Abstract
Objectives
Dietary calcium and oxalate intake influences the development of calcium oxalate kidney stones. We hypothesized that an imbalance in the amount of calcium and oxalate in meals might impact gastrointestinal oxalate absorption and urinary oxalate excretion.
Methods
A study was conducted with ten non-stone forming adults placed on controlled diets with daily calcium and oxalate contents of 1000 mg and 750 mg, respectively. Subjects consumed a balanced calcium/oxalate ratio diet for one week, observed a minimum one week washout period, and subsequently consumed an imbalanced calcium/oxalate ratio diet for one week. Urine specimens were collected on the last four days of each diet. Outcome measures included urinary creatinine, calcium, and oxalate as well as the Tiselius index for assessing urinary calcium oxalate supersaturation.
Results
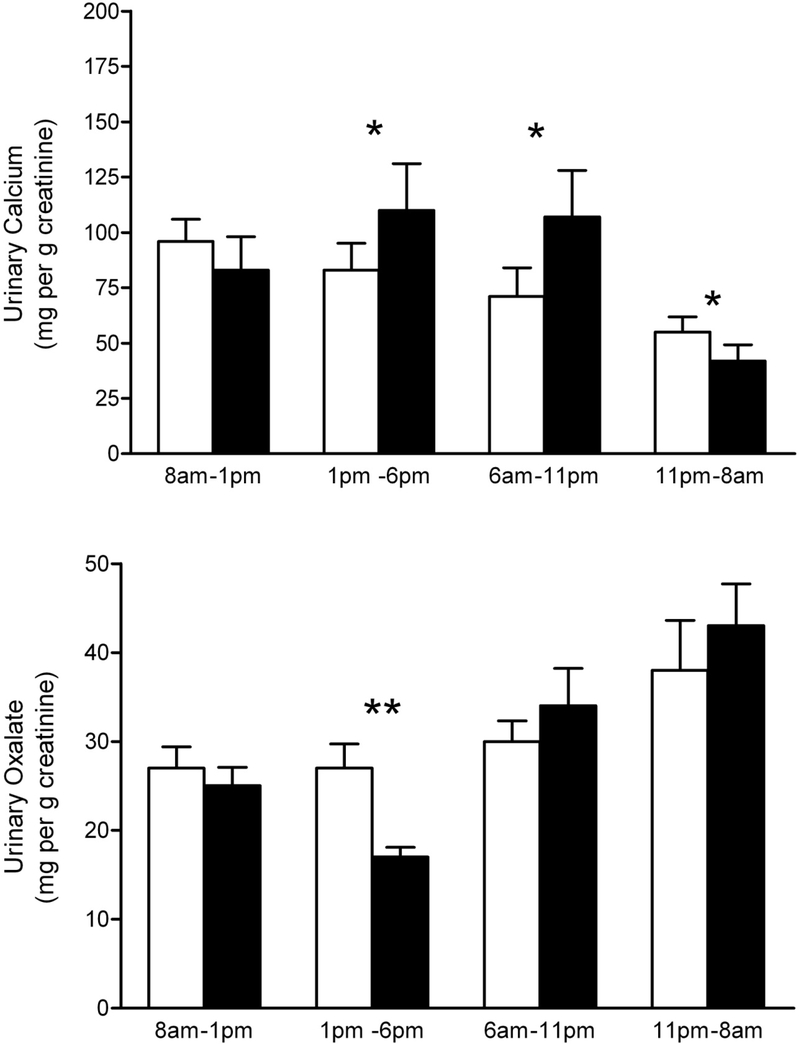
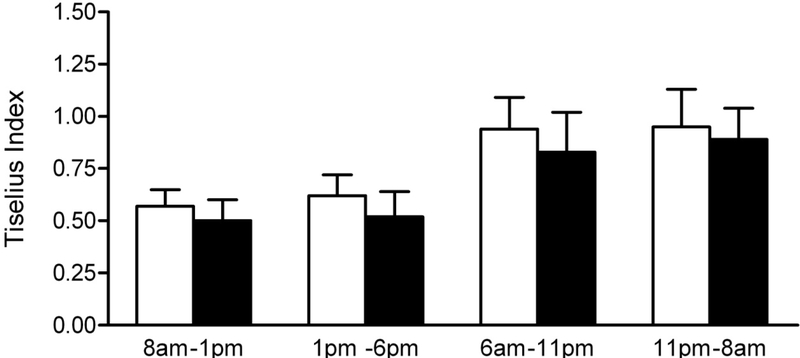
Total daily calcium excretion, oxalate excretion, and Tiselius index were similar between balanced and imbalanced dietary phases. There were significant differences in calcium excretion (mg/g creatinine) between balanced and imbalanced diets in the 1 – 6 p.m. (83.1 vs. 110.2, p<0.04), 6 – 11 p.m. (71.3 vs. 107.2, p<0.02), and 11 p.m. – 8 a.m. collections (55.0 vs. 41.8, p<0.02). There was significantly higher oxalate excretion on the balanced diet in the 1 – 6 p.m. time period (28.1 vs. 16.7, p<0.01). There were no differences in the Tiselius Index in these collections.
Conclusions
These results demonstrate that the sequence of ingesting relatively large amounts of oxalate does not significantly impact calcium oxalate stone risk if the recommended daily quantity of dietary calcium is consumed.
Keywords: Calcium, Oxalate, Nephrolithiasis, Diet
Introduction
Kidney stones are a common problem, affecting at least 5% of adults in the United States. 1 Calcium oxalate stones are the most common type, accounting for about 80% of all kidney stones, and increased urinary calcium and oxalate excretion promote the formation of such stones. 2, 3 Three large, epidemiologic cohort studies have demonstrated that limited dietary calcium consumption and elevated dietary oxalate intake are independent risk factors for stone formation.4–7 It has been demonstrated that approximately 50% of urinary oxalate is derived from dietary sources. Therefore, the amount of oxalate in one’s diet can significantly impact stone risk. 8
Curhan et al. have suggested that the amount of oxalate absorbed from the gastrointestinal tract is influenced by the amount of dietary calcium ingested, as calcium is thought to crystallize with oxalate, limiting its absorption and promoting its elimination via the fecal stream.4 As a result, the ratio of dietary calcium and oxalate consumption in meals could theoretically influence stone risk. We hypothesized that stone risk would be greater on a normal calcium/high oxalate diet if the amounts ingested were imbalanced in daily meals, as the imbalance would allow more soluble oxalate to be absorbed in the gastrointestinal tract. This has real world application, as many people consume moderate amounts of calcium and limited oxalate during breakfast and lunch and a large amount of oxalate relative to calcium at dinner.
Methods
After obtaining approval by the Institutional Review Board, ten healthy, non-stone forming adults (8 females, 2 males, mean age 30.8 ± 3.3 years, mean BMI 22.5 ± 2.8 kg/m2) were recruited and consented to participate in this study. Prior to their participation subjects were on self selected diets. During the study they consumed controlled diets with total daily calcium and oxalate contents of 1000 mg and 750 mg, respectively, at designated times (breakfast, 8 a.m.; lunch, 1 p.m.; dinner, 6 p.m.). This amount of calcium is the recommended dietary allowance for this age group, and the latter, while relatively high, is consistent with the amount of oxalate in a large spinach salad. The high oxalate content will accentuate the contribution of dietary oxalate to urinary oxalate excretion and diminish the contribution of endogenous oxalate synthesis. The balanced diet contained 333 mg of calcium and 250 mg of oxalate in all three daily meals, while the imbalanced diet contained 400 mg of calcium and 20 mg of oxalate at breakfast and lunch, and 200 mg of calcium and 710 mg of oxalate at dinner. Two different meal plans were alternated every other day during both phases to provide variety and aid in compliance. Daily meals were prepared in the metabolic kitchen of the Clinical Research Unit. Patients consumed a balanced calcium/oxalate ratio diet for one week, observed a minimum one week washout period, and then were placed on an imbalanced calcium/oxalate ratio diet for one week. No urinary collections were obtained during the washout phase. The average nutritional composition the diets are listed in Table 1. While the sodium intake was higher than what may be recommended for a kidney stone former, it approximates the average daily intake of an adult American and increased the palatability of meals for participants. Fluid intake was held constant at 1 ml/kcal per day for both dietary phases. Voided urine specimens were collected on the last four days of each diet and were subdivided into four collection periods: 8 a.m. – 1 p.m., 1 p.m. – 6 p.m., 6 p.m. – 11 p.m., and 11 p.m. – 8 a.m. Three days were allowed for equilibration and this is more than adequate based on recent unpublished work which we have done. Obtaining collections over 4 days helps to diminish the variability in daily calcium and oxalate excretion that is observed even on controlled diets. Urinary creatinine, calcium, oxalate, magnesium, and citrate were measured using the Beckman C5E Analyzer. Urine volume was also recorded (Table 2). The Tiselius index was calculated to estimate the calcium oxalate supersaturation in urine.9 Differences in outcome measures were determined using two-way repeated measures ANOVA and paired Student’s t tests.
Table 1 –
Average nutrient content of each diet
| Nutrient | Balanced Diet Average | Imbalanced Diet Average |
|---|---|---|
| Calories | 2511 | 2514 |
| Magnesium (mg) | 323 | 244 |
| Phosphorus (mg) | 1595 | 1482 |
| Sodium (mg) | 3833 | 3997 |
| Vitamin C (IU) | 210 | 157 |
| % Protein | 16 | 16 |
| % Fat | 30 | 30 |
| % Carbohydrate | 54 | 54 |
| Fluids (L) | 2.5 | 2.5 |
Table 2 –
Average Urine Volumes (ml)
| Collection Period | Balanced Phase | Imbalanced Phase |
|---|---|---|
| 8 a.m. – 1 p.m. | 675.5 | 640.8 |
| 1 p.m. – 6 p.m. | 510.6 | 492.3 |
| 6 p.m. – 11 p.m. | 348.1 | 511.7 |
| 11 p.m. – 8 a.m. | 539.3 | 531.2 |
Results
The mean excretions of calcium (109 ± 44, 112 ± 48 mg) and oxalate (44 ± 10, 45 ± 9 mg), and the Tiselius index for calcium oxalate supersaturation (3.1 ± 1.3, 2.7 ± 1.4) were similar between the balanced and imbalanced diet phases, respectively, when calculated over a 24 hr period. However, significant differences were observed in both calcium and oxalate excretion in the subdivided collections (Figure 1 and Table 3). Surprisingly, the imbalance in calcium and oxalate intake at 6 pm, did not influence oxalate excretion until the collection 19 – 24 hrs later. There was no difference in the calculated Tiselius index during any period. A time–related effect was observed with oxalate excretion and the Tiselius index being their highest and calcium excretion its lowest in the 11 pm – 8 am collection, on both balanced and imbalanced diets.
Figure 1.
Daily subdivided urine collection outcome measures. White bars, balanced diet. Black bars, imbalanced diet. Data expressed as mean ± SEM (N = 10). Single asterisk, p<0.05. Two asterisks, p<0.01.
Table 3 –
Subdivided collection ANOVA results
| Parameter | Degrees of Freedom | F value | P value |
|---|---|---|---|
| Urinary calcium excretion | |||
| -Balanced vs. imbalanced | 1 | 2.547 | 0.145 |
| -Change over time | 3 | 7.806 | <0.001 |
| -Interaction between diet type and time | 3 | 6.769 | 0.002 |
| Urinary oxalate excretion | |||
| -Balanced vs. imbalanced | 1 | 0.290 | 0.603 |
| -Change over time | 3 | 17.103 | <0.001 |
| -Interaction between diet type and time | 3 | 8.124 | <0.001 |
| Tiselius index | |||
| -Balanced vs. imbalanced | 1 | 1.991 | 0.192 |
| -Change over time | 3 | 8.146 | <0.001 |
| -Interaction between diet type and time | 3 | 0.0780 | 0.971 |
Comments
Interactions between dietary calcium and oxalate in the gastrointestinal tract are thought to influence the formation of calcium oxalate kidney stones. The ratio of calcium to oxalate is an important determinant of how much calcium oxalate crystallizes and how much calcium and oxalate is soluble and available for absorption. Studies of large cohorts of health professionals have demonstrated that reduced calcium consumption is a risk factor for the incident development of kidney stones.4–6 Furthermore, carefully controlled dietary studies and a randomized controlled study have shown that reduced calcium consumption increases urinary oxalate excretion.8–10 Based on this evidence it is reasonable to conclude that reducing calcium intake will increase the amount of soluble oxalate available for absorption.
Most studies examining the relationship between dietary calcium and oxalate have studied how much calcium and oxalate are excreted in urine in a 24 hour period. Such analyses provide an average determination of what may be large changes in excretions throughout that period. For instance, loading studies indicate that peaks in absorption and urinary excretion of both calcium and oxalate occur two to six hours after their ingestion.11–15 This time scale is compatible with this absorption occurring in the small intestine. Thus, twenty-four hour collections with three meals per day reflect three four hour periods of meal-induced ion absorption and twelve hours with lower ion absorption. If meals differ in their calcium and oxalate contents, it will create further variability in meal-influenced excretions. In a study of calcium oxalate stone formers, Ahlstrand et al confirmed this variable pattern in hourly urine collections.16 Given this information, we hypothesized that maintaining the same ratio of oxalate and calcium during each meal of the day would limit kidney stone risk as compared to consumption of these substances in an imbalanced manner.
Our results confirmed that the excretion of calcium and oxalate relative to creatinine varied throughout the day on both balanced and imbalanced diets. Notably, the excretion of calcium was lowest in the overnight fasting period of 11 p.m. – 8 a.m., whereas oxalate excretion was highest. With dietary imbalance, calcium excretion was significantly higher during the 1 p.m. – 6 p.m. and 6 p.m. – 11 p.m. periods. The meals at 8 a.m. and 1 p.m. in the imbalanced diet collectively contained 134 mg more calcium and 460 mg less oxalate than in the balanced diet. These changes in urinary calcium excretion are consistent with responses to changes in dietary calcium and oxalate that we recently reported.9 What is surprising is the extension of the effect to the period five to ten hours after the meals at 8 a.m. and 1 p.m. The high oxalate, low calcium meal at 6 p.m. on the imbalanced diet would be expected to exert the opposite response and decrease calcium excretion. A significant decrease in oxalate excretion was observed in the period 1 p.m. – 6 p.m. Some decrease in urinary oxalate excretion would be anticipated as it was observed 19 – 24 hours after the oxalate-rich meal, but the decrease of ~30% from the previous collection from 8 a.m. – 1 p.m. was much larger than expected. For instance, we have previously reported that when changing from a self-selected diet (150 – 200 mg oxalate/day) to a zero oxalate diet, urinary oxalate excretion decreases by only 19% in 24 hours.8 The bulk of the ingested oxalate should be in the colon 19 – 24 hours post ingestion and less sensitive to changes in absorption triggered by the calcium ingestion at 8 a.m. and 1 p.m. One possible hypothesis is that a significant portion of the 400 mg of calcium ingested at 8 a.m. and some of that ingested at 1 p.m. had reached the colon during the 1 p.m. – 6 p.m. period and suppressed oxalate absorption during that period. The ratio of gastrointestinal calcium to oxalate will determine the amount of calcium oxalate that is crystalline and unavailable for absorption and the amount of free oxalate available for uptake. It is also possible that these dietary factors could influence the gastrointestinal transport of calcium and oxalate whether it be via paracellular or transcellular routes.
Despite the changes in calcium and oxalate excretion produced by the imbalanced diet, calcium oxalate supersaturation as assessed by the Tiselius Index was not affected. The increased calcium excretion during the 1– 6 p.m. period during dietary imbalance was offset by a significantly lower oxalate excretion resulting in a level of calcium oxalate supersaturation similar to the balanced diet. The significantly higher calcium excretion during the 6–11 p.m. period of the imbalanced phase was counterbalanced by an increased urine volume relative to the balanced phase resulting in similar levels of calcium oxalate supersaturation. The significantly higher calcium excretion during the balanced 11 p.m. – 8 a.m. period was probably offset by a lower although not statistically significant oxalate excretion. Collectively, these results demonstrate that if an individual in this age group consumes at least the recommended amount of dietary calcium in a day, the stone risk impact of moderate to high dietary oxalate intake is not influenced by an imbalance in dietary calcium and oxalate intake.
Our results also demonstrate that excretions of urinary analytes and levels of supersaturation are not static. This has been demonstrated by other investigators. Ahlstrand and colleagues examined twenty-four hour urine collections in calcium oxalate stone formers.16 Similar to our results, they found a higher mean excretion of calcium during daytime hours. Our findings also mirrored theirs in that urine volume was lowest at night and in the early morning hours. We found urinary oxalate excretion increased throughout the day to a peak concentration at night, while their investigations revealed its highest concentration after meals and its lowest concentration at night and in the early morning. The discordance in these results is most likely a consequence of subjects in their study potentially consuming varied diets.
This study has certain limitations. Participants were healthy, non-stone forming adults, and it is possible that the response would be different in stone formers. The sample size was small due to the high costs of diet-controlled studies and the rigors such studies impose on participants. It is possible that different results would be obtained if the oxalate-rich meal was consumed at breakfast or lunch, rather than dinner. More frequent urine collections may also have allowed a better dynamic assessment of responses.
Conclusions
In conclusion, these results demonstrate that the sequence of consuming a moderate to large amount of food derived oxalate does not significantly impact calcium oxalate stone risk if the recommended daily quantity of dietary calcium is eaten.
Acknowledgments
Research Support: National Institutes of Health Grants R01 DK62284 and M01 RR07122
Footnotes
Conflicts of Interest: Jessica N. Lange – none
Kyle D. Wood – none
Patrick W. Mufarrij – none
Michael F. Callahan – none
Linda Easter – none
John Knight – none
Ross P. Holmes – none
Dean G. Assimos – none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 63: 1817–23, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Coe FL, Parks JH and Asplin JR The pathogenesis and treatment of kidney stones. N Engl J Med. 327: 1141–52, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Taylor EN and Curhan GC Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol. 3: 1453–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curhan GC, Willett WC, Rimm EB, et al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 328: 833–8, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Curhan GC, Willett WC, Knight EL, et al. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 164: 885–91, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 126: 497–504, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Taylor EN and Curhan GC Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. 18: 2198–204, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Holmes RP, Goodman HO and Assimos DG Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 59: 270–6, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J, Knight J, Easter LH, et al. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol. 186: 135–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 346: 77–84, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Heller HJ, Stewart A, Haynes S, et al. Pharmacokinetics of calcium absorption from two commercial calcium supplements. J Clin Pharmacol. 39: 1151–4, 1999. [PubMed] [Google Scholar]
- 12.Karkkainen MU, Lamberg-Allardt CJ, Ahonen S, et al. Does it make a difference how and when you take your calcium? The acute effects of calcium on calcium and bone metabolism. Am J Clin Nutr. 74: 335–42, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Holmes RP, Ambrosius WT and Assimos DG Dietary oxalate loads and renal oxalate handling. J Urol. 174: 943–7; discussion 947, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Prenen JA, Boer P and Dorhout Mees EJ Absorption kinetics of oxalate from oxalate-rich food in man. Am J Clin Nutr. 40: 1007–10, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Knight J, Holmes RP and Assimos DG Intestinal and renal handling of oxalate loads in normal individuals and stone formers. Urol Res. 35: 111–7, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlstrand C, Larsson L and Tiselius HG Variations in urine composition during the day in patients with calcium oxalate stone disease. J Urol. 131: 77–81, 1984. [DOI] [PubMed] [Google Scholar]