Abstract
Translational neuroprotective and drug development studies need to be gauged against well-characterized functional outcomes, including motor, sensory and cognitive domains. Since intracerebral hemorrhage (ICH) causes dramatic neurological and cognitive deficits in humans, we hypothesized that ICH would result in prolonged motor-sensory and learning/memory deficits in rats. Neurological tests of sensorimotor functions were performed before ICH, 1-3 days and 10 weeks after ICH. Water maze, open field, and rotarod performance was tested 2 and 8 weeks after ICH. Early neurological evaluations revealed significant deficits, with almost full recovery by 10 weeks. The water maze revealed significant learning (but not motor) deficits at 2 weeks, but by 8 weeks, the learning deficits had diminished and significant motor deficits had emerged, coinciding with a drop in activity. The injured hemisphere showed significant atrophy at sacrifice. Therefore, ICH produced detectable cognitive and motor deficits in rats that evolved over a 10-week period, and thereby provides a suitable baseline for analysis of future therapeutic interventions following hemorrhagic stroke.
Keywords: Hemorrhage, collagenase, learning, memory, motor
1. Introduction
Intracerebral hemorrhage (ICH) is a devastating form of brain injury that accounts for 15-20% of all strokes (Broderick et al., 1993; Broderick et al., 1999; Qureshi et al., 2001). An approximate 50% survival rate leaves those patients with prolonged neurological deficits and significant brain atrophy (Broderick et al., 1999; Skriver and Olsen, 1986), and only 20% of patients becoming independent by 6 months (Gebel and Broderick, 2000). ICH in any brain region can be associated with cognitive impairment, and almost half of all subcortical strokes result in long-term cognitive deficits (Nys et al., 2007). In fact, the cognitive deficits described by patients are among the most prominent and troubling symptoms across several types of cerebrovascular injury (King et al., 2006; Nys et al., 2007; Thajeb et al., 2007).
ICH most commonly occurs in the sub-cortical basal ganglia (striatum) region, which is best known for its role in motor tasks. Recent neuropsychological findings have also indicated an important role for the striatum in learning and memory (Benke et al., 2003; El Massioui et al., 2007; Ragozzino, 2007; Sridharan et al., 2006). In fact, lesions to this region have also been shown to cause significant cognitive disability in patients (Bhatia and Marsden, 1994; Hochstenbach et al., 1998; Su et al., 2007; Werring et al., 2004) especially in the areas of executive functioning and visual perception (Nys et al., 2007).
Based on the recommendations of the STIAR report (1999), preclinical neuroprotective and drug development studies need to be gauged against behavioral assessments, including motor, sensory and cognitive functions. Experimental animal models of ICH have successfully demonstrated several sensory and motor tasks as appropriate measures of treatment end-points (Clarke et al., 2007; Hua et al., 2002; MacLellan et al., 2006a; MacLellan et al., 2006b), but long-term behavioral studies with cognitive components are lacking. We therefore hypothesized that intracerebral hemorrhage in rats would produce long-term deficits across several cognitive, motor and sensory tasks.
2. Results
Behavioral Outcomes
By 2 weeks after ICH, none of the rats had obvious physical or motor abnormalities, and all appeared normal when handled throughout the following experiments. All rats blinked their eyes when approached by a cotton swab (suggesting functional vision) and could right themselves from their back at every time-point measured.
ICH activity levels declined with age (Fig. 1A). A significant treatment × time point interaction (F(1,26)=7.2, p<0.02) revealed that ICH rats spent less time moving at 8 weeks than 2 weeks (post hoc p<0.00003). However, body weights never differed between groups (Fig. 1B). There were no differences detected for the rotarod at 2 or 8 weeks using baseline acceleration parameters, but when tested using faster acceleration parameters at 8 weeks, ICH rats fell significantly more quickly than control rats (F(1,26)=5.4, p<.03; data not shown).
Fig. 1 – Declining level of activity with age in spite of steady weight gain.
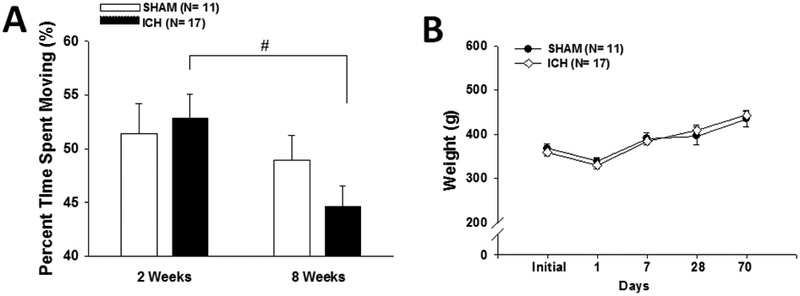
Both graphs show mean +/− standard error of the mean. (A) No difference between sham and ICH rats was observed at either time-point, however a significant treatment × time point interaction (# p<0.00003) revealed that ICH rats spent less time moving at 8 weeks than they did at 2 weeks. (B) No difference in weight was observed at any time-point measured.
ICH rats had a dramatic bias toward turning to the right (ipsilateral to the ICH) while swimming that evolved over time (F(1,26)=4.7, p<0.05), whereas control rats had no turning preference (Fig. 2A). ICH rats also swam more slowly than control rats (F(1, 26)=13.5, p<0.002), and a treatment × time point interaction analysis (F(1,26)=4.8, p<0.04) showed that ICH rats swam more slowly at 8 weeks compared to 2 weeks (post hoc p<0.006) (Fig. 2B). Because of the differences in swimming speed, swim distance, rather than escape latency, was used for assessing task acquisition in the cued and spatial water maze.
Fig. 2 -. Water maze motor measures deteriorate with age.
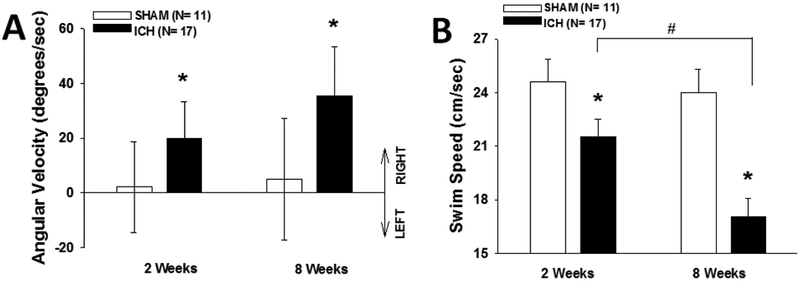
(A) Mean +/− 95% confidence intervals. Sham rats had no bias toward turning left versus right whilst swimming, but ICH rats were significantly biased toward turning to the right at both 2 and 8 weeks (* p<0.05). (B) Mean +/− standard error of the mean. ICH rats swam more slowly than sham rats (* p<0.002), and ICH rats swam more slowly at 8 weeks than at 2 weeks (# p<0.006).
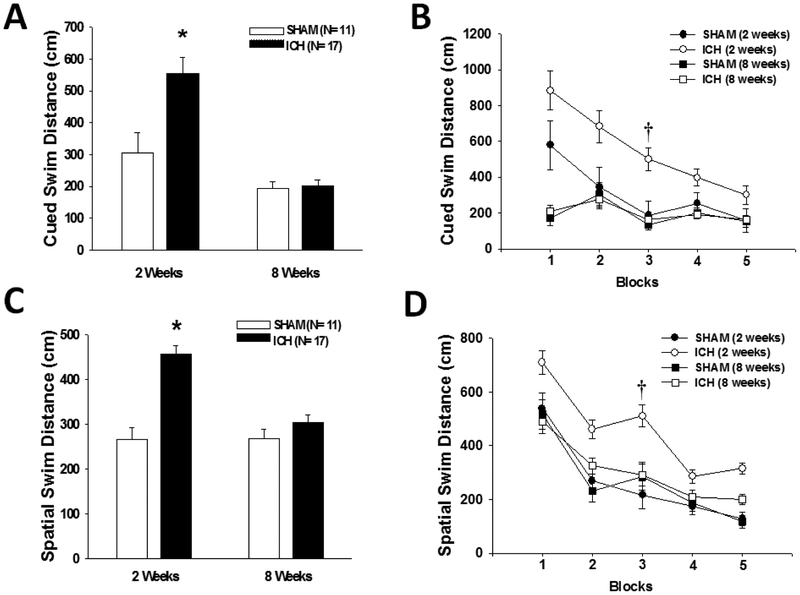
Water maze cued learning performance (Fig. 3A and 3B) showed that ICH rats swam further before finding the visible escape platform (F(1,26)=8.8, p<0.007). A treatment × time point interaction (F(1,26)=8.0, p<0.009) revealed that the difference was only present at 2 weeks (post hoc p<0.005).
Fig. 3 -. Water maze performance improves with age.
All graphs show mean +/− standard error of the mean. (A and B) Cued learning: ICH rats swam further before finding the visible escape platform († p<0.005), but the difference only existed at 2 weeks (* p<0.05). (C and D) Spatial learning: ICH rats swam further before finding the submerged escape platform († p<0.0003), but the difference only existed at 2 weeks (* p<0.05)
Water maze spatial learning performance (Fig. 3C and 3D) showed that ICH rats swam further before finding the submerged escape platform (F(1,26)=19.7, p<0.0002). A significant treatment × time point interaction (F(1,26)=21.9, p<0.00009) revealed that the difference only existed at 2 weeks (post hoc p<0.0003). No significant differences were detected in spatial memory performance during the probe trials (data not shown).
Neurological Outcomes
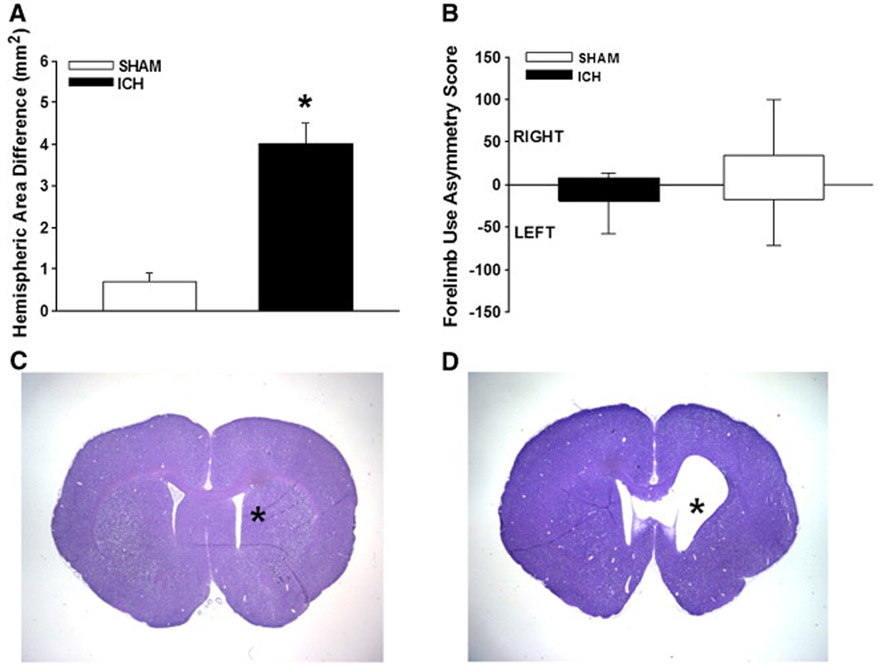
Prior to and after the other behavioral tests, the animals were subjected to a series of neurological tests (forelimb placing, forelimb use asymmetry, and a neurological test battery). For both the neurological test battery (Fig. 4A) and forelimb-placing (Fig. 4B) tests, all ICH rats were significantly worse compared to sham animals over the first three days (p<0.001). By 10 weeks, however, all animals had recovered neurological test battery scores, while trends toward late forelimb placing deficits continued to persist (p=0.06). The forelimb use asymmetry test (Fig. 5B) failed to show differences at 10 weeks.
Fig. 4 -. Neurological outcomes.
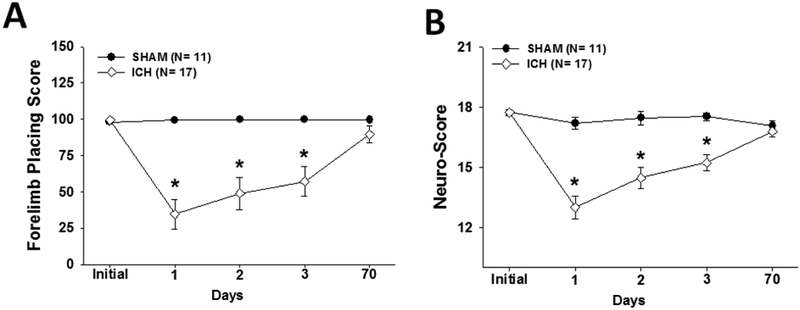
Both graphs show mean +/− standard error of the mean. (A) Forelimb placing and (B) Neurological test battery scores at baseline (pre-ICH), 1, 2, 3 and 70 days after ICH induction. ICH rats performed significantly worse compared to shams over the first three days (* p<0.001) with no significant differences at baseline or 10 weeks later.
Fig. 5 -. Long-term cerebral atrophy and forelimb-use asymmetry outcomes.
Both graphs show mean +/− standard error of the mean. (A): ICH rats had significant brain atrophy compared to shams (* p<0.001). (B): Forelimb use asymmetry at 10 weeks after ICH revealed no differences. (C and D) Representative photos of cresyl violet stained coronal brain sections obtained at 10 weeks after ICH showing that (D) ICH animals had visible atrophy near cerebral ventricles, whereas (C) shams had no discernable cerebral tissue loss. Asterisk (*) indicates site of needle-track.
Behavioral Correlation Matrix
A correlation matrix including all measures with significant main effects (swim speed, turn bias, cued swim distance, spatial swim distance) revealed that overall spatial water maze performance was correlated with overall cued water maze performance (r= 0.61, p<0.0001). Because cued (visible platform) performance deficits suggest potential non-associative (e.g., sensorimotor and/or motivational) or non-spatial (e.g., general associative learning) factors, the deficits observed in the spatial (submerged platform) task may have been caused by the same factors that contributed to the cued deficit. To control for this possibility, an analysis of covariance (ANCOVA) was performed, in which overall cued swim distance was covaried out of the overall spatial swim distance effect. The difference in spatial swim distance was still significant (F(1,24)=15.4, p<0.0007). Cued and spatial swim distance at the 2 week time point were also correlated (r=.51, p<.005). As with the overall (main) effects, even when controlling for cued swim distance at 2 weeks, spatial swim distance at 2 weeks was still significant F(1,25)=16.8,p<.0004). Although turn bias was not correlated with spatial performance, this variable was not measured for the cued phase. It is possible, therefore, that circling was less prominent during the cued phase, and that the ANCOVA procedure may not have completely isolated those circling effects. Percent time moving in the open field, rotarod, and swim speed data from the 8-week time point were also analyzed in a correlation matrix. Open field percent time moving was correlated with water maze swim speed (r= 0.47, p<0.01). However, the swimming speed difference was still significant even after controlling for percent time moving (F(1,25)=14.1, p<0.001).
Histological examination
Hemispheric area quantification 10 weeks after ICH revealed significant atrophy in the injured hemisphere (4.02 ± 2.1 vs. 0.7 ± 0.48 mm2, p<0.001, Fig. 5).
3. Discussion
Though several other studies have shown that sensory and motor tasks are appropriate measures of outcome after ICH (Clarke et al., 2007; Hua et al., 2002; MacLellan et al., 2006a; MacLellan et al., 2006b), this is the first study to provide a rigorous, comprehensive and longitudinal analysis of the cognitive (cued learning, spatial learning, long-term and short-term memory) and sensorimotor-neurological (activity levels, turn bias, swim speed, neurological test battery scoring and fore-limb use) deficits that are associated with this model. It is interesting to note that this model produced no observable differences (compared to sham) in spontaneous open field activity levels at 2 and 8 weeks. This lack of differences was also reflected in the general observation of home-cage and handling activity, and corroborates a similar finding by others at 4 weeks (MacLellan et al., 2009). However, testing activity levels in an open field are not stressful, motivation-driven tasks like the water maze. Thus, future tests of therapeutic efficacy could possibly employ stress-inducing procedures (such as restraint or mild shock; Schaefer et al., 2000) before testing activity that may better enhance the detection of deficits. Post-ICH stress could mimic the psychosocial stresses experienced by humans following stroke, and thus provide a more realistic assessment-model.
We are the first to report a significant drop in activity between the 2 and 8 week testing periods in those animals receiving ICH, whereas there was no such difference in the sham animals. Coincidently, there was also a degeneration of water maze swim speed and turning bias parameters over the same period of time, whereas the shams showed no such trend. In rats, the age-related changes in (exploratory) activity and locomotor ability have been attributed to losses of dopaminergic neurons within the mesencephalic substantia nigra (SN) (Emerich et al., 1993; Sanchez et al., 2008). This region has a plethora of interconnections with the rostral striatum (Bjorklund and Dunnett, 2007), and accelerated degeneration of the SN forms the pathophysiological basis of Parkinson’s disease (PD, a neurodegenerative disorder associated with movement disorders). Recent evidence suggests that PD leads to reciprocal structural and functional reorganizations of the basal ganglia (Bartels and Leenders, 2008; Obeso et al., 2008), which may contribute to the deficits in activity, locomotion and postural stability seen in patients with PD (Chastan et al., 2009). Taken together, the decreased levels of motor measures (activity, swim speed and turn bias) could be an indication of progressive dysfunctional structural re-organization of the damaged basal ganglia circuits following hemorrhagic injury to that area and could be contributing to the prolonged neurological morbidities seen after ICH in patients. Although others have shown cerebral ischemia to cause delayed neuronal cell-death in the substantia nigra pars reticulata (Forno, 1983; Nakane et al., 1992; Saji et al., 1994; Tamura et al., 1990), this is the first report showing behavioral signs of possible delayed injury to the SN after ICH, and future studies should pursue this further.
The collagenase injection model of ICH in adult male rats was associated with severe cued and spatial learning deficits at 2 weeks post-ICH. Although the deficit in cued navigation at 2 weeks adds a confounding factor to measurements of the rats’ spatial learning abilities, an analysis of covariance (ANCOVA) mathematically controlled for variance in spatial performance contributed by factors associated with cued performance. Even after controlling for deficits in the cued task, the ICH rats’ performance on the spatial task was still significantly impaired at 2 weeks.
The lack of significant spatial learning deficits at the 8-week time point may have resulted from a test history effect. The rats had experienced a similar test 6 weeks earlier (albeit with different spatial locations and cues), and may have learned the necessary strategies for solving the task, thus masking potentially subtle differences. However, we believe this not to be the case, since others have found similar results 8 weeks after induction of less severe ICH (using half the dose of collagenase used in this study; MacLellan et al., 2009). Nonetheless, future longitudinal tests of therapeutic efficacy should include more difficult water maze parameters for subsequent testing. However, it is worth noting that this situation closely approximates the human condition, in which practice effects are commonly seen, and indeed, successfully used as therapeutic rehabilitation strategies. Alternatively, because subtle, but not significant, differences in spatial water maze performance were observed at the 8-week time point, statistical power may have been insufficient to detect subtle differences. Although the repeated-measures design employed for this study produced data with relatively tight variance, future studies of therapeutic efficacy should use larger sample sizes to ensure the reliable detection of long-term cognitive deficits.
While the spatial deficits had somewhat dissipated by 8 weeks, sensorimotor coordination deficits were only detected by the rotarod when more difficult parameters were used at 8 weeks. Future tests of therapeutic efficacy using the rotarod should therefore employ parameters that will ensure adequate, but not excellent, performance from the control group. This strategy will allow for the detection of subtle deficits in the experimental groups.
The neurological test battery scoring system used was based on a series of tests for assessing outcomes after middle cerebral artery occlusion in rats (Garcia et al., 1995). It bears similarities to other neurological scoring systems used after ICH (Altumbabic et al., 1998; Clark et al., 1998), and variations have been used recently to successfully assess treatments in rats after ICH (Titova et al., 2007), transient global cerebral ischemia (Ostrowski et al., 2008), subarachnoid hemorrhage (Sugawara et al., 2009) and surgically-induced brain injury (Yamaguchi et al., 2007). This, however, is the first report using this neurological scoring system at early and late time-points after ICH. The modified form of this test is shown in table 1.
Table 1.
Neurological Evaluation after ICH
| Score | ||||
|---|---|---|---|---|
| 3 | 2 | 1 | 0 | |
| Spontaneous Activity (5 min in typical cage) | Moved around, explored environment and reached the upper rim ≥ 3 sides of cage | Moved hesitatingly around, explored, and reached the upper rim < 3 sides of cage | Severely affected rat did not rise-up at all. and barely moved in the cage | Rat did not move at all |
| Symmetry of Limb Movement (suspended by tail) | All limbs extended symmetrically | Limbs on one side outstretched less (or more slowly) than the other | Limbs on one side showed minimal movement | One side did not move at all (paretic) |
| Forelimb Outstretching (suspended by tail, forepaws to table) | Both forelimbs outstretched, walking symmetrically on forepaws | Impaired forepaw walking with asymmetric outstretch | Limbs on one side showed minimal movement | One side did not move at all (paretic) |
| Climbing (45° angle on gripping surface) | Climbed to the top easily with a firm grip | Asymmetric or impaired climbing or did not grip the wire as strongly | Failed to climb or circled instead | |
| Axial Sensation (mild stimulus to the trunk from behind) | Equally startled to the stimulus on both sides of trunk | Reacted more slowly on one side compared to other | Did not respond to stimulus on one side | |
| Vibrissae Proprioception (gentle touch from behind | Equally turned head to cotton-wisp on both sides | Reacted more slowly on one side compared to other | Did not respond to stimulus on one side | |
Table showing the neuro-scoring system adopted and modified for the functional assessment of rats after ICH
Since the collagenase model of intracerebral hemorrhage is known to produce functional impairments that most severely affect the control of the distal forelimb and paw (Clarke et al., 2007), we decided to assess the late outcomes in both forelimb use asymmetry and forelimb placement. Since asymmetrical forelimb use had already been shown to be deficient in the collagenase ICH model of rats at 1 week (Clarke et al., 2005; MacLellan and Colbourne, 2005), 3 weeks (Clarke et al., 2005), 4 weeks (Auriat and Colbourne, 2009; DeBow et al., 2003; Fingas et al., 2007; MacLellan and Colbourne, 2005), 6 weeks (Auriat and Colbourne, 2009), and 2, 5 and 8 weeks (Nguyen et al., 2008), we did not assess an earlier time-point. Although an autologous blood injection model of ICH resulted in persistent deficits in forelimb placement at 8 weeks (with recovery by 3 months) and forelimb use asymmetry at 12 weeks (Hua et al., 2006), we observed the opposite result with our collagenase model. Forelimb use asymmetry completely recovering by 10 weeks, but residual trends in forelimb placement deficiency remaining at 10 weeks after injury. This is the first collagenase ICH report of forelimb placement and forelimb use asymmetry this long after injury. The incomplete recovery of forelimb placement is an important finding for future therapeutic applications, whereas the asymmetry result should direct future studies to assess time-points at 8 weeks (Nguyen et al., 2008) or earlier.
4. Conclusion
The collagenase model of ICH produced detectable cognitive and motor deficits in adult rats that evolved over a 10 week time period, and provides a suitable baseline for the analysis of therapeutic intervention strategies following stroke.
5. Experimental procedures
5.1. Intracerebral Hemorrhage
All the procedures used for these studies were in compliance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Loma Linda University. Aseptic technique was used for all surgeries. Rats were allowed free access to food and water and divided into sham-operated controls (n=11) and ICH (n=17) groups. Adult male Sprague-Dawley rats (290–395g; Harlan, Indianapolis, IN, USA) were used in the present study. Since neurobiological maturity is acquired at around 10-12 weeks in rats (Quinn, 2005), we chose adult animals that were at least 12 weeks old at the time of injury. Rats were anaesthetized with isoflurane and placed prone in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Then, using a collagenase injection ICH model similar to that described previously in rats (Rosenberg et al., 1990), the following stereotactic coordinates were used to localize the right basal ganglia: 0.2 mm anterior, 5.6 mm ventral and 2.9 mm lateral to the bregma. A posterior cranial burr hole (1 mm) was drilled over the right cerebral hemisphere, into which a 27 gauge needle was inserted at a rate of 1 mm/min. A microinfusion pump (Harvard Apparatus, Holliston, MA, USA) infused the bacterial collagenase (VII-S; Sigma, St Louis, MO, USA; 0.2 U in 1 μL saline) through a Hamilton syringe at a rate of 0.2 μL/min. The needle remained in place for an additional 10 min after injection to prevent “back-leakage”. To maintain a core temperature within 37.0 ± 0.5°C, an electronic thermostat-controlled warming blanket was used throughout the operation. After needle removal, the burr hole was sealed with bone wax, the incision sutured closed, and the animal allowed to recover. Sham surgeries consisted of needle insertion alone.
5.3. Behavioral testing
At 2 weeks and 8 weeks post-ICH, animals were subjected to a 10-day battery of tests that assess a variety of behavioral domains. Before testing on day 1, each animal was placed on its back to observe whether it could turn itself over, and eye blink reflexes were tested by holding the rat while slowly approaching its eyes with a cotton swab. All animals passed the initial evaluation and the 10 days of testing are described below.
5.4. Open field
General activity level and movement patterns were assessed on days 2 and 4 (of the 10-day battery at weeks 2 and 8 after ICH) using procedures similar to previously published protocols (Hartman et al., 2001; Hartman et al., 2005a; Hartman et al., 2005b). This test involves observation of an animal for 30 minutes in opaque open-topped plastic boxes (49 cm long, 35.5 cm wide, 44.5 cm tall). The animals’ movements were recorded by an overhead camera and analyzed by a computerized tracking system to determine the percentage of the trial that was spent moving versus resting (Noldus Ethovision).
5.5. Rotarod
The rotarod (Columbus Instruments) is a test of sensorimotor coordination and balance that consists of a rotating horizontal cylinder (7 cm diameter) divided into 9.5 cm-wide lanes. When placed into a lane, the animal must continuously walk forward to avoid falling off the cylinder. Latency to fall off is detected and recorded by a photobeam circuit. Rats were tested on days 1, 3, and 5 (of the 10-day battery at weeks 2 and 8 after ICH). Two consecutive trials, in which the cylinder started at 4 RPM and accelerated by 2 RPM every 5 seconds, were administered per day. To control for a potential learning effect of previous rotarod exposure, an additional set of more difficult trials was added to the 8-week time point, in which the cylinder started at 10 RPM and accelerated by 2 RPM every 5 seconds.
5.6. Water Maze
This rodent learning and memory test (Morris, et al., 1982; Sutherland, et al., 1982; Hartman et al., 2001; Hartman et al., 2005a; Hartman et al., 2005b; Hartman et al., 2006) was administered on test days 6-10 (of the 10-day battery at weeks 2 and 8 after ICH). Briefly, this test of spatial navigation learning requires finding a hidden (submerged) platform in a pool of water using visual cues from around the room. It consists of a metal pool (110 cm diameter) in a well-lit room filled to within 15 cm of the upper edge with water made opaque by the addition of white non-toxic tempera paint. The pool contains a platform (11 cm diameter) that the animal can step onto. For each trial, an animal was placed nose against the wall into the water at one of four release points and allowed to find the platform. All trials lasted a maximum of 60 seconds, at which point the animal was manually guided to the platform. An overhead camera recorded the swim path, allowing for quantification of swim distance, escape latency, proximity to target, swim speed, and left/right turn bias by a computerized tracking system (Noldus Ethovision). Generally, as performance improves, escape latency and swim path length decrease. The cued water maze was tested on day 6 (of the 10-day battery at weeks 2 and 8 after ICH). This is the “visible-platform” task, used primarily as a control to assess sensorimotor and/or motivational deficits that could affect performance during the spatial water maze task. For this task, the surface of the escape platform was visible (5 mm above the surface of the water), and a 20 cm tall pole capped by a tennis ball was placed on top of the platform to make its location even more obvious. The animals were tested for 10 trials per day in 5 blocks of 2 consecutive trials with a 10-minute inter-block interval. The location of the platform changed for each block of trials. The animals were released into the pool opposite the location of the platform for that trial and allowed to remain on the platform for 5 seconds after finding it (or being guided to it). None of the animals displayed behaviors inappropriate for spatial water maze testing (including spinning, thygmotaxic navigation around the perimeter of the pool, or inability to swim). The spatial water maze was tested on days 7-10 (of the 10-day battery at weeks 2 and 8 after ICH). For this task, the surface of the escape platform was submerged 1 cm below the surface of the water, requiring the animal to find the platform based on its relationship to spatial cues in the room rather than direct visualization. The animals were given 10 trials per day in 5 blocks of 2 consecutive trials with a 10-minute inter-block interval. The location of the platform remained the same throughout each day, and changed for the next day. Three locations were tested. After finding (or being guided to) the platform, the animals were allowed to remain on it for 10 seconds. A different set of platform locations and spatial cues was used for the 2 week and 8 week time points. At the beginning of the next day, each animal was given a 60 second “probe” trial, in which the platform was removed from the water maze. The total number of times that the animal crossed over the former location of the platform was recorded, as well as the amount of time spent searching the target quadrant. An hour later, the platform was placed back into the pool in a new location, and the next set of 10 trials was administered.
5.7. Neurological testing
Forelimb placing tests and neurological test battery scoring were performed at baseline (pre-ICH), 24, 48, and 72 hours and 10 weeks after ICH, and forelimb use asymmetry was assessed 70 days after ICH, prior to sacrifice for the histological analysis.
5.8. Forelimb Placing
For vibrissae-elicited forelimb placing testing (Hua et al., 2002), the rats were held by their torsos and moved gently up and down before the testing to facilitate muscle relaxation and minimize any struggling movements. Trials that elicited high levels of resistance, muscle straining, struggling, or placing of any limbs onto the examiner’s hand were not counted. Each forelimb received independent testing by brushing the ipsilateral vibrissae onto the corner-edge of a tabletop. Most animals are able to place the vibrissae-stimulated forelimb quickly onto the countertop, but brain injury can produce deficits in placement of the forelimb contralateral to the injury. For each test day, the rats were given a total of 10 trials per forelimb, and the percentage of successful trials was calculated.
5.9. Neurological test battery
The scoring system (Garcia et al., 1995) consisted of six tests (spontaneous activity, symmetrical limb movement, forelimb outstretching, climbing, axial sensation and vibrissae proprioception) with possible scores of 0–3 (0=worst; 3=best). The minimum score was 0 and the maximum was 18. The full assessment with modifications suitable for ICH is provided in Table 1.
5.10. Forelimb Use Asymmetry
At 10 weeks after ICH, animals were subjected to an assessment of forelimb use during exploratory activity using methods described previously (Hua et al., 2002). Briefly, video of each rat in a transparent cylinder (20cm diameter × 30cm height) was recorded for 10 minutes. Behavior was scored according to following criteria: (1) the independent use of either forelimb (left or right) to make contact with the cylinder wall during a full rearing, to perform a weight-shifting maneuver, or to simply regain the center of gravity while (lateral) stepping vertically along the inside of the wall, and (2) simultaneous use of both forelimbs to make contact with the cylinder wall during a full rearing and alternating (lateral) stepping movements along the inside of the wall. Behavior was quantified by recording the number of times the unimpaired (ipsilateral) forelimb was used as a percentage of, (l): total number of limb contacts with the wall, (C): number of limb contacts using the forelimb contralateral to the injury expressed as a percentage of total limb contacts, (B): number of limb contacts using both (bilateral) forelimbs, expressed as a percentage of total limb contacts along the inside of the wall. The overall forelimb use asymmetry score was calculated as follows= [I/(I+C+B)]-[C/(I+C+B)].
5.11. Histology
At 10 weeks after ICH, the animals were deeply anesthetized and transcardially perfused with ice-cold PBS (60 mL, 0.01 mol/L, pH 7.4), followed by 4% formaldehyde solution. The brains were quickly removed, post-fixed in fresh 4% formaldehyde solution at 4°C overnight, and immersed in 30% sucrose until they sank. The brains were then embedded, and horizontal sections (10 μm thick) were cut and placed onto polylysine coated glass slides (Richard Allen Scientific) with the use of a cryostat (CM3050S; Lecia Microsystems; Kusaka et al., 2004), and Nissl staining was performed as previously described using 0.5% cresyl violet (Ostrowski et al., 2006). The area of preserved ipsilateral cortex was measured with image analysis software (Image J software, National Institutes of Health, version 1.40). Area of injury was calculated using the following formula: [Total area of R hemisphere – (Injured area + ventricle)]/(Total area of L Hemisphere – Ventricle).
5.12. Statistical Analysis
An α-level of .05 was used for all statistical significance tests. To avoid violating the assumption that differences between levels of repeated measures must not be correlated across subjects, the p-values for such repeated-measures analyses reflected the conservative Huynh-Feldt adjustment to the degrees of freedom. Open field percent time moving data were analyzed with a treatment (control, ICH) × time point (2 weeks, 8 weeks) × day (1st, 2nd) repeated-measures ANOVA. Rotarod fall latency data were analyzed with a treatment (control, ICH) × time point (2 weeks, 8 weeks) × day (1st, 2nd) × trial (1st, 2nd) repeated-measures ANOVA. The more difficult set of rotarod trials (administered at 8 weeks) was analyzed with a treatment (control, ICH) × trial (1st, 2nd) repeated-measures ANOVA. The forelimb placing, forelimb use asymmetry, and neurological tests were analyzed using t-tests at each time point. Water maze motor data (swim speed, relative angular velocity / turn bias) were taken from the three probe trials at each time point (since these trials are all consistently 60 s long) and analyzed with treatment (control, ICH) × time point (2 weeks, 8 weeks) × spatial location (1st, 2nd, 3rd) repeated-measures ANOVAs. Because of clear confounds between swim speed and escape latency, only swim path distance data were analyzed for the water maze learning trials. Water maze cued learning swim distance data were averaged into five blocks of two trials each and analyzed with a treatment (control, ICH) × time point (2 weeks, 8 weeks) × block (blocks 1-5) repeated-measures ANOVA. Water maze spatial learning swim distance data were averaged into five blocks of two trials each and analyzed with a treatment (control, ICH) × time point (2 weeks, 8 weeks) × spatial location (1st, 2nd, 3rd) × block (blocks 1-5) repeated-measures ANOVA. Water maze probe trial data (percentage of the trial spent searching the probe quadrant and number of target location crossings) were analyzed using treatment (control, ICH) × time point (2 weeks, 8 weeks) × spatial location (1st, 2nd, 3rd) repeated-measures ANOVAs. Significant ANOVA interactions were further explored using the conservative Scheffe post hoc test. Correlations were determined using the Pearson’s r test. Correlation matrices were generated for three sets of averaged behavioral variables: those with overall main effects, those with effects seen at the 2 week time point, and those with effects seen at the 8 week time point. An analysis of covariance (ANCOVA) was used to determine whether the observed effects were still significant after controlling for the variance contributed by the correlated variables.
Acknowledgements
This work was funded by NIH grants NS45694, NS53407 and NS43338 to JHZ.
Abbreviations:
- STIAR
Stroke Therapy Academic Industry Roundtable
- ICH
Intracerebral hemorrhage
- PBS
Phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1999. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 30, 2752–8. [DOI] [PubMed] [Google Scholar]
- Altumbabic M, Peeling J, Del Bigio MR, 1998. Intracerebral hemorrhage in the rat: effects of hematoma aspiration. Stroke. 29, 1917–22; discussion 1922-3. [DOI] [PubMed] [Google Scholar]
- Auriat AM, Colbourne F, 2009. Delayed rehabilitation lessens brain injury and improves recovery after intracerebral hemorrhage in rats. Brain Res. 1251, 262–8. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL, 2008. Parkinson's disease: The syndrome, the pathogenesis and pathophysiology. Cortex. [DOI] [PubMed] [Google Scholar]
- Benke T, Delazer M, Bartha L, Auer A, 2003. Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase. 9, 70–85. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD, 1994. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 117 ( Pt 4), 859–76. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB, 2007. Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott T, Tomsick T, Miller R, Huster G, 1993. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 78, 188–91. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Adams HP Jr., Barsan W, Feinberg W, Feldmann E, Grotta J, Kase C, Krieger D, Mayberg M, Tilley B, Zabramski JM, Zuccarello M, 1999. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 30, 905–15. [DOI] [PubMed] [Google Scholar]
- Chastan N, Westby GW, Yelnik J, Bardinet E, Do MC, Agid Y, Welter ML, 2009. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson's disease. Brain. 132, 172–84. [DOI] [PubMed] [Google Scholar]
- Clark W, Gunion-Rinker L, Lessov N, Hazel K, 1998. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 29, 2136–40. [DOI] [PubMed] [Google Scholar]
- Clarke J, Herzberg G, Peeling J, Buist R, Corbett D, 2005. Dietary supplementation of omega-3 polyunsaturated fatty acids worsens forelimb motor function after intracerebral hemorrhage in rats. Exp Neurol. 191, 119–27. [DOI] [PubMed] [Google Scholar]
- Clarke J, Ploughman M, Corbett D, 2007. A qualitative and quantitative analysis of skilled forelimb reaching impairment following intracerebral hemorrhage in rats. Brain Res. 1145, 204–12. [DOI] [PubMed] [Google Scholar]
- DeBow SB, Davies ML, Clarke HL, Colbourne F, 2003. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 34, 1021–6. [DOI] [PubMed] [Google Scholar]
- El Massioui N, Cheruel F, Faure A, Conde F, 2007. Learning and memory dissociation in rats with lesions to the subthalamic nucleus or to the dorsal striatum. Neuroscience. 147, 906–18. [DOI] [PubMed] [Google Scholar]
- Emerich DF, McDermott P, Krueger P, Banks M, Zhao J, Marszalkowski J, Frydel B, Winn SR, Sanberg PR, 1993. Locomotion of aged rats: relationship to neurochemical but not morphological changes in nigrostriatal dopaminergic neurons. Brain Res Bull. 32, 477–86. [DOI] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F, 2007. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol. 208, 277–84. [DOI] [PubMed] [Google Scholar]
- Forno LS, 1983. Reaction of the substantia nigra to massive basal ganglia infarction. Acta Neuropathol. 62, 96–102. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ, 1995. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 26, 627–34; discussion 635. [DOI] [PubMed] [Google Scholar]
- Gebel JM, Broderick JP, 2000. Intracerebral hemorrhage. Neurol Clin. 18, 419–38. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM, 2001. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp Neurol. 170, 326–44. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Izumi Y, Bales KR, Paul SM, Wozniak DF, Holtzman DM, 2005a. Treatment with an amyloid-beta antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer's disease. J Neurosci. 25, 6213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Lee JM, Zipfel GJ, Wozniak DF, 2005b. Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res. 1043, 48–56. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM, 2006. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 24, 506–15. [DOI] [PubMed] [Google Scholar]
- Hochstenbach J, van Spaendonck KP, Cools AR, Horstink MW, Mulder T, 1998. Cognitive deficits following stroke in the basal ganglia. Clin Rehabil. 12, 514–20. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G, 2002. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 33, 2478–84. [DOI] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G, 2006. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 104, 305–12. [DOI] [PubMed] [Google Scholar]
- King JT Jr., DiLuna ML, Cicchetti DV, Tsevat J, Roberts MS, 2006. Cognitive functioning in patients with cerebral aneurysms measured with the mini mental state examination and the telephone interview for cognitive status. Neurosurgery. 59, 803–10; discussion 810–1. [DOI] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J, 2004. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 286, H2442–51. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Colbourne F, 2005. Mild to moderate hyperthermia does not worsen outcome after severe intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 25, 1020–9. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Auriat AM, McGie SC, Yan RH, Huynh HD, De Butte MF, Colbourne F, 2006a. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab. 26, 1031–42. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Gyawali S, Colbourne F, 2006b. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behav Brain Res. 175, 82–9. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Langdon KD, Churchill KP, Granter-Button S, Corbett D, 2009. Assessing cognitive function after intracerebral hemorrhage in rats. Behav Brain Res. 198, 321–8. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JN, O'Keefe J, 1982. Place navigation impaired in rats with hippocampal lesions. Nature. 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Nakane M, Teraoka A, Asato R, Tamura A, 1992. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke. 23, 328–32. [DOI] [PubMed] [Google Scholar]
- Nguyen AP, Arvanitidis AP, Colbourne F, 2008. Failure of estradiol to improve spontaneous or rehabilitation-facilitated recovery after hemorrhagic stroke in rats. Brain Res. 1193, 109–19. [DOI] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ, 2007. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 23, 408–16. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Marin C, Rodriguez-Oroz C, Blesa J, Benitez-Temino B, Mena-Segovia J, Rodriguez M, Olanow CW, 2008. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol. 64 Suppl 2, S30–46. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Tang J, Zhang JH, 2006. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 37, 1314–8. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, Corleone D, Tang J, Zhang JH, 2008. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 29, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R, 2005. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 21, 775–7. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF, 2001. Spontaneous intracerebral hemorrhage. N Engl J Med. 344, 1450–60. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, 2007. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 1121, 355–75. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M, 1990. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 21, 801–7. [DOI] [PubMed] [Google Scholar]
- Saji M, Cohen M, Blau AD, Wessel TC, Volpe BT, 1994. Transient forebrain ischemia induces delayed injury in the substantia nigra reticulata: degeneration of GABA neurons, compensatory expression of GAD mRNA. Brain Res. 643, 234–44. [DOI] [PubMed] [Google Scholar]
- Sanchez HL, Silva LB, Portiansky EL, Herenu CB, Goya RG, Zuccolilli GO, 2008. Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience. 154, 1598–606. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Wong ST, Wozniak DF, Muglia LM, Liauw JA, Zhuo M, Nardi A, Hartman RE, Vogt SK, Luedke CE, Storm DR, Muglia LJ, 2000. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 20, 4809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver EB, Olsen TS, 1986. Tissue damage at computed tomography following resolution of intracerebral hematomas. Acta Radiol Diagn (Stockh). 27, 495–500. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Prashanth PS, Chakravarthy VS, 2006. The role of the basal ganglia in exploration in a neural model based on reinforcement learning. Int J Neural Syst. 16, 111–24. [DOI] [PubMed] [Google Scholar]
- Su CY, Chen HM, Kwan AL, Lin YH, Guo NW, 2007. Neuropsychological impairment after hemorrhagic stroke in basal ganglia. Arch Clin Neuropsychol. 22, 465–74. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Jadhav V, Ayer R, Chen W, Suzuki H, Zhang JH, 2009. Thrombin inhibition by argatroban ameliorates early brain injury and improves neurological outcomes after experimental subarachnoid hemorrhage in rats. Stroke. 40, 1530–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ, 1982. Spatial mapping: Definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 31, 271–276. [DOI] [PubMed] [Google Scholar]
- Tamura A, Kirino T, Sano K, Takagi K, Oka H, 1990. Atrophy of the ipsilateral substantia nigra following middle cerebral artery occlusion in the rat. Brain Res. 510, 154–7. [DOI] [PubMed] [Google Scholar]
- Thajeb P, Thajeb T, Dai D, 2007. Cross-cultural studies using a modified mini mental test for healthy subjects and patients with various forms of vascular dementia. J Clin Neurosci. 14, 236–41. [DOI] [PubMed] [Google Scholar]
- Titova E, Ostrowski RP, Sowers LC, Zhang JH, Tang J, 2007. Effects of apocynin and ethanol on intracerebral haemorrhage-induced brain injury in rats. Clin Exp Pharmacol Physiol. 34, 845–50. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jager HR, 2004. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 127, 2265–75. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH, 2007. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 61, 1067–75; discussion 1075-6. [DOI] [PubMed] [Google Scholar]