Abstract
Objective
To observe the safety and short-term efficacy of apatinib in the treatment of recurrent, metastatic cervical cancer in patients who have already received more than two kinds of comprehensive treatment.
Methods
Forty-eight patients with recurrent or metastatic cervical cancer after radiotherapy or surgery who received apatinib between June 2016 and June 2017 were involved in this study. These patients experienced progression after first-line or second-line chemotherapy. There were 38 patients with cervical squamous cell carcinoma, 8 with adenocarcinoma, and 2 with adenosquamous carcinoma. Progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (AEs) were reviewed and evaluated.
Results
All patients had complete follow-up records, and the median follow-up time was 14.5 months (5.5–20.5 months). Among the 48 patients, 14.58% achieved a partial response and 52.08% achieved stable disease. The overall response rate and disease control rate were 14.58% and 66.67%, respectively. The median time that the 48 patients received oral apatinib was 8.2 months. The median PFS was 4.6 months (95% confidence interval [CI]=3.31–5.26) and OS was 13.9 months (95% CI=8.37–17.96). The main apatinib-related adverse reactions were leukopenia (37.5%), neutropenia (41.67%), hemorrhage (37.5%), hypertension (33.33%), proteinuria (12.5%), fatigue (37.5%), and hand-foot syndrome (27.08%). Most of them were grade 1–2, and no drug-related death occurred.
Conclusions
Apatinib can improve the disease control rate of recurrent and metastatic cervical cancer when chemotherapy has failed, and the treatment is well tolerated. This represents that apatinib may be a new treatment option for metastatic cervical cancer patients.
Keywords: Cervix Neoplasms, Apatinib Mesylate, Molecular Targeted Therapy, Drug Toxicity
INTRODUCTION
Cervical cancer is the third leading cause of cancer-related death in females, and there were an estimated 527,600 new cervical cancer cases and 265,700 deaths worldwide in 2012 [1]. In recent years, the incidence of cervical cancer has significantly increased, and it is gradually affecting younger women [2]. Although improved treatments consisting of surgery, radiotherapy, and chemotherapy have prolonged the survival time and improved the quality of life of patients, there remain limited options for patients with metastatic cancers, especially those with persistent or recurrent disease after platinum-based chemoradiotherapy. Women diagnosed with locally advanced or metastatic carcinoma of the cervix have very poor prognosis, with a 5-year survival for patients with stage IV disease between 5% and 15% [3]. The widely accepted standard chemotherapy regimen for the treatment of metastatic, recurrent, or persistent cervical cancer is paclitaxel combined with cisplatin as proposed by the Gynecologic Oncology Group (GOG) [4]. However, this chemotherapy is not highly effective [5], and patients who are physically weakened after repeated treatments cannot tolerate the side effects caused by long-term use of chemotherapy drugs. Moreover, if patients are resistant to platinum-based chemotherapeutic drugs, they face a situation in which no effective drug is available. Hence, clinical treatment of advanced and recurrent cervical cancer remains difficult, and a highly effective, simple, and well-tolerated treatment is urgently needed.
In recent years, the emergence and development of targeted drugs, especially anti-angiogenesis agents, has been encouraging. Vascular endothelial growth factor (VEGF) is a key mediator of tumor angiogenesis, a process that correlates directly with the extent of disease and inversely with survival. There have been several clinical studies conducted to investigate the efficacy of anti-angiogenesis agents such as bevacizumab, sunitinib, and gefitinib. The results show that these drugs, especially bevacizumab, combined with chemotherapy drugs significantly increases the overall survival (OS) of advanced or recurrent cervical cancer, but there have been few studies on monotherapy with VEGF inhibitors, and most of these drugs have presented poor efficacy [6,7,8].
Apatinib, a novel oral small molecule tyrosine kinase inhibitor that mainly targets vascular endothelial growth factor receptor (VEGFR-2) within cells, was approved by the China Food and Drug Administration for use as a single agent in patients with metastatic gastric or gastroesophageal junction adenocarcinoma after second-line chemotherapy failure [9]. In addition, apatinib has demonstrated good safety, tolerability, and efficacy in the treatment of advanced solid tumors such as colorectal, liver, non-small cell lung, ovarian, non-Hodgkin's lymphomas, and bone soft tissue sarcoma in clinical studies [10,11,12,13,14,15,16]. However, no clinical studies with detailed data have investigated the efficacy of apatinib in the treatment of cervical cancer. Herein, a retrospective series analysis was performed to evaluate the efficacy and toxicity of apatinib in recurrent, metastatic cervical cancer after failure of chemotherapy.
MATERIALS AND METHODS
1. General information
From June 2016 to June 2017, a total of 48 patients with recurrent or metastatic cervical cancer who received apatinib treatment in Affiliated Cancer Hospital of Zhengzhou University were enrolled in this study. All patients had underwent more than 2 comprehensive treatments and could not sustain surgery and radiation and were resistant to platinum-based chemotherapeutic agents or refused to continue chemotherapy. Patients were required to have at least one measurable lesion and a Karnofsky Performance Score ≥70. Before treatment, patients were confirmed to have a normal electrocardiogram and no intestinal obstruction, active bleeding, circulatory failure, or other serious complications, and there were no previous heart, liver, kidney, brain, or hematopoietic system diseases. Patients with a propensity for bleeding in the digestive tract, uncontrollable hypertension, coagulation abnormalities, who were receiving thrombolytic or anticoagulant drugs, who were urine protein positive, or had bilirubin ≥1.25 times the upper limit of normal were excluded. The median age was 58 (range, 32–75) years. Pathological diagnosis confirmed 38 patients with cervical squamous cell carcinoma, 8 with adenocarcinoma, and 2 with adenosquamous carcinoma. Among these patients, 15 had undergone surgery, radiotherapy, and chemotherapy, and 31 had undergone radiotherapy and chemotherapy. Most of the patients had multi-site metastases, including 33 patients with pelvic recurrence, 27 patients with retroperitoneal and/or mediastinal lymph nodes and/or supraclavicular lymph node metastasis, 11 patients with lung metastasis, and 7 patients with hepatic metastasis. Patient characteristics are listed in Table 1. The study was approved by the Institutional Review Board of our hospital and was conducted in compliance with the Declaration of Helsinki and local regulatory requirements. The approved number of the Institutional Review Board is 2018120. All patients provided written informed consent.
Table 1. Clinical characteristics of the study population (n=48).
| Characteristic | No. of patients (%) | |
|---|---|---|
| Age (yr) | ||
| <50 | 21 (43.75) | |
| ≥50 | 27 (56.25) | |
| Pathological type | ||
| Squamous carcinoma | 38 (79.16) | |
| Adenocarcinoma | 8 (16.67) | |
| Adenosquamous carcinoma | 2 (4.17) | |
| Prior treatment | ||
| Surgery, radiotherapy, and chemotherapy | 15 (31.25) | |
| Radiotherapy and chemotherapy | 31 (64.58) | |
| Metastatic site | ||
| Pelvic | 33 (68.75) | |
| Retroperitoneal, mediastinal, and supraclavicular lymph nodes | 27 (56.25) | |
| Lung | 11 (22.92) | |
| Liver | 7 (14.58) | |
| History of hypertension | 18 (37.5) | |
2. Treatment protocols
Apatinib was given at a dose of 500 mg once a day, and the median time of apatinib administration was 8.2 (range 3 to 14) months. There was no chemotherapy or radiotherapy during oral administration. Apatinib was administrated until disease progression, unacceptable toxicity, or death. A dose reduction to no less than 250 mg per day was allowed, and dose increases were not allowed.
3. Response evaluation criteria
The primary objective of this study was to observe the progression-free survival (PFS), OS, and adverse drug reactions. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were assessed according to the RECIST version 1.1 response evaluation criteria in solid tumors [17]. The apatinib-related adverse events (AEs) were graded from 0 to 4 based on the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0) [18]. Gynecological examinations were performed before each cycle of treatment. After each cycle of treatment, magnetic resonance imaging or positron emission tomography-computed tomography examinations were performed to evaluate the efficacy, and adverse reactions were recorded. The Cox regression model was used to estimate the survival curves of OS and PFS.
4. Follow-up
All patients were followed up to disease progression or death. PFS refers to the time interval between the administration of apatinib and tumor progression or death that was not caused by tumors. OS was defined as the duration from the time of apatinib treatment to the date of the patient's death by any cause or the last day of the follow-up period. The last follow-up date was May 15, 2018. The follow-up time of the whole group was 5.5 to 20.5 months, and the median follow-up duration was 14.5 months.
RESULTS
1. Treatment situation
The median time of treatment with apatinib in the 48 patients was 8.2 months. When high blood pressure, hematuria, hematochezia, or myelosuppression occurred, the dose of apatinib was reduced to 250 mg per day. Eleven patients required dose reductions for toxicity: hypertension (n=4), hematuria (n=1), hematochezia (n=1), and myelosuppression (n=5). Apatinib was discontinued in 24 patients due to the progression of the disease and in 3 patients because of physical decline.
2. Adverse effects
Adverse reactions were observed from apatinib treatment until death or 8 weeks after drug withdrawal. Adverse reactions associated with apatinib in 40 patients included leukopenia, neutropenia, hypertension, proteinuria, hemorrhage, fatigue, and hand-foot syndrome (Table 2). The most common grade 3 treatment-related non-hematologic adverse reactions included hypertension (6.25%), fatigue (2.08%), and hemorrhage (4.17%), and there was 1 patient each observed to have hematuria and hematochezia. Except for 2 cases of grade 4 leukopenia, 2 cases of grade 4 neutropenia, and 1 case of grade 4 hypertension, other grade 4 toxicities and drug-related deaths were not observed.
Table 2. Apatinib-related adverse events.
| Adverse event | No. of patients (%) | Total | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Hypertension | 8 (16.67) | 4 (8.33) | 3 (6.25) | 1 (2.08) | 16 |
| Proteinuria | 4 (8.33) | 2 (4.17) | 0 | 0 | 6 |
| Hemorrhage | 10 (20.83) | 6 (12.5) | 2 (4.17) | 0 | 18 |
| Fatigue | 13 (27.08) | 4 (8.33) | 1 (2.08) | 0 | 18 |
| Hand-foot syndrome | 11 (22.92) | 2 (4.16) | 0 | 0 | 13 |
| Leukopenia | 9 (18.75) | 4 (8.33) | 3 (6.25) | 2 (4.17) | 18 |
| Neutropenia | 11 (22.92) | 4 (8.33) | 3 (6.25) | 2 (4.17) | 20 |
3. Efficacy
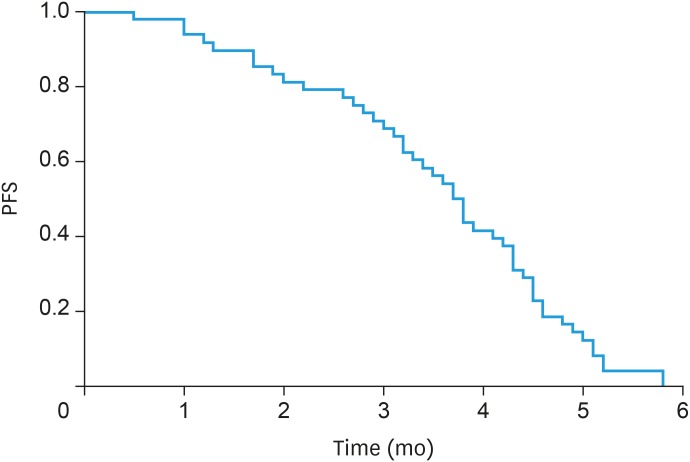
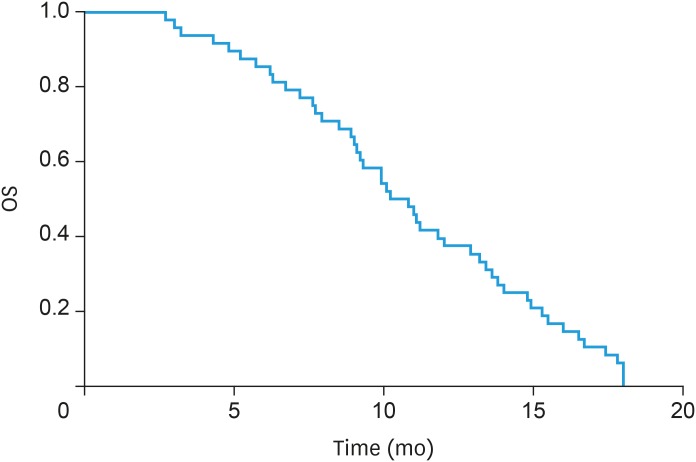
All patients had complete follow-up records and all the responses were therefore evaluable. Seven patients achieved PR, 25 patients had SD, and 16 patients reported PD, but CR was not achieved in any patient. The overall response rate (ORR) and disease control rate (DCR) were 14.58% (7/48) and 66.67% (32/48), respectively (Table 3). The median apatinib treatment time for all patients was 8.2 months, median PFS was 4.6 months (95% confidence interval [CI]=3.31–5.26; Fig. 1), and OS was 13.9 months (95% CI=8.37–17.96; Fig. 2).
Table 3. Overall tumor response in patients.
| Parameter | No. of patients (%) (n=48) |
|---|---|
| CR | 0 |
| PR | 7 (14.58) |
| SD | 25 (52.08) |
| PD | 16 (33.33) |
| ORR | 7 (14.58) |
| DCR | 32 (66.67) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate.
Fig. 1. PFS curve.
PFS, progression-free survival.
Fig. 2. OS curve.
OS, overall survival.
DISCUSSION
The main treatments for patients with recurrent or metastatic cervical cancer are surgery, radiotherapy, and chemotherapy. However, the anatomy and pelvic tissue elasticity of patients are significantly changed after repeated treatments, which cause great difficulty for operations, or postoperative complications seriously affect patient quality of life. If there is a recurrence or metastasis in the primary radiotherapy area shortly after the first radiotherapy, another radiation treatment may result in severe complications, and the tumor may be insensitive to radiation. More importantly, if patients resistant to platinum-based chemotherapeutic drugs in the process of chemotherapy, they will face a situation where no effective drug is available. In addition, the efficacy of the standard treatments is generally not good, which presents a great challenge to gynecologists.
Increasing research on targeted drugs shows that they have good efficacy in patients with recurrent and metastatic cervical cancer. In GOG 240, the study group used bevacizumab in combination with chemotherapy to prolong the survival of patients with refractory, recurrent, or metastatic cervical cancer for approximately 4 months. The median OS was 17 months, the median PFS was 8.2 months, the response rate was 48%, and the patient's mortality rate was reduced by 29% [19]. However, combined treatment with multiple drugs results in major adverse reactions and poor tolerance, and it is difficult to maintain treatment. In another phase II study of sunitinib monotherapy [7], 19 patients with locally advanced or metastatic cervical cancer who received first-line chemotherapy and had a pathological type of squamous or adenocarcinoma were treated. No patient achieved clinical remission after treatment, and the median disease-free survival (DFS) was 3.5 months; hence the treatment was not effective. There are several other VEGF inhibitors that are also being studied in patients with advanced cervical cancer. A phase II clinical trial (VEG105281) [20,21] compared the efficacy of pazopanib and lapatinib monotherapies as a second-line or above treatment for stage IVB resistant or recurrent cervical cancer. The results showed that the pazopanib group (n=74) had a longer median PFS compared to the lapatinib group (n=78) (18.1 vs. 17.1 weeks, p<0.05). The OS was 49.7 weeks and 44.1 weeks in the pazopanib group and lapatinib group, respectively, but there was not a statistically significant difference between the groups. More targeted therapeutic drugs are still being explored for the treatment of cervical cancer.
Apatinib is an oral small molecule anti-angiogenic drug that is effective against multiple solid tumors. Twenty-nine patients with platinum resistance and recurrent ovarian cancer received 500 mg oral apatinib daily in a phase II clinical study [16]. The median PFS and the median OS were 5.1 months (95% CI=3.8–6.5) and 14.5 months (95% CI=12.4–16.4). A study of apatinib in cervical cancer cells showed that apatinib can induce G0/G1 phase arrest in cervical cancer cells, inhibit the proliferation of cervical cancer cells in a dose-dependent manner, and significantly reduce the expression levels of cyclin D3 mRNA and cyclin D3 protein (p<0.01) [22]. Another in vitro study showed that apatinib combined with radiotherapy can significantly arrest the G0/G1 phase of the cell cycle, but with no significant apoptosis induction [23].
In this study, the effective rate and DCR of 48 patients receiving apatinib monotherapy was 14.58% and 66.67%, respectively. Among them, 7 patients had PR, 25 had SD, and 16 had PD. The median PFS was 4.6 months, and the median OS was 13.9 months. This efficacy is similar to that of other oral targeting drugs for cervical cancer, but lower than that of bevacizumab. The reason may be related to the benefits of combining bevacizumab and chemotherapeutic drugs, though the adverse reactions caused by combined chemotherapies are serious, and many patients receiving multiple treatments cannot tolerate them. Hence, apatinib may be used as a palliative treatment for cervical cancer patients unable to tolerate chemotherapy. Although the treatment efficacy in this study was low, the disease control rate was high and apatinib delayed disease progression.
Safety is also critical in the treatment of patients with recurrent and metastatic cervical cancer. Apatinib-related adverse reactions mainly include hypertension, hemorrhage, anemia, fatigue, hand-foot syndrome, and proteinuria [9,24,25]. In this study, adverse reactions associated with apatinib in the 48 patients were mainly hypertension, proteinuria, hemorrhage, fatigue, hand-foot syndrome, and leukopenia. Grade 3–4 adverse reactions were few, and only 1 patient experienced grade 4 hypertension of nonhematologic toxicity. The grade 3 toxicities included hypertension in 3 cases (6.25%), hemorrhage in 2 cases (4.17%, bladder bleeding in 1 case, rectal bleeding in 1 case), and fatigue in 1 case (2.08%). The most common grade 3–4 hematologic toxicities included leucopenia (10.41%) and neutropenia (10.41%), yet no drug-related deaths were observed. The GOG 240 study showed that patients given bevacizumab are more prone to grade 3 and 4 AEs, including bleeding (5%), thrombosis/embolism (9%), and gastrointestinal fistula (9%) [19]. Monk et al. found that the major grade 3 AE of pazopanib and lapatinib was diarrhea, of which the incidence rate in the pazopanib group was 11% and that of the lapatinib group was 13%; grade 4 adverse reactions were 9% in the lapatinib group and 12% in the pazopanib group [20]. The most common treatment-related AEs from apatinib administered to patients with ovarian cancer are hand-foot syndrome (51.7%), hypertension (34.6%), and nausea and vomiting (31%) [22]. The high incidence of bleeding in this study may be due to cystitis and proctitis bleeding after radiotherapy, and the other side effects were mild, indicating that apatinib may have a favorable safety profile for treating recurrent and metastatic cervical cancer.
In conclusion, apatinib monotherapy may be feasible for the treatment of relapsed and metastatic cervical cancer after chemotherapy failure, and it has certain advantages in terms of disease control rate and delaying disease progression. The patients' tolerance to the drug was good, the incidence of serious AEs was low, and the overall adverse reactions were mild, especially in patients with weak constitutions unable to tolerate chemotherapy. The recommended dose of apatinib is 500 mg daily. Although the sample size of this study was limited, it provides preliminary results indicating that apatinib may be appropriate for the treatment of recurrent and metastatic cervical cancer. Further prospective, large-scale phase II trials are in progress.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: W.L.
- Data curation: Y.X.
- Formal analysis: X.Y., Y.X.
- Investigation: C.H., W.L., Y.X.
- Project administration: X.Y., W.L.
- Validation: X.Y., C.H., W.L., Y.X.
- Visualization: X.Y., W.L., Y.X.
- Writing - original draft: X.Y.
- Writing - review & editing: X.Y., C.H., W.L.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Changing demographics of cervical cancer in the United States (1973–2008) Gynecol Oncol. 2012;126:330–333. doi: 10.1016/j.ygyno.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 4.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay HJ, Tinker A, Winquist E, Thomas G, Swenerton K, Oza A, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Sharma DN, Rath GK, Julka PK, Gandhi AK, Jagadesan P, Kumar S. Role of gefitinib in patients with recurrent or metastatic cervical carcinoma ineligible or refractory to systemic chemotherapy: first study from Asia. Int J Gynecol Cancer. 2013;23:705–709. doi: 10.1097/IGC.0b013e31828b1699. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Liao Z, Zhao J, Zhao G, Li X, Du X, et al. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget. 2017;8:64471–64480. doi: 10.18632/oncotarget.16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Liu X, Yang S, Zhang X, Shi Y. Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol. 2018;14:264–269. doi: 10.1111/ajco.12834. [DOI] [PubMed] [Google Scholar]
- 12.Liang L, Wang L, Zhu P, Xia Y, Qiao Y, Wu J, et al. A pilot study of apatinib as third-line treatment in patients with heavily treated metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:e443–9. doi: 10.1016/j.clcc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Kong Y, Sun L, Hou Z, Zhang Y, Chen P, Cui Y, et al. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget. 2017;8:105596–105605. doi: 10.18632/oncotarget.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Li J, Xie Q, Diao L, Gai L, Yang W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: an observational study. Cancer Biol Ther. 2018;19:198–204. doi: 10.1080/15384047.2017.1416275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Xiao S, Zhang L, Li X, Fu X, Wang X, et al. An open label, single-armed, exploratory study of apatinib (a novel VEGFR-2 tyrosine kinase inhibitor) in patients with relapsed or refractory non-Hodgkin lymphoma. Oncotarget. 2018;9:16213–16219. doi: 10.18632/oncotarget.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao M, Deng G, Luo S, Zhou J, Chen L, Yang J, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2018;148:286–290. doi: 10.1016/j.ygyno.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. CTCAE v4.0, Common Terminology Criteria for Adverse Events. Washington, D.C.: U.S. Department of Health and Human Services; 2019. May 28, [Google Scholar]
- 19.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 21.Monk BJ, Pandite LN. Survival data from a phase II, open-label study of pazopanib or lapatinib monotherapy in patients with advanced and recurrent cervical cancer. J Clin Oncol. 2011;29:4845. doi: 10.1200/JCO.2011.38.8777. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Li J, Li J. Effect of apatinib on inhibiting proliferation and increasing chemosensitivity of cervical cancer cells. Prog Obstet Gynecol. 2017;11:806–809. [Google Scholar]
- 23.Liu W, Zhang J. Influence of apatinib combined radiotherapy on HeLa cell cycle and apoptosis in vitro. Acta Univ Med Anhui. 2018;53:55–58. [Google Scholar]
- 24.Wang L, Liang L, Yang T, Qiao Y, Xia Y, Liu L, et al. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: Clinical Trial/Experimental Study. Medicine (Baltimore) 2017;96:e9053. doi: 10.1097/MD.0000000000009053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Shi M, Huang C, Liu X, Xiong JP, Chen G, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol. 2012;15(Suppl):7548. [Google Scholar]