INTRODUCTION
Identification of reliable Helicobacter pylori eradication therapy has proved difficult, in part because brief exposure of H. pylori to commonly used antimicrobials such as macrolides, nitroimidazoles or quinolones often results in resistance (bystander effect). Most treatment studies and meta-analyses contains major flaws preventing generalisability that making reliable treatment recommendations and guidelines an illusion (box 1).
Box 1. Helicobacter pylori treatment illusions.
-
►
Most apparently well done treatment studies and meta-analyses are valid.
-
►
Studies reporting one regimen as superior to another can generally be believed.
-
►
Meta-analyses identifying the best H. pylori treatment regimen can generally be believed.
-
►
Treatment results without susceptibility testing are generally valid.
-
►
High overall cure rates validate use of successive low cure rate first, second and third-line treatments.
-
►
Increasing the number of antibiotic to 3 or 4 (eg, concomitant or quintuple therapies) is a rational approach to overcoming resistance.
-
►
Commercially available regimens have generally been optimised.
Development of H. pylori therapy differs from other infectious diseases. Since the advent of antibiotics, infectious diseases therapy has been susceptibility based, whereas most H. pylori treatment guidelines recommend susceptibility testing only after two empiric therapy failures. Increased penicillin resistance in the 1970s prompted rapid changes in recommendations and the development of antimicrobial surveillance programme to regularly update recommendations thus allowing empirical therapies to remain effective.1 Despite increasing resistance, H. pylori treatment guidelines have continued to recommend increasingly ineffective therapies and most new empiric therapies consist of variations using those same drugs (eg, sequential therapy). Treatment success has focused on comparisons between regimens irrespective of cure rates and without consideration of the antibiotic susceptibility profile of the infection, thus producing illusions of success. For example, sequential therapy consists of 5 days of dual proton pump inhibitor (PPI)-amoxicillin therapy followed by 5 days of PPI-clarithromycin and metronidazole triple therapy (Bazzoli’s triple therapy).2 Interestingly, sequential therapy was never compared with Bazzoli’s triple therapy. Studies repeatedly attempted to prove superiority of sequential therapy over triple therapy until more than 1000 patients were studied.3 Meta-analyses compiled these results and together formed the basis for recommendations and guidelines. In regions where metronidazole or metronidazole-clarithromycin resistance is common, sequential therapy produced poor results and was eventually abandoned.4 If a susceptibility-based approach had been used, potential limitations of treatment regimens could have been pinpointed, thereby destroying illusions, obviating testing for superiority and preventing studies where failure was inevitable.
ILLUSIONS OF VALID H. PYLORI TREATMENT STUDIES
Many, even most, of technically well-done clinical H. pylori trials and meta-analyses are fatally flawed. Studies judged excellent in terms of randomisation, blinding, size, and so on often involved groups that differed in antibiotic susceptibility (box 2). Valid results require treatment groups be equivalent in all important variables. The fact that studies showing that a regimen proven to cure ≥95% of susceptible infections (eg, 14-day triple therapy) was inferior to one that cured approximately 94% in the same population (eg, 10-day sequential therapy) were paradoxical was ignored.4 When effectiveness of one treatment is more affected by resistance than another, the results are applicable only to the study population and cannot be generalised. Thus, even if successfully concluded, the trial cannot disturb clinical equipoise and ‘convincingly resolve the dispute among clinicians’.5 Feinstein called this type of research ‘fastidious trials’ which while “designed to resolve some theoretical question, fail to satisfy the second ethical requirement of clinical research, since the special conditions of the trial .... render it useless for influencing clinical decisions”.5
Box 2. Clues to probable invalid Helicobacter pylori treatment studies.
-
►
Poor cure rates with therapies that produce excellent results with susceptible infections.
-
►
One treatment superior to another when both produce excellent results with susceptible infections.
-
►
Treatment results devoid of susceptibility results (results are not generalisable).
-
►
Cure rates below 80% with a reliable therapy.
-
►
Poor results without a proposed mechanism.
Valid studies attempt to answer an important unanswered question. Many published studies provide sample size calculations that confirmed that the outcome could be accurately predicted and it was unclear whether this information was shared honestly with the subjects (ie, a requirement for informed consent).6 Freedman noted ‘if a physician knows that these treatments are not equivalent, ethics requires that the superior treatment be recommended’.5
Few would accept the results of a comparison of therapies of a urinary tract or skin infection reporting that an excellent therapy proved to be inferior for treatment of resistant organisms.7 In contrast, the H. pylori literature is replete with superiority claims and meta-analyses when one or both otherwise excellent regimens produced poor results due to resistance.8 Comparative trials in the infectious disease literature are confined to infections susceptible to the regimens tested and employ regimens that produce good to excellent cure rates. The analyses are based on non-inferiority.8,9 I have been unable to find studies with other infectious diseases that included a population known to have significant resistance to the drug used. In contrast to most digestive disease problems, treatment failures of previously successful antibiotic regimens can almost always be attributed to resistance organisms, poor choice of doses, duration of therapy, and so on.
The Infectious Disease Society of America has grappled with the problem of trying to do an ethical antimicrobial superiority trial.9 Their conclusion was that “active-controlled superiority studies of antibacterial agents are ethical to conduct only if 1) the control (ie, the comparator drug) is active against most, or all, of the bacterial strains likely to be encountered in the study; 2) all available drugs that could be used as comparators for the study are inadequately active against the strains likely to be encountered, such that there is no alternative effective therapy possible; or 3) the infection under study is almost universally non-fatal, such that rescue therapy can be instituted rapidly enough to preclude serious sequelae upon recognition that the strain causing the infection is resistant to the comparator drug (eg, uncomplicated urinary tract infection). The susceptibility of etiologic bacteria is almost never known at the time an infected patient is enrolled in a clinical trial that evaluates initial antimicrobial treatment. Therefore, the comparator drugs chosen for study in antibacterial clinical trials are selected because they are anticipated to be effective against all, or almost all, strains likely to be encountered during conduct of the study. Yet, antibacterial therapy is generally so effective when treating infections caused by susceptible bacteria, it is unlikely that investigational therapy can achieve superiority to a marketed comparator drug when the infections under study are susceptible to both drugs”.9 Based on these criteria, few H. pylori treatment trials would be considered ethical.
HOW ILLUSIONS ARISE
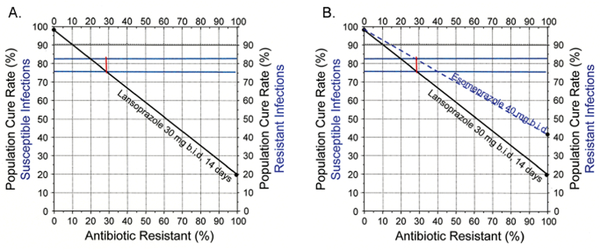
It has been suggested that second-generation PPIs produced better cure rates than first generation PPIs with triple therapy.10 That conclusion was based on studies in populations where resistance produced relatively poor cure rates. Figure 1 shows a theoretical non-inferiority trial that randomised 1600 subjects to compare a first and second-generation PPI in triple therapy (ie, 30 mg lansoprazole (equivalent of 27 mg of omeprazole) vs 40 mg esomeprazole (equivalent to 64 mg of omeprazole)) both given twice a day for 14 days.11 Susceptibility results were available after completion. The sample size was based on published cure rates with susceptible infections (approximately 98% for both regimens). PPI plus amoxicillin dual therapy (ie, with clarithromycin resistant infections) was known to be responsive to PPI effectiveness, cure rates with resistant infections in western populations being approximately 20%. Overall, the trial produced cure rates of 76% versus 82%, p = 0.004 for lansoprazole versus esomeprazole and the authors concluded that second generation PPIs produced superior results with triple therapy.
Figure 1.
Effect of resistance on cure rates with two different proton pump inhibitors. First, the cure rates achieved by the studies (76% and 82%) are drawn horizontally (in green). (A) shows the results with lansoprazole based on 98% cured with all susceptible and 20% with clarithromycin resistant infections. Randomisation ensured that the proportion with resistance is similar between groups, thus the point there the cure rate crosses the 76% cure rate identifies the mean proportion with resistance for both groups. (B) Because the groups were drawn from the same population, the proportion with resistance is the same. The cure rate for the more potent PPI must cross the cure rate line (82%) at the same proportion with resistance. When extended, it shows the cure rate with resistant infections was ~40%.
The results are plotted on an H. pylori treatment nomogram (figure 1).12 The cure rates with susceptible strains were approximately 98%. The differences between regimens relate to differences in the prevalence of clarithromycin resistance in the population (range 0% to 20%). Because the trial results are population specific, they were not generalisable. A valid comparison of first and second-generation PPIs would include accounting for susceptibility, duration of therapy, doses and relative effectiveness and each type of antisecretory therapy. For example, while the outcome for 7-day clarithromycin triple therapy with susceptible infections might prove superior with 40 mg compared with 20 mg of omeprazole, it could be non-inferior with 14-day therapy.
UNRAVELLING ILLUSIONARY RESULTS
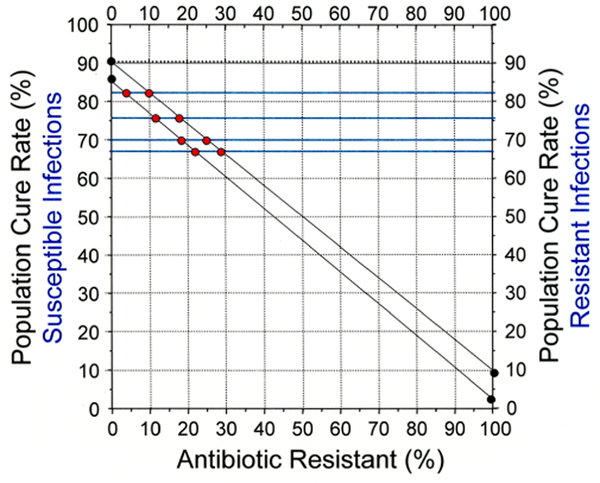
Despite the absence of susceptibility-specific results, the approximate prevalence of resistance and other results can often be determined using simple formulas13 or an Hp-nomogram.12 Our example uses data from a specific meta-analysis14; however, hundreds of others would have served equally well. We examine results of 10 day PPI—lev ofloxacin—amoxicillin triple therapy in western populations. Levofloxacin resistance is all or none (ie, resistance leaves only PPI—amoxicillin dual therapy). The cure rate with 10-day therapy with susceptible infections likely ranges between 80% and 90%, and 14-day therapy is required to reliably achieve cure rates >95% with susceptible infections.4 The 4 trials of 10-day therapy produced cure rates of 67%, 70%, 76% and 83%, suggesting that levofloxacin resistance was present although the proportion varied among trials. We assume the cure rate with 10-day therapy is above 83%, the highest cure rate reported. The cure rate with the PPI—amoxicillin component with 10-day therapy is estimated between 0% and 10%.4 Figure 2 shows an H. pylori treatment nomogram based on levofloxacin susceptible and resistant cure rates that encompass the expected range (ie, 85% and 3% and 80% and 10%). Individual data are plotted where individual cure rate cross the two population cure rates (figure 2). The model shows levofloxacin resistance ranged from ~3 to ~30% and differed among studies. The plots confirm that these four studies each produced population-specific results and thus could not be combined into a meaningful meta-analysis. The meta-analysis has been cited in three recent treatment guidelines.15–17
Figure 2.
Cure rates with 10-day levofloxacin triple therapy unravelled. The cure rates 67%, 70%, 76% and 83% are drawn horizontally in green. The estimated maximum and minimum cure rates with susceptible (85% and 80% and resistant strains 10% and 3%) are marked and connected with solid lines. The results for each of the four studies is then marked where the cure rates cross the population cure rate for different proportions of resistance. The results show that each of the four studies varied in relation to the proportion with levofloxacin resistance. Combining the results would result in a Helicobacter pylori meta-analysis.
ILLUSION OF HIGH CUMULATIVE HIGH CURE RATES AS A MEASURE OF SUCCESS
Publications have reported success in achieving high-population cure rates by implementing three different multidrug treatment regimens as first through third- line regimens (eg, references18–21). This strategy of successive treatments with low cure rate regimens produces an illusion of success. For example, successive use of three different regimens each with a 75% cure rate would provide an overall 98% treatment success (provided all patients continued) and require 131 courses of therapy (table 3), a cure rate of 60% require 156 treatments and a 50% cure rate require 175 treatments. Depending on the number of antibiotics used per regimen and the number of different antibiotics used, the number of antibiotics may not necessarily be increased but the total antibiotic exposure will (ie, days of use times number of individuals). Antimicrobial resistance correlates with antibiotic usage and multiple treatments should be avoided.22 The use of many different antibiotics also amplifies the problem antimicrobial misuse.1,23 Achieving the highest cure possible on the first try reduces population exposure to antibiotics, decreases the risk of side effects, the probability of drop-outs or being lost to follow-up.
Table 3.
Number of treatments required to achieve high cure rates with the strategy of sequential use of a first, second and third-line low cure rate (75% to 50%) therapies
| cure rate | Therapy | Total | ||
|---|---|---|---|---|
| Number cured | Initial | Second | Third | % |
| 75% cured | 100 | 25 | 6 | 131 |
| Number cured | 75 | 19 | 4.5 | 98.5% |
| 60% cured | 100 | 40 | 16 | 156 |
| Number cured | 60 | 24 | 9.6 | 93.6% |
| 50% cured | 100 | 50 | 25 | 175 |
| Number cured | 50 | 25 | 12.5 | 87.5% |
THE MULTIPLE ANTIBIOTIC SUCCESS ILLUSION
One principle of antibiotic use is to use the most effective therapy with the narrowest spectrum for the shortest time.24 One approach to the lack of susceptibility testing has been to increase the number of antibiotics to three or more with the hope that the patient’s infection will be susceptible to some combination (eg, concomitant or quintuple therapies).1,25 Success produces the multiple antibiotic illusion. Another version is treatment success despite the high prevalence of resistance as seen with vonoprazan clarithromycin triple therapy in Japan.23 In that instance, the results with PPI and vonoprazan with susceptible strains were essentially identical.26 In the overall population, the high cure rate with vonoprazan and amoxicillin obscured the fact that approximately 80% of patients achieve no possible benefit from clarithromycin. This currently results in approximately 1 million unnecessary antibiotic regimens per year amid falling cure rates as resistance continues to increase.1 Meta-analyses comparing vonoprazan and PPI triple therapies are Hp-shmeta-analyses.27 Current pragmatic approaches of attempting to deal the problem of resistance without susceptibility testing, are poorly thought out and ensure that most, if not all, subjects receive at least one unnecessary antibiotic which contributes to global antibiotic resistance.1,23 Our alternate names for concomitant three antibiotic and quintuple four antibiotic therapies are ‘hope’ and ‘prayer’, respectively 1,23
ILLUSION OF OPTIMUM OR OPTIMISED THERAPY
‘Optimised’ has been used to describe H. pylori therapies. We define optimised as a therapy with reliably excellent results which cannot be improved by changing dose, formulation, duration, and so on. It should also be the most cost-effective. Most optimised therapies will reliably achieve ≥95% cure rates with susceptible infections in adherent patients. Investigators and companies often succumb to the illusion they can dictate to H. pylori how it should respond to therapy (thus, 7-day triple, 10-day sequential or 10-day bismuth quadruple therapy (Pylera)).7,28 This approach may achieve ‘good enough’ but rarely achieves optimal results.4,7 Ten-day Pylera therapy is an example; with metronidazole susceptible infections, 7-day bismuth quadruple is likely sufficient, whereas with resistant strains 14 days is best (ie, the average patient receives too much or too little).7,29,30 Optimal therapies can only be identified by experiment and not by committee.31
THE MASTER ILLUSION: HP-SHMETA-ANALYSES
Shmeta-analysis was coined by Shapiro in his discussion of the limitations of meta-analysis for observational studies.32 We define an Hp-shmeta-analysis as an H. pylori meta-analysis that produced erroneous conclusions.8 An Hp-shmeta-analysis is a special form of meta-analysis typically used with clinical trials in which the proportion with resistance is unknown (ie, apples versus oranges-type comparison) (box 3).8,32 A number of such studies are combined to produce a Hp-shmeta-analysis. In many instances, at least one group achieved unacceptable cure rates (eg, <80%). The results are expressed as OR or HR which are prominently displayed, obscuring the actual cure proportions. As a result, the goal of these Hp-shmeta-analyses appears to identify the better of two bad therapies or the least worst therapy. Most meta-analyses of H. pylori therapy are Hp-shmeta-analyses which are also commonly used to support H. pylori guidelines.
Box 3. Characteristics a Helicobacter pylori shmeta-analysis.
-
►
Methodology: meta-analysis.
-
►
Study type: H. pylori therapy comparison.
-
►
Study groups: inappropriately matched for resistance.
-
►
Results: invalid.
-
►
Conclusions: misleading and often clinically useless.
SUMMARY
While the details of therapy are important (drugs, doses, duration, etc), the most important detail is to ensure that the organism is susceptible to the antibiotics used. Most comparative studies and meta-analyses report illusions of reliable data, based on trials in which antimicrobial resistance is present but not taken into account which make the results trial specific and useless for informing clinical decisions. Comparisons among trials where differences are dependent on the presence of antibiotic resistance populations are invalid. Because study-specific results cannot be generalised, they should not be published, quoted nor used for meta-analyses. Both the recent ACG and Toronto treatment guidelines are largely based on Hp-shmeta-analyses.15,17 The Maastricht guidelines are the least flawed, more nuanced and provide more data related to the effects of resistance.16 Illusions arose because gastroenterologists have attempted to develop treatments in the absence of susceptibility data. In ‘My Fair Lady’, Henry Higgins asked “Why can a woman not be more like a man?”, we ask “Why can gastroenterologists not be more like infectious disease doctors?”.
Acknowledgements
The author thanks Bich Dang and Aylin Tansel for insights into treatment of infectious diseases and meta-analysis, respectively.
Funding DYG is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
competing interests DYG is a consultant for RedHill Biopharma regarding novel Helicobacter pylori therapies and has received research support for culture of H. pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies.
provenance and peer review Commissioned; internally peer reviewed.
REFERENCES
- 1.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol 2017;14:383–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzoli F, Zagari RM, Fossi S, et al. Short-term low- dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 1994;6:773–8. [Google Scholar]
- 3.Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003;17:719–26. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY, Lee YC, Wu MS, Ms W. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014;12:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman B Equipoise and the ethics of clinical research. N Engl J Med 1987;317:141–5. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin Gastroenterol Hepatol 2010;8:1032–6. [DOI] [PubMed] [Google Scholar]
- 7.Dang BN, Graham DY. It is time to rethink H. pylori therapy. J Gastrointestin Liver Dis 2017;26:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016;14:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infectious Diseases Society of America. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 2012;55:1031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2012;36:414–25. [DOI] [PubMed] [Google Scholar]
- 11.Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009;65:19–31. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Hp-normogram GDY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2016;21:85–90. [DOI] [PubMed] [Google Scholar]
- 13.Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol 2014;8:21–8. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 2006;23:35–44. [DOI] [PubMed] [Google Scholar]
- 15.Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016;151:51–69. [DOI] [PubMed] [Google Scholar]
- 16.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection- the Maastricht V/Florence consensus report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 17.Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212–39. [DOI] [PubMed] [Google Scholar]
- 18.Rokkas T, Sechopoulos P, Robotis I, et al. Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am J Gastroenterol 2009;104:21–5. [DOI] [PubMed] [Google Scholar]
- 19.Gasbarrini A, Ojetti A, Armuzzi V, et al. Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment Pharmacol Ther 2000;14:79–83. [DOI] [PubMed] [Google Scholar]
- 20.Gisbert JP, Gisbert JL, Marcos S, et al. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment Pharmacol Ther 2008;27:346–54. [DOI] [PubMed] [Google Scholar]
- 21.Graham DY, Calvet X. Guide regarding choice of second-line therapy to obtain a high cumulative cure rate. Helicobacter 2012;17:243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34–42. [DOI] [PubMed] [Google Scholar]
- 23.Shiotani A, Lu H, Dore MP, et al. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med 2017;84:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branquinho D, Almeida NMP, Gregorio C, et al. Twelve-day quintuple regime containing four antibiotics as a rescue therapy for Helicobacter pylori eradication in the central region of Portugal. RevEsp Enferm Dig. In Press. 2017;109:109. [DOI] [PubMed] [Google Scholar]
- 26.Graham DY Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 2017;66:384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther 2017;46:106–14. [DOI] [PubMed] [Google Scholar]
- 28.Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013;381:205–13. [DOI] [PubMed] [Google Scholar]
- 29.Dore MP, Lu H, Graham DY Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Lee SY How to effectively use Bismuth Quadruple Therapy: The good, the bad, and the ugly. Gastroenterol Clin North Am 2015;44:537–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham DY Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol 2009;7:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro S Meta-analysis/Shmeta-analysis. Am J Epidemiol 1994;140(9):771–8. [DOI] [PubMed] [Google Scholar]