Abstract
Objective
Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) confers similar outcomes as primary debulking surgery and chemotherapy. Little is known about patients who receive NACT but do not undergo debulking surgery. Our aim was to characterize these patients.
Methods
We prospectively identified patients with newly diagnosed stage III/IV ovarian cancer treated with NACT from 7/1/15–12/1/17. Fisher exact and Wilcoxon rank-sum tests were used to compare clinical characteristics by surgical status. The Kaplan-Meier method was used to estimate survival outcomes. Log-rank test and Cox proportional hazards model were applied to assess the relationship of covariates to outcome, and time-dependent covariates were applied to variables collected after diagnosis.
Results
Of 224 women who received NACT, 162 (72%) underwent IDS and 62 (28%) did not undergo surgery. The non-surgical group was older (p<0.001), had higher Charlson comorbidity index (CCI; p<0.001), lower albumin levels (p=0.007), lower Karnofsky performance scores (p<0.001), and were more likely to have dose reductions in NACT (p<0.001). Reasons for no surgery included poor response to NACT (39%), death (15%), comorbidities (24%), patient preference (16%), and loss to follow-up (6%). The no surgery group had significantly worse overall survival (OS) than the surgery group (hazard ratio=3.34; 95% confidence interval=1.66–6.72; p<0.001), after adjustment for age, CCI, and dose reductions.
Conclusions
A significant proportion of women treated with NACT do not undergo IDS, and these women are older, frailer, and have worse OS. More studies are needed to find optimal therapies to maximize outcomes in this high-risk, elderly population.
Keywords: Ovarian Cancer, Neoadjuvant Therapy, Cytoreduction Surgical Procedures, Survival, Elderly
INTRODUCTION
Primary cytoreductive/debulking surgery (PDS) followed by chemotherapy traditionally has been considered standard management for newly diagnosed, advanced ovarian cancer, and multiple studies have shown that degree of cytoreduction is the best predictor of outcomes [1-6]. Given the rationale that preoperative chemotherapy may increase the likelihood of an optimal debulking, 4 large randomized studies comparing neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) with PDS have been conducted [7-10]. The results of two of these studies have been fully published, and both have shown that NACT followed by IDS is non-inferior to PDS followed by chemotherapy [8,10].
These studies have been criticized for their comparatively poorer surgical outcomes and survival, as well as patient selection [11]. Although the majority of the patients in both studies had stage III disease and an ECOG performance status of 0 or 1, many believe that NACT is most beneficial in patients with extensive unresectable disease and poor performance status and multiple comorbidities [8,10,11]. Despite these concerns, the use of NACT and IDS in ovarian cancer has increased since 2010 [12]. In a joint clinical practice guideline published in 2016 by the Society of Gynecologic Oncology (SGO) and the American Society of Clinical Oncology (ASCO), an expert panel recommended NACT for women with high perioperative risk and low likelihood of achieving an optimal debulking [13]. The use of NACT is also increasing in the elderly, and survival appears to be similar between those treated with NACT and those undergoing PDS in some studies [14].
As a result, multiple clinical factors and their associations with outcomes in patients with ovarian cancer who received NACT have been studied to improve patient selection. Some have suggested that age [15], comorbidities [15], functional status [16], cancer antigen 125 (CA-125) level [17,18], and type of chemotherapy and number of cycles [19,20] may influence surgical morbidity and survival. However, studies are conflicting [21], and although many predictive models [22,23] have been proposed, no one has been widely adopted.
Although many different prognostic markers have been examined, little is known about patients who receive NACT but do not undergo IDS. These patients and their reasons for deferring IDS may provide valuable information to optimize patient selection for NACT and thereby improve outcomes. We sought to evaluate the clinical characteristics, reasons for deferring IDS, and outcomes of patients who received NACT but did not undergo IDS.
METERIALS AND METHODS
1. Patient selection
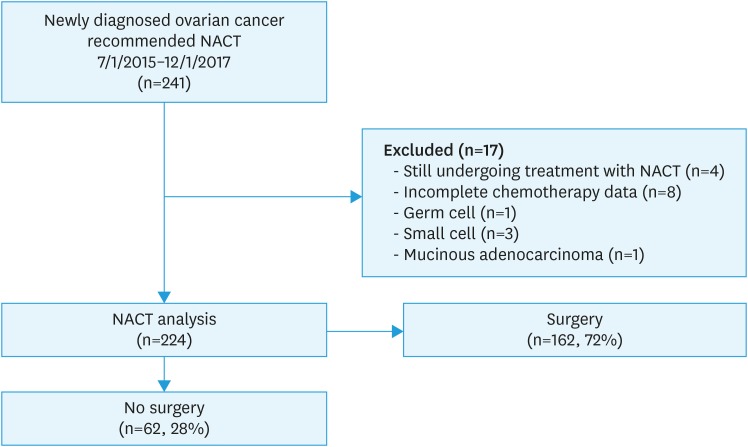
From July 1, 2015 until December 1, 2017, we prospectively identified 241 women with newly diagnosed, pathologically verified ovarian, fallopian tube, or primary peritoneal cancer who sought medical and surgical care at Memorial Sloan Kettering Cancer Center (MSK) and were recommended to receive NACT. Women were excluded if they were still undergoing treatment at the end of data abstraction (n=4), had incomplete chemotherapy data (n=8), or had a histology revealing germ cell (n=1), small cell (n=3) or mucinous adenocarcinoma (n=1), leaving 224 women who were included in this analysis (Fig. 1). We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines to report our findings.
Fig. 1. Patient selection. Two hundred forty-one patients with newly diagnosed ovarian cancer were seen between 7/1/2015 and 12/1/2017 and recommended to receive NACT. Of these, 224 were included in this analysis.
NACT, neoadjuvant chemotherapy.
2. Data collection
Clinical data were abstracted and verified from the electronic medical record from March 1, 2018 to July 1, 2018 by 2 independent reviewers (YL and OF). Patients were identified prospectively via the center's ovarian database, which tracks all patients seen with an ovarian complaint from the time of initial visit. Patient age was defined in years from date of pathological diagnosis. Stage was defined at pathological diagnosis based on imaging using International Federation of Gynecology and Obstetrics (FIGO) staging [24]. Charlson comorbidity index (CCI), a composite score measuring comorbidities in 12 areas, which has been shown to be predictive of mortality [25,26], was calculated based on comorbidities present at pathological diagnosis. CA-125 levels, serum albumin levels, and Karnofsky performance score (KPS) were collected at the time of first chemotherapy treatment from the medical record. BRCA testing status and results were abstracted from the medical record. NACT regimens and doses were documented.
Dose reductions in NACT were defined as any reductions in chemotherapy occurring before IDS in the surgery group or change of therapy in the non-surgical group and included any of the following: 1) carboplatin area under the curve (AUC) <5; 2) weekly paclitaxel <80 mg/m2; 3) every 3-week paclitaxel <175 mg/m2; or 4) any single-agent treatment for one or more treatment cycles. They were further categorized as baseline dose reductions or dose reductions occurring after the first cycle but before IDS in the surgical group or therapy change in the non-surgical group. Toxicities were extracted from the medical oncology notes during NACT and were further categorized into 1) neuropathy, 2) myelosuppression, or 3) other.
Surgical status was defined based on having undergone or not having undergone IDS at any point during treatment with first-line chemotherapy. Surgical and medical oncology notes were reviewed to determine the indication for NACT and primary reason for deferring IDS. Indications for NACT were categorized into the following groups: 1) extent of disease not amenable to surgery; 2) patient comorbidity preventing surgery; 3) both extent of disease and patient comorbidity; or 4) other, which was mostly due to venous thromboembolic disease. There were no standardized criteria to determine extent of disease; however, both imaging and/or laparoscopic assessment were used by the surgeon to make that determination. The decision to proceed with IDS after NACT was based on response to preoperative chemotherapy seen on interval computerized tomography (CT) scan and was left to the discretion of the treating surgeon. Reasons for not undergoing IDS were categorized into 1 of 5 categories: death during NACT, inadequate response to NACT, patient comorbidities, patient refusal, or other. Those with inadequate response were further characterized as having 1) platinum-refractory disease/progression if they experienced disease progression via CT imaging during NACT, 2) stable/mixed response if CT imaging showed stable disease or overall mixed response during NACT, or 3) a response insufficient for surgery if CT imaging during NACT showed any response as documented by the electronic medical record but were still ineligible for surgery. In general, our institution's practice is to continue chemotherapy for platinum-refractory disease rather than perform debulking surgery. For those with patient comorbidities as their reason for deferring IDS, the specific, major medical contraindication was collected. Cause of death was also collected and defined as disease-related, NACT-related, or other. All research was conducted under MSK Institutional Review Board protocol 17-430.
3. Statistical analysis
The Fisher exact and Wilcoxon rank-sum tests were used to compare clinical characteristics based on surgical status. Reasons for not undergoing surgery and their frequencies were reported. Overall survival (OS) was defined from date of pathologic diagnosis to death (all-cause) or last follow-up for those still alive. The Kaplan-Meier method was used to estimate the median OS and OS rate. The log-rank test and Cox proportional hazards (CoxPH) model were used to assess the relationship of covariates to outcome. The CoxPH model with time-dependent covariates was applied to variables collected after diagnosis including surgery versus no surgery. This methodology precluded generation of survival curves or estimations of median OS/OS rate by surgical status using the Kaplan-Meier method.
RESULTS
1. Patient characteristics
Of the 224 women identified, 162 (72%) had undergone IDS and 62 (28%) had not. Of the 224, 40 (18%) women underwent diagnostic laparoscopy with or without biopsy at MSK prior to initiation of NACT. Of those, 36 (90%) women went on to interval debulking, and 4 (10%) women did not undergo surgery. The non-surgical group was older (median age, 76.5 vs. 66 years; p<0.001) and more likely to have disease of histology other than high-grade serous (p=0.05). The 2 groups had similar stage at diagnosis (p=0.52). The non-surgical group also had a higher CCI (p<0.001), lower albumin level (p=0.007), and lower KPS (p<0.001) at the initiation of NACT. There were no differences in CA-125 levels at the start of chemotherapy between the groups (p=0.29). Indications for NACT were significantly different amongst the 2 groups. A NACT approach for patients in the surgical group was more likely due to disease extent, whereas in the non-surgical group, it was due to patient comorbidity or both disease extent and patient comorbidity (p<0.001; Table 1).
Table 1. Patient characteristics by surgery status.
| Variable | Total (n=224) | No Surgery (n=62) | Surgery (n=162) | p* | |
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| Median (mean) | 69 (68.1) | 76.5 (74.5) | 66 (65.7) | ||
| Range | 42–92 | 42–92 | 43–87 | ||
| Race (12 missing) | 0.69 | ||||
| White | 173 (82%) | 47 (84%) | 126 (81%) | ||
| Non-white | 39 (18%) | 9 (16%) | 30 (19%) | ||
| CA-125 | 0.29 | ||||
| Median (mean) | 964 (3,272) | 590 (3,426) | 1,135 (3,218) | ||
| Range | 4–38,600 | 18–34,200 | 4–38,600 | ||
| Histology | 0.05 | ||||
| High-grade serous | 211 (94%) | 55 (89%) | 156 (96%) | ||
| Other | 13 (6%) | 7 (11%) | 6 (4%) | ||
| Clinical stage at diagnosis | 0.52 | ||||
| III | 66 (29%) | 16 (26%) | 50 (31%) | ||
| IV | 158 (71%) | 46 (74%) | 112 (69%) | ||
| KPS at cycle 1 (65 missing) | <0.001 | ||||
| Median (mean) | 80 (78) | 70 (71.3) | 80 (80.7) | ||
| Range | 40–100 | 40–90 | 50–100 | ||
| BRCA mutations | <0.001 | ||||
| BRCA 1 or 2 mutation | 35 (16%) | 7 (11%) | 28 (17%) | ||
| No mutation | 128 (57%) | 25 (40%) | 103 (64%) | ||
| No recorded testing | 61 (27%) | 30 (48%) | 31 (19%) | ||
| Albumin at cycle 1 (1 missing) | 0.007 | ||||
| Median (mean) | 3.7 (3.6) | 3.5 (3.4) | 3.8 (3.7) | ||
| Range | 1.8–4.9 | 1.8–4.6 | 2.3–4.9 | ||
| CCI | <0.001 | ||||
| Median (mean) | 9 (8.9) | 10 (10.2) | 8 (8.5) | ||
| Range | 6–14 | 6–14 | 6–12 | ||
| Indication for NACT | <0.001 | ||||
| Extent of disease | 130 (58%) | 17 (27%) | 113 (70%) | ||
| Patient comorbidities | 23 (10%) | 15 (24%) | 8 (5%) | ||
| Both | 61 (27%) | 29 (47%) | 32 (20%) | ||
| Other | 10 (5%) | 1 (2%) | 9 (5%) | ||
| NACT: weekly paclitaxel/carboplatin | <0.001 | ||||
| Yes | 155 (69%) | 32 (52%) | 123 (76%) | ||
| No† | 69 (31%) | 30 (48%) | 39 (24%) | ||
| Dose reductions before IDS/therapy change | <0.001 | ||||
| Yes | 84 (38%) | 47 (76%) | 37 (23%) | ||
| No | 140 (62%) | 15 (24%) | 125 (77%) | ||
| Cycles before IDS/therapy change | <0.001 | ||||
| Median (mean) | 4 (4.5) | 6 (5.3) | 4 (4.2) | ||
| Range | 1–18 | 1–18 | 2–7 | ||
CA-125, cancer antigen 125; CCI, Charlson comorbidity index; IDS, interval debulking surgery; KPS, Karnofsky performance status; NACT, neoadjuvant chemotherapy.
*Obtained using the Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables; ¢ÓIncludes paclitaxel/carboplatin every 3 weeks, IV/IP chemotherapy, and other (13 single-agent carboplatin, 1 carboplatin/liposomal pegylated doxorubicin, 1 carboplatin/gemcitabine, and 1 weekly paclitaxel/carboplatin + nivolumab on protocol).
The majority of women (69%) were treated with weekly paclitaxel/carboplatin. Forty-six women (21%) were treated with every 3-week paclitaxel/carboplatin, and 7 (3%) were treated with combination intravenous and intraperitoneal therapy. The remaining 16 women received other regimens, including single-agent carboplatin (n=13), carboplatin/liposomal pegylated doxorubicin (n=1), carboplatin/gemcitabine (n=1), and weekly paclitaxel/carboplatin + nivolumab on protocol (n=1). Only 6 patients in the surgery group who initially received platinum/taxane-based therapy had their NACT regimen changed; 3 were switched to carboplatin/gemcitabine, and 3 were switched to carboplatin/liposomal pegylated doxorubicin. The non-surgical group was more likely to receive a regimen other than weekly paclitaxel/carboplatin (p<0.001). Ten women received NACT regimens that included bevacizumab, and of these, 8 were in the surgery group and 2 were in the non-surgical group. Sixteen women (14 in the surgical group and 2 in the non-surgical group) received poly (ADP-ribose) polymerase (PARP) versus placebo maintenance after chemotherapy on protocol.
In those treated with weekly paclitaxel/carboplatin as compared with other regimens, there were no differences in proportion of patients experiencing toxicity during NACT (30% vs. 36% respectively; p=0.439). In those experiencing toxicities, rates of neuropathy were similar between those receiving weekly paclitaxel/carboplatin compared with other regimens (49% vs. 48%). Although the weekly paclitaxel/carboplatin group had higher “other” toxicities (36% vs. 16%) and the other NACT regimens were associated with more myelosuppression (36% vs. 15%), these differences were not significant (p=0.072). Median cycles of NACT prior to IDS or change due to progression in the whole cohort was 4 (range, 1–18) and was significantly higher in the non-surgical group (median, 6; range, 1–18) compared to the surgical group (median, 4; range, 2–7), p<0.001. Patients in the non-surgical group were more likely to have dose reductions in NACT, and these dose reductions were more likely to occur at baseline (p<0.001) (Table 1).
2. Rationale for not undergoing IDS
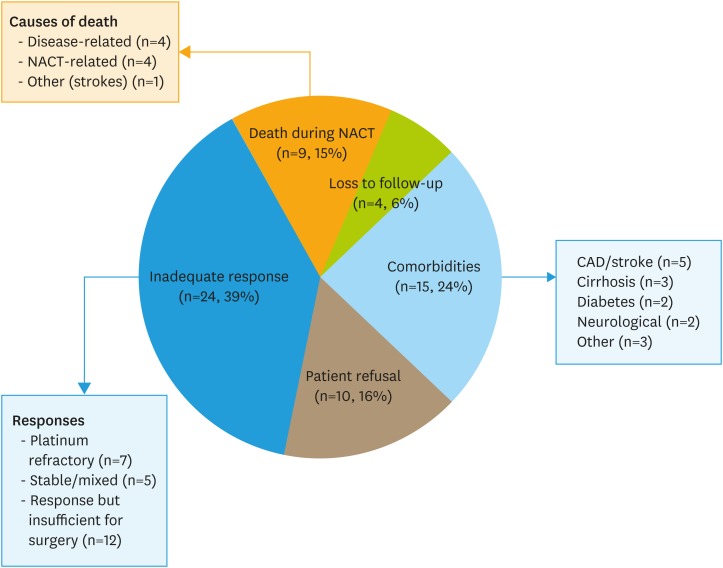
Reasons for not undergoing surgery included poor response to NACT (39%), death during NACT (15%), comorbidities (24%), patient preference (16%), and loss to follow-up (6%). Of the 9 women who died during NACT, 4 died of complications of their disease, 4 died due to treatment-related complications (neutropenic sepsis), and 1 died of another reason (stroke). Of those with a poor response to chemotherapy, 7 were platinum refractory and experienced disease progression during NACT, 5 had stable disease or a mixed response to NACT, and 12 had at least a partial response to NACT, but their burden of disease was still too great to outweigh the risks of surgery. In those in whom comorbidities prohibited surgery, most were due to cardiovascular disease or stroke, cirrhosis/liver disease, severe diabetes, or neurological compromise (Fig. 2).
Fig. 2. Reasons for not receiving surgery. Reasons for not receiving surgery included inadequate response to NACT (39%), including death during NACT (15%), baseline patient comorbidities (24%), patient refusal (16%), and loss to follow-up (6%).
NACT, neoadjuvant chemotherapy.
3. Survival analysis
Median follow-up was 16.3 months (range, 0.8–37.8 months). Among all 244 women, there were 60 deaths. The median OS was not reached, and the 1-year OS rate was 86.1% (95% confidence interval [CI]=80.4%–90.2%). There were 32 observed deaths in the non-surgical group and 28 observed deaths in the surgical group. Time-dependent analysis of the surgical variable prohibited generation of stratified median OS, 1-year OS rates and survival curves.
On univariate analysis, age, CCI, KPS, albumin, and dose reductions in NACT were significantly associated with OS (p<0.05) and were included in multivariate models examining associations between surgical status and OS. CCI, KPS, and albumin were significantly correlated (p=0.01), and as the KPS and albumin variables had significant amounts of missing data, CCI was used in multivariate models. Stage at diagnosis, presence of BRCA 1/2 mutations, and choice of NACT (weekly paclitaxel/carboplatin vs. other) were not significantly associated with OS (Table 2).
Table 2. Univariate overall survival analysis.
| Variables | No. | Deaths | HR (95% CI) | p | |
|---|---|---|---|---|---|
| Entire cohort | 224 | 60 | - | - | |
| Age (by 5-year increase) | - | - | 1.19 (1.05–1.34) | 0.005 | |
| CCI (1-unit increase) | - | - | 1.32 (1.13–1.55) | <0.001 | |
| BRCA 1 or 2 mutation | 0.067* | ||||
| Negative | 128 | 30 | 1 | ||
| Positive | 35 | 4 | 0.39 (0.14–1.11) | ||
| Stage | 0.066* | ||||
| III | 66 | 12 | 1 | ||
| IV | 158 | 48 | 1.8 (0.95–3.39) | ||
| KPS at cycle 1 (1-unit increase)† | - | - | 0.96 (0.93–0.98) | <0.001 | |
| Albumin at cycle 1 (1-unit increase)† | - | - | 0.29 (0.18–0.47) | <0.001 | |
| NACT regimen† | 0.207 | ||||
| Weekly paclitaxel/carboplatin | 155 | 40 | 1 | ||
| Other | 69 | 20 | 1.42 (0.82–2.43) | ||
| Dose reduction† | 0.006 | ||||
| No | 140 | 32 | 1 | ||
| Yes | 84 | 28 | 2.03 (1.22–3.38) | ||
| Surgery (IDS)† | <0.001 | ||||
| Yes | 162 | 28 | 1 | ||
| No | 62 | 32 | 3.99 (2.31–6.89) | ||
CCI, Charlson comorbidity index; CI, confidence interval; HR, hazard ratio; IDS, interval debulking surgery; KPS, Karnofsky performance status; NACT, neoadjuvant chemotherapy.
*Obtained using the log-rank test; all the other p-values were obtained using the Cox proportional hazards model; ¢ÓThese variables (KPS, albumin, NACT regimen, dose reduction, and IDS) were modeled through time-dependent methodology.
In multivariate models, the non-surgical group compared with the surgical group had significantly worse OS (hazard ratio [HR]=3.34; 95% CI=1.66–6.72; p<0.001) after adjustment for age, CCI, and dose reductions (Table 3).
Table 3. Multivariate overall survival analysis.
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Age (by 5-year increase) | 0.987 | 0.821–1.188 | 0.8925 |
| CCI (1-unit increase) | 1.173 | 0.898–1.53 | 0.2414 |
| Dose reduction in NACT (Yes vs. No) | 0.872 | 0.436–1.744 | 0.698 |
| No surgery vs. surgery | 3.344 | 1.664–6.72 | <0.001 |
All variables with a p-value <0.05 in the univariate setting were considered in the multivariate model (Karnofsky performance status and albumin were not considered due to high correlation with CCI and missing data).
CCI, Charlson comorbidity index; CI, confidence interval; HR, hazard ratio; NACT, neoadjuvant chemotherapy.
DISCUSSION
In our study, a significant proportion of the women undergoing treatment with NACT for newly diagnosed ovarian cancer did not undergo debulking surgery. These women were older, frailer, and more likely to have dose reductions in NACT compared to the surgical group. The most common reasons for not undergoing surgery were poor response to NACT, including death during NACT, patient comorbidities, and preferences. For the non-surgical group, there was a >3-fold increase in all-cause mortality, even after adjustment for age, comorbidities, and dose reductions in NACT.
In the European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group study, 670 women with ovarian cancer, mostly with stage IIIC/IV disease, were randomized to NACT followed by IDS or PDS followed by chemotherapy. In the intention-to-treat (ITT) analysis, NACT was non-inferior to PDS with respect to OS (HR=0.98; 90% CI=0.84–1.13; p=0.01 for non-inferiority). Of those randomized to the NACT arm (n=334), 326 (98%) received NACT and 295 (88%) underwent IDS. Of the 336 randomized to PDS, 315 (94%) underwent surgery [10]. The CHORUS study randomized 552 women to NACT and IDS or PDS followed by chemotherapy. An ITT analysis found that NACT/IDS was non-inferior with respect to OS (HR=0.87; 90% CI=0.72–1.05), with a median OS of 22.6 months in the PDS group compared with 24.1 months in the NACT group. Of the 274 women randomized to NACT, 253 (92%) received primary chemotherapy and 217 (79%) underwent IDS. Of the 276 assigned to PDS, 251(91%) underwent surgery [8].
In our single-center retrospective review, we found a lower IDS rate (72%), with 28% of women receiving NACT but not undergoing surgery. This may reflect a higher burden of disease at diagnosis and more comorbidities in this non-clinical trial population. In our study population, 71% of all the women had stage IV disease at diagnosis, and our median CCI was 9 for the entire cohort. Stage at diagnosis, however, did not differ by surgical status in our cohort. In addition, as we are a tertiary geriatric referral center, many of our patients (46%) were older than 70 years of age, and our oldest patient was 92 in the non-surgical group and 87 in the surgical group.
Our IDS rate is higher than that of a recently published study using Surveillance, Epidemiology and End Results-linked Medicare data to examine 9,016 women with ovarian cancer treated from 2002–2011, which found an IDS rate of 39% in the 2,638 women who received NACT [27]. The median OS for the no-surgery group was 10 months compared to 36.3 months for the surgery group, which supports our finding of a >3-fold risk in mortality in the no-surgery group independent of other clinical factors. Our higher IDS rate may represent more specialized care at a National Cancer Institute-designated cancer center and practice modifications to facilitate surgery. For example, our institution does not limit preoperative NACT cycles, and the median number of preoperative cycles was 4 (range, 2–7). In addition, their study represented a heterogeneous population and found racial differences in surgery, with a higher proportion of White patients receiving surgery compared to Blacks and Hispanics. Our population is predominantly White (82%), which may contribute to our higher IDS rate.
Among the non-surgical group, the majority (55%) had a poor response to NACT. Of those, half had primary refractory disease and either died or progressed during NACT. These patients likely represent a group with a different and aggressive underlying disease phenotype with a poor prognosis at baseline. They may derive the most benefit from the addition of novel therapies or clinical trials, including bevacizumab [28] and checkpoint inhibitors [29], in the frontline setting, as our study revealed limited incorporation of these therapies into NACT.
The remaining women experienced stable disease or some response to NACT; however, their burden of disease was still considered too extensive to warrant a debulking surgery. Of the remaining women, the majority were not recommended for debulking surgery due to underlying comorbidities, mostly cardiovascular disease or liver disease. These patients likely would not have been candidates for PDS. A large portion of patients (16%) chose to not pursue surgery. Most of these patients cited fear of surgical risk or wanting to maintain quality of life as their main reason for deferring surgery. Finally, a small proportion of patients (6%) were lost to follow-up and potentially decided to pursue treatment with a local oncologist.
The non-surgical group was also on average 8.8 years older than the surgical group, and many studies have identified age as an important prognostic factor [15,30]. Although 46% of the entire cohort was age 70 or older, 74% of patients in the non-surgical group was 70 years of age or older compared with 36% in the surgery group (p<0.001). GOG study 273 looked at 212 women aged 70 or older treated with either paclitaxel every 3 weeks and carboplatin or carboplatin alone as NACT or after PDS. In the group receiving NACT, only 53% went on to undergo surgery. Although the non-surgical group had lower OS and progression-free survival on univariate analysis compared to the surgical group, this difference disappeared after adjustment for performance status, stage, age, and treatment regimen [19].
In contrast, our study found that the non-surgical group had a >3-fold increase in mortality. This was independent of age, comorbidity as represented by CCI, and reductions in NACT on multivariate analysis. This suggests that although these patients were older, sicker and received different treatments, these factors do not completely explain the increase in mortality and that surgery may offer a mortality benefit. Our study differed from GOG study 273 in that we included women under 70 years of age, and the majority of women in both groups (surgical and non-surgical) were treated with weekly paclitaxel and carboplatin and received more than 4 cycles of treatment. This more aggressive treatment, coupled with surgery in a younger population, may explain the survival benefit of treatment plans that include surgery.
Our findings that comorbidity, as assessed by CCI, KPS and albumin, is prognostic for survival is substantiated by multiple studies in the literature [15,18,30]. Unlike other studies [17], CA-125 was not significantly different between the two groups, although those who underwent surgery had a higher median CA-125 level. Although the non-surgical group was more likely to have disease of histology other than high-grade serous, the overall number of other histologies was small. This association should be investigated further in larger studies. Body mass index at initiation of NACT did not vary between the 2 groups (rank sum p=0.8; data not shown). Stage at diagnosis has been shown to be a robust predictor of survival [31], and even though stage was not significantly predictive of survival in our study, the hazard ratio and p-value show a trend towards worse survival for those with stage IV disease.
Although the surgical group was more likely to receive weekly paclitaxel and carboplatin over other regimens, this regimen was not associated with improved OS. However, dose reductions in NACT were more likely to occur in the non-surgical group (p<0.001), and they were significantly associated with worse OS. For the non-surgical group, these dose reductions were more likely to occur at baseline (58% vs. 11%; p<0.001).
Overall, 16% of women had a BRCA 1 or 2 mutation, and the presence of a mutation showed a potential trend towards improved OS (p=0.067), which is consistent with the literature [32]. Although guidelines recommend BRCA testing in all women diagnosed with ovarian cancer [32], the non-surgical group had more women with no recorded testing (48%) compared with the surgical group (19%). The cause of this is unclear but should be investigated further, particularly given the growing use of targeted therapies such as PARP inhibitors in the frontline setting [33,34] and the need for earlier BRCA testing.
Future studies should focus on strategies to increase the proportion of women who can undergo IDS and optimize medical therapies in those who cannot undergo IDS. Our study revealed that a large majority of these women had primary refractory disease or progressed early on during NACT, and these women may be salvaged by the addition of bevacizumab or other novel agents to their NACT. Early BRCA testing may also provide opportunities for targeted therapies as well. Others were medically frail or refused surgery, and efforts to improve medical optimization, particularly in the geriatric population are needed. A recent study highlighted the importance of phenotypic frailty measurements in selection of appropriate and potentially less aggressive chemotherapy regimens [35].
In addition, some women, particularly older, frailer patients with more comorbidities, may truly not benefit from surgery. OV1741 is an NRG-sponsored concept seeking to randomize women 70 years or older with newly diagnosed ovarian cancer receiving NACT to surgery or no surgery after 3 cycles of NACT. This study, which will assess survival, should help to definitively answer the question of the benefit of surgery in this older, more comorbid population.
In our single-center, retrospective review, a significant proportion of women receiving NACT did not undergo IDS. These women were older, had more comorbidities, and received reduced doses of NACT. Even after adjusting for these factors, there was a >3-fold increase in mortality for the non-surgical group. Future studies should focus on the early identification of these women and the optimization of their medical treatments.
ACKNOWLEDGEMENTS
We would like to thank the ovarian database team and all the women who underwent treatment at Memorial Sloan Kettering Cancer Center (MSK).
Footnotes
Funding: Memorial Sloan Kettering Cancer Center is supported in part by the National Institutes of Health/National Cancer Institute Core grant P30 CA008748.
Conflict of Interest: There are no potential conflicts of interest related to this work. Outside the submitted work, Dr. Iasonos reports personal fees from Mylan; Dr. Chi reports personal fees from Bovie Medical Co. (medical advisory board and stock options), Verthermia Inc. (medical advisory board and stock options), C Surgeries (shareholder), and Intuitive Surgical Inc (stock ownership); Dr. O'Cearbhaill reports personal fees from Clovis (medical advisory board) and Tesaro (medical advisory board); Dr. Konner reports personal fees from Clovis (guest speaker), AstraZeneca (medical advisory board), and Immunogen (medical advisory board); Dr. Aghajanian reports personal fees from Tesaro (medical advisory board), Immunogen (medical advisory board), Mateon Therapeutics (steering committee) and Cerulean Pharma (medical advisory board), grants and personal fees from Clovis (medical advisory board) and Genentech (steering committee), and grants from AbbVie (steering committee) and Astra Zeneca; and Dr. Long Roche reports personal fees from Intuitive Surgical (travel). The other authors have no potential conflicts of interest to disclose.
- Conceptualization: L.Y.L., F.O.T., Z.Q., I.A., C.D.S., Z.O., S.Y., G.G.J., B.V.A., O'C.R.E., K.J.A., A.C., L.R.K., T.W.P.
- Data curation: L.Y.L., F.O.T., Z.Q., I.A., L.R.K.
- Formal analysis: Z.Q., I.A.
- Funding acquisition: L.R.K., T.W.P.
- Investigation: L.Y.L., F.O.T., Z.Q., I.A., C.D.S., Z.O., S.Y., G.G.J., B.V.A., O'C.R.E., K.J.A., L.R.K., T.W.P.
- Methodology: L.Y.L., F.O.T., Z.Q., I.A., C.D.S., Z.O., S.Y., G.G.J., B.V.A., O'C.R.E., K.J.A., A.C., L.R.K., T.W.P.
- Project administration: L.Y.L., F.O.T., L.R.K., T.W.P.
- Resources: L.R.K., T.W.P.
- Software: L.Y.L., F.O.T., Z.Q., I.A.
- Supervision: L.R.K., T.W.P.
- Validation: L.Y.L., F.O.T., Z.Q., I.A.
- Visualization: L.Y.L., L.R.K., T.W.P.
- Writing - original draft: L.Y.L., T.W.P.
- Writing - review & editing: L.Y.L., F.O.T., Z.Q., I.A., C.D.S., Z.O., S.Y., G.G.J., B.V.A., O'C.R.E., K.J.A., A.C., L.R.K., T.W.P.
References
- 1.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 2.Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 5.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Wallace S, Kumar A, Mc Gree M, Weaver A, Mariani A, Langstraat C, et al. Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol. 2017;145:21–26. doi: 10.1016/j.ygyno.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 9.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 11.Kang S. Neoadjuvant chemotherapy for ovarian cancer: do we have enough evidence? Lancet. 2015;386:223–224. doi: 10.1016/S0140-6736(14)62259-5. [DOI] [PubMed] [Google Scholar]
- 12.Mueller JJ, Zhou QC, Iasonos A, O'Cearbhaill RE, Alvi FA, El Haraki A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140:436–442. doi: 10.1016/j.ygyno.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol Oncol. 2016;143:3–15. doi: 10.1016/j.ygyno.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer LA, He W, Sun CC, Zhao H, Wright AA, Suidan RS, et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: rates of use and effectiveness. Gynecol Oncol. 2018;150:451–459. doi: 10.1016/j.ygyno.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suidan RS, Leitao MM, Jr, Zivanovic O, Gardner GJ, Long Roche KC, Sonoda Y, et al. Predictive value of the age-adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2015;138:246–251. doi: 10.1016/j.ygyno.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tinquaut F, Freyer G, Chauvin F, Gane N, Pujade-Lauraine E, Falandry C. Prognostic factors for overall survival in elderly patients with advanced ovarian cancer treated with chemotherapy: results of a pooled analysis of three GINECO phase II trials. Gynecol Oncol. 2016;143:22–26. doi: 10.1016/j.ygyno.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Won E, Hurria A, Feng T, Mohile S, Owusu C, Klepin HD, et al. CA125 level association with chemotherapy toxicity and functional status in older women with ovarian cancer. Int J Gynecol Cancer. 2013;23:1022–1028. doi: 10.1097/IGC.0b013e318299438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Liu N, Zhang A, Bao X. Potential risk factors associated with prognosis of neoadjuvant chemotherapy followed by interval debulking surgery in stage IIIc-IV high-grade serous ovarian carcinoma patients. J Obstet Gynaecol Res. 2018;44:1808–1816. doi: 10.1111/jog.13710. [DOI] [PubMed] [Google Scholar]
- 19.von Gruenigen VE, Huang HQ, Beumer JH, Lankes HA, Tew W, Herzog T, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer - an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;144:459–467. doi: 10.1016/j.ygyno.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips A, Sundar S, Singh K, Nevin J, Elattar A, Kehoe S, et al. Complete cytoreduction after five or more cycles of neo-adjuvant chemotherapy confers a survival benefit in advanced ovarian cancer. Eur J Surg Oncol. 2018;44:760–765. doi: 10.1016/j.ejso.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 21.Cioffi R, Bergamini A, Rabaiotti E, Petrone M, Pella F, Ferrari D, et al. Neoadjuvant chemotherapy in high-risk ovarian cancer patients: role of age. Tumori. 2019;105:168–173. doi: 10.1177/0300891618792468. [DOI] [PubMed] [Google Scholar]
- 22.Son JH, Chang K, Kong TW, Paek J, Chang SJ, Ryu HS. A study of clinicopathologic factors as indicators for early prediction of suboptimal debulking surgery after neoadjuvant chemotherapy in advanced ovarian cancer. J Obstet Gynaecol Res. 2018;44:1294–1301. doi: 10.1111/jog.13653. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Deng W, Tew W, Bender D, Mannel RS, Littell RD, et al. Pre-operative assessment and post-operative outcomes of elderly women with gynecologic cancers, primary analysis of NRG CC-002: An NRG oncology group/gynecologic oncology group study. Gynecol Oncol. 2018;150:300–305. doi: 10.1016/j.ygyno.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prat J FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 27.Taylor JS, He W, Harrison R, Zhao H, Sun CC, Lu KH, et al. Disparities in treatment and survival among elderly ovarian cancer patients. Gynecol Oncol. 2018;151:269–274. doi: 10.1016/j.ygyno.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YL, Zamarin D. Combination immune checkpoint blockade strategies to maximize immune response in gynecological cancers. Curr Oncol Rep. 2018;20:94. doi: 10.1007/s11912-018-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. 2012;125:25–30. doi: 10.1016/j.ygyno.2011.12.423. [DOI] [PubMed] [Google Scholar]
- 31.van de Laar R, IntHout J, Van Gorp T, Verdonschot S, van Altena AM, Gerestein CG, et al. External validation of three prognostic models for overall survival in patients with advanced-stage epithelial ovarian cancer. Br J Cancer. 2014;110:42–48. doi: 10.1038/bjc.2013.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiderpass E, Tyczynski JE. Epidemiology of patients with ovarian cancer with and without a BRCA1/2 mutation. Mol Diagn Ther. 2015;19:351–364. doi: 10.1007/s40291-015-0168-x. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Martin A, Backes FJ, Baumann KH, Chase DM, Fehr MK, Coleman RL, et al. A randomized, double-blind phase III trial of niraparib maintenance treatment in patients with HRD+ advanced ovarian cancer after response to front-line platinum-based chemotherapy. J Clin Oncol. 2016;34(15 suppl):TPS5606 [Google Scholar]
- 34.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 35.Hay CM, Donovan HS, Campbell GB, Taylor SE, Wang L, Courtney-Brooks M. Chemotherapy in older adult gynecologic oncology patients: can a phenotypic frailty score predict tolerance? Gynecol Oncol. 2019;152:304–309. doi: 10.1016/j.ygyno.2018.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]