Kansas City, Newark, Philadelphia, and San Francisco | 2013–2014 | N = 86 | Concerns (clinical, system-level, cost, and behavioral): patients' daily adherence, drug resistance, side effects of long-term use, monitoring, insurance/cost, risk compensation | Perceived benefits: add levels of protection and protection for HIV-negative partners; additional education |
| Hakre (2016),S8 quantitative | San Antoni, TX | 2015 | N = 403 | Side effects (67%), discomfort with prescribing drugs without clear evidence (60%), low adherence (54%), PrEP efficacy (51%) | |
| Hoffman (2016),S32 qualitative | New York City regions | Late 2012 to early 2013 | N = 30 | Close follow-up and provide adherence support to patients; lack of skills of risk assessment among PCPs; “purview paradox” regarding PrEP care implementation | Specific skills and support (e.g., knowledge of medication, skills in risk assessment, and ability to monitor and adherence support) |
| Karris (2014),S40 quantitative | Online | June to July 2013 | N = 573 | 77% were worried about adherence and the risk for future resistance, 57% were concerned about cost and reimbursement issues, 53% did not want to use potentially toxic drugs in healthy persons, and 53% felt there was insufficient evidence for the efficacy of real-world PrEP; other concerns included risk compensation, a lack of resources and information, limited resource allocation, and personal ideology | Patients had risk factors (e.g., discordant relationship, unprotected sex, and IDU, conceive desire for discordant couples, IPV and sex workers), patient request |
| Krakower (2014),S33 qualitative | Boston | May to June 2012 | N = 39 | Concerns: PrEP effectiveness in real-world settings; unintended consequences (e.g., risk compensation, medication toxicity); a belief that PrEP provision would be more feasible in primary care settings (“purview paradox”) | Patients' motivation and normative guidance (e.g., CDC) |
| Krawkower (2015),S41 quantitative | New England | September to December 2013 | N = 184 | Major barriers: time constraints (9%), insurance coverage (32%), lack of patient request (26%), limited number of PrEP candidates (15%), no awareness of guidance (25%), lack of training (35%), and unaware of PrEP (20%) | Older age, male, and non-white race |
| Krakower (2016),S9–S11 quantitative | Boston, MA | 2015 | N = 32 | Lack of insurance/copay; risk compensation | |
| Krakower (2016),S9–S11 quantitative | Online | September 2014 | N = 1,191 | Clinicians who deferred ART were less likely than those who recommended early ART to discuss and prescribe PrEP | |
| Krakower (2017),S34 qualitative | Boston/MA | September 2013 to August 2014 | N = 31 | Patients who strongly opposed, who are ambivalent of using PrEP; concerns about substance use and medication adherence; lack of sufficient knowledge of PrEP prescription | Patients who engaged in risky behaviors and were interested in using PrEP; who are at extremely high risk; patient-centered approach to decision-making |
| Mullins (2016/2015),S35,S36 qualitative | Nationwide | October 2012 to April 2013 | N = 15 | Confidentiality, legality of minors consenting to PrEP without parental involvement, ability of minors to understand the risks/benefits of PrEP, the possible impact of PrEP on bone accrual, off-label use of PrEP medication in minors, and the high costs associated with PrEP use | Attitude and adaptation of PrEP guidance; educating communities and other clinicians about PrEP, ensuring adequate financial resources, and infrastructure for delivering PrEP, developing formal guidance on effective behavioral interventions that should be delivered with PrEP, and gaining personal experience with prescribing PrEP |
| Mullins (2017),S12 quantitative | 14 US locations | January and April 2014 | N = 56 | Urban practicing location, discussed PrEP with patients, patient aged 18 years or older and who are heterosexual with HIV+ partners and MSM; practicing in suburban (vs. urban), lower knowledge about CDC PrEP guidance, lower endorsement of cost and insurance factors impacting clinician likelihood of prescribing PrPE; patients who are heterosexual with HIV+ partners, MSM, and transgender MSM (vs. heterosexual with multiple partners) | |
| Scherer (2014),S13 quantitative | New York City | October 2013 | N = 145 | Only 10% of respondents were able to identify the US DHHS recommendation regarding reproductive options for serodiscordant couples | |
| SeidmanS14 (2016), quantitative | Nationwide | 2015 | N = 495 | Lack of training on PrEP use | |
| Shaeer (2014),S15 quantitative | Florida | March to July 2013 | N = 225 | Major concerns: cost (12%), development of HIV resistance (25%), risk compensation (27%), lack of information regarding PrEP (10%), poor adherence (13%), adverse effect (6%) | |
| Smith (2015),S16 quantitative | Online | 2009–2015 | N = 1,500 (2009); N = 1,504 (2010); N = 1,503 (2012); N = 251 (2012, pharmacist); N = 1,507 (2013); N = 1,508 (2014); N = 1,751 (2015) | Clinicians working in larger groups (vs. smaller), aged 45–54 years (vs. older), were less likely to be willing to prescribe PrEP | OBGYN (vs. family practitioners), and ever prescribed nPEP or ARTs were more likely to be willing to prescribe PrEP |
| Spector (2015),S37 qualitative | New York City | 2013 | N = 36 | Challenges in delivering PrEP (e.g., providers had limited information about PrEP, no qualified medical staff to prescribe PrEP, eligibility determination, and making referrals to PrEP prescribers without supporting practice/policy); concerns about PrEP safety/side effects | Providers support the introduction of PrEP in outpatient substance abuse treatment |
| Tellalian (2013),S17 quantitative | Online | April to September 2011 | N = 189 | Concerns for drug resistance development, risk compensation behaviors, and poor medical adherence | A patient with seropositive partner would most influence their decision on prescribe PrEP |
| Tripathi (2012),S18 quantitative | South Carolina and Mississippi | September 2006 to January 2008 | N = 360 | The majority of the providers were concerned about the safety, efficacy, and cost of PrEP; the willingness of providers to prescribe PrEP in the future increased with greater knowledge about PrEP, older age, white race/ethnicity, and belief that PrEP would empower women and/or that it would be effective in preventing new HIV transmission | |
| Unni (2016),S19 quantitative | Utah | January and February 2015 | N = 251 | Beliefs about capabilities and social/peer influence were significant predictors of intention to counsel | |
| Walsh (2017)S20/Petroll (2017),S21 quantitative | Nationwide | July 2014 to May 2015 | N = 280 | With information about PrEP, attitudes toward PrEp and confidence in PrEP-related skills, all related to PrEP discussion and prescription among PCPs (based upon IMB model) | With information about PrEP, attitudes toward PrEP and confidence in PrEP-related skills, all related to PrEP discussion and prescription among PCPs (based upon IMB model) |
| Weiser (2017), S22 quantitative |
Nationwide | 2013–2014 | N = 935 | Patients who are IDU (1%) | Patients who are MSM (74%), WSM (30%), MSW (23%), and discordant couple (23%). Providers who are male, lesbian/bisexual/gay, and more HIV caseload |
| Wood (2018),S25 quantitative | Online | May 2016 | N = 735 | Concerns are adherence, costs, uncomfortable discussing PrEP, perceived patients at a low risk, side effect, patients seeking for PrEP elsewhere, and renal insufficiency | To have print material available for guidelines, assessment for PrEP candidates, and PrEP efficacy as well as information on insurance/financial resources |
| White (2012)S23; Mimiaga (2014),S24 quantitative | Massachusetts | Baseline: 2010; follow-up: 2011 | N = 178 (pretest) and N = 115 (post-test) | Ongoing concerns included potential drug resistance (93%), decreased funds for other forms of HIV prevention (88%), medication side effects (83%), and limited data regarding PrEP's clinical efficacy (75%) | CDC guidance will have greatest impact on their willingness to prescribe PrEP (96%) |
ART, antiretroviral therapy; IDUs, injecting drug users; IMB, information-motivation-behavioral skills; IPV, intimate partner violence; MSW, men who have sex with women; nPEP, nonoccupational postexposure prophylaxis; OBGYN, obstetrics-gynecology; PWID, people who inject drugs; WSM, women who have sex with men.
Discussion
In this study, we systematically reviewed and rigorously synthesized estimates of PrEP awareness, willingness to prescribe, patient consultation, and PrEP prescription practices among HCPs in the United States. We further quantified the different roles of PCPs and IDs as well as physicians and APRNs/PAs in the cascade. Our findings have crucial implications for enhancing the PrEP implementation cascade for stakeholders, including patients, HCPs, and policymakers.
These study findings reveal several notable discrepancies regarding PrEP implementation. First, a discrepancy between overall awareness and the actual prescription was substantial among HCPs. Our data reveal that the pooled prevalence of PrEP awareness (68%) was similar to the willingness to prescribe (66%), but it was almost three times higher than the prevalence of actual prescription (24%). HCPs have encountered barriers embedded within different levels during their practice, which may hinder the prescription of PrEP for HIV prevention despite the general willingness to prescribe the medication. Although the discrepancy between willingness to prescribe and actual prescribing is large, this discrepancy is similar to the well-recognized concept of clinical inertia or failing to initiate intervention or intensify it appropriately. Clinical inertia has been observed across a range of interventions.100
Second, although the overall trend is increasing prevalence across stages and the peak of PrEP implementation was observed in 2014, there appears to be a downward trend from 2014 to 2015 on willingness, and perhaps consultations and prescriptions. We also found that the PrEP implementation cascade for the most recent years after FDA approval (2013–2015) was the most optimal. This downturn in the PrEP cascade may be the result of a lack of training or supporting programs for HCPs who were interested in but unable to provide PrEP care.
Third, significant disparities of the PrEP implementation cascade were observed across regions. For instance, the lowest provider-reported prescription prevalence was identified in the south, a region that has among the highest HIV incidence and prevalence.101 Although several risk factors including poverty, lack of health insurance coverage, mistrust with HCPs, and internalized and structural stigma may contribute to the HIV epidemic in the south,30,102 suboptimal engagement of HCPs in PrEP care may further exacerbate HIV disparities in this region. In addition to identified regional disparities based upon our analysis, our data echoed the underutilization of PrEP care in the United States from providers' perspective with previously published data from PrEP users' perspective.15
Fourth, we found several differences in provider-reported PrEP implementation by health care specialty. Although the “purview paradox” prevails in the existing literature,29,49,51,52 significant distinctions across each stage of PrEP cascade were observed between the actual practice of PCPs and IDs after rigorous assessments. Our review of the literature indicates that a lack of experience and knowledge is reported as the major barriers hindering PCPs from providing PrEP care. Although the feasibility of PrEP provision in primary care settings has been demonstrated by previous research,35,82 the gap of the trajectory of PrEP diffusion from early adopters (e.g., ID/HIV specialists) to early/late majorities is still significant.50,103 The purview paradox predicts low PrEP provision by both PCPs and IDs. Resolution of the paradox could be that both PCPs and IDs equally increase PrEP provision, or it could be resolved by one of the two specialties “owning” PrEP provision, thereby one specialty would increase more than the other. The dynamic breakdown of ORs between specialties by years showed the purview paradox is being resolved gradually, although progress is needed requiring sustained effort from all stakeholders.
In addition to health care specialty, we assessed PrEP implementation by the health care profession—physicians and APRNs/PAs. Most published studies emphasized the role of physicians in PrEP implementation.46,62–64,71,81 However, our study revealed that although awareness of PrEP among APRNs/PAs was lower than physicians, the odds of prescribing PrEP among APRNs/PAs were 50% higher than physicians. Despite a recently published article advocating for APRNs to accelerate PrEP scale-up,104 the role of nurses in PrEP implementation has been overlooked in the extant literature.46,62–64,71,81 A recent study indicated that the supply of APRNs increased more rapidly than physician supply, especially in rural areas, which can offset the shortage of physician supply in the United States.105 Therefore, APRNs may be in the ideal position for PrEP care implementation.104
In addition to these identified discrepancies, “patients' request” is consistently quoted as a critical factor related to the PrEP implementation.49,80,82 “Patient-centered” care models in which both patients and health providers can make mutual decisions regarding PrEP use are essential for optimal PrEP care implementation in clinical settings.65,97,106 Further, as pharmacists usually have direct interactions with patients, their role in PrEP uptake and adherence is crucial.37,42,86,107 More research is urgently needed to explore the engagement of pharmacists in the PrEP care implementation model.
Strengths
This study has several strengths. First, it is the first to quantitatively evaluate the pooled prevalence of each specific stage along the PrEP implementation cascade. Besides, this study contains a reasonably large number of studies with a considerable sample size, affording substantial power to detect the outcomes.108 Second, DerSimonian–Laird random-effects modeling has been employed to account for heterogeneity across studies.57 Also, we used both I2 and corresponding 95% CI to assess heterogeneity to account for the biased estimates of studies in the meta-analysis.109,110 Third, we used metan to calculate effect sizes and metaprop to calculate the pooled proportions based upon the binominal nature of the data. In addition, meta-regression was employed to assess the association between study-level demographics and PrEP implementation cascade among HCPs as well as to better explain the heterogeneity of included studies.111,112 Likewise, we filled a research gap by considering weighted sample sizes to account for potentially inflated type I errors in published studies while assessing each specific stage along the cascade.113 Fourth, we quantified the different roles of PCPs and IDs as well as physicians and APRNs/PAs in the cascade.
Limitations
Our findings should be interpreted with caution, considering a few caveats. First of all, our meta-analysis reveals significant publication biases throughout studies that may be subject to inflated type I errors. Second, there was high heterogeneity across studies due to different design, populations, and settings among study participants, which may lead to biased pooled estimates. Third, data on PrEP implementation by certain specialties are scarce. No PrEP implementation-related information was available specifically for OBGYN or reproductive health care specialists, a subgroup that has been suggested as ideal for PrEP prescription.114–116 Fourth, as the categorization and measurement of several variables may vary across studies, we had to group participants arbitrarily, which may have affected the precision and validity of crucial estimates. For instance, some studies categorized APRNs and PAs as one category with no information provided to assess each profession separately. Last, due to the limited number of studies, we cannot conduct analyses while controlling time-varying effects. However, our trend analyses have revealed crucial patterns for the PrEP cascade across years.
Conclusions
Utilizing synthesized data from 36 studies representing ∼20,000 HCPs in the United States, this is the first study to date to report pooled proportions along each stage of the PrEP implementation cascade. We found that significant discrepancies exist between relatively high provider awareness and willingness to prescribe PrEP on the one hand, and low prevalence of PrEP consultation and prescribing on the other. Our results point to opportunities to expand PrEP provision by further engaging a more extensive range of health care professions and specialties and focusing on regions with disparities between HIV incidence and PrEP uptake. These findings can help guide future research and policy to address identified discrepancies. As one of the key pillars in the strategic initiative that has been proposed by the US Department of Health and Human Services, PrEP plays a crucial role in ending the HIV epidemic in the United States.117
Supplementary Material
Acknowledgments
We sincerely thank Mr. Daniel Trout who worked as a librarian at the University of Rochester Miner Library and helped us with key words development as well as a comprehensive and iterative process to evaluate inclusiveness and relevancy of potentially included studies.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the University of Rochester Center for AIDS Research (P30A1078498) and School of Nursing at University of Rochester Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the sponsor who had no role in the design or conduct of the study, the writing of this report, or its submission for publication.
Supplementary Material
References
- 1. Centers for Disease Control and Prevention (CDC). HIV in the United States and Dependent Areas. Atlanta, GA: CDC; 2019 [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection in the United States and Dependent Areas. Atlanta, GA: CDC, 2017. 2018 [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC). HIV Prevention Works. 2017. Available at: www.cdc.gov/hiv/policies/hip/works.html (Last accessed September5, 2019).
- 4. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delaugerre C, Antoni G, Mahjoub N, et al. Assessment of HIV screening tests for use in preexposure prophylaxis programs. J Infect Dis 2017;216:382–386 [DOI] [PubMed] [Google Scholar]
- 6. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: An observational cohort study. Lancet HIV 2017;4:e402–e410 [DOI] [PubMed] [Google Scholar]
- 8. Morton JF, Celum C, Njoroge J, et al. Counseling framework for HIV-serodiscordant couples on the integrated use of antiretroviral therapy and pre-exposure prophylaxis for HIV prevention. J Acquir Immune Defic Syndr 2017;74(Suppl 1):S15–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailey JL, Molino ST, Vega AD, Badowski M. A review of HIV pre-exposure prophylaxis: The female perspective. Infect Dis Ther 2017;6:363–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC). Preventing New HIV Infections. 2018. Available at: www.cdc.gov/hiv/guidelines/preventing.html (Last accessed January30, 2019).
- 11. US Preventive Services Task Force (USPSTF). Draft Recommendation Statement: Prevention of Human Immunodeficiency Virus (HIV) Infection: Pre-Exposure Prophylaxis. 2018. Available at: www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis (Last accessed April30, 2019).
- 12. Owens DK, Davidson KW, Krist AH, et al. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA 2019;321:2203–2213 [DOI] [PubMed] [Google Scholar]
- 13. Smith DK, Van Handel M, Wolitski RJ, et al. Vital signs: Estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. J Miss State Med Assoc 2015;56:364–371 [PubMed] [Google Scholar]
- 14. Mera RM, Palmer B, Mayer G, Magnuson D, Rawlings K. FTC/TDF (Truvada) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States: 2013–2015. TUAX0105LB. 21st International AIDS Conference, Durban, 2016 [Google Scholar]
- 15. AIDSVu. Mapping PrEP: First Ever Data on PrEP Users Across the U.S. 2019. Available at: https://aidsvu.org/prep (Last accessed January30, 2019).
- 16. Jenness SM, Maloney KM, Smith DK, et al. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. Am J Epidemiol 2018;188:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marks SJ, Merchant RC, Clark MA, et al. Potential healthcare insurance and provider barriers to pre-exposure prophylaxis utilization among young men who have sex with men. AIDS Patient Care STDS 2017;31:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMahan VM, Martin A, Garske L, et al. Development of a targeted educational intervention to increase pre-exposure prophylaxis uptake among cisgender men and transgender individuals who have sex with men and use methamphetamine in Seattle (WA, USA). Sex Health 2019;16:139–147 [DOI] [PubMed] [Google Scholar]
- 19. Schueler K, Ferreira M, Nikolopoulos G, et al. Pre-exposure prophylaxis (PrEP) awareness and use within high HIV transmission networks. AIDS Behav 2019;23:1893–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang YA, Zhu W, Smith DK, Harris N, Hoover KW. HIV preexposure prophylaxis, by race and ethnicity—United States, 2014–2016. MMWR Morb Mortal Wkly Rep 2018;67:1147–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, McMahon J, Simmons J, Brown LL, Nash R, Liu Y. Suboptimal HIV pre-exposure prophylaxis awareness and willingness to use among women who use drugs in the United States: A systematic review and meta-analysis. AIDS Behav 2019;23:2641–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Footer KHA, Lim S, Rael CT, et al. Exploring new and existing PrEP modalities among female sex workers and women who inject drugs in a U.S. city. AIDS Care 2019;31:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain JP, Strathdee SA, Patterson TL, et al. Perceived barriers to pre-exposure prophylaxis use and the role of syndemic factors among female sex workers in the Mexico-United States border region: A latent class analysis. AIDS Care 2019;4:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS 2017;31:731–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dolwick Grieb SM, Desir F, Flores-Miller A, Page K. Qualitative assessment of HIV prevention challenges and opportunities among Latino immigrant men in a new receiving city. J Immigr Minor Health 2015;17:118–124 [DOI] [PubMed] [Google Scholar]
- 26. Okoro ON, Whitson SO. HIV risk and barriers to care for African-born immigrant women: A sociocultural outlook. Int J Womens Health 2017;9:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Auerbach JD, Banyan A, Riordan M. Will and should women in the U.S. use PrEP? Findings from a focus group study of at-risk HIV negative women in Oakland, Memphis, San Diego, and Washington, DC. Paper presented at: XIX International AIDS Conference, Washington, DC, July27, 2012 [Google Scholar]
- 28. Bien CH, Patel VV, Blackstock OJ, Felsen UR. Reaching key populations: PrEP uptake in an urban health care system in the Bronx, New York. AIDS Behav 2017;21:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinto RM, Berringer KR, Melendez R, Mmeje O. Improving PrEP implementation through multilevel interventions: A synthesis of the literature. AIDS Behav 2018;22:3681–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kerr JC, Valois RF, Diclemente RJ, et al. HIV-related stigma among African-American youth in the Northeast and Southeast US. AIDS Behav 2014;18:1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siegler AJ, Bratcher A, Weiss KM, Mouhanna F, Ahlschlager L, Sullivan PS. Location location location: An exploration of disparities in access to publicly listed pre-exposure prophylaxis clinics in the United States. Ann Epidemiol 2018;28:858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold T, Brinkley-Rubinstein L, Chan PA, et al. Social, structural, behavioral and clinical factors influencing retention in pre-exposure prophylaxis (PrEP) care in Mississippi. PLoS One 2017;12:e0172354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel RR, Mena L, Nunn A, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS One 2017;12:e0178737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krakower D, Mayer KH. What primary care providers need to know about preexposure prophylaxis for HIV prevention: A narrative review. Ann Intern Med 2012;157:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS 2012;7:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petroll AE, Walsh JL, Owczarzak JL, McAuliffe TL, Bogart LM, Kelly JA. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav 2017;21:1256–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruno C, Saberi P. Pharmacists as providers of HIV pre-exposure prophylaxis. Int J Clin Pharm 2012;34:803–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sowicz TJ, Teitelman AM, Coleman CL, Brawner BM. Considerations for implementing oral preexposure prophylaxis: A literature review. J Assoc Nurses AIDS Care 2014;25:496–507 [DOI] [PubMed] [Google Scholar]
- 39. Phanuphak N, Sungsing T, Jantarapakde J, et al. Princess PrEP program: The first key population-led model to deliver pre-exposure prophylaxis to key populations by key populations in Thailand. Sex Health 2018;15:542–555 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz K, Ferrigno B, Vining S, et al. PrEP communications accelerator: A digital demand creation tool for sub-Saharan Africa. Sex Health 2018;15:570–577 [DOI] [PubMed] [Google Scholar]
- 41. Przybyla S, LaValley S, St Vil N. Health care provider perspectives on pre-exposure prophylaxis: A qualitative study. J Assoc Nurses AIDS Care 2019;30:630–638 [DOI] [PubMed] [Google Scholar]
- 42. Przybylaa S, Parksb K, Bleasdalec J, Sawyerd J, Morsee D. Awareness, knowledge, and attitudes towards human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) among pharmacy students. Curr Pharm Teach Learn 2019;11:352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ports KA, Barnack-Tavlaris JL, Syme ML, Perera RA, Lafata JE. Sexual health discussions with older adult patients during periodic health exams. J Sex Med 2014;11:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seftel AD. Re: What we don't talk about when we don't talk about sex(1): Results of a national survey of U.S. obstetrician/gynecologists. J Urol 2012;188:1265–1266 [DOI] [PubMed] [Google Scholar]
- 45. Wimberly YH, Hogben M, Moore-Ruffin J, Moore SE, Fry-Johnson Y. Sexual history-taking among primary care physicians. J Natl Med Assoc 2006;98:1924–1929 [PMC free article] [PubMed] [Google Scholar]
- 46. Silapaswan A, Krakower D, Mayer KH. Pre-exposure prophylaxis: A narrative review of provider behavior and interventions to increase PrEP implementation in primary care. J Gen Intern Med 2017;32:192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turner L, Roepke A, Wardell E, Teitelman AM. Do you PrEP? A review of primary care provider knowledge of PrEP and attitudes on prescribing PrEP. J Assoc Nurses AIDS Care 2018;29:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhatia R, Modali L, Lowther M, et al. Outcomes of preexposure prophylaxis referrals from public STI clinics and implications for the preexposure prophylaxis continuum. Sex Transm Dis 2017;45:50–55 [DOI] [PubMed] [Google Scholar]
- 49. Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV providers' perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: A qualitative study. AIDS Behav 2014;18:1712–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krakower DS, Mayer KH. The role of healthcare providers in the roll out of preexposure prophylaxis. Curr Opin HIV AIDS 2016;11:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffman S, Guidry JA, Collier KL, et al. A clinical home for preexposure prophylaxis: Diverse health care providers' perspectives on the “purview paradox.” J Int Assoc Provid AIDS Care 2016;15:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee SS, Petersen E. Overcoming “purview paradox” to make way for the effective implementation of PrEP in preventing HIV transmission. Int J Infect Dis 2018;77:105–106 [DOI] [PubMed] [Google Scholar]
- 53. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151:264–269, w264. [DOI] [PubMed] [Google Scholar]
- 55. Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint—Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys Ther 2009;89:873–880 [PubMed] [Google Scholar]
- 56. PRISMA Group. PRISMA: Transparent Reporting of Systematic Reviews and Meta-Analyses. 2015. Available at: www.prisma-statement.org (Last accessed September5, 2019).
- 57. Borenstein M, Hedges L, Rothstein H. Meta-Analysis Fixed Effect vs. Random Effects. 2007. Available at: www.meta-analysis.com/downloads/M-a_f_e_v_r_e_sv.pdf (Last accessed August30, 2018).
- 58. Borenstein M, Hedges LV, Higgins J, Rothstein H.. Introduction to Meta-Analysis. Hoboken, NJ: Wiley; 2009 [Google Scholar]
- 59. Littell JH, Corcoran J, Pillai V.. Systematic Reviews and Meta-Analysis. New York: Oxford University Press; 2008 [Google Scholar]
- 60. Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch Public Health 2014;72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Walsh JL, Petroll AE. Factors related to pre-exposure prophylaxis prescription by U.S. primary care physicians. Am J Prev Med 2017;52:e165–e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mimiaga MJ, White JM, Krakower DS, Biello KB, Mayer KH. Suboptimal awareness and comprehension of published preexposure prophylaxis efficacy results among physicians in Massachusetts. AIDS Care 2014;26:684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. White JM, Mimiaga MJ, Krakower DS, Mayer KH. Evolution of Massachusetts physician attitudes, knowledge, and experience regarding the use of antiretrovirals for HIV prevention. AIDS Patient Care STDS 2012;26:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Calabrese SK, Magnus M, Mayer KH, et al. Putting PrEP into practice: Lessons learned from early-adopting U.S. providers' firsthand experiences providing HIV pre-exposure prophylaxis and associated care. PLoS One 2016;11:e0157324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Calabrese SK, Magnus M, Mayer KH, et al. “Support your client at the space that they're in”: HIV pre-exposure prophylaxis (PrEP) prescribers' perspectives on PrEP-related risk compensation. AIDS Patient Care STDS 2017;31:196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mullins TL, Zimet G, Lally M, Kahn JA. Adolescent human immunodeficiency virus care providers' attitudes toward the use of oral pre-exposure prophylaxis in youth. AIDS Patient Care STDS 2016;30:339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mullins TLK, Zimet G, Lally M, Xu J, Thornton S, Kahn JA. HIV care providers' intentions to prescribe and actual prescription of pre-exposure prophylaxis to at-risk adolescents and adults. AIDS Patient Care STDS 2017;31:504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Adams LM, Balderson BH. HIV providers' likelihood to prescribe pre-exposure prophylaxis (PrEP) for HIV prevention differs by patient type: A short report. AIDS Care 2016;28:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bacon O, Gonzalez R, Andrew E, et al. Brief report: Informing strategies to build PrEP capacity among San Francisco Bay Area clinicians. J Acquir Immune Defic Syndr 2017;74:175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blackstock OJ, Moore BA, Berkenblit GV, et al. A cross-sectional online survey of HIV pre-exposure prophylaxis adoption among primary care physicians. J Gen Intern Med 2017;32:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. military services—2014–2016. MMWR Morb Mortal Wkly Rep 2018;67:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blumenthal J, Jain S, Krakower D, et al. Knowledge is power! Increased provider knowledge scores regarding pre-exposure prophylaxis (PrEP) are associated with higher rates of PrEP prescription and future intent to prescribe PrEP. AIDS Behav 2015;19:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Calabrese SK, Earnshaw VA, Underhill K, Hansen NB, Dovidio JF. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): Assumptions about sexual risk compensation and implications for access. AIDS Behav 2014;18:226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Castel AD, Feaster DJ, Tang W, et al. Understanding HIV care provider attitudes regarding intentions to prescribe PrEP. J Acquir Immune Defic Syndr 2015;70:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clement ME, Seidelman J, Wu J, et al. An educational initiative in response to identified PrEP prescribing needs among PCPs in the Southern U.S. AIDS Care 2018;30:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Edelman EJ, Moore BA, Calabrese SK, et al. Primary care physicians' willingness to prescribe HIV pre-exposure prophylaxis for people who inject drugs. AIDS Behav 2017;21:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Flash C, Landovitz R, Giler RM, et al. Two years of Truvada for pre-exposure prophylaxis utilization in the US. J Int AIDS Soc 2014;17(Suppl 3):19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hakre S, Blaylock JM, Dawson P, et al. Knowledge, attitudes, and beliefs about HIV pre-exposure prophylaxis among US Air Force Health Care Providers. Medicine (Baltimore) 2016;95:e4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karris MY, Beekmann SE, Mehta SR, Anderson CM, Polgreen PM. Are we prepped for preexposure prophylaxis (PrEP)? Provider opinions on the real-world use of PrEP in the United States and Canada. Clin Infect Dis 2014;58:704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krakower DS, Beekmann SE, Polgreen PM, Mayer KH. Diffusion of newer HIV prevention innovations: Variable practices of frontline infectious diseases physicians. Clin Infect Dis 2016;62:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krakower DS, Maloney KM, Grasso C, Melbourne K, Mayer KH. Primary care clinicians' experiences prescribing HIV pre-exposure prophylaxis at a specialized community health centre in Boston: Lessons from early adopters. J Int AIDS Soc 2016;19:21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Krakower DS, Oldenburg CE, Mitty JA, et al. Knowledge, beliefs and practices regarding antiretroviral medications for HIV prevention: Results from a survey of healthcare providers in New England. PLoS One 2015;10:e0132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scherer ML, Douglas NC, Churnet BH, et al. Survey of HIV care providers on management of HIV serodiscordant couples—Assessment of attitudes, knowledge, and practices. AIDS Care 2014;26:1435–1439 [DOI] [PubMed] [Google Scholar]
- 85. Seidman D, Carlson K, Weber S, Witt J, Kelly PJ. United States family planning providers' knowledge of and attitudes towards preexposure prophylaxis for HIV prevention: A national survey. Contraception 2016;93:463–469 [DOI] [PubMed] [Google Scholar]
- 86. Shaeer KM, Sherman EM, Shafiq S, Hardigan P. Exploratory survey of Florida pharmacists' experience, knowledge, and perception of HIV pre-exposure prophylaxis. J Am Pharm Assoc (2003) 2014;54:610–617 [DOI] [PubMed] [Google Scholar]
- 87. Smith DK, Mendoza MC, Stryker JE, Rose CE. PrEP awareness and attitudes in a national survey of primary care clinicians in the United States, 2009–2015. PLoS One 2016;11:e0156592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tellalian D, Maznavi K, Bredeek UF, Hardy WD. Pre-exposure prophylaxis (PrEP) for HIV infection: Results of a survey of HIV healthcare providers evaluating their knowledge, attitudes, and prescribing practices. AIDS Patient Care STDS 2013;27:553–559 [DOI] [PubMed] [Google Scholar]
- 89. Tripathi A, Ogbuanu C, Monger M, Gibson JJ, Duffus WA. Preexposure prophylaxis for HIV infection: Healthcare providers' knowledge, perception, and willingness to adopt future implementation in the southern US. South Med J 2012;105:199–206 [DOI] [PubMed] [Google Scholar]
- 90. Unni EJ, Lian N, Kuykendall W. Understanding community pharmacist perceptions and knowledge about HIV preexposure prophylaxis (PrEP) therapy in a Mountain West state. J Am Pharm Assoc (2003) 2016;56:527..e521–532.e521. [DOI] [PubMed] [Google Scholar]
- 91. Weiser J, Garg S, Beer L, Skarbinski J. Prescribing of human immunodeficiency virus (HIV) pre-exposure prophylaxis by HIV medical providers in the United States, 2013–2014. Open Forum Infect Dis 2017;4:ofx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wood BR, McMahan VM, Naismith K, Stockton JB, Delaney LA, Stekler JD. Knowledge, practices, and barriers to HIV preexposure prophylaxis prescribing among Washington State medical providers. Sex Transm Dis 2018;45:452–458 [DOI] [PubMed] [Google Scholar]
- 93. Finocchario-Kessler S, Champassak S, Hoyt MJ, et al. Pre-exposure prophylaxis (PrEP) for safer conception among serodifferent couples: Findings from healthcare providers serving patients with HIV in seven US cities. AIDS Patient Care STDS 2016;30:125–133 [DOI] [PubMed] [Google Scholar]
- 94. Collier KL, Colarossi LG, Sanders K. Raising awareness of pre-exposure prophylaxis (PrEP) among women in New York City: Community and provider perspectives. J Health Commun 2017;22:183–189 [DOI] [PubMed] [Google Scholar]
- 95. Mullins TL, Lally M, Zimet G, Kahn JA. Clinician attitudes toward CDC interim pre-exposure prophylaxis (PrEP) guidance and operationalizing PrEP for adolescents. AIDS Patient Care STDS 2015;29:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One 2012;7:e40603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Krakower DS, Ware NC, Maloney KM, Wilson IB, Wong JB, Mayer KH. Differing experiences with pre-exposure prophylaxis in Boston among lesbian, gay, bisexual, and transgender specialists and generalists in primary care: Implications for scale-up. AIDS Patient Care STDS 2017;31:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Doblecki-Lewis S, Jones D. Community federally qualified health centers as homes for HIV preexposure prophylaxis: Perspectives from South Florida. J Int Assoc Provid AIDS Care 2016;15:522–528 [DOI] [PubMed] [Google Scholar]
- 99. Spector AY, Remien RH, Tross S. PrEP in substance abuse treatment: A qualitative study of treatment provider perspectives. Subst Abuse Treat Prev Policy 2015;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reach G. Clinical Inertia: A Critique of Medical Reason. Cham: Springer; 2015 [Google Scholar]
- 101. Centers for Disease Control and Prevention (CDC). HIV in the Southern United States. Atlanta, GA: CDC; 2016 [Google Scholar]
- 102. Reif S, Pence BW, Hall I, Hu X, Whetten K, Wilson E. HIV diagnoses, prevalence and outcomes in nine southern states. J Commun Health 2015;40:642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rogers E. Diffusion of Innovations. New York: Free Press, 2003. [Google Scholar]
- 104. Nelson LE, McMahon JM, Leblanc NM, et al. Advancing the case for nurse practitioner-based models to accelerate scale-up of HIV pre-exposure prophylaxis. J Clin Nurs 2019;28:351–361 [DOI] [PubMed] [Google Scholar]
- 105. Xue Y, Smith JA, Spetz J. Primary care nurse practitioners and physicians in low-income and rural areas, 2010–2016. JAMA 2019;321:102–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. John SA, Rendina HJ, Starks TJ, Grov C, Parsons JT. Decisional balance and contemplation ladder to support interventions for HIV pre-exposure prophylaxis uptake and persistence. AIDS Patient Care STDS 2019;33:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Clauson KA, Polen HH, Joseph SA, Zapantis A. Role of the pharmacist in pre-exposure chemoprophylaxis (PrEP) therapy for HIV prevention. Pharm Pract (Granada) 2009;7:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Hoboken, NJ: Wiley, 2009 [Google Scholar]
- 109. von Hippel PT. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med Res Methodol 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods 2017;8:5–18 [DOI] [PubMed] [Google Scholar]
- 111. Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int J Clin Pract 2009;63:1426–1434 [DOI] [PubMed] [Google Scholar]
- 112. Stanley TD, Doucouliagos H. Meta-regression approximations to reduce publication selection bias. Res Synth Methods 2014;5:60–78 [DOI] [PubMed] [Google Scholar]
- 113. Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications, 2001 [Google Scholar]
- 114. Savasi V, Mandia L, Laoreti A, Cetin I. Reproductive assistance in HIV serodiscordant couples. Hum Reprod Update 2013;19:136–150 [DOI] [PubMed] [Google Scholar]
- 115. ACOG Committee Opinion no 595. Committee on Gynecologic Practice: Preexposure prophylaxis for the prevention of human immunodeficiency virus. Obstet Gynecol 2014;123:1133–1136 [DOI] [PubMed] [Google Scholar]
- 116. Hong JN, Farel CE, Rahangdale L. Pharmacologic prevention of human immunodeficiency virus in women: Practical approaches for the obstetrician and gynecologist. Obstet Gynecol Surv 2015;70:284–290 [DOI] [PubMed] [Google Scholar]
- 117. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: A plan for the United States. JAMA 2019;321:844–845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.