Abstract
Objective: Neutrophil extracellular traps (NETs) are associated with impaired wound healing in diabetes. This study evaluates the association between NET-specific markers and wound healing among diabetic foot ulcer (DFU) patients treated in a multidisciplinary setting.
Approach: Clinical data of diabetic patients with active foot ulcers who presented to our team between January 1, 2016 and June 30, 2017 were recorded. The diabetic ulcer severity score (DUSS) and wound, ischemia, and foot infection (WIfI) score were calculated. NET-specific markers in plasma and wound tissues were tested. The capacity for plasma and platelets to prime neutrophils to release NETs was assessed. The prognostic value of NET-specific markers for wound healing was evaluated.
Results: NET-specific markers were significantly higher in DFU patients than in diabetic patients without DFU or healthy controls and were found to correlate positively with DUSS or WIfI score. Elastase levels in ulcer tissue significantly increased in wounds with infections and delayed healing. Higher levels of NET release were observed after the stimulation of plasma or platelets from ulcer-related vessels than from nonulcer-related vessels of the DFU patients. Citrullinated histone 3 (citH3) was identified as a risk factor for wound healing impairment and amputation. The patients with the highest quartile of citH3 levels presented significantly lower healing rates and higher amputation rates than those with the lower three quartiles.
Innovation: This study extended current knowledge of NETs on wound healing in DFU patients.
Conclusion: NET-specific markers negatively correlated with wound healing in DFU patients, and citH3 is a potential marker.
Keywords: neutrophil extracellular traps, diabetic foot ulcers, wound healing, multidisciplinary setting
Guanhua Xue, MD, PhD.

Zhichun Gu, MD.

Introduction
Wound healing is impaired in diabetes, and people at greatest risk of ulceration can easily be identified by careful clinical examination of the feet.1 Diabetic foot ulcer (DFU) is estimated to affect 25% of the diabetic patient population during their lifetime and result in significant morbidity and mortality risks.1,2 The economic burden of DFU care is substantial.3 The treatment of DFU is complex and often involves the expertise of a variety of specialists to improve wound healing and amputation-free survival.4,5 With the advent of the multidisciplinary limb preservation team, understanding a wound's prognosis is important to help patients set realistic expectations for wound healing and can also help guide cost-benefit analyses to determine the cost-effectiveness of an aggressive limb salvage attempt. Currently, available tools for predicting the wound healing of DFU mainly focus on clinical scores. The new diabetic ulcer severity score (DUSS) categorizes DFU patients into subgroups of certain severity levels and similar clinical outcomes and predicts probabilities for healing, amputation and the need for surgery and hospitalization.6 The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system correlates cost of care and predicts wound healing in DFU patients treated in a multidisciplinary setting.7,8
Inflammation is a typical feature of the wound healing process, and neutrophils are recruited early to the wound bed.9 Recently, the role of neutrophils in DFUs has taken a new direction with the involvement of neutrophil extracellular traps (NETs). Hyperglycemia can promote NET release, revealing a link between neutrophils, inflammation, and tissue damage in diabetes.10 In addition, infection is common in DFU patients and NETs may constitute a natural response against infection. Activated neutrophils release NETs composed of granular proteins/enzymes and nuclear material. Then, NETs entrap and remove bacteria by using a sticky extracellular network loaded with bactericidal proteins.11,12 NETosis is a combined process of citrullination of histone 3 that is mediated by protein arginine deiminase 4 (PAD4), massive chromatin decondensation, and the nuclear localization of granular enzymes (e.g., elastase).13 It has been found that NET induction can cause tissue damage and the inhibition of NETosis by PAD4 knockout and that the disruption of NETs with deoxyribonuclease I (DNase I) can accelerate wound healing in DFU cases.14 NETs have been reported as prognostic markers of acute ischemic stroke, severe burn injury, community-acquired pneumonia, and post-traumatic sepsis.15–17
Clinical Problem Addressed
To date, rapid laboratory markers for wound healing and adverse outcomes in DFU patients are still lacking. Assessments of NETs in DFU patients are scarce and particularly those on the evaluation of NET-specific markers. The aims of this study were to evaluate the presence of NETs by using citrullinated histone 3 (citH3), cell-free DNA (cfDNA), and nucleosomes in plasma and elastase in ulcer tissue as markers and to study the potential association between NET-specific markers and wound healing and adverse outcomes among DFU patients treated in a multidisciplinary setting with 1 year of follow-up data.
Materials and Methods
Study cohort
This retrospective study was conducted on the basis of a prespecified database. All diabetic patients with at least one active foot ulcer who presented to our multidisciplinary team between January 1, 2016 and June 30, 2017 were included in this study. Patients with traumatic amputation, Buerger's disease, vasculitis, and acute arterial occlusion and those who underwent lower extremity amputation for any other reason were excluded. Patients with irreversible acute hepatic or renal failure, advanced malignancies were also excluded. In total, 198 DFU patients were finally included in this study. Demographics, comorbidities, laboratory testing data, and wound information were recorded on admission and updated at each subsequent visit. All surgical procedures, including revascularizations (open and endovascular), wound interventions (wound debridement and coverage), and adverse outcomes (minor and major amputations), were also recorded and updated in real time. The DUSS and WIfI score were calculated for each patient. In addition, 60 diabetic patients without foot ulcers and 60 healthy controls were recruited. The multidisciplinary service for DFU care and measurement protocols of NET markers had been established before we initiated this study. There were no major changes in our clinical practice during the study period. The study was approved by the Institutional Review Board. All patients provided informed consent for the collection and maintenance of their demographic and wound data. Separate informed consent was gathered from each patient for collection and analysis of peripheral blood and tissue samples.
Multidisciplinary management of DFU
Our multidisciplinary management of DFU involves an integrated service provided by a vascular surgeon, endocrinologist, physician assistant, prosthetist, and wound care nurse. This service operates on an inpatient and outpatient basis and is designed to optimize wound healing. Patients are seen by the service team with each inpatient admission and weekly outpatient clinic visit in an integrated fashion and are followed up at frequent intervals for ongoing wound assessment and intervention. Consultations from other specialists, including plastic surgery, orthopedic foot and ankle, and infectious disease specialists, are given as needed. Patients are educated about their disease and are provided with wound care instruction and supplies and home care when appropriate.
At their initial assessment, all patients undergo noninvasive vascular testing to assess for ischemic disease, radiography or magnetic resonance imaging to assess for osteomyelitis, and laboratory testing to assess for glycemic control. For DFU patients with peripheral artery disease, an endovascular first strategy was adopted in our center. Patients with peripheral artery disease were identified through noninvasive testing and underwent endovascular interventions whenever possible. However, open endarterectomy or thrombectomy is recommended for disease of common femoral artery. When endovascular revascularization is not possible (e.g., patients allergic to iodine, patients with severe coagulation disorders, infection at the puncture site, renal dysfunction) or fails, open revascularization (e.g., embolectomy, endarterectomy, and artery bypass surgery) is performed. Once vascular status was optimized, wound care was individualized to each patient. All devitalized or infected tissue was surgically debrided. Bones with chronic osteomyelitis were typically surgically excised. In wounds in which osseous and tendinous structures were exposed, either secondary delayed closure or a xenograft was employed. Once a new dermal layer was generated, continued wound care or split-thickness skin grafting was performed to facilitate wound closure. Wound care also focused on offloading and the regulation of edema with multilayer compression, various foam dressings, and vacuum sealing drainage. Antibiotic therapy is determined based on the culture data. All patients have complete blood tests, including blood count with differential, erythrocyte sedimentation rate, C-reactive protein, and procalcitonin testing. In our clinical experience, the values of more specific inflammatory markers (i.e., combined C-reactive protein and procalcitonin) might be of value in discriminating diabetic foot infection and could help to ensure a more rational use of antibiotic agents. Continuous antibiotic therapy is used until there is evidence that the infection has resolved but not necessarily until a wound has healed. The criterion for infection eradication includes regression of local and systemic infection signs, complete removal of infected and necrotic tissue, normal level of inflammatory markers in blood tests, and negative results of two consecutive cultures of wound tissue and blood. Empirically, patients with osteomyelitis involving the forefoot or proximal typically receive 6 weeks of antibiotics. Wounds without osteomyelitis and those limited to the phalanx usually receive 2 weeks of antibiotics.
After revascularization and wound optimization, patients are observed regularly on an outpatient basis to assess wound progression, medication compliance, glycemic control, and vascular status. Once wound healing is achieved, patients are seen in continued follow-up meetings that allow for early intervention in the case of new ulcer development.
Sample collection
Peripheral blood was collected from all patients on their initiation of our multidisciplinary DFU management program. For patients receiving endovascular intervention, manual aspiration of blood was performed with a heparinized catheter after the wiring of the ulcer-related artery. Blood samples from nonulcer-related healthy vessels of DFU patients and from control individuals were obtained during primary intervention or diagnostic catheterization, respectively. Plasma was collected immediately after the centrifugation of blood obtained with a heparinized catheter at 1,000 g for 10 min followed by supernatant centrifugation at 15,000 g for 15 min and was stored at −80°C until analysis. All procedures used for the collection and centrifugation of plasma were performed at 0°C. The isolation of neutrophils was performed by using Polymorphprep (Axis-Shield) following the manufacturer's protocol. Purity and viability of neutrophil was assessed by Diff–Quik and Trypan blue stain, respectively (both >95%). RPMI 1640 plus 1% FBS was used as the culture medium for all reactions. The tissue biopsy was performed at the wound center on initial treatment in the clinic.
Markers of NETs
Nucleosomes were measured with the Cell Death Detection ELISAPLUS kit (Roche, Madrid, Spain) according to the manufacturer's instructions. The determination of citH3 was performed as previously described.18 In brief, plasma samples were mixed with a monoclonal mouse anti-histone biotinylated antibody in a streptavidin-coated plate. A rabbit histone 3 (Abcam, MA) antibody was used in the second phase. Detection was performed with a peroxidase-linked antibody (GE Biosciences, Barcelona, Spain). Values were normalized to a pool of samples from normal subjects, which was included in all microplates. Values are expressed as individual absorption values. The cell-free double-stranded DNA was measured after phenol extraction by using a Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, MA). Elastase concentrations in the tissue were measured by using commercially available ELISA kits.
In vitro test of NET release
Purified neutrophils (1 × 106) isolated from healthy controls were incubated for 3 h at 37°C in 5% CO2 and then treated with 6% platelet-free plasma isolated from ulcer-related arteries and nonulcer-related healthy vessels of DFU patients or from control individuals. They were also treated with platelets derived from patients or healthy controls individually in a ratio of 1:50 for 3 h. The myeloperoxidase-DNA (MPO-DNA) complex was used as a quantified marker of NETs release with a capture ELISA. For the capture antibody, 5 μg/mL anti-MPO mAb (Abcam) was coated onto 96-well plates (dilution 1:500 in 50 μL) overnight at 4°C. After three rounds of rinsing, 20 μL of the samples was added with a 80-μL incubation buffer containing a peroxidase-labeled anti-DNA mAb (Cell Death ELISAPLUS, dilution 1:25; Roche, Madrid, Spain). The plate was incubated for 2 h and shaken at 300 rpm at room temperature. After three rounds of rinsing, peroxidase substrate (100 μL) was added. Absorbance at 405-nm wavelength was measured after 20 min of incubation at room temperature in the dark. Values for soluble NET formation are expressed as percentage increases in absorbance above the control.
NETs were visualized by immunofluorescence confocal microscopy as previously described.19 Samples were stained by using antihuman neutrophil elastase (Abcam) and antihuman myeloperoxidase (BD Bioscience, CA) antibodies. Primary antibodies were detected with the following secondary antibodies: Alexa Fluor 488-conjugated donkey anti-mouse and Alexa Fluor 568-conjugated donkey anti-rabbit (both from Invitrogen). Visualization was performed with a Nikon ECLIPSE Ti microscope (Tokyo, Japan). The percentage of NET-releasing cells was determined by examining 200 cells with a double-blind experimental procedure.
Statistical analysis
Continuous variables were defined as of the interquartile range (P25–P75) when they were not normally distributed. Wilcoxon rank-sum tests were used for independently abnormally distributed samples. Groups for categorical variables were analyzed with chi-squared or adjusted chi-squared tests. Logistic regression models were applied to test associations between the confounders and wound healing or major amputations. Spearman correlations were used to test correlations between DUSS, WIfI scores, nucleosomes, citH3, and cfDNA. Receiver operating characteristic (ROC) curves and area under curves (AUC) were used to analyze the prognostic effects for wound healing. Kaplan-Meier curves were then constructed to identify differences in wound healing and amputation results. SPSS software (version 21.0; SPSS, Inc., Chicago, IL) was used for statistical analysis. Statistical significance was accepted at a p-value of 0.05.
Results
Baseline characteristics
Baseline characteristics of the cohort are summarized in Table 1. The mean age was 72 years, and 45.5% of the patients were male. Patients with unhealed wounds had a higher prevalence of peripheral arterial disease (PAD, 95.2% vs. 75.6%, p = 0.01) and infection (85.7% vs. 6.4%, p < 0.01) than patients with healed wounds. The patients' demographics, smoking history, medication use, and comorbidities were similar between the two groups. There were also no significant differences in the patients' baseline or nadir hemoglobin A1C values, wound areas and depths, and revascularization approaches across the two groups. Significantly increased periods from wound onset to initial assessment through the multidisciplinary service (3.4 [2.1–5.0] vs. 2.8 [1.5–4.5] months, p = 0.04), lower rates of direct angiosome perfusion (45.2% vs. 78.2%, p < 0.01), and higher DUSS or WIfI score (4 [3.4–4.0] vs. 2 [1.4–3.2], 4 [3.6–4.2] vs. 3 [2.2–4.3], p < 0.01) were found in patients with unhealed wounds than in patients with healed wounds (Table 1).
Table 1.
Clinical and wound characteristics of patients overall and stratified by healed versus not healed at 1-year follow-up
| Characteristic | Overall (n = 198) | Healed (n = 156) | Not Healed (n = 42) | p |
|---|---|---|---|---|
| Age, years | 72 (62.1–79.0) | 71 (61.1–79.0) | 73 (64.3–81.8) | 0.26 |
| Male sex, n (%) | 90 (45.5) | 72 (46.2) | 18 (42.9) | 0.84 |
| Hemoglobin A1c, n (%) | ||||
| Baseline | 8.1 (7.0–9.1) | 8.1 (7.2–9.1) | 8.0 (6.6–9.0) | 0.33 |
| Nadir | 6.8 (5.7–8.0) | 6.8 (5.8–7.9) | 6.9 (5.7–8.0) | 0.67 |
| Comorbidities, n (%) | ||||
| Hypertension | 90 (45.5) | 71 (45.5) | 19 (45.2) | 1.00 |
| Dyslipidemia | 106 (53.5) | 83 (53.2) | 23 (54.8) | 1.00 |
| Coronary artery disease | 100 (50.5) | 80 (51.3) | 20 (47.6) | 0.80 |
| Congestive heart failure | 87 (44.0) | 68 (43.6) | 19 (45.2) | 0.99 |
| Peripheral arterial disease | 158 (79.8) | 118 (75.6) | 40 (95.2) | 0.01 |
| Chronic kidney disease | 88 (44.4) | 72 (46.2) | 16 (38.1) | 0.45 |
| Stroke | 96 (48.5) | 80 (51.3) | 16 (38.1) | 0.18 |
| Smoking, n (%) | 102 (51.5) | 84 (53.9) | 18 (42.9) | 0.28 |
| Medication use, n (%) | ||||
| Aspirin | 111 (56.1) | 89 (57.1) | 22 (52.4) | 0.71 |
| Clopidogrel | 77 (38.9) | 60 (38.5) | 17 (40.5) | 0.96 |
| Warfarin | 20 (10.1) | 19 (12.2) | 1 (2.4) | 0.11 |
| Novel anticoagulants | 8 (4.0) | 4 (2.6) | 4 (9.5) | 0.11 |
| Statin | 93 (47.0) | 70 (44.9) | 23 (54.8) | 0.33 |
| Diabetic medication, n (%) | ||||
| Oral only | 90 (45.5) | 74 (47.4) | 16 (38.1) | 0.37 |
| Insulin | 108 (54.6) | 82 (52.6) | 26 (61.9) | 0.37 |
| Wound area, cm2 | 13.5 (7.1–20.1) | 13.2 (7.1–19.4) | 15.9 (7.7–21.3) | 0.36 |
| Wound depth, cm | 1.0 (0.5–1.5) | 1.0 (0.5–1.5) | 0.7 (0.3–1.3) | 0.07 |
| Infection, n (%) | 46 (23.2) | 10 (6.4) | 36 (85.7) | <0.01 |
| Time from onset to assessment, months | 3.5 (2.0–5.0) | 3.4 (2.1–5.0) | 2.8 (1.5–4.5) | 0.04 |
| Revascularization approach, n (%) | ||||
| Endovascular | 149 (75.3) | 112 (71.8) | 37 (88.1) | 0.09 |
| Open | 9 (4.6) | 6 (3.9) | 3 (7.1) | 0.62 |
| Direct angiosome perfusion, n (%) | 141 (71.2) | 122 (78.2) | 19 (45.2) | <0.01 |
| DUSS | 2 (1.2–3.2) | 2 (1.4–3.2) | 4 (3.4–4.0) | <0.01 |
| WIfI score | 3 (2.3–4.4) | 3 (2.2–4.3) | 4 (3.6–4.2) | <0.01 |
DUSS, diabetic ulcer severity score; WIfI score, wound, ischemia, and foot infection score.
Excess NETs were identified in patients with DFU
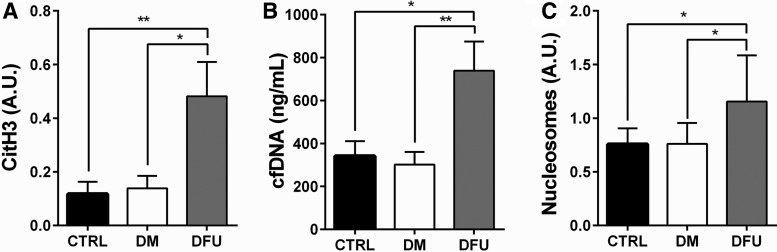
Biomarkers of NETs were determined in the bloodstream of patients with DFU; then, they were compared with matched diabetic patients without DFU and matched controls without diabetes. Circulating levels of cfDNA, citH3, and nucleosomes were significantly increased in DFU patients when compared with diabetic patients without DFU and the healthy controls (Fig. 1).
Figure 1.
Circulating NETosis biomarkers in patients with DFU. Circulating levels of citH3 (A), cfDNA (B), and nucleosomes (C) of DFU patients were significantly higher than those of diabetic patients without foot ulcers and of the healthy controls. The results are expressed as the means ± standard deviations for each group. *p < 0.05, **p < 0.01. cfDNA, cell-free DNA; citH3, citrullinated histone 3; CTRL, control; DFU, diabetic foot ulcer; DM, diabetes mellitus.
NETs content is associated with impaired wound healing in DFU patients
Circulating levels of cfDNA, citH3, and nucleosomes correlate positively with the DUSS or WIfI score of patients, respectively (Fig. 2A, B). Neutrophil elastase in tissue extracts of wound biopsies taken from DFU patients was quantified. Neutrophil elastase, the prototypical NET marker, was found at significantly higher levels in wounds not healed than in wounds healed over 1 year (p < 0.05). Infected wounds presented significantly higher elastase levels than noninfected wounds (p < 0.05). However, no significant difference was detected in DFU patients with ≥1 patent infrapopliteal arteries when compared with patients with no patent infrapopliteal arteries (Fig. 2C). Levels of citH3 in plasma and elastase in wound tissue progressively increased with the DUSS (Fig. 2D).
Figure 2.
NETs content is associated with impaired wound healing in DFU patients. Circulating levels of cfDNA, citH3, and nucleosomes correlate positively with the DUSS (A) and WIfI score (B) of DFU patients. (C) Levels of elastase were significantly higher in wounds healed than in wounds not healed after 1 year. Infected wounds had significantly higher elastase levels than noninfected wounds. No significant difference was detected in DFU patients with ≥1 patent infrapopliteal arteries when compared with patients with no patent infrapopliteal arteries. (D) Levels of citH3 in plasma and elastase in wound tissue progressively increased according to the DUSS. Concentrations of citH3 and elastase in patients with DUSS of 4 are compared with those of patients with DUSS of <4. *p < 0.05, **p < 0.01. DUSS, diabetic ulcer severity score; NETs, neutrophil extracellular traps; WIfI, wound, ischemia, and foot infection.
The microenvironment in patients with DFU primes neutrophils to release NETs
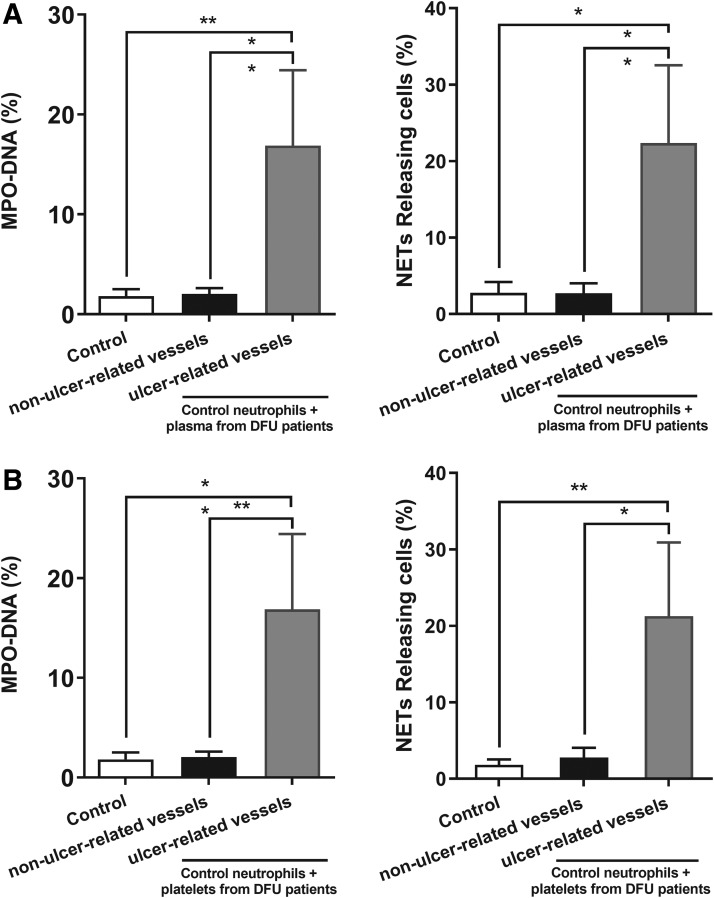
Significantly increased levels of MPO-DNA complex and percentages of NET-releasing cells were observed in control neutrophils stimulated with plasma (Fig. 3A) or platelets (Fig. 3B) from ulcer-related vessels when compared with nonulcer-related vessels of DFU patients in vitro (p < 0.05). This finding illustrates that the microenvironment of DFU patients can cause neutrophils to release NETs.
Figure 3.
The microenvironment in patients with DFU primes control neutrophils to release NETs. Significant elevation in MPO-NDA complex levels and percentage of NETs releasing cells are observed in control neutrophils stimulated with plasma (A) or platelets (B) from ulcer-related vessels when compared with those of nonulcer-related vessels of DFU patients in vitro. The results are expressed as the means ± standard deviations with three rounds of experiments conducted for each sample. *p < 0.05, **p < 0.01. MPO-DNA, myeloperoxidase-DNA.
NETs are markers of wound healing and adverse outcomes in DFU patients
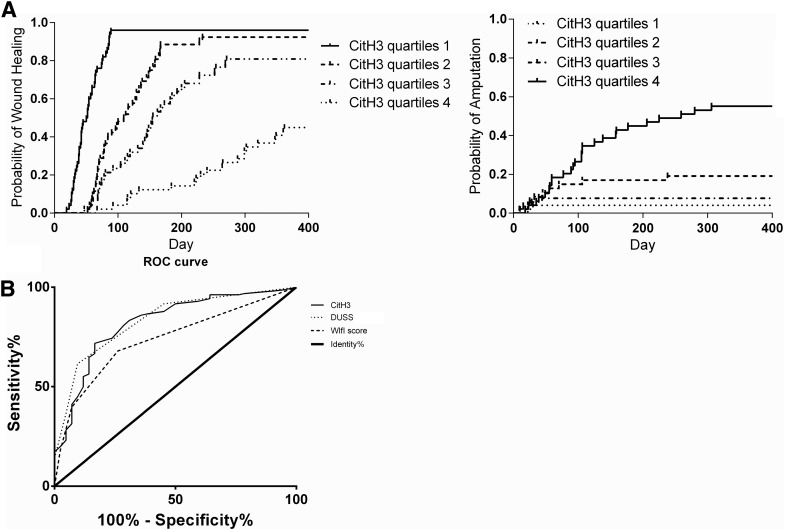
The overall healing rate of this cohort was measured at 78.8% at 12 months. Major and minor amputations were required for 11.1% and 10.1% of limbs overall over the course of the study period. The citH3 was a marker of wound healing, with higher levels requiring more healing time (p < 0.01). Patients with the highest quartile of citH3 levels presented significantly lower healing rates and higher amputation rates by 12 months than patients with the lower three quartiles of citH3 (p < 0.01, Fig. 4A). Based on the ROC curve, citH3 (AUC = 0.84 [95% CI 0.76–0.90]) was a more accurate marker of wound healing by 12 months than the WIfI score (AUC = 0.73 [95% CI 0.66–0.82]) (Fig. 4B).
Figure 4.
(A) Kaplan-Meier curves comparing wound healing and amputation results for DFU patients stratified by citH3 levels. The citH3 was predictive of wound healing and amputation. Patients with the highest quartile of citH3 levels had significantly lower healing rates and higher amputation rates after 12 months than patients with the lower three quartiles (p < 0.05). (B) ROC curve for predictors of wound healing. The citH3 was a more accurate marker wound healing after 12 months than the WIfI score. ROC, receiver operating characteristic.
Correlation between NETs and risks of delayed wound healing in patients with DFU
From a univariate analysis, PAD results, wound depths, direct angiosome perfusion results, DUSSs, WIfI score, and circulating levels of citH3, cfDNA, nucleosomes, and elastase were identified as potential risk factors of wound healing. Through multivariate logistic regression modeling, circulating levels of citH3 were found to be a risk factor for wound healing impairment and major amputations by 12 months (Table 2).
Table 2.
Analysis of risk factors associated with wound healing and major amputation over 1 year
| Characteristics | Wound Healing |
Major Amputation |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis |
Multivariable Model |
Univariate Analysis |
Multivariable Model |
|||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Baseline hemoglobin A1c | 1.16 (0.87–1.55) | 0.32 | 0.72 (0.49–1.06) | 0.10 | ||||
| Nadir hemoglobin A1c | 0.95 (0.73–1.23) | 0.70 | 0.94 (0.67–1.31) | 0.72 | ||||
| Peripheral arterial disease | 0.16 (0.04–0.67) | 0.01 | 0.43 (0.09–2.11) | 0.30 | 0.97 (0.04–0.92) | 0.99 | ||
| Wound area | 0.98 (0.93–1.03) | 0.33 | 0.99 (0.93–1.06) | 0.78 | ||||
| Wound depth | 1.91 (1.05–3.51) | 0.04 | 1.77 (0.88–3.57) | 0.11 | 0.46 (0.20–1.03) | 0.06 | ||
| Infection | 0.07 (0.04–0.12) | 0.89 | 0.98 (0.02–0.94) | 0.99 | ||||
| Time from onset to assessment | 1.19 (0.98–1.45) | 0.08 | 0.84 (0.65–1.08) | 0.17 | ||||
| Direct angiosome perfusion | 4.34 (2.12–8.89) | <0.01 | 1.03 (0.38–2.8) | 0.95 | 0.11 (0.04–0.31) | <0.01 | 1.36 (0.32–5.77) | 0.68 |
| DUSS | 0.23 (0.14–0.39) | <0.01 | 2.53 (0.35–4.35) | 0.21 | 2.65 (6.59–8.82) | <0.01 | 0.75 (0.10–5.08) | 0.14 |
| WIfI score | 0.33 (0.20–0.55) | <0.01 | 2.03 (0.85–4.85) | 0.11 | 9.70 (2.46–38.3) | 0.01 | 1.81 (0.29–11.2) | 0.52 |
| citH3 | 0.07 (0.04–0.12) | <0.01 | 0.07 (0–1.27) | 0.003 | 0.99 (0.83–4.94) | <0.01 | 0.89 (0.50–10.6) | 0.02 |
| cfDNA | 0.99 (0.99–1.00) | <0.01 | 0.22 (0–4.61) | 0.73 | 1.00 (0.98–1.01) | 0.05 | 1.00 (0.99–1.02) | 0.89 |
| Nucleosomes | 0.02 (0.01–0.09) | <0.01 | 0.38 (0.01–10.4) | 0.57 | 3.71 (1.09–12.7) | 0.04 | 0.64 (0.02–18.7) | 0.79 |
| Elastase | 0.01 (0–0.03) | <0.01 | 0.20 (0.07–0.57) | 0.07 | 4.41 (0.5–38.7) | 0.18 | ||
cfDNA, cell-free DNA; citH3, citrullinated histone 3.
Discussion
DFU is one of the most serious complications of diabetes. These wounds are recalcitrant to healing due to poor circulation in narrowed vessels, neuropathy leading to a loss of pain sensation, and an inefficient immune response that is unable to control wound infections.20 Multidisciplinary teams with essential skills in limb salvaging for DFU have been created at a number of institutions with favorable outcomes.4,5,21 Through our multidisciplinary service for DFU, a vascular surgeon, podiatrist, endocrinologist, and wound care nurse are involved in every clinic visit and coordinate with infectious disease, plastic surgery, and orthopedic foot and ankle specialists as needed on a case-by-case basis. On the initiation of an integrated interdisciplinary surgical team for DFU care, a 1-year major amputation rate of 3–5% is reported, with a 46% reduction in below-knee amputations and a 50% reduction in major amputations is found.5,22,23 Our team is responsible for the amputation service of diabetic foot in our city and several neighboring provinces. As a tertiary referral center for PAD and diabetic foot, our team has a better chance of treating extremely complex cases with high risk of amputation. Both these outpatient referrals and inpatient consultations of DFU were included in the study. Therefore, an overall amputation rate of roughly 10% was achieved.
The WIfI score can independently predict wound healing in DFU patients treated in a multidisciplinary setting.23,24 However, this scoring system is predictive of wound healing only when specific baseline and wound characteristics are accounted for. A plasma marker that can be easily and quickly tested to stratify patients with different risks of amputation is desperately needed. Recent reports have documented increased circulating NET-related biomarkers in diabetes cases.25 This is the first study to demonstrate that markers of NETs have prognostic value in DFU patients. Neutrophil chromatin decondensation taking place before the release of NETs is mediated by PAD4, which catalyzes the citrullination of histone 3.26 Thus, citH3 appears to be the most specific marker of NETs, and it has been used to test for the presence of NETs in plasma. Circulating markers of NETs, and especially citH3, have been found to constitute a useful prognostic marker in patients with acute ischemic stroke.16 In addition, NETs represent a novel marker for outcome and a possible target for adjunct treatments of pneumonia.27 It has been shown that increased NETosis and delayed wound healing in diabetic mice and patients occur due to PAD4 overexpression.14,28 Our study confirms that citH3 but not cfDNA and nucleosome is a risk factor for wound healing impairment and major amputation.
The current lack of understanding of cellular and molecular mechanisms underlying impaired wound healing in diabetes precludes new therapeutic strategies beyond those of glucose control, revascularization, and traditional wound care. The mechanism underlying excessive NET formation in DFU patients and associations with disease severity is still unclear. NETosis is a physiological response to infection, and the primary function of NET is to entrap bacteria.12 However, excessive NETosis, which promotes tissue damage and cytotoxic injury, in diabetic patients with foot ulcers is the problem and can be pathologic.29,30 Therefore, a finely tuned balance of NETosis may be important to allow for tissue repair. This balance is altered in diabetes and leads to impaired wound healing. Neutrophil elastase has been shown to degrade peptide growth factors and, therefore, impede healing.31 Moreover, abnormalities in coagulation and fibrinolytic pathways may also contribute to the development of DFUs in diabetic patients.
In this study, plasma or platelets obtained from ulcer-related vessels in DFU patients were found to be able to induce neutrophils from healthy controls to release NETs in vitro. The thrombogenic signal occurring during atherothrombosis is released by NETosis during acute myocardial infarction.32 It is unknown whether NET-associated microvascular thrombosis plays a role in altered wound healing in diabetic people. Excessive NETs may directly damage keratinocytes, as they can be toxic to cells.33 Alternatively, the balance of interactions of NETs with macrophages may also play a role since NETs activate pro-inflammatory cytokine production in macrophages.34 NETs may sustain inflammation and block tissue repair signals generated when macrophages digest apoptotic neutrophils.35 Future work must determine how neutrophil activation and NET formation contribute to impaired wound healing in DFU.
A higher prevalence of PAD and infection is found in patients with unhealed ulcers than in patients with healed ulcers at a 1-year follow-up meeting. PAD is a significant component of the diabetic foot. Diabetic patients should be assessed for lower limb arterial disease. There is a higher incidence of major, previous and new-onset cardiovascular events in diabetic patients with foot ulcers than in those without such complications.36 The severity of PAD significantly influences the outcomes of DFUs in terms of wound healing, major amputations, and mortality.37 Infection is another major risk factor for DFU development. In the literature, infections are seen in 35–50% of DFUs.38 In this study, we found infected wounds to present significantly increased NET content levels when relative to noninfected wounds.
Some limitations should be considered in the interpretation of our results. Our results were obtained from a single-center cohort. Potential selection biases and a lack of long-term outcomes are not negligible. In our hospital, patients with a higher likelihood of limb salvaging may have been referred by outside providers for outpatient evaluation to our multidisciplinary service. In addition, we report major outcomes observed at 1 year. Our treatment may have simply delayed rather than prevented major amputations. Continued enrollment and observations will assist with our understanding of how diabetes affects wound healing rates over time. However, specific mechanisms of NET in circulation or ulcer tissues promoting tissue damage and organ failure have not been addressed in this study. Further prospective study and pathophysiologic research is needed to validate this association between NETs expression level and wound healing impairment as well as to determine whether our results are reflective of differences in the disease's pathophysiologic mechanisms among patients with DFU relative to all patients with critical limb ischemia.
In conclusion, among DFU patients receiving multidisciplinary diabetic foot care, markers of NETs negatively correlate with wound healing and limb amputation and citH3 is a potential marker that could be helpful to monitor DFU healing. The microenvironment around the foot ulcer primes neutrophils to release NETs that contribute to impaired wound healing. Our observations extend current knowledge on NETs and suggest that NETs have detrimental effects on wound healing in DFU cases. Accordingly, NET targeting (e.g., PAD4 inhibitor, DNase I, N-acetylcysteine) could attenuate impaired wound healing and result in a lower incidence of adverse outcomes in DFU patients.
Innovation
Laboratory markers of wound healing in patients with DFUs are lacking. NETs are associated with impaired wound healing in diabetes. However, data on NETs in clinical patients are scarce and particularly those on the evaluation of NET-specific markers. This study found that NETs-specific markers might predict the wound healing impairment in patients with DFU. It is also found that the microenvironment around the foot ulcer primes neutrophils to release NETs that might contribute to the impaired wound healing. Thus, targeting NETs might attenuate the impaired wound healing and result in a declined incidence of amputation.
Key Findings
NETs-specific markers correlated well with delayed wound healing and limb amputation among DFU patients treated in a multidisciplinary setting.
The microenvironment around the foot ulcer primes neutrophils to release NETs that contribute to the impaired wound healing.
NETs have detrimental effects in wound healing of DFU patients and targeting NETs could attenuate the impaired wound healing and result in a declined incidence of adverse outcomes.
Acknowledgments and Funding Sources
This work was supported by Grants from the National Science Foundation of China (Grant No. 81700423 and No. 81873526), Clinical Research Innovation and Cultivation Fund of Renji Hospital (Grant No. PYIII-17-003), Shanghai Outstanding Young Doctor Training Program from Shanghai Municipal Commission of Health and Family Planning (to S.Y.), Shanghai Jiaotong University Medical Engineering Cross Fund (Grant No. YG2016QN57), scientific research project of Shanghai municipal commission of health and family planning (Grant No. 20164Y0058), and Shanghai Municipal Education Commission-Gaoyuan Nursing Grant Support (Grant No. hlgy16004kyx).
Abbreviations and Acronyms
- AUC
area under curves
- cfDNA
cell-free DNA
- citH3
citrullinated histone 3
- DFU
diabetic foot ulcer
- DNase I
deoxyribonuclease I
- DUSS
diabetic ulcer severity score
- MPO-DNA
myeloperoxidase-DNA
- NETs
neutrophil extracellular traps
- PAD
peripheral arterial disease
- PAD4
protein arginine deiminase 4
- ROC
receiver operating characteristic
- WIfI
wound, ischemia, and foot infection
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Shuofei Yang, MD, PhD, is a senior resident in the department of vascular surgery at Renji Hospital. Zhichun Gu, MD, is an experienced clinical pharmacist in the department of pharmacy at Renji Hospital. Can Lu, MD, is a master student of the Wound Healing Program in the School of Medicine, Shanghai Jiaotong University. Ting Zhang, MSN, is a research nurse in the department of vascular surgery at Renji Hospital. Xiangjiang Guo, MD, PhD, is an attending physician with the Wound Healing Program at Renji Hospital. Guanhua Xue, MD, PhD, is the Associate Professor of Surgery and of the Director of Section of Wound Healing and Tissue Repair at Renji Hospital. Lan Zhang, MD, PhD, is the Professor of Surgery and of the Director of Section of Wound Healing and Tissue Repair at Renji Hospital.
References
- 1. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 2. Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current Challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018;41:645–652 [DOI] [PubMed] [Google Scholar]
- 3. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 4. Fitzgerald RH, Mills JL, Joseph W, Armstrong DG. The diabetic rapid response acute foot team: 7 essential skills for targeted limb salvage. Eplasty 2009;9:e15. [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong DG, Bharara M, White M, Lepow B, Bhatnagar S, Fisher T, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev 2012;28:514–518 [DOI] [PubMed] [Google Scholar]
- 6. Beckert S, Witte M, Wicke C, Konigsrainer A, Coerper S. A new wound-based severity score for diabetic foot ulcers: a prospective analysis of 1,000 patients. Diabetes Care 2006;29:988–992 [DOI] [PubMed] [Google Scholar]
- 7. Weaver ML, Hicks CW, Canner JK, Sherman RL, Hines KF, Mathioudakis N, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing better than direct angiosome perfusion in diabetic foot wounds. J Vasc Surg 2018;68:1473–1481 [DOI] [PubMed] [Google Scholar]
- 8. Hicks CW, Canner JK, Karagozlu H, Mathioudakis N, Sherman RL, Black JH 3rd, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system correlates with cost of care for diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2018;67:1455–1462 [DOI] [PubMed] [Google Scholar]
- 9. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005;15:599–607 [DOI] [PubMed] [Google Scholar]
- 10. Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015;52:497–503 [DOI] [PubMed] [Google Scholar]
- 11. Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ 2011;18:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 13. Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010;207:1853–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015;21:815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margraf S, Logters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock 2008;30:352–358 [DOI] [PubMed] [Google Scholar]
- 16. Valles J, Lago A, Santos MT, Latorre AM, Tembl JI, Salom JB, et al. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb Haemost 2017;117:1919–1929 [DOI] [PubMed] [Google Scholar]
- 17. Altrichter J, Zedler S, Kraft R, Faist E, Mitzner SR, Sauer M, et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur J Trauma Emerg Surg 2010;36:551–557 [DOI] [PubMed] [Google Scholar]
- 18. Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013;33:2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp 2010; DOI: 10.3791/1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 21. Mathioudakis N, Hicks CW, Canner JK, Sherman RL, Hines KF, Lum YW, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2017;65:1698–1705 e1 [DOI] [PubMed] [Google Scholar]
- 22. Vartanian SM, Robinson KD, Ofili K, Eichler CM, Hiramoto JS, Reyzelman AM, et al. Outcomes of neuroischemic wounds treated by a multidisciplinary amputation prevention service. Ann Vasc Surg 2015;29:534–542 [DOI] [PubMed] [Google Scholar]
- 23. Mathioudakis N, Hicks CW, Canner JK, Sherman RL, Hines KF, Lum YW, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2017;65:1698–1705.e1 [DOI] [PubMed] [Google Scholar]
- 24. Hicks CW, Canner JK, Mathioudakis N, Sherman R, Malas MB, Black JH 3rd, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg 2018;68:1096–1103 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Xiao Y, Zhong L, Ye D, Zhang J, Tu Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with beta-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014;63:4239–4248 [DOI] [PubMed] [Google Scholar]
- 26. Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 2013;110:8674–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ebrahimi F, Giaglis S, Hahn S, Blum CA, Baumgartner C, Kutz A, et al. Markers of neutrophil extracellular traps predict adverse outcome in community-acquired pneumonia: secondary analysis of a randomised controlled trial. Eur Respir J 2018;51:pii: [DOI] [PubMed] [Google Scholar]
- 28. Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes 2016;65:1061–1071 [DOI] [PubMed] [Google Scholar]
- 29. Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015;125:1948–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 2018;18:134–147 [DOI] [PubMed] [Google Scholar]
- 31. Yager DR, Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32 [DOI] [PubMed] [Google Scholar]
- 32. Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J 2015;36:1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014;15:1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349:316–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011;11:519–531 [DOI] [PubMed] [Google Scholar]
- 36. Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. J Diabetes Res 2015;2015:268390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brechow A, Slesaczeck T, Münch D, Nanning T, Paetzold H, Schwanebeck U, et al. Improving major amputation rates in the multicomplex diabetic foot patient: focus on the severity of peripheral arterial disease. Ther Adv Endocrinol Metab 2013;4:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25 [DOI] [PubMed] [Google Scholar]