Abstract
The Fourth Edition of the Guidelines for Treatment of Uterine Body Neoplasm was published in 2018. These guidelines include 9 chapters: 1. Overview of the guidelines, 2. Initial treatment for endometrial cancer, 3. Postoperative adjuvant therapy for endometrial cancer, 4. Post-treatment surveillance for endometrial cancer, 5. Treatment for advanced or recurrent endometrial cancer, 6. Fertility-sparing therapy, 7. Treatment of uterine carcinosarcoma and uterine sarcoma, 8. Treatment of trophoblastic disease, 9. Document collection; and nine algorithms: 1-3. Initial treatment of endometrial cancer, 4. Postoperative adjuvant treatment for endometrial cancer, 5. Treatment of recurrent endometrial cancer, 6. Fertility-sparing therapy, 7. Treatment for uterine carcinosarcoma, 8. Treatment for uterine sarcoma, 9. Treatment for choriocarcinoma. Each chapter includes overviews and clinical questions, and recommendations, objectives, explanation, and references are provided for each clinical question. This revision has no major changes compared to the 3rd edition, but does have some differences: 1) an explanation of the recommendation decision process and conflict of interest considerations have been added in the overview, 2) nurses, pharmacists and patients participated in creation of the guidelines, in addition to physicians, 3) the approach to evidence collection is listed at the end of the guidelines, and 4) for clinical questions that lack evidence or clinical validation, the opinion of the Guidelines Committee is given as a “Recommendations for tomorrow”.
Keywords: Clinical Practice Guideline, Endometrial Cancer, Treatment, Gestational Trophoblastic Disease, Uterine Sarcoma
INTRODUCTION
Endometrial cancer is a major gynecologic malignancy that has the highest rate among patients with gynecologic malignant tumors in Japan. The age-adjusted prevalence of endometrial cancer in 2014 was 16.0 per 100,000 females, and this has increased about fourfold in the past 30 years [1,2]. However, the age-adjusted mortality rate in 2017 was 2.0, which is lower than the rates for cervical and ovarian cancers [1].
The 1st edition of the Guidelines for Treatment of Uterine Body Cancer was published by the Japan Society of Gynecological Oncology (JSGO) in 2006, and was revised in the 2nd edition in 2009 [3]. In the second revision, 3 chapters on treatment of mesenchymal tumors such as leiomyosarcoma and treatment of serous carcinoma and clear cell carcinoma were added [3]. In the 3rd edition in 2013, treatments for trophoblastic disease were added, and the target disease was expanded [4]. The current 4th revision does not have major changes from the 3rd version. The new version includes nine chapters with the following contents, and 9 algorithms.
Chapter 1: Overview of the guidelines, recommendation process, and conflict of interest.
Chapter 2: Initial treatment for endometrial cancer; hysterectomy and clinical significance of pelvic lymph node dissection and paraaortic lymph node dissection; minimally invasive surgery (MIS) based on endoscopic (laparoscopic and robot-assisted) surgery and sentinel lymph node biopsy; and optimal treatment for endometrial cancer found after hysterectomy or after inadequate staging surgery.
Chapter 3: Postoperative adjuvant treatment for endometrial cancer; and superiority of chemotherapy over radiation therapy based on the actual clinical situation in Japan.
Chapter 4: Post-treatment surveillance for endometrial cancer; and hormone replacement therapy.
Chapter 5: Treatment for advanced or recurrent endometrial cancer; adjuvant chemotherapy is often used for a case with recurrence risk, and many patients are not chemo-naïve at the time of recurrence.
Chapter 6: Fertility-sparing treatment; treatment for atypical endometrial hyperplasia (AEH) and endometrioid carcinoma G1, followed by infertility treatment and treatment for recurrent cases.
Chapter 7: Treatment for uterine carcinosarcoma and uterine sarcoma; initial treatment and treatment for recurrent cases.
Chapter 8: Treatment for trophoblastic disease (added in 3rd edition); choriocarcinoma, invasive mole, persistent trophoblastic disease, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT).
Chapter 9: Document collections, including literature search terms.
Most treatment for uterine body neoplasms including endometrial cancer (excluding trophoblastic diseases) involves surgical therapy, including total hysterectomy as initial treatment. Methods of hysterectomy and the need for pelvic/paraaortic lymphadenectomy will mainly be discussed. Chemotherapy or radiotherapy is added as needed in cases with a high risk of postoperative recurrence. This also affects the choice of treatment for recurrent cancer. Hormone therapy is used for recurrence or in fertility preservation therapy. Immunotherapy has yet to be included in the guidelines. Chemotherapy is the main treatment for unresectable advanced uterine cancers and trophoblastic diseases.
CHAPTER 1: OVERVIEW OF THE GUIDELINES
1. How to use the guidelines
We describe one criterion for selecting a better treatment method for uterine body neoplasms in Japan, and show evidence for the suggested approach. This does not limit treatment to that described in the Guidelines. The aims of the guidelines are as follows:
1) To define appropriate treatment for endometrial cancer, carcinosarcoma/sarcoma, and trophoblastic disease.
2) Reduce disparities in treatment approaches among institutions.
3) Improve the safety of these treatments and the prognosis of patients.
4) Reduce physical, psychological and economic burdens on patients by using appropriate treatment.
5) Improve mutual understanding between medical staff and patients.
2. Intended audience
These Guidelines are intended for practicing physicians engaged in treatment of patients with endometrial cancer, carcinosarcoma/sarcoma, and trophoblastic diseases.
3. Diseases addressed by these guidelines
Diseases addressed by the guidelines include AEH, endometrial cancer, uterine carcinosarcoma, uterine sarcoma, and trophoblastic tumor, and recurrence of these diseases.
4. Notes on using these guidelines
1) There are fewer randomized controlled trials of treatment of uterine body tumor compared to lung, breast, and colorectal cancers. Some items cannot be defined in the guidelines at the evidence level.
2) Some evidence in Europe and the United States is unacceptable in Japan due to differences in background, while some treatment that is common in Japan differs from that in Europe and the United States. In such cases, the content is prioritized using the current consensus in Japan.
3) Therapy is often difficult to administer under the Japanese medical care insurance system. In this regard, the guidelines follow the Committee on Clinical Practice Guidelines for Use of Anticancer Agents of the Japan Society of Clinical Oncology (JSCO).
5. Literature retrieval
In this revision, we asked the Japan Medical Library Association (JMLA) to prepare literature search terms for a systematic database search. The specific literature retrieval method was as follows.
1) The Formulation Committee selected an article using keywords related to the clinical question (CQ), and the JMLA then prepared relevant search terms and conducted a comprehensive literature search. If a large number of articles were found, the keywords were changed and more were added after review by the Formulation Committee and the JMLA. The Formulation Committee examined the retrieved articles and finally identified about 20 important articles.
2) Articles in PubMed, the Japan Medical Abstract Society, and the Cochrane Library from January 2011 to December 2016 were covered in the search. Articles published before 2011 that were cited in previous editions of the guidelines and are needed for recommendations are used as references. Articles published after January 2017 were examined separately and some were used as references.
6. Procedure for creation of the guidelines
To create these guidelines, the Guidelines Formulation Committee and Evaluation Committee were independently established within the Committee for Treatment Guidelines for Uterine Body Neoplasms that was established by the Guidelines Committee of the JSGO. The Chair of the Guidelines Committee was concurrently Chair of the Committee for Treatment Guidelines for Uterine Cancer and Chair of the Guidelines Formulation Committee. Revision of the Guidelines took place from October 2016 to June 2018, after 5 meetings of the Guidelines Formulation Committee, a consensus meeting, and a period for public comment.
7. Evidence levels and recommendation grades
1) Collected evidence was evaluated for quality using the criteria of the JSCO and its Committee of Clinical Practice Guidelines for the Use of Anticancer Agents [5,6]. However, some contents were modified in line with these guidelines (Table 1).
2) Strengths of recommendations in our guidelines were also determined using the recommendation criteria of the JSCO and its Committee of Clinical Practice Guidelines for the Use of Anticancer Agents [5,6]. These were modified with reference to the Guide 2007 Minds Practice Guidelines (Table 2) [7].
3) We referred to the Guide 2014 Minds Practice Guidelines to determine recommendation grades [8]. Judgment of recommendation grades was made based on agreement of the Guidelines Committee, based on the evidence level, effect, concerns with the reference study, implementation of the treatment regimen, and coverage by insurance in Japan.
4) If it was difficult to reach a decision, the recommendation grade was decided by a vote.
Table 1. Classification of evaluation criteria for evidence quality.
| Level | Description |
|---|---|
| Level I | Evidence from meta-analyses of multiple RCTs or evidence from multiple RCTs. |
| Level II | Evidence from at least one RCT or evidence from multiple well-designed controlled studies without randomization. |
| Level III | Evidence obtained from at least one other type of well-designed quasi-experimental study, or evidence obtained from well-designed, non-experimental descriptive studies, such as comparative studies, correlation studies, or case studies. |
| Level IV | Expert committee reports or opinions and/or clinical experiences of respected authorities. |
RCT, randomized controlled trial.
Table 2. Classification of recommendation categories.
| Grade | Description |
|---|---|
| Grade A | Treatment is strongly recommended. |
| Grade B | Treatment is recommended. |
| Grade C1 | Treatment can be considered, or is suggested, but the evidence is insufficient. |
| Grade C2 | Treatment is not recommended without sufficient scientific evidence. |
| Grade D | Treatment is not recommended because utility and efficacy have not been shown and the treatment may be harmful. |
8. Disclosure of information
These guidelines are published as a pamphlet and are shown on the homepages of JSGO, JSCO and Minds to facilitate widespread use.
9. Responsibility for treatment
The JSGO bears responsibility for the content and descriptions of these guidelines. However, the final decision to use the guidelines should be made by the individual user. Thus, the responsibility for the treatment outcomes is directly attributed to the person in charge.
10. Revision
1) These guidelines are continuously being revised by the Committee for Treatment Guidelines for Uterine Body Neoplasms based on medical advances and changes.
2) Evidence that is newly accumulated after preparation of these guidelines is saved in a database.
3) Information on clinical problems occurring with use of these guidelines is being collected.
4) Revisions are considered by the Guidelines Formulation Committee and Evaluation Committee based on new evidence and information. Opinions from academic societies, groups and JSGO members are also widely sought.
5) After these processes, the Committee for Treatment Guidelines for Uterine Cancer will develop a revised version with the approval of the JSGO.
11. Funding
Preparation of these guidelines was funded by the JSGO only. No assistance was provided by other organizations or companies.
12. Conflicts of interest
1) The Board of the Society Conflict of Interest Committee confirmed the absence of any conflicts of interest. Thirteen members (4 in the Guidelines Formulation Committee and 9 in the Guidelines Evaluation Committee) had conflicts of interest due to work or social activity with a company, but none of these conflicts of interest conditions were judged to have exceeded the acceptable range.
2) The contents of these Guidelines are based on the consensus of the Guideline Committee and thus are unaffected by any interest associated with specific groups or products.
13. Summary of recommendations
Each chapter comprises CQs, recommendations, background, objectives, explanations, and references. This article summarizes the guidelines in a question-and-answer format. Recommendations from each chapter are listed below, under their respective chapter titles.
14. Algorithms
The guidelines contain the following 9 algorithms:
1) Initial treatment for endometrial cancer considered to be stage I or II preoperatively (Fig. 1).
2) Initial treatment for the patients who are confirmed to be endometrial cancer after hysterectomy and for cases with an intermediate or high risk of postoperative recurrence despite a presumed low risk preoperatively (Fig. 2).
3) Initial treatment for endometrial cancer considered to be stage III or IV preoperatively (Fig. 3).
4) Postoperative adjuvant treatment for endometrial cancer (Fig. 4).
5) Treatment of recurrent endometrial cancer (Fig. 5).
6) Fertility-sparing therapy for AEH and endometrioid carcinoma (corresponding to G1) (Fig. 6).
7) Treatment for uterine carcinosarcoma (Fig. 7).
8) Treatment for uterine sarcoma (Fig. 8).
9) Treatment for choriocarcinoma (Fig. 9).
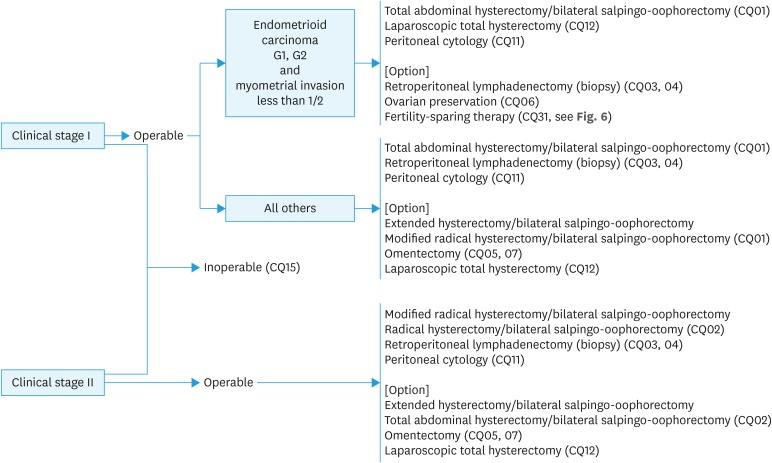
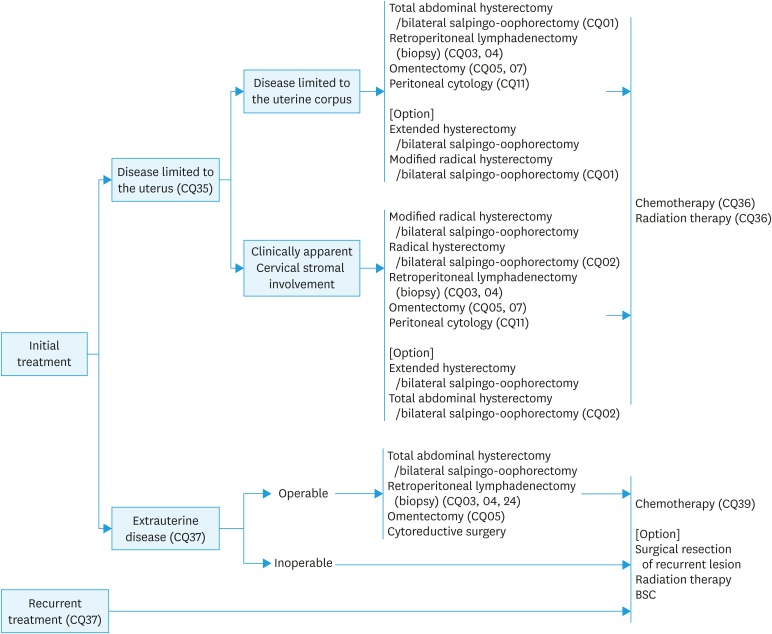
Fig. 1. Initial treatment for patients with endometrial cancer considered to be stage I or II preoperatively. Surgical staging is estimated before surgery by pathological diagnosis and diagnostic imaging (CQ08). Pelvic and para-aortic lymphadenectomy/lymph node biopsy and omentectomy are considered, in addition to total hysterectomy with bilateral salpingo-oophorectomy, for serous carcinoma or clear cell carcinoma (CQ07). Radiation therapy (CQ15) is considered when surgery cannot be performed.
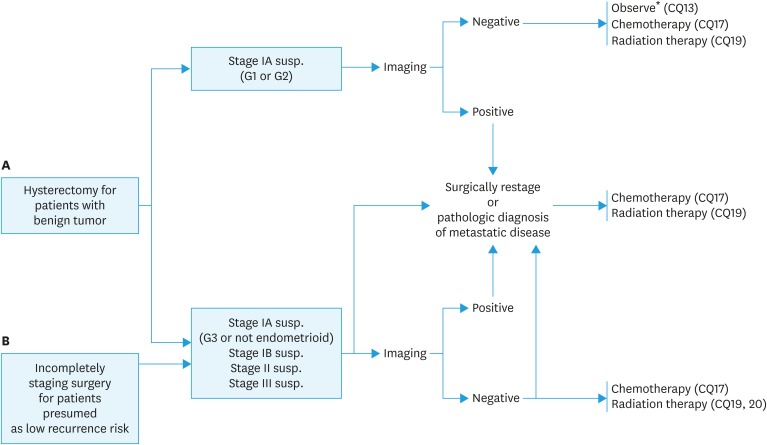
Fig. 2. Initial treatment for (A) patients who are confirmed to be endometrial cancer after hysterectomy, and (B) patients diagnosed with an intermediate to high risk of recurrence after surgery performed with a presumed low risk of recurrence.
*In the 2017 NCCN guidelines, observation is possible for patients with a tumor of <2 cm, LVSI negative, and a presumed low recurrence risk.
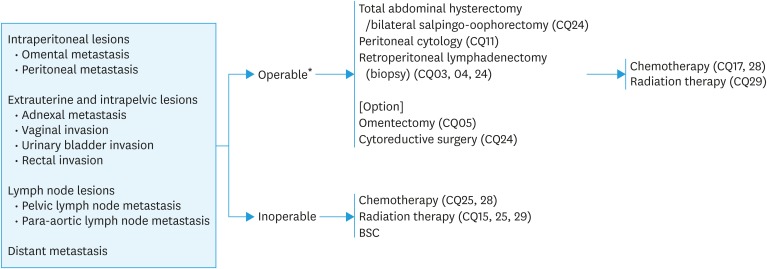
Fig. 3. Initial treatment for patients with endometrial cancer considered to be stage III or IV preoperatively.
*If the general condition is not worse, this refers to all patients in stage III and patients who can undergo hysterectomy and cytoreductive surgery in stage IV (CQ24).
BSC, best supportive care.
Fig. 4. Postoperative adjuvant treatment for endometrial cancer.
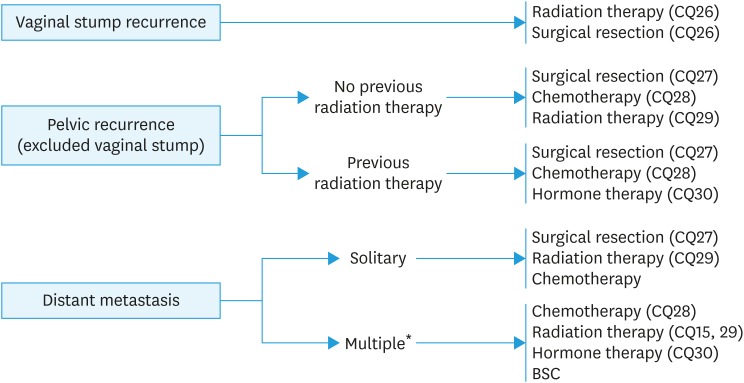
Fig. 5. Treatment of recurrent endometrial cancer.
BSC, best supportive care.
*Resection should also be considered for cases with a few small lung metastases (CQ27).
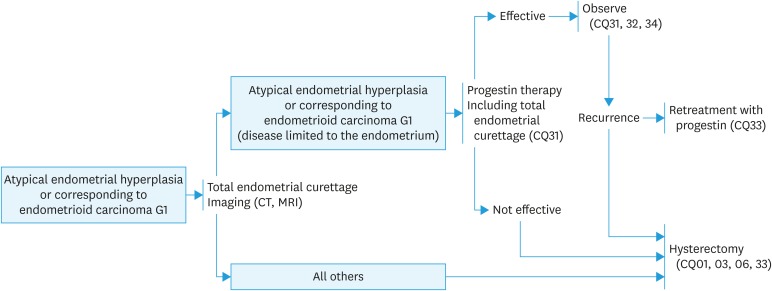
Fig. 6. Strategies for fertility-sparing therapy for atypical endometrial hyperplasia and endometrioid adenocarcinoma (corresponding to G1).
CT, computed tomography; MRI, magnetic resonance imaging.
Fig. 7. Treatment for uterine carcinosarcoma.
BSC, best supportive care.
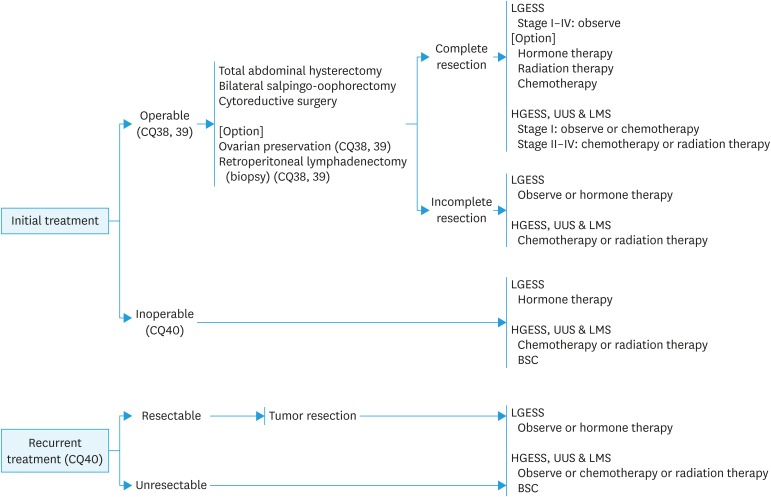
Fig. 8. Treatment for uterine sarcoma.
LGESS, low grade endometrial stromal sarcoma HGESS, high grade endometrial stromal sarcoma; UUS, undifferentiated uterine sarcoma; LMS, leiomyosarcoma; BSC, best supportive care.
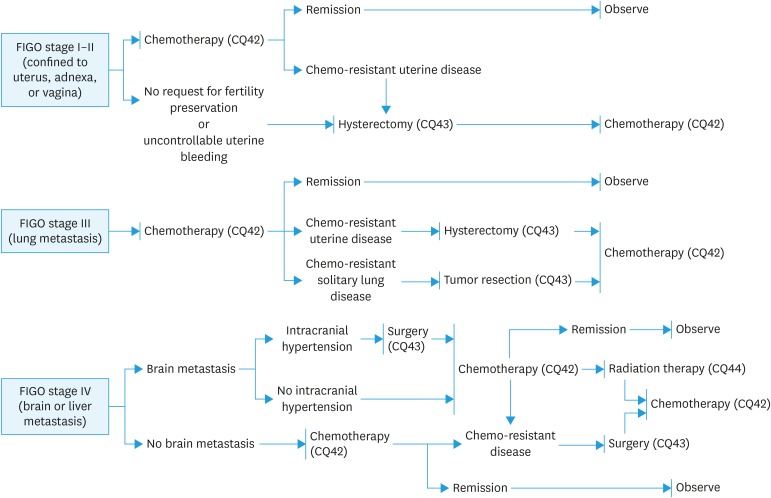
Fig. 9. Treatment for choriocarcinoma.
FIGO, International Federation of Gynecology and Obstetrics.
CHAPTER 2: INITIAL TREATMENT FOR ENDOMETRIAL CANCER (INCLUDING HISTOLOGIC VARIANT TYPE)
CQ01. Which surgical techniques for hysterectomy are recommended for patients thought to be in stage I preoperatively?
Recommendation:
1. Abdominal total hysterectomy or extended hysterectomy (extrafascial technique) are recommended (Grade B).
2. Modified radical hysterectomy is also suggested (Grade C1).
[See Fig. 1]
CQ02. Which surgical hysterectomy techniques are recommended for patients thought to be in stage II?
Recommendation:
Radical hysterectomy or modified radical hysterectomy is suggested (Grade C1).
[See Fig. 1]
CQ03. What are the benefits of pelvic lymphadenectomy?
Recommendation:
1. Required to determine the exact surgical staging (Grade A).
2. Suggested for intermediate-risk or high-risk patients (Grade C1).
3. Omission of this procedure is suggested for some low-risk patients (Grade C1).
[See Fig. 1]
CQ04. What are the benefits of para-aortic lymphadenectomy (biopsy)?
Recommendation:
1. Required to determine the exact surgical staging (Grade A).
2. Suggested for intermediate-risk or high-risk patients (Grade C1).
3. Omission of this procedure is suggested for low-risk patients (Grade C1).
[See Fig. 1]
CQ05. Is omentectomy necessary?
Recommendation:
1. Searching the omentum by careful ocular inspection and palpation is necessary in all cases (Grade A).
2. Strongly recommended for cases with suspected omentum metastasis during surgery (Grade A).
3. Suggested if deeper myometrial invasion, cytology, Grade 3 endometrioid carcinoma or non-endometrioid carcinoma is expected, or positive intraoperative peritoneal cytology or macroscopic extrauterine disease is found during surgery, even if no gross omentum metastasis is detected (Grade C1).
[See Fig. 1]
CQ06. Is ovarian preservation possible?
Recommendation:
1. In principle, bilateral salpingo-oophorectomy is conducted to determine the exact surgical staging during initial treatment (Grade A).
2. Ovarian preservation is considered after the risks are explained to young patients with G1 endometrioid carcinoma and superficial myometrial invasion (Grade C1).
[See Fig. 1]
CQ07. What surgical technique is recommended for serous carcinoma and clear cell carcinoma?
Recommendation:
1. Total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
2. Pelvic and para-aortic lymphadenectomy (lymph node biopsy) and omentectomy are also recommended (Grade C1).
[See Fig. 1]
CQ08. When is preoperative diagnostic imaging useful for estimating staging?
Recommendation:
1. Evaluation of myometrial invasion and cervical invasion by preoperative MRI is strongly recommended (Grade A).
2. Evaluation of lymph node metastases or distant metastases by preoperative imaging such as computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET)/CT is strongly recommended (Grade A).
CQ09. Is intraoperative frozen-section diagnosis useful to determine the optimal operative method?
Recommendation:
1. May be useful for predicting high-risk disease for which pelvic and para-aortic lymphadenectomy or omentectomy is appropriate (Grade C1).
2. Not recommended for definite diagnosis of histological type, histological grade, and myometrial invasion (Grade C2).
CQ10. Is it possible to omit lymphadenectomy based on the result of sentinel node biopsy?
Recommendation:
Omission of lymphadenectomy in patients with negative sentinel lymph node metastasis can be considered in the context of a clinical trial by a team proficient in the procedure and with strong assistance from a pathologist (Grade C1).
CQ11. Should intraoperative peritoneal cytology be conducted?
Recommendation:
CQ12. What is the indication for laparoscopic surgery?
Recommendation:
1. Laparoscopic surgery is recommended for cases with AEH or a low risk of recurrence in presumed stage I endometrial cancer (Grade B).
2. Laparoscopic surgery is considered for cases with an intermediate or high recurrence risk in presumed stage I or II endometrial cancer (Grade C1).
3. Laparoscopic surgery is not recommended for advanced endometrial cancer (Grade C2).
[See Fig. 1]
Additional statement
1) This surgery requires a board-certified gynecologic oncologist of the JSGO who is skilled in surgical procedures, or the assistance of both of a qualified surgeon of the Japan Society of Gynecologic and Obstetric Endoscopy and Minimally Invasive Therapy and a board-certified gynecologic oncologist.
2) The decision on the operative method in laparoscopic surgery is made according to the basic policy described in CQ01, CQ03, and CQ04.
CQ13. How should patients with confirmed endometrial cancer after hysterectomy be treated?
Recommendation:
1. Appropriate additional treatment including reoperation is recommended for cases thought to have an intermediate to high risk of recurrence (Grade B).
2. Observation is also possible for cases thought to have a low risk of recurrence (Grade C1).
[See Fig. 2]
CQ14. What treatment is recommended for cases with an intermediate or high risk of postoperative recurrence despite a presumed low risk preoperatively?
Recommendation:
After diagnostic imaging, appropriated additional treatment is suggested based on the exact surgical staging following the reoperation (Grade C1).
[See Fig. 2]
CQ15. What is the indication for definitive radiation therapy?
Recommendation:
Radiation therapy is an option when surgery is undesirable because of advanced age or complications (Grade C1).
[See Fig. 3]
CHAPTER 3: POSTOPERATIVE ADJUVANT THERAPY FOR ENDOMETRIAL CANCER (INCLUDING HISTOLOGIC VARIANT TYPE)
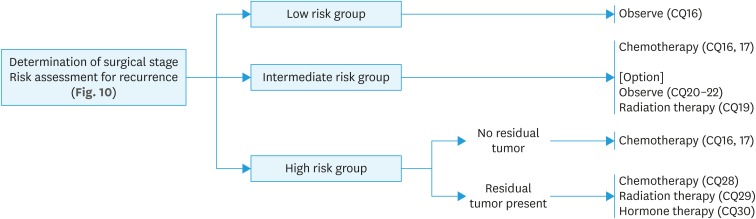
CQ16. What are the indications and methods recommended for postoperative chemotherapy?
Recommendation:
1. Postoperative adjuvant chemotherapy is recommended for high-risk patients* (Grade B).
2. Postoperative adjuvant chemotherapy is suggested for intermediate-risk patients* (Grade C1).
3. Postoperative adjuvant chemotherapy is not recommended for low-risk patients* (Grade D).
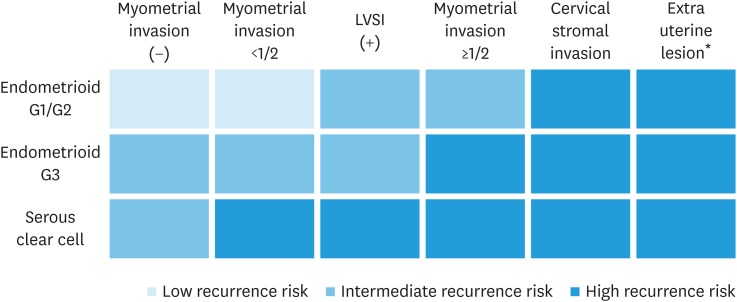
*Classification of postoperative risk of recurrence of endometrial cancer is shown in Fig. 10.
Fig. 10. Classification of postoperative risk of recurrence of endometrial cancer.
LVSI, lymphovascular space invasion.
*Adnexa, uterine serosa, vagina, cardinal ligament, lymph node, bladder, rectum, intraperitoneal and distant metastasis.
CQ17. What drugs are recommended for postoperative chemotherapy?
Recommendation:
1. Chemotherapy with adriamycin (doxorubicin hydrochloride) and cisplatin is recommended for high-risk patients (Grade B).
2. Taxane-based and platinum-based drug combination therapy are also suggested (Grade C1).
3. Regimens for high-risk patients are also recommended for intermediate-risk patients (Grade C1).
CQ18. Is hormone therapy effective as postoperative adjuvant therapy?
Recommendation:
Postoperative progesterone therapy is not recommended (Grade D).
CQ19. What are the indications for postoperative radiotherapy?
Recommendation:
CHAPTER 4: POST-TREATMENT SURVEILLANCE FOR ENDOMETRIAL CANCER
CQ20. What intervals are recommended for post-treatment surveillance?
Recommendation:
Standard intervals between routine follow-up appointments are as follows (Grade C1):
1. Every 1 to 4 months for the first 1 to 3 years after the first treatment
2. Every 6 months for the fourth and fifth years after the first treatment
3. Annually from the sixth year after the first treatment
[See Fig. 4]
CQ21. Should pelvic examination and vaginal vault cytology be performed in post-treatment follow-up?
Recommendation:
1. Pelvic examination should be performed to detect intra-pelvic recurrence (Grade A).
2. Vaginal vault cytology can be used to detect vaginal stump recurrence (Grade C1).
[See Fig. 4]
CQ22. Should measurement of serum tumor markers or diagnostic imaging be performed in post-treatment follow-up?
Recommendation:
1. Measurement of cancer antigen (CA) 125 or CA 19-9 as serum tumor markers should be considered in post-treatment follow-up (Grade C1).
2. Based on the risk of recurrence in each case, diagnostic imaging methods such as chest X-ray and CT are considered to be appropriate.
3. When recurrence is suspected clinically, diagnostic imaging methods such as CT, MRI and PET-CT are recommended for detection of recurrent lesions.
[See Fig. 4]
CQ23. Is hormone replacement therapy recommended after treatment?
Recommendation:
Hormone replacement therapy after treatment can be considered after the benefits and risks are explained to the patient (Grade C1).
CHAPTER 5: TREATMENT FOR ADVANCED OR RECURRENT ENDOMETRIAL CANCER
CQ24. Is surgery recommended for patients considered to be stage III or IV preoperatively?
Recommendation:
1. Surgery is recommended for cases considered to be stage III (Grade B).
2. Surgery is suggested whenever hysterectomy and cytoreduction are possible for cases considered to be stage IV (Grade C1).
[See Fig. 3]
CQ25. Is neoadjuvant therapy recommended for patients with advanced cancer that is difficult to resect or for those who undergo incomplete surgery?
Recommendation:
Neoadjuvant chemotherapy is recommended for a locally invasive tumor that is difficult to resect or for a distant metastasis that cannot be resected completely.
[See Fig. 3]
CQ26. What is recommended for vaginal stump recurrence?
Recommendation:
1. Radiotherapy is recommended (Grade B).
2. Surgical resection should also be considered (Grade C1).
CQ27. Is surgery recommended for recurrent cancer without a vaginal stump?
Recommendation:
1. Surgical resection is suggested for an isolated recurrent lesion that can be resected completely (Grade C1).
2. Resection should also be considered for cases with a few small lung metastases (Grade C1).
[See Fig. 5]
CQ28. Is chemotherapy recommended for patients with advanced or recurrent cancer that cannot be resected or for those who undergo incomplete surgery?
Recommendation:
1. Chemotherapy (doxorubicin/cisplatin) is recommended for advanced cancer (Grade B).
2. Paclitaxel/carboplatin or paclitaxel/doxorubicin/cisplatin therapy are also considered for advanced cancer because of their efficacy and safety (Grade C1).
3. Doxorubicin/cisplatin therapy, paclitaxel/carboplatin therapy or monotherapy are considered for recurrent cancer based on the condition of the patient and previous treatment (Grade C1).
CQ29. Is radiotherapy recommended for patients with advanced or recurrent cancer that cannot be resected or for those who undergo incomplete surgery without vaginal stump recurrence?
Recommendation:
1. Radiation therapy is considered for advanced cancer that cannot be resected or after incomplete surgery for local control or as a palliative procedure (Grade C1).
2. Radiation therapy is considered as a palliative option for recurrent cancer without a vaginal stump for local control (Grade C1).
CQ30. Is hormone therapy recommended for advanced and recurrent cancer?
Recommendation:
CHAPTER 6: FERTILITY-SPARING THERAPY
CQ31. What treatment is recommended for patients with AEH or G1 endometrioid carcinoma who desire for fertility preservation?
Recommendation:
1. Progesterone therapy is suggested for AEH (Grade C1).
2. Progesterone therapy is also suggested for G1 endometrioid carcinoma that is thought to be localized in the endometrium (Grade C1).
[See Fig. 6]
CQ32. What are suitable follow-up periods and examinations?
Recommendation:
Endometrial biopsy and transvaginal ultrasonography are suggested every 3 months (Grade C1).
[See Fig. 6]
CQ33. What treatments are recommended for patients with a residual tumor or a recurrent lesion after fertility preservation therapy?
Recommendation:
1. Total hysterectomy is recommended (Grade B).
2. If a patient strongly desires preservation of fertility, retreatment with progesterone can be considered for a recurrent lesion, but only under strict control (Grade C1).
[See Fig. 6]
CQ34. Is ovulation induction permissible in patients after fertility preservation therapy?
Recommendation:
Induction of ovulation for pregnancy can be considered (Grade C1).
[See Fig. 6]
CHAPTER 7: TREATMENT OF UTERINE CARCINOSARCOMA AND UTERINE SARCOMA
CQ35. What surgical techniques are recommended for uterine carcinosarcoma?
Recommendation:
1. Based on endometrial cancer, total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
2. Pelvic, para-aortic lymphadenectomy (biopsy) and omentectomy are also suggested (Grade C1).
[See Fig. 7]
CQ36. What adjuvant therapy is recommended for uterine carcinosarcoma?
Recommendation:
1. When adjuvant chemotherapy is selected, regimens including ifosfamide, platinum-based drugs, and paclitaxel are suggested (Grade C1).
2. Radiation therapy (whole-pelvis external-beam irradiation) is also suggested (Grade C1).
[See Fig. 7]
CQ37. What treatment methods are recommended for advanced and recurrent uterine carcinosarcoma?
Recommendation:
1. If total hysterectomy and cytoreductive surgery are possible, surgical treatment is suggested for advanced uterine carcinosarcoma (Grade C1).
2. Regimens including ifosfamide, platinum-based drugs, and paclitaxel are suggested as chemotherapy for advanced or recurrent disease (Grade C1).
3. If an isolated recurrent lesion can be resected completely, surgical resection is suggested (Grade C1).
[See Fig. 7]
CQ38. What surgical methods and postoperative adjuvant therapy are recommended for uterine leiomyosarcoma?
Recommendation:
1. Complete extraction including total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
2. Chemotherapy is suggested as adjuvant therapy (Grade C1).
[See Fig. 8]
CQ39. What surgical methods and adjuvant therapy are recommended for endometrial stromal sarcoma (ESS)?
Recommendation:
1. Total hysterectomy with bilateral salpingo-oophorectomy is recommended (Grade B).
2. Pelvic and para-aortic lymphadenectomy (biopsy) or cytoreductive surgery is also suggested (Grade C1).
3. For stage I low-grade ESS, adjuvant therapy is not recommended (Grade D).
4. When adjuvant therapy is considered to be necessary for high-grade ESS or undifferentiated uterine sarcoma, chemotherapy is suggested (Grade C1).
[See Fig. 8]
CQ40. What treatments are recommended for unresectable advanced or recurrent ESS/leiomyosarcoma?
Recommendation:
1. Surgical resection is suggested for a completely resectable recurrent lesion (Grade C1).
2. Chemotherapy should also be considered (Grade C1).
3. Hormonal therapy is suggested for patients with low-grade ESS (Grade C1).
4. Radiation therapy should also be considered for the purpose of palliative care (Grade C1).
[See Fig. 8]
CHAPTER 8: TREATMENT OF TROPHOBLASTIC DISEASE
CQ41. What chemotherapy is recommended for an invasive mole, clinically invasive mole, or post-molar persistent human chorionic gonadotropin (hCG)?
Recommendation:
Monotherapy with methotrexate or actinomycin D is recommended (Grade B).
CQ42. What chemotherapy is recommended for choriocarcinoma?
Recommendation:
A multidrug regimen including methotrexate, actinomycin D, and etoposide is desirable (Grade B).
[See Fig. 9]
CQ43. What are the indications for surgery for choriocarcinoma?
Recommendation:
1. Surgical resection is suggested for a uterine lesion or metastatic lesion associated with chemoresistance (Grade C1).
2. Surgical resection is also suggested for a uterine lesion in which hemorrhage is difficult to control or for brain metastasis with symptoms of intracranial hypertension (Grade C1).
[See Fig. 9]
CQ44. Is radiotherapy effective for choriocarcinoma?
Recommendation:
Radiotherapy is suggested for brain metastases, but the indication should be carefully considered (Grade C1).
[See Fig. 9]
CQ45. What treatments are recommended for cases with PSTT or ETT?
Recommendation:
1. Total hysterectomy is suggested for patients with a tumor limited to the uterus (Grade C1).
2. Combination therapy with surgical treatment including total hysterectomy and chemotherapy are suggested for patients with a metastatic lesion (Grade C1).
CQ46. How should patients with persistent low-positive hCG be treated?
Recommendation:
1. Detection of the lesion and confirmation of real hCG are recommended (Grade B).
2. If there is no obvious lesion, but low unit real hCG persists for a long time, strict follow-up is suggested. (Grade C1).
ACKNOWLEDGMENTS
We thank the Japan Society of Obstetrics and Gynecology, the Japan Association of Obstetricians and Gynecologists, the Japanese Gynecologic Oncology Group, the Japan Society of Clinical Oncology, and the Japanese Society for Therapeutic Radiology and Oncology for their comments and contributions throughout the project.
Participating contributors list
-
Members of the Guidelines Formulation Committee (alphabetical order)
Atsushi Arakawa, Hiroyuki Fujiwara, Kenichi Harano, Kiyoshi Hasegawa, Kazuhiko Hayashi, Haruko Iwase,Yuko Kaneyasu, Noriko Kato, Hiroaki Kobayashi, Eiji Kondo, Yoshiaki Kawano, Tomoyuki Nagai, Hitoshi Niikura, Shin Nishio, Fumitaka Saito, Shu Soeda, Nobuyuki Susumu, Kazuhiro Takehara, Munetaka Takekuma, Yoshito Terai, Yasuhisa Terao, Takafumi Toita, Hirokazu Usui, Hidekazu Yamada, Eiko Yamamoto, Hiroyuki Yanai, Hidemitchi Watari.
-
Members of the Guidelines Evaluation Committee (alphabetical order)
Daisuke Aoki, Takayuki Enomoto, Takuma Fujii, Junzo Hamanishi, Yasuyuki Hasuo, Junji Hongo, Masaharu Hukunaga, Hitoshi Ikushima, Kazuhiko Ino, Atsuo Itakura, Kiyoshi Ito, Hiroyuki Kanao, Hidenori Kato, Naoki Kawamura, Hiroshi Kobayashi, Kaneyuki Kubushiro, Satoru Kyo, Kouji Matsumoto, Kouji Matsumoto, Yoshiki Mikami, Yo Miyahara, Mika Mizuno, Shouji Nagao, Satoru Nakayama, Kaei Nasu, Aiko Okamoto, Masahide Omichi, Takashi Onda, Mikiko Sato, Ritsuko Saito, Tadahiro Shoji, Atsuko Sone, Kenzo Sonoda, Koutaro Sueoka, Nao Suzuki, Kiyoshi Takamatsu, Takashi Uno, Yoshihito Yokoyama
Patient group Katoreanomori.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Methodology: M.M., N.S.
- Project administration: M.M., N.S.
- Supervision: T.T., K.Y., K.H., A.D.
- Visualization: Y.W.
- Writing - original draft: Y.W., K.H., Y.H., H.K., F.H.
- Writing - review & editing: Y.W., M.M., N.S.
References
- 1.Cancer Registry and Statistics (JP) Cancer Information Service [Internet] Tokyo: National Cancer Center; [cited 2019 May 1]. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html. [Google Scholar]
- 2.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32. doi: 10.3802/jgo.2017.28.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagase S, Katabuchi H, Hiura M, Sakuragi N, Aoki Y, Kigawa J, et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol. 2010;15:531–542. doi: 10.1007/s10147-010-0138-6. [DOI] [PubMed] [Google Scholar]
- 4.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016;21:419–434. doi: 10.1007/s10147-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 5.Ariyoshi H. Guideline for correct use of antineoplastic agents (draft). General theory. Gan To Kagaku Ryoho. 2002;29:970–977. [PubMed] [Google Scholar]
- 6.Ochiai K, Okamoto A, Katsumata N. Guidelines for proper use of antineoplastic agents. Gynecologic cancer. Gan To Kagaku Ryoho. 2002;29:1047–1054. [PubMed] [Google Scholar]
- 7.Fukui T, Yoshida M, Yamaguchi N. Minds Guidance of Practice Guidelines 2007. Tokyo: Igakushoin; 2007. [Google Scholar]
- 8.Yamaguchi N, Morizane T, Kojimabara N, Yoshida M, Fukui T. Minds Guidance of Practice Guidelines 2014. Tokyo: Igakushoin; 2014. [Google Scholar]