Abstract
Based on emerging data and current knowledge regarding high-risk human papillomavirus (hrHPV) testing as a primary screening for cervical cancer, the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology support the following scientific facts:
• Compared to cytology, hrHPV screening has higher sensitivity and detects more cases of high-grade cervical intraepithelial neoplasia.
• Qualified hrHPV testing can be considered as an alternative primary screening for cervical cancer to the current cytology method.
• The starting age of primary hrHPV screening should not be before 25 years because of possible overtreatment in this age, which has a high human papillomavirus (HPV) prevalence but rarely progresses to cancer. The screening interval should be no sooner than every 3 years and no longer than every 5 years.
• Before the introduction of hrHPV screening in Korea, research into comparative effectiveness of primary hrHPV screening for cervical cancer should be conducted to determine the appropriate HPV assay, starting age, and screening interval.
Keywords: Uterine Cervical Neoplasms, Cancer Screening Tests, Human Papillomavirus DNA Tests
HIGH-RISK HUMAN PAPILLOMAVIRUS (hrHPV) INFECTION AND CARCINOGENESIS IN THE CERVIX UTERI
The human papillomaviruses (HPVs) consist of a heterogeneous group of capsid-enclosed double-stranded DNA viruses from the Papillomaviridae family that have a histological tropism for squamous epithelium [1]. The HPV genome is composed of the following 3 major regions: the early (E) region encoding nonstructural proteins, the late (L) region encoding the 2 capsid proteins, and the noncoding long control region that regulates viral replication and gene expression [2,3]. E5, E6, and E7 directly promote cellular transformation and alter pathways related to the immune response. The most notable activity of E6 is degradation of the tumor suppressor protein p53 via the proteasome pathway, and the E7 protein binds to the hypophosphorylated form of retinoblastoma protein and promotes its degradation via the ubiquitin-proteasome pathway.
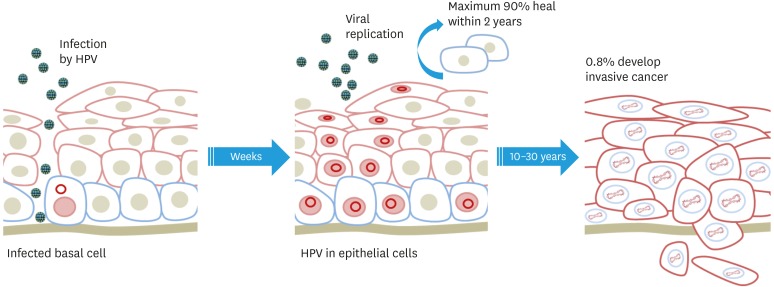
Recently, more than 170 HPV types have been isolated and characterized [4]. Among them, the International Agency for Research on Cancer Monographs classified these hrHPV (HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, and HPV59) as group 1 carcinogens for cervical cancer [5,6,7]. HPV16 and HPV18 are the most common carcinogenic types within this group and are responsible for approximately 50% and 20% of cervical cancer, respectively [8]. The major steps in cervical carcinogenesis are HPV infection in cervical basal cells, progression to a precancerous lesion, and cancer invasion (Fig. 1).
Fig. 1. Progression of cervical disease after human papillomaviruses infection.
HPV, human papillomavirus.
SCREENING STRATEGIES FOR HPV-ASSOCIATED CERVICAL DISEASE
Cervical cancer is the 4th most frequent cancer and the 4th leading cause of cancer death in women, with an estimated 570,000 cases and 311,000 deaths in 2018 worldwide [9]. In Korean women, cervical cancer is the 7th most common malignancy, and the incidence rate is still higher than that in other developed countries [10]. Based on cytology-based screening (Papanicolaou smear or liquid-based cytology) for cervical cancer, the incidence and associated mortality of cervical cancer have continued to decrease worldwide [11,12,13]. Since the introduction of the national cervical cancer screening program in 1999 in Korea, the incidence rate of cervical cancer has also steadily decreased from 16.3/100,000 to 9.1/100,000 in 2015 [14]. In 2015, the National Cervical Cancer Screening Guideline Development Committee, which is composed of experts from the Korean Society of Gynecologic Oncology, the Korean Society for Cytopathology, the Korean Society for Preventive Medicine, and the Korean Academy of Family Medicine, recommended a cytology-based screening for cervical cancer every 3 years in women older than 20 years old [15]. However, this cytology-based screening has a sensitivity of 51%–53% in detecting high-grade cervical intraepithelial neoplasia (CIN) [16,17,18].
Cytology depends on the morphological analysis of cervical exfoliated cells. Compared to cytology, HPV testing does not depend on morphological analysis and is commonly based on the detection of HPV DNA or mRNA. With the understanding of the causal relationship between hrHPV infection and cervical carcinogenesis, the Atypical Squamous Cells of Undetermined Significance/Low Grade Squamous Intraepithelial Lesion Triage Study showed that reflex hrHPV testing of the cytological category of “atypical squamous cells of undetermined significance” (ASCUS) resulting in a triage of colposcopy can be a feasible alternative to cytology alone [19]. The HPV triage test has been considered the preferred management for women with ASCUS on cytology since the early 2000s [20]. In 2003, the US Food and Drug Administration (US-FDA) approved the use of HPV testing as a reflex test in women over 21 years with ASCUS and as an adjunctive test in women over 30 years. Currently, the US-FDA approves the use of Cobas® HPV (Roche Diagnostics, Basel, Switzerland) and Onclarity® HPV (BD, Franklin Lakes, NJ, USA) as primary screening tests for cervical cancer. In Korea, Cobas® HPV has been approved as a primary screening test for cervical cancer. However, in 2015, the National Cervical Cancer Screening Guideline Development Committee stated that the existing evidence regarding the advantages and disadvantages of primary HPV testing is very low and the level of evidence regarding the effects of HPV/cytology co-testing is moderate [15].
SCIENTIFIC EVIDENCE OF hrHPV TESTING AS A PRIMARY SCREENING
The first randomized controlled trial (RCT) found that HPV screening had a higher sensitivity (95%) in detecting high-grade CIN than cytology (55%) [21]. RCTs discussed in this study are summarized in Table 1. Four European RCTs (Swedescreen, Population-Based Screening Study Amsterdam, A Randomized Trial in Screening to Improve Cytology [ARTISTIC], and New Technologies for Cervical Cancer Screening [NTCC]) showed that earlier HPV-based screening in patients detects persistent high-grade CIN with higher sensitivity than cytology, thus the incidence of high-grade CIN was lower after HPV screening than after cytology [22,23,24,25,26,27,28,29]. Furthermore, a pooled analysis of these European RCTs found that HPV screening provides 60%–70% greater protection against cervical cancers than cytology [30]. In the USA, the Addressing THE Need for Advanced HPV Diagnostics (ATHENA) trial showed higher sensitivity in detecting CIN3+ (CIN3 and cervical cancer) in the HPV primary strategy group than in the cytology strategy group (76.1% vs. 47.8%) in 2015 [31]. Women in the HPV primary strategy group underwent colposcopy if they were found positive for HPV16 or HPV18 (or underwent reflex cytology if other types of HPV were positive) in the ATHENA trial.
Table 1. Results of randomized controlled trials of high-risk human papillomavirus screening, with or without co-testing.
| Study | No. of participants | Ages included (yr) | Screening interval (yr) | Arms | Criteria for immediate colposcopy | Absolute detection (%) | Colposcopy referral rate (%) | Follow-up period (maximum, yr) | |
|---|---|---|---|---|---|---|---|---|---|
| CIN3+ | Cancer | ||||||||
| Swedescreen [22] | 12,527 | 32–38 | 3 | Conventional cytology | ASCUS+ | 55/6,270 (0.9) | NR | NR | Mean: 4.1 |
| hrHPV with conventional cytology | ASCUS+ | 72/6,257 (1.2) | NR | NR | |||||
| POBASCAM [23,24,25] | 44,938 | 29–61 | 5 | Conventional cytology | HSIL+ | 150/20,106 (0.7) | 6/20,109 (0.03) | NR | 9.0 |
| hrHPV with conventional cytology | HSIL+ | 171/19,999 (0.9) | 12/19,999 (0.06) | NR | |||||
| ARTISTIC [26,27] | 24,510 | 20–64 | 3 | LBC | HSIL+ | 81/6,124 (1.3) | 4/6,124 (0.07) | 320/6,124 (5.2) | 4.5 |
| hrHPV with LBC | HSIL+ | 233/18,386 (1.3) | 5/18,386 (0.03) | 1,247/18,386 (6.8) | |||||
| NTCC [28,29] | 49,196 | 25–60 | 3 | Conventional cytology | ASCUS+ or LSIL+ | 33/24,535 (0.1) | NR | 679/25,435 (2.8) | 7.0 |
| hrHPV | hrHPV+ | 97/24,661 (0.4) | NR | 1,936/24,661 (7.9) | |||||
| HPV FOCAL [44,45,46,47] | 19,000 | 25–65 | 4 | LBC with hrHPV triage | ASCUS+ and hrHPV+ (or, for cytology only, ASC-H or LSIL+) | 41/9,408 (0.4) | NR | 290/9,408 (3.1) | 4.0 |
| hrHPV with LBC triage | hrHPV+ and ASCUS+ | 67/9,540 (0.7) | NR | 544/9,540 (5.7) | |||||
| FINNISH [48] | 203,425 | 25–65 | 5 | Conventional cytology | LSIL+ | 118/65,784 (0.2) | 9/65,784 (0.01) | 755/65,784 (1.1) | 5.0 |
| hrHPV with conventional cytology triage | hrHPV+ and LSIL+ | 195/66,410 (0.3) | 17/66,410 (0.03) | 796/66,410 (1.2) | |||||
| Compass [49] | 4,995 | 25–64 | 2.5 | LBC | ASC-H+/HSIL+ | 1/995 (0.1) | 0/995 (0) | 27/995 (2.7) | 2.5 |
| 5 | hrHPV with LBC triage | HPV16/18+, other hrHPV+ with LSIL or ASC-H+ or p16/Ki-67+ | 30/4,000 (0.8) | 0/4,000 (0) | 154/4,000 (3.8) | 5.0 | |||
| ATHENA [31] | 40,901 | ≥25 | 3 | Cytology | ASCUS+ or hrHPV+ | 179/45,156 (0.4) | NR | 1,934/45,156 (4.3) | 3.0 |
| HPV primary | 294/52,651 (0.6) | NR | 3,769/52,651 (7.2) | ||||||
| Hybrid strategy* | 240/82,994 (0.3) | NR | 3,097/82,994 (3.7) | ||||||
ARTISTIC, A Randomized Trial in Screening to Improve Cytology; ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; ATHENA, Addressing THE Need for Advanced HPV Diagnostics; CIN, cervical intraepithelial neoplasia; hrHPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; HPV, human papillomavirus; HPV FOCAL, Human Papillomavirus for Cervical Cancer Screening; LBC, liquid-based cytology; LSIL, low-grade squamous intraepithelial lesion; NR, not reported; NTCC, New Technologies for Cervical Cancer Screening; POBASCAM, Population-Based Screening Study Amsterdam.
*A hybrid strategy uses the cytology strategy for women 25–29 years old and co-testing with both cytology and HPV (pooled 14 genotypes) in women ≥30 years.
Three-year risks for CIN3+ following a negative result in hrHPV screening or HPV/cytology co-testing were significantly lower than those of cytology alone in the ATHENA trial and Gage's study [31,32]. A US Preventive Services Task Force (US-PSTF) systematic review also found that hrHPV screening detected CIN3+ with a higher rate than cytology. However, HPV/cytology co-testing did not increase the detection rate of CIN3+ [33]. Instead, hrHPV screening and HPV/cytology co-testing both increased the number of diagnostic colposcopies [33].
Randomized trials for primary HPV screening have not yet been published in Korea. In 2016, Choi et al. [34] retrospectively compared the clinical performance of primary HPV screening, HPV/cytology co-testing, and cytology alone using 1,000 cervical samples. The sensitivity was calculated using CIN2+ with colposcopy biopsy as the gold standard, and the sensitivities of primary HPV screening, HPV/cytology co-testing, and cytology alone were 71.7%, 72.5%, and 63.8%, respectively.
OTHER CURRENT RECOMMENDATIONS: STARTING AGE AND SCREENING INTERVAL
Guidelines for cervical cancer screening in several countries are summarized in Table 2. The National Institute for Public Health and the Environment in the Netherlands revealed that the HPV screening for women aged 30–60 years every 5 years was the primary screening method in their national cervical cancer screening program [35]. Moreover, in Australia, the Papanicolaou test performed biannually for women aged 18–69 years has been replaced by HPV screening performed once in 5 years for women aged 25–74 years [36]. However, the ideal starting age and screening interval of primary HPV screening are still under investigation. The Society for Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology issued interim guidelines recommending primary HPV screening as an acceptable approach in women 25–65 years old based on the ATHENA trial [37]. Although disease detection increases, there are concerns about potential disadvantages, such as unwarranted diagnostic colposcopies, of primary hrHPV screening before the age of 25 years. Several diseases detected in this age group can be safely treated up until the age of 30 years [38,39,40]. Therefore, general guidelines have recommended less aggressive management for cervical abnormalities in these age groups, and most RCTs enrolled their study populations based on this evidence [41].
Table 2. Guidelines for cervical cancer screening in different countries.
| Country | Screening ages (yr) | Primary screening test and interval | Use of hrHPV screening | |
|---|---|---|---|---|
| Australia [36] | 25–69 | hrHPV screening with partial HPV genotyping and reflex LBC triage every 5 yr | - | |
| Canada [50,51] | 25–69 | Cytology every 3 yr | With regional variation and rollout of primary HPV screening in pilot studies | |
| England [52,53] | 25–49 | hrHPV screening every 3 yr | - | |
| 50–64 | hrHPV screening every 5 yr | - | ||
| Germany [52,54] | ≥20 | Cytology annually | HPV primary testing in implementation, HPV triage testing [55] | |
| Netherlands [35] | 30–64 | hrHPV screening every 5 yr | - | |
| Singapore [56] | 25–29 | Cytology every 3 yr | - | |
| 30–69 | hrHPV screening every 5 yr | - | ||
| Sweden [52] | 23–50 | hrHPV screening every 3 yr | - | |
| 51–60 | hrHPV screening every 5 yr | - | ||
| USA | ||||
| ACS/ASCCP/ASCP (2012) [57] | 21–29 | Cytology every 3 yr | - | |
| 30–65 | Co-testing every 5 yr (preferred) | - | ||
| Cytology every 3 yr | ||||
| Interim guidance (2015) [58] | ≥25 | - | hrHPV screening with genotyping | |
| US-PSTF (2018) [42] | 21–29 | Cytology every 3 yr | - | |
| 30–65 | Cytology every 3 yr (preferred) | - | ||
| hrHPV screening every 5 yr (preferred) | ||||
| Co-testing every 5 yr | ||||
ACS, American Cancer Society; ASCP, American Society for Clinical Pathology; ASCCP, American Society for Colposcopy and Cervical Pathology; hrHPV, high-risk human papillomavirus; HPV, human papillomavirus; LBC, liquid-based cytology; US-PSTF, US Preventive Services Task Force.
Gage et al. [32] compared the 3- and 5-year risks of cervical cancer in women with negative hrHPV screening and negative HPV/cytology co-testing. The 3-year risk in women with a hrHPV-negative result was lower than the 5-year risk in women with a cytology-negative/hrHPV-negative co-testing result (0.011% vs. 0.014%, p=0.21). These results show that hrHPV screening with a 3-year interval is at least as effective as a 5-year interval co-testing. The guidelines issued in September 2018 by the US-PSTF confirmed a similar protocol, recommending HPV screening or HPV/cytology co-testing every 5 years for women aged 30–65 years [42]. In the US-PSTF systematic review, a microsimulation model suggested similar life-years achieved by HPV screening with 3- and 5-year intervals, but in a 3-year interval, several tests and procedures were required [43]. A pooled analysis of European RCTs also showed that 5-year intervals for HPV screening were safer than 3-year intervals for cytology [30]. However, 3 (Swedescreen, ARTISTIC, NTCC) of the 4 European RCTs utilized 3-year screening intervals and follow-up data based on the ATHENA trial [22,27,29,31].
CONCLUSION
For almost 2 decades, scientific evidence from large-scale epidemiological studies has established the diagnostic and preventive value of primary hrHPV screening for high-grade CIN and cervical cancer. However, there are still several challenges in the introduction of hrHPV screening in Korea. First, direct cost-effectiveness comparisons among primary hrHPV screening, cytology, and HPV/cytology co-testing are required. Comparative effectiveness studies that consider the starting age, screening interval, and follow-up visits for primary hrHPV screening are also necessary. These studies may be time-consuming and will likely require significant effort. Nevertheless, the scientific evidence for hrHPV screening should be strongly considered, and we should consider integrating hrHPV screening with published screening and treatment guidelines and comprehensively discuss this new strategy to healthcare providers and patients.
ACKNOWLEDGMENTS
This position statement is developed by the Position Statement Writing Committee including Miseon Kim (committee secretary), Young-Han Kim (representative from the Korean Society of Maternal Fetal Medicine), Yong Beom Kim (affiliate of the scientific committee of the Korean Society of Obstetrics and Gynecology [KSOG]), Jayeon Kim (representative from the Korean Society for Reproductive Medicine), Jae-Weon Kim (committee chair), Mi Hye Park (affiliate of the scientific committee of KSOG), Joo Hyun Park (representative from the Korean Society of Gynecologic Endocrinology), Jeong Ho Rhee (affiliate of the scientific committee of KSOG), Myong Cheol Lim (representative from the Korean Society of Gynecologic Oncology [KSGO]) and Joon-Seok Hong (secretary of the scientific committee of KSOG). The position statement was reviewed and approved by all committee members.
We would like to extend our gratitude to the KSGO advisors (Sunghoon Kim, Young-Tak Kim, Young Tae Kim, Jeong-Yeol Park, Dong Hoon Suh, Seung-Hyuk Shim, Keun Ho Lee, Jae-Kwan Lee, Sung Jong Lee, Soo Young Hur), advisors from the Korean Society for Laboratory Medicine (Seung-Man Park and Eun-Hee Nah), and the Korean Academy of Family Medicine advisor (Bumjo Oh) who provided insight and expertise that significantly assisted this position statement.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.T.W., K.M., K.Y.B., K.J.W.
- Investigation: K.T.W., K.M.
- Methodology: K.T.W., K.M., K.Y.B., K.J.W.
- Project administration: K.Y.B., K.J.W.
- Supervision: K.Y.B., K.J.W.
- Writing - original draft: K.T.W., K.M., K.Y.B., K.J.W.
- Writing - review & editing: K.T.W., K.M., K.Y.H., K.Y.B., K.J., K.J.W., P.M.H., P.J.H., R.J.H., L.M.C., H.J.S.
References
- 1.Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40:1–13. doi: 10.1007/s11262-009-0412-8. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien PM, Campo MS. Papillomaviruses: a correlation between immune evasion and oncogenicity? Trends Microbiol. 2003;11:300–305. doi: 10.1016/s0966-842x(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 4.zur Hausen H, Gissmann L, Schlehofer JR. Viruses in the etiology of human genital cancer. Prog Med Virol. 1984;30:170–186. [PubMed] [Google Scholar]
- 5.Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984;3:1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 10.Lim MC, Won YJ, Ko MJ, Kim M, Shim SH, Suh DH, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol. 2019;30:e38. doi: 10.3802/jgo.2019.30.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45:2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung KW, Won YJ, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min KJ, Lee YJ, Suh M, Yoo CW, Lim MC, Choi J, et al. The Korean guideline for cervical cancer screening. J Gynecol Oncol. 2015;26:232–239. doi: 10.3802/jgo.2015.26.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 17.Stoler MH, Schiffman M Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 18.Wright TC, Jr, Stoler MH, Behrens CM, Sharma A, Sharma K, Apple R. Interlaboratory variation in the performance of liquid-based cytology: insights from the ATHENA trial. Int J Cancer. 2014;134:1835–1843. doi: 10.1002/ijc.28514. [DOI] [PubMed] [Google Scholar]
- 19.Solomon D, Schiffman M, Tarone R ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293–299. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 20.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ ASCCP-Sponsored Consensus Conference. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 21.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 22.Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 23.Bulkmans NW, Rozendaal L, Snijders PJ, Voorhorst FJ, Boeke AJ, Zandwijken GR, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94–101. doi: 10.1002/ijc.20076. [DOI] [PubMed] [Google Scholar]
- 24.Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78–88. doi: 10.1016/S1470-2045(11)70296-0. [DOI] [PubMed] [Google Scholar]
- 25.Dijkstra MG, van Zummeren M, Rozendaal L, van Kemenade FJ, Helmerhorst TJ, Snijders PJ, et al. Safety of extending screening intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow-up of population based randomised cohort in the Netherlands. BMJ. 2016;355:i4924. doi: 10.1136/bmj.i4924. [DOI] [PubMed] [Google Scholar]
- 26.Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al. ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess. 2009;13:1–150. doi: 10.3310/hta13510. [DOI] [PubMed] [Google Scholar]
- 27.Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672–682. doi: 10.1016/S1470-2045(09)70156-1. [DOI] [PubMed] [Google Scholar]
- 28.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008;100:492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 29.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 30.Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 31.Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189–197. doi: 10.1016/j.ygyno.2014.11.076. [DOI] [PubMed] [Google Scholar]
- 32.Gage JC, Schiffman M, Katki HA, Castle PE, Fetterman B, Wentzensen N, et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106:dju153. doi: 10.1093/jnci/dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melnikow J, Henderson JT, Burda BU, Senger CA, Durbin S, Weyrich MS. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320:687–705. doi: 10.1001/jama.2018.10400. [DOI] [PubMed] [Google Scholar]
- 34.Choi JW, Kim Y, Lee JH, Kim YS. The clinical performance of primary HPV screening, primary HPV screening plus cytology cotesting, and cytology alone at a tertiary care hospital. Cancer Cytopathol. 2016;124:144–152. doi: 10.1002/cncy.21632. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Public Health and the Environment (NL) Cervical cancer screening programme [Internet] Bilthoven: National Institute for Public Health and the Environment; 2019. [cited 2019 Aug 26]. Available from: https://www.rivm.nl/en/cervical-cancer-screening-programme. [Google Scholar]
- 36.Department of Health (AU) National Cervical Screening Program [Internet] Canberra: Department of Health; 2017. [cited 2019 Aug 26]. Available from: http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/cervical-screening-1. [Google Scholar]
- 37.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Benard VB, Watson M, Castle PE, Saraiya M. Cervical carcinoma rates among young females in the United States. Obstet Gynecol. 2012;120:1117–1123. doi: 10.1097/aog.0b013e31826e4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 40.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 41.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1–27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 42.US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- 43.Kim JJ, Burger EA, Regan C, Sy S. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706–714. doi: 10.1001/jama.2017.19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogilvie GS, van Niekerk DJ, Krajden M, Martin RE, Ehlen TG, Ceballos K, et al. A randomized controlled trial of Human Papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial) BMC Cancer. 2010;10:111. doi: 10.1186/1471-2407-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook DA, Mei W, Smith LW, van Niekerk DJ, Ceballos K, Franco EL, et al. Comparison of the Roche cobas® 4800 and Digene Hybrid Capture® 2 HPV tests for primary cervical cancer screening in the HPV FOCAL trial. BMC Cancer. 2015;15:968. doi: 10.1186/s12885-015-1959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogilvie GS, Krajden M, van Niekerk D, Smith LW, Cook D, Ceballos K, et al. HPV for cervical cancer screening (HPV FOCAL): Complete Round 1 results of a randomized trial comparing HPV-based primary screening to liquid-based cytology for cervical cancer. Int J Cancer. 2017;140:440–448. doi: 10.1002/ijc.30454. [DOI] [PubMed] [Google Scholar]
- 47.Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: The HPV FOCAL randomized clinical trial. JAMA. 2018;320:43–52. doi: 10.1001/jama.2018.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leinonen MK, Nieminen P, Lönnberg S, Malila N, Hakama M, Pokhrel A, et al. Detection rates of precancerous and cancerous cervical lesions within one screening round of primary human papillomavirus DNA testing: prospective randomised trial in Finland. BMJ. 2012;345:e7789. doi: 10.1136/bmj.e7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canfell K, Caruana M, Gebski V, Darlington-Brown J, Heley S, Brotherton J, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: Results of the Compass pilot randomised trial. PLoS Med. 2017;14:e1002388. doi: 10.1371/journal.pmed.1002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickinson J, Tsakonas E, Conner Gorber S, Lewin G, Shaw E, Singh H, et al. Recommendations on screening for cervical cancer. CMAJ. 2013;185:35–45. doi: 10.1503/cmaj.121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzo AE, Feldman S. Update on primary HPV screening for cervical cancer prevention. Curr Probl Cancer. 2018;42:507–520. doi: 10.1016/j.currproblcancer.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Chrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. Cervical cancer screening programs in Europe: the transition towards HPV vaccination and population-based HPV testing. Viruses. 2018;10:E729. doi: 10.3390/v10120729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Public Health England. Cervical screening: implementation guide for primary HPV screening [Internet] London: Public Health England; 2019. [cited 2019 Oct 20]. Available from: https://www.gov.uk/government/publications/cervical-screening-primary-hpv-screening-implementation/cervical-screening-implementation-guide-for-primary-hpv-screening. [Google Scholar]
- 54.Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer. 2018;142:44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 55.Bujan Rivera J, Klug SJ. Cervical cancer screening in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61:1528–1535. doi: 10.1007/s00103-018-2835-7. [DOI] [PubMed] [Google Scholar]
- 56.The Society for Colposcopy and Cervical Pathology of Singapore. Management guidelines for cervical screening & preinvasive disease of the cervix [Internet] Singapore: The Society for Colposcopy and Cervical Pathology of Singapore; 2019. [cited 2019 Oct 20]. Available from: https://www.sccps.org/wp-content/uploads/2019/03/CSS-Clinical-Mgt-Guidelines-2019_March-Release.pdf. [Google Scholar]
- 57.American Society for Colposcopy and Cervical Pathology. Screening guidelines [Internet] Rockville, MD: ASCCP; [cited 2019 Oct 20]. Available from: http://www.asccp.org/screening-guidelines. [Google Scholar]
- 58.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330–337. doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]