Abstract
Purpose of Review
The paper aims to discuss the global trends in gastric cancer incidence in relation to important factors involved in the pathogenesis of gastric cancer.
Recent Findings
Despite a significant worldwide decline, gastric cancer remains a common cause of cancer death. The decline has been multifactorial and preceded the fall in Helicobacter pylori prevalence. The initial decline was associated with changes in food preservation and availability, especially of fresh fruits and vegetables, followed by a decline in the primary etiologic factor, H. pylori. Gastric cancer incidence remains high in East Asia, intermediate in Latin America, and low in developed countries. Significant racial/ethnic variability exists.
Summary
The rapid decline in incidence in East Asia will continue as primary and secondary prevention strategies are implemented. The incidence in Latin America is unlikely to decline significantly over the next few decades given high H. pylori prevalence in the young. Ultimately, global H. pylori eradication will be needed to largely eliminate gastric cancer.
Keywords: Gastric cancer, Disparities, Helicobacter pylori, Epidemiology, Risk factors, Natural history
Introduction
Gastric cancer incidence has declined significantly worldwide over the past half-century. Nevertheless, gastric cancer remains a global health problem as the fifth leading cancer and third most common cause of cancer-related deaths worldwide [1••]. Even today, gastric cancer incidence and mortality remain disproportionately high in East Asia, Latin America and Eastern Europe and within specific subgroups in the USA. Much of the reduction in gastric cancer incidence occurred coincident with economic improvements resulting in improved sanitation, hygiene, clean water supplies, and advances in food preservation, variety, and availability. In regions with currently high gastric cancer incidence, primary and secondary prevention strategies have helped reduce gastric cancer mortality [2••, 3••, 4].
Here, we review temporal changes, regional variations, and racial/ethnic disparities (specifically within the USA) in gastric cancer incidence and mortality. We focus on non-cardia gastric cancers and exclude adenocarcinoma of the esophagus related to Barrett’s esophagus. We also discuss putative causes for global changes and variations in gastric cancer burden across populations. Finally, we present primary and secondary prevention strategies with proven effectiveness and propose future directions for reducing gastric cancer incidence and fatality among populations that still experience a high burden of gastric cancer.
Gastric Cancer Subtypes
Gastric adenocarcinoma accounts for approximately 90–95% of all stomach cancers, with lymphoma, leiomyosarcoma, gastrointestinal stromal tumor, and neuroendocrine tumors accounting for the remainder [5]. Gastric adenocarcinoma, although frequently discussed as a singular entity, actually encompasses two anatomic subtypes, cardia gastric cancer and non-cardia gastric cancer (here called gastric cancer), with distinct clinical and epidemiological characteristics. Cardia gastric cancer is defined as an adenocarcinoma in the proximal stomach located within 5 cm of and involving the gastroesophageal junction [6]. Cardia gastric cancer is associated with obesity, long-standing gastroesophageal reflux, and possibly tobacco smoking. Within the USA, it has the highest incidence among Caucasian men. Cardia cancer has a clinical course and epidemiology similar to that of esophageal adenocarcinoma [7] and a worse long-term survival than that of noncardia gastric cancer [8•]. Cardia gastric cancer incidence has remained stable or increased within specific subgroups [9].
Typical non-cardia gastric cancers encompass tumors arising more distally and are etiologically associated with Helicobacter pylori infection, tobacco smoking, and high dietary salt intake. A small proportion is related to EBV infection. Overall, non-cardia cancer has declined worldwide and is subtyped histologically into intestinal and diffuse types using the Lauren classification [10, 11]. Both types are associated with H. pylori infection which is involved with progressive and long-standing mucosal inflammation, followed by mucosal atrophy ultimately culminating in intraepithelial and finally advanced neoplasia. Gastric cancer is associated with well-defined precancerous lesions of mucosal atrophy and metaplasia. These can be readily identified endoscopically and confirmed by histology. Diffuse-type gastric cancer is strongly associated with loss of E-cadherin expression. Most diffuse-type cancers are also associated with atrophic gastritis but a definite precancerous lesion has not been identified. Diffuse-type gastric cancer includes signet ring adenocarcinoma which is also associated with non-H. pylori-associated hereditary gastric cancer. These are typically diagnosed in younger patients and carry a poor prognosis [10].
Gastric Cancer Pathogenesis and Risk Factors
Most cases of gastric cancers are sporadic and etiologically related with H. pylori infection. H. pylori is a class I carcinogen [12] that is estimated to account for approximately 89% gastric cancer cases (at least 95% of those in high gastric cancer incidence areas) [13]. H. pylori virulence, differences in host inflammatory response to infection, and specific environmental exposures additionally influence gastric cancer risk.
A strong association between gastric atrophy and gastric cancer has long been observed [14••]. The discovery of H. pylori in 1983 and subsequent demonstration that it was the main cause of gastritis culminated the long search for the cause of gastritis which had long been known to be tightly associated with gastric atrophy and with gastric cancer. H. pylori infections are usually acquired during childhood and typically remain latent for decades. Over the duration of a latent H. pylori infection, progressive gastric mucosal damage may occur resulting in progressive gastric atrophy which is associated with the development of metaplastic epithelia; first, as a progressive lawn of pseudopyloric metaplasia (now called spasmolytic polypeptide expressing mucosa or SPEM). Patches of intestinal metaplasia may then appear within the atrophic area. These islands of intestinal metaplasia give rise to the misnomer of a multifocal process [15]. Foci of dysplasia may appear within the lawn of atrophic mucosa which may then progress to invasive gastric neoplasia. In most developed countries, gastric cancer is a relatively rare event with only 1 to 3% of H. pylori infections progressing to gastric cancer [16]. In other countries, such as Japan and Korea, and in parts of China, the lifetime risk of gastric cancer is much higher and often may exceed 10%. The country or region-wide risk of gastric cancer directly relates to the proportion of the population that develops atrophic gastritis and the rate in which it develops [17]. Once atrophic gastritis is present, the annual risk of developing gastric cancer ranges increases to about 1% [15, 18].
H. pylori infection is considered a necessary but insufficient cause of gastric cancer as there are other factors that modulate the risk. For example, in India and tropical countries there is discordance between a relatively high H. pylori infection prevalence and low gastric cancer incidence (gastric cancer age-adjusted incidence in India: 7.8/100,000 and 6.1/100,000, respectively among males and females). In contrast, in Japan and China where H. pylori prevalence is also high, the gastric cancer incidence ranges from 62 to 69/100,000 and 26/100,000, respectively, among males and females [19•].
While H. pylori virulence and the host response to infection modulate gastric cancer risk [10], the dominant factor is environmental, most likely related to diet [20]. There has been considerable effort expended attempting to identify specific H. pylori virulence factors that increase the risk of gastric cancer. None have appeared, and overall, the data support the notion that H. pylori virulence factors that have been associated with gastric cancer share the common property of causing an increased host inflammatory response that underlies the risk increase in gastric cancer [21••]. The H. pylori virulence factors most often identified are the vacuolating cytotoxin, VacA, and the cytotoxin-associated antigen, CagA. However, even the most avirulent H. pylori strains have been associated with development of gastric cancer and peptic ulcer; the presence of the most virulent strains approximately doubles the risk [21••]. Host factors associated with an increased risk of gastric cancer are those associated with an increased inflammatory response to the infection. One example is the pro-inflammatory interleukin (IL)-1. Some IL-1 genotypes are associated with an increased risk of gastric cancer whereas others are associated with reduced inflammation and reduced risk [21••]. For example, those infected with a virulent H. pylori strain and a pro-inflammatory IL-1 have been reported to have up to an 87-fold higher risk of gastric cancer [22].
Temporal Tends in Gastric Cancer Incidence and Mortality
Until the second half of the twentieth century, most gastric cancer epidemiological data came from local or regional epidemiological observations and fledgling local cancer registries predominantly in Northern America and Western Europe. Formal cancer registries developed more widely in the mid-1900s and global-level cancer tracking started with the launch of the International Association of Cancer Registries (IACR) in 1966 [23]. Since then, various estimation methods have been used to derive global cancer statistics from national, regional, or local-level cancer registry data [24]. A well-recognized limitation in global cancer data has been a paucity of high-quality, and in some cases any, data from low- and middle-income countries leading to potential underrepresentation of trends within those regions. Though a persistent problem, the number of countries included in global cancer estimations has recently expanded considerably from 29 to 184 [23, 25••].
Gastric cancer was common in the nineteenth century, and in the first half of the twentieth century, it was the most common cause of cancer death in Western countries [20]. For example, in 1919, James Ewing, who provided some of the earlier summary statistics of gastric cancer prevalence, reported that gastric cancer constituted 10% of 4131 cancer cases in Vienna, to as high as 41.5% of 27,511 cancers Switzerland; he also reported a relatively high mortality rate based on the 1912 US Census findings of 46,534 cancer-related deaths, 39.8% of which were stomach and liver in origin [26].
A decade later, as part of the Schorstein Lecture, Sir Arthur Hurst highlighted the continued high incidence and mortality of gastric cancer commenting that “every year about sixteen thousand people die in Great Britain from cancer of the stomach. It is the cause of death in 5 out of every 100 people dying after the age of 40, and accounts for about one-third of all deaths from carcinoma” [27]. Contemporary reports from regions within the USA also documented high gastric cancer incidence and mortality [28].
Gastric cancer continued to be the leading cause of cancer and cancer-related death until 1975. While the overall incidence did decrease over that time period, there was significant geographic variability [29]. Between 1930 and 1955, gastric cancer age-adjusted mortality fell in the USA, the Netherlands, and Canada. Conversely, in Japan during that same time period, gastric cancer incidence remained stable and cancer mortality actually rose [30]. By 1972, the standardized gastric cancer incidence rate per 100,000 among persons 35 to 64 years ranged from 14 to 35 in the USA, 31 to 67 in Europe, 110 to 160 in USSR, and 155 to164 in Japan [31••].
In 1975, gastric cancer had declined to where it represented the second leading cause of cancer worldwide, after lung cancer. This change was the result of both a rise in lung cancer and decline in gastric cancer incidence [32•]. From 1980 to 2011, gastric cancer mortality rates continued to fall worldwide although with considerable regional variation in the direction and magnitude of change [33•]. Japan, Korea, the European Union (EU), and Russia had the most significant declines in gastric cancer mortality between 2000 and 2009 (estimated annual percent changes [EAPC] in men and women, respectively: Japan −3.1 and −3.9%; Korea −4.1 and −4.7%; EU −3.7 and −3.4%; Russia −3.0% in both sexes). In Japan, Korea, Russia, and the UK, the greatest reduction in mortality rates was observed among persons aged 35 to 64 years of age [33•]. Meanwhile, there was a leveling off in mortality rates in the USA and France. Notably, Japan, Korea, and Russia are among the countries with the greatest reductions in gastric cancer mortality over 1980 to 2011. They were also those with a higher proportion of non-cardia gastric cancer compared to cardia gastric cancer.
At present, gastric cancer is the fifth most incident cancer and the third leading cause of cancer deaths worldwide [34]. In 2012, the global age-standardized gastric cancer incidence rate was 12.1/100,000 [32•]. The total number of newly diagnosed gastric cancer cases was estimated at 952,000 representing 6.8% of all newly diagnosed malignancies and accounting for 8.8% of cancer-related deaths [34]. Incidence of gastric cancer occurs approximately twice as frequently in men compared to that in women with most cases occurring after the age of 60 [10, 32•]. Despite the decline in incidence, gastric cancer still has a dismal, 20%, 5-year survival rate [35••, 36] and high case fatality rate of74.5% throughout most of the world [19]. Gastric cancer is a main contributor to disability-adjusted life-year burden and early deaths from cancer and has a significant impact on overall health and life expectancy throughout the world [37].
Current Geographic Trends in Gastric Cancer Incidence and Mortality
There are significant regional differences in current gastric cancer incidence and mortality. The highest rates are observed in East Asia, Eastern and Central Europe, and South and Central America. Gastric cancer rates are also significantly lower in more economically developed regions of the world than in less developed (age-standardized incidence rate [ASIR] per 100,000: men = 15.6 vs. 18.1; ASIR women = 6.7 vs. 7.8; age-standardized mortality rate [ASMR] per 100,000: men = 9.2 vs. 14.4; ASMR women 4.2 vs. 6.5) [34]. More than 70% of gastric cancer cases occur in less-developed countries.
Currently, East Asia carries most of the world’s gastric cancer burden. In 2012, China, Japan, and Korea accounted for 60% of all newly diagnosed gastric cancer. Gastric cancer ASIRs in Korea, Mongolia, Japan, and China remain among the highest in the world, estimated at 41.8, 23.5, 29.9, and 22.7 per 100,000 respectively [32•]. Gastric cancer mortality rates are also high in East Asia (24 and 9.8 per 100,000 men and women, respectively) with the greatest number of cumulative gastric cancer deaths occurring in the Republic of Korea and Japan [32•]. Gastric cancer survival tends to be better in East Asia than in North America or Europe [38•]. For example, 5-year survival rates of 67 and 69% for primary gastric cancer have been reported in Korea and Japan, respectively [39•, 40]. Screening programs in East Asia that result in early detection of gastric cancer explain some of these differences. For example, greater than 50% of cancers are diagnosed at an early stage in Japan [41] as opposed to approximately 27% in the USA. Differences in tumor biology and gastric cancer subtype (where the East has a higher proportion of non-cardia gastric cancer than the West) may also contribute to survival differences.
Central/Eastern Europe has the second highest gastric cancer rates after Eastern Asia with estimated ASIR of 13.5/100,000 and ASMR of 10.9/100,000. South and Central America have intermediate ASIRs (10.3 and 9.3 per 100,000 respectively) and ASMRs. Outliers within the region include Guatemala and Costa Rica, which have gastric cancer rates that compete with Eastern Asia with estimated ASIRs of 23.7 and 17.3 per 100,000 respectively [32•].
Western Europe, Northern Europe, and North America have intermediate to low rates of gastric cancer (ASIR: 6.3, 5.4, and 4 per 100,000 respectively). The USA specifically has an ASIR of 3.9/100,000 and the lowest gastric cancer mortality rate in the world (ASMR: 2.7 and 1.5 deaths per 100,000 in men and women, respectively) [32•]. In the USA, 5-year survival from gastric cancer has improved somewhat over the last 30 years from 15 to 29% [42]. However, the gastric cancer prognosis for the individual patient remains dismal as gastric cancer is typically diagnosed at an advanced stage (73% of cases) [43].
There are also differences in the anatomic subtype of gastric cancer that effect incidence and mortality. Globally and through much of East/Central Asia and Eastern Europe, non-cardia gastric cancer is the predominant anatomic subtype. Conversely, in North America and Western Europe, cardia gastric cancer is the predominant subtype due to the fact that non-cardia gastric cancer incidence has declined and cardia gastric cancer rates have remained stable or possibly increased [44••, 45–46].
Gastric Cancer Variability Within the USA
While gastric cancer burden is relatively low in the USA, there are persistent and significant ethnic/racial disparities in gastric cancer incidence and mortality. The few studies to examine gastric cancer by anatomic subtype have also shown that non-cardia gastric cancer specifically is disproportionately high among non-White populations [47, 48••] and indigenous populations [49•].
Non-White populations have nearly twice the incidence and mortality of gastric cancer compared to non-Hispanic White populations [47, 48••, 50••, 51–52•]. The Surveillance, Epidemiology, and End Results Program (SEER) reported ASIRs of 10.5, 10.6, and 10.2 per 100,000 in Hispanics, Asians/Pacific Islanders, and Blacks, respectively, versus 5.7 per 100,000 in non-Hispanic Whites over 2000–2014 [52•]. Similarly, a large US population study in a single health system in Southern California reported a 40–50% increased risk of gastric cancer in Hispanics (odds ratios [OR] 1.4), non-Hispanic Blacks (OR 1.5), and Asians (OR 1.5) compared to that in non-Hispanic Whites [51]. Gastric cancer mortality rates are the lowest among non-Hispanic Whites (ASMR 2.5 per 100,000) while double this rate was observed among all other ethnic/racial groups in 2010–2014 (5.3, 5.5, and 5.9 per 100,000 Hispanics, Asians/Pacific Islanders, and Blacks, respectively) [52•].
Indigenous populations within the USA are also disproportionately affected by gastric cancer. The estimated ASIR was much higher than observed in the general population at 30.8 and 9.4 per 100,000 Alaskan Inuit/Yupik/Inupiat men and American Indian/Alaskan Native men respectively over the 1990s to 2000s [49•]. The USA, however, is not unique in having such striking contrasts in gastric cancer patterns between indigenous and non-indigenous populations. Significantly higher gastric cancer burden is also observed among the indigenous populations of Canada, Northern Europe, Australia, New Zealand, and Russia as well [49•].
Gastric cancer disparities between subgroups in the USA have been observed as far back as the 1960s [30]. Ethnic/racial disparities in gastric cancer trends in the USA may be partly explained by an immigration effect, where individuals from higher gastric cancer incident regions of the world such as Eastern Asia, Eastern Europe, and Latin America account for higher rates. But, an immigration effect does not completely explain the trend given the disproportionately high gastric cancer rates observed among indigenous populations as well. Socioeconomic disparities are likely a strong contributing factor, mediated by greater exposure to H. pylori infection, risky environmental exposures, and barriers in accessing medical care [53••, 54•, 55••]. However, further work needs to be done to identify the root causes and rational prevention methods for the disparate gastric cancer trends observed in the USA. Also, an unexpected trend has been highlighted by recent data indicating a rise in non-cardia gastric cancer among young White men in the USA. Two separate analyses of SEER data noted unexpected increases in non-cardia gastric cancer among young White persons over the 1970s–2000s: one observed an increase among young Whites aged 25–39 years [56•] and the second, an unexpected increase in corpus cancers among young (25–39 years) and middle-aged Whites (40–59 years) [57••]. The significance of and reasons for this increase are still unclear.
Putative Mechanisms for the Decline in Gastric Cancer
H. pylori prevalence partially accounts for the observed trends in gastric cancer incidence over time and across populations. However, the cause for global declines and differences in gastric cancer incidence is multifactorial and related to trends in dietary, tobacco, and other environmental exposures in addition to H. pylori infection.
Countries and populations that have experienced marked improvements in public sanitation (linked to economic development) and access to clean water supplies have seen the most significant reductions in H. pylori infection. This is reflected by the “birth cohort effect” whereby H. pylori rates have been declining among younger generations in most regions of the world. Countries that have experienced the largest birth cohort effect include Korea, Japan, and the USA where observed H. pylori prevalence rates are markedly lower among younger compared to that among older age groups, reflecting a drop in acquisition of infection with successive generations. In Korea, H. pylori prevalence was estimated at 60% among 60-year-old versus 20% among 20-year-old persons in 2005 [58]; in Japan, cross-sectional studies have estimated H. pylori prevalence at 80% among pre-1950s-born persons versus 5% among 1980s-born persons [59••]; and in the USA, H. pylori prevalence was estimated at 40% among older adults versus 20% among younger adults in 1999–2000 [58]. On the other hand, Central and South American countries have not experienced a significant birth cohort effect [58] which is reflected in high H. pylori prevalence rates estimated at 70–85% among all age groups in a cross-sectional study conducted from 2009 to 2012 across Mexico, Honduras, Costa Rica, Nicaragua, Chile, and Colombia [60••].
Environmental Factors and Gastric Cancer Incidence
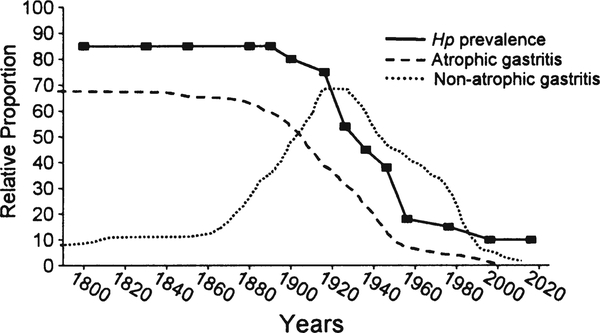
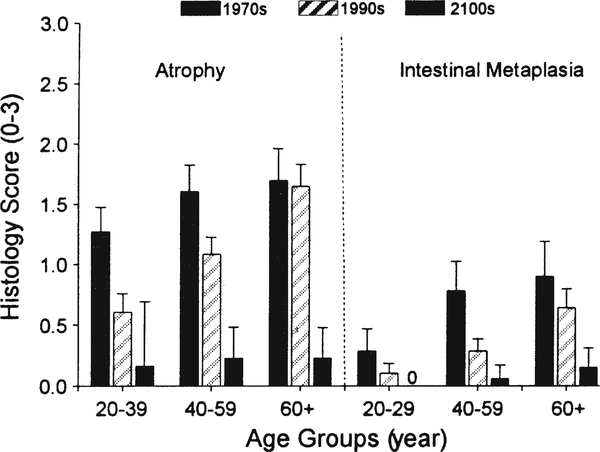
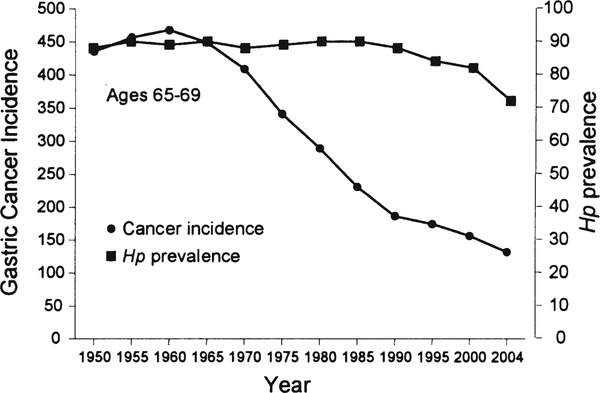
Earlier reductions in gastric cancer incidence were not related to a change in H. pylori prevalence but more likely related to changes in diet [20••]. This is best seen in the West where high rates of gastric cancer and gastric ulcer were rapidly replaced by duodenal ulcer as the primary H. pylori-related disease (Fig. 1) [20••]. Gastric ulcer and gastric cancer are both related to progressive gastric injury and the development of atrophic gastritis. In contrast, duodenal ulcer is related to non-atrophic gastritis with high acid secretion and relative “protection” against gastric cancer. This change in the pattern of disease was therefore a reflection of the change in the pattern of gastric damage associated with H. pylori infections. This difference in the pattern of gastritis is also reflected in the differences in gastric cancer incidence between regions where fresh fruits and vegetables are available year round as opposed to those with seasonal diets. Reductions in gastric cancer risk are associated with diets rich in fruits, vegetables, fish, and whole grains rather than processed meats, refined grains, and high-fat products [61, 62••]. In the USA and Europe, the nineteenth and early twentieth centuries experienced marked changes in food production, processing, preservation, and especially transportation, such that in the second half of the twentieth century, H. pylori infection was in marked decline and fresh fruits and vegetables were available year round [20••]. This change was recently vividly illustrated by a study in Japan that evaluated gastric histology and H. pylori prevalence over a recent 40-year period. Despite the presence of H. pylori infection, there was a marked decrease in the rate of development and prevalence of atrophic changes (Fig. 2) [63••]. This change was also reflected in the rapid change in gastric cancer incidence between 1965 and 1995 where it fell to approximately 60% in every age group irrespective of the prevalence of H. pylori within that group (Fig. 3) [64••, 65•]. It is also important to note that these changes occurred despite no changes in virulence of the predominant strain of H. pylori or in host genetics.
Fig. 1.
Changes in H. pylori prevalence among an asymptomatic White US population. Data from reference [71] for the birth cohorts born between 1916 and 1976, the data before and after, are estimated. The plot illustrates that until the late 1800s, most adults have H. pylori infection and atrophic gastritis. Late in the 1800s, the pattern of gastritis and the most common H. pylori-related disease changed from atrophic gastritis, gastric ulcer, and gastric cancer to a non-atrophic duodenal ulcer pattern and then with further decline in H. pylori prevalence, all H. pylori-related diseases tended to disappear
Fig. 2.
Mean ± 95% confidence intervals of atrophy and intestinal metaplasia scores of corpus mucosal biopsies in H. pylori-positive patients according to age group. Both mucosal atrophy and metaplasia in the corpus significantly decreased in time period setting in all age group and comparisons between all subgroups were significant (p < 0.05). Note the age-related increase between groups but the time-related decrease within groups. From reference [63••], with permission from John Wiley and Sons.
Fig. 3.
Changes in the incidence of gastric cancer and H. pylori infection among Japanese men aged 65–69 during the latter half of the twentieth century. Data from reference [64••]. Figure from reference [65•], with permission from Elsevier
Other important factors included a fall in salt consumption and patterns in tobacco smoking [63••]. A recent study evaluated the effect of tobacco smoking on gastric cancer incidence in 118 countries and found that the proportion of gastric cancers attributable varied by geographic region and sex with the highest attributable fractions observed among men in Eastern Asia and women in Western Europe. Importantly, the authors predicted an increase in the absolute number of gastric cancers attributable to smoking by the year 2020. This highlights a potential need to target smoking as a gastric cancer prevention strategy in regions where smoking is highly prevalent [66••].
In the USA and other developed countries, the change in the prevalence of the type of H. pylori-related diseases expressed as a rise in the incidence of duodenal ulcer was then noted to decline such that the incidence of all H. pylori-related diseases declined [20••]. This change reflected the progressive decline in H. pylori prevalence related to progressive improvements in sanitation, access to clean water, and improved household hygiene [20••]. A microsimulation model using National Health and Nutrition Examination Survey (NHANES) and National Health Interview Survey (NHIS) data estimated that between 1978 and 2008, the US gastric cancer incidence decreased 60% with the change in H. pylori prevalence alone accounting for 43% of the observed decline and the reduction in smoking for additional 3% [67].
Effect of Primary and Secondary Prevention Strategies on Gastric Cancer
Future efforts against gastric cancer likely consist of a combination of primary and secondary prevention strategies. The actual strategy utilized will depend on the resources available and the prevalence of H. pylori-related diseases. The report of an effective vaccine to reduce or prevent H. pylori infection suggests that it will be possible to extend H. pylori eradication to the developing world where it is most prevalent [68]. Meta-analyses have confirmed that eradication of H. pylori infection is effective in reducing gastric cancer incidence [69, 70]. Secondary preventive strategy using endoscopic screening and surveillance of those with high-risk histology is being performed in Japan and Korea with some success [2••, 3••, 4]. We predict that worldwide H. pylori eradication will soon be possible and will make gastric cancer a vanishing rare disease.
Conclusions
Gastric cancer incidence has declined dramatically throughout the world largely as a consequence of economic improvements that have brought about improved food preservation, availability, improved sanitation, access to clean water, and improved household hygiene which further led to a fall in H. pylori acquisition and a decline in prevalence among subsequent generations. Japan, Korea, and China currently carry the highest burden of gastric cancer. Overall, declining H. pylori prevalence and active gastric cancer screening and surveillance have resulted in reduced gastric cancer incidence and mortality. Tobacco smoking is emerging as an increasingly significant modifiable risk factor to target in the context of gastric cancer prevention.
Reductions in gastric cancer seen worldwide have not been universal. Central and South America both have intermediate to high rates of gastric cancer and have not yet experienced a significant decline in H. pylori prevalence in any age group. Even within the USA, a region with low gastric cancer burden, specific ethnic/racial groups and indigenous persons in the country experience a disproportionate burden of disease. Immigration effect and poverty may be contributing social factors to gastric cancer disparities across the world and within individual countries, but this needs to be understood more fully. Finally, primary and secondary prevention efforts among high-risk groups warrant consideration in the USA given the relative success of these interventions observed in East Asia.
Key Points.
-
(1)
Gastric cancer incidence has declined worldwide but remains the fifth cause of cancer and the third cause of cancer deaths globally. However, there are significant differences in its incidence across the world and within ethnic/racial subgroups in developed countries.
-
(2)
East Asia currently carries the highest gastric cancer burden; however, the risk is declining as the societies become westernized. Latin America is an outlier in that it has intermediate rates of gastric cancer but a decline is not predicted given the persistence of risk factors.
-
(3)
Reductions in gastric cancer are largely the consequence of improvements in sanitation, food preservation, and availability as well as a decline in H. pylori infections. Reduction in tobacco smoking remains an important modifiable risk factor in gastric and other cancers.
Acknowledgments
Support Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center. Dr. Balakrishnan is supported in part by a prevention grant from the Cancer Prevention and Research Institute of Texas-CPRIT (PP160089).
David Graham is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of H. pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Ashish Sharma, Maya Balakrishnan, and Rollin George declare no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148(4):719–31.e3. doi: 10.1053/j.gastro.2015.01.040.Excellent review that discusses the effects of H. pylori infection and challenges, benefits, and potential downsides of its eradication.
- 2.••.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. doi: 10.1136/gutjnl-2015-309252.Global consensus on gastritis which discusses classification of chronic gastritis and duodenitis and defines H. pylori infection as an infectious disease that should be cured whenever encountered.
- 3.••.Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. Journal of gastroenterology. 2014;49(1):1–8. doi: 10.1007/s00535-013-0897-8.Excellent review of the Japanese experience in H. pylori eradication as an approach to reducing gastric cancer deaths.
- 4.Asaka M A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132(6):1272–6. doi: 10.1002/ijc.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman M. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 9th ed 2014 Philadelphia, PA: Saunders Elsevier; 2010. p. 887–906. [Google Scholar]
- 6.Washington K 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 7.Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29(4):645–54. [DOI] [PubMed] [Google Scholar]
- 8.•.Amini N, Spolverato G, Kim Y, Squires MH, Poultsides GA, Fields R, et al. Clinicopathological features and prognosis of gastric cardia adenocarcinoma: a multi-institutional US study. J Surg Oncol. 2015;111(3):285–92. doi: 10.1002/jso.23799.This study compares the clinicopathological characteristics and the prognosis of gastric cardia adenocarcinoma (GCA) versus non-cardia adenocarcinoma. Key findings were that disease-free survival and overall survival were similar between patients with GCA versus non-cardia adenocarcinoma. Long term outcomes were worse with GCA and early stage disease.
- 9.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96(18):1383–7. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]
- 10.Marques-Lespier JM, Gonzalez-Pons M, Cruz-Correa M. Current perspectives on gastric cancer. Gastroenterol Clin N Am. 2016;45(3):413–28. doi: 10.1016/j.gtc.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauren P The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta pathol Microbiol Scand. 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 12.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC monographs on the evaluation of carcinogenic risks to humans. 1994;61:1–241.
- 13.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136(2):487–90. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 14.••.Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45(1):1–8. doi: 10.1007/s00535-009-0117-8.Review of the historical relation of gastric cancer and atrophic gastritis with special emphasis on the role of acid secretion in predicting outcome. Much of this data was unknown to early H. pylori investigators causing significant delay in understanding the correlations.
- 15.Rugge M Gastric cancer risk in patients with Helicobacter pylori infection and following its eradication. Gastroenterol Clin N Am. 2015;44(3):609–24. doi: 10.1016/j.gtc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Peek RM Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208(2):233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 17.Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;35(Suppl 12):90–7. [PubMed] [Google Scholar]
- 18.de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12(1):1–15. doi: 10.1111/j.1523-5378.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 19.•.Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–60. doi: 10.1111/apt.12814.A comprehensive review article that covered literature since early 2000s regarding epidemiology and strategies in prevention of non cardia gastric cancer. It specifically also discusses how H. pylori eradication in high gastric cancer regions can lead to a decline in the incidence of this highly lethal disease.
- 20.••.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20(18):5191–204. doi: 10.3748/wjg.v20.i18.5191.Review of the natural history of H. pylori-related diseases, peptic ulcer, and gastric cancer throughout time with special emphasis on the effect of the changes in the patterns of gastritis on disease presentation and prevalence
- 21.••.Miftahussurur M, Yamaoka Y, Graham DY. Helicobacter pylori as an oncogenic pathogen, revisited. Expert reviews in molecular medicine. 2017;19:e4. doi: 10.1017/erm.2017.4.Review of the role of H. pylori in gastric carcinogenesis particularly in relation to H. pylori virulence factors and inflammation.
- 22.Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94(22):1680–7. [DOI] [PubMed] [Google Scholar]
- 23.Whelan SL. International Association of Cancer Registries—a history. Asian Pac J Cancer Prev. 2010;10 [Google Scholar]
- 24.Antoni S, Soerjomataram I, Moller B, Bray F, Ferlay J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull World Health Organ. 2016;94(3):174–84. doi: 10.2471/blt.15.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J cancer. 2015;136(5):E359–E86. doi: 10.1002/ijc.29210.Discussion of the worldwide incidence and mortality from 27 major cancers and for all cancers combined for 2012 and gastric cancer trends worldwide.
- 26.Ewing J Carcinoma of the stomach Neoplastic diseases. Philadelphia: W.B: Saunders Company; 1919. p. 605–39. [Google Scholar]
- 27.Hurst A Schorstein lecture on the precursors of carcinoma of the stomach. Lancet. 214(5542):1023–8. doi: 10.1016/S0140-6736(01)09840-3. [DOI] [Google Scholar]
- 28.Sullivan PD, Christine B, Connelly R, Barrett H. Analysis of trends in age-adjusted incidence rates for 10 major sites of cancer. Am J Public Health. 1972;62(8):1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ. 1984;62(2):163–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Wynder EL, Kmet J, Dungal N, Segi M. An epidemiological investigation of gastric cancer. Cancer. 1963;16:1461–96. [DOI] [PubMed] [Google Scholar]
- 31.Doll R Cancer in five continents. Proc R Soc Med. 1972;65(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S , Mathers C , Rebelo M , Parkin DM , Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 13/03/2017 Available from: http://globocan.iarc.fr, accessed on day/month/year Accessed March 13 2017.This publication nicely summarizes the worldwide incidence and mortality from 27 major cancers and for all cancers combined up until 2012. Stomach cancer was the 5th most common cancer by incidence with the 3rd highest mortality rate.
- 33.•.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. European journal of cancer (Oxford, England: 1990). 2014;50(7):1330–44. doi: 10.1016/j.ejca.2014.01.029.This paper documents mortality rates from gastric cancer decreased by 3% per year in Europe, Japan, and Korea and by 2% per year for North America and major Latin countries between 1980 and 2011.
- 34.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 35.••.Lui FH, Tuan B, Swenson SL, Wong RJ. Ethnic disparities in gastric cancer incidence and survival in the USA: an updated analysis of 1992–2009 SEER data. Dig Dis Sci. 2014;59(12):3027–34. doi: 10.1007/s10620-014-3275-3.Summary of SEER data from 1992–2009 showing ethnic/racial disparities exist in the USA with regard to gastric cancer incidence and survival.
- 36.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(5):700–13. doi: 10.1158/1055-9965.epi-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380(9856):1840–50. doi: 10.1016/s0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 38.•.Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22(9):2965–71. doi: 10.1245/s10434-015-4388-4.This paper highlights that Asians have better overall survival compared to Caucasians with respect to non cardia gastric cancer. The mean difference in 5 yr survival was 12% (range- 9% for stage IIIc to 31% for stage I). This trend was shown to persist despite adjustment for age, gender, tumor grade, and number of examined and positive lymph nodes.
- 39.•.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1): 18–28. doi: 10.1016/j.gie.2016.02.028.This review article discusses who should be screened for gastric cancer in the United States and how? Key take home points are that individuals who are immigrants to United States from regions associated with a high risk of gastric cancer (East Asia, Russia, or South America) or who have a family history of gastric cancer should be considered for gastric cancer screening. Authors suggested that those with findings of atrophic gastritis or intestinal metaplasia on screening endoscopy should undergo surveillance endoscopy every 1 to 2 years.
- 40.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 41.Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer sub-types in Europe. Eur J Gastroenterol Hepatol. 2010;22(6):669–78. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 42.Tinmouth J, Green J, Ko YJ, Liu Y, Paszat L, Sutradhar R, et al. A population-based analysis of esophageal and gastric cardia adenocarcinomas in Ontario, Canada: incidence, risk factors, and regional variation. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011;15(5):782–90. doi: 10.1007/s11605-011-1450-9. [DOI] [PubMed] [Google Scholar]
- 43.Powell J, McConkey CC. The rising trend in oesophageal adenocarcinoma and gastric cardia. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 1992;1(3):265–9. [DOI] [PubMed] [Google Scholar]
- 44.••.Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol. 2011;106(11):1978–85. doi: 10.1038/ajg.2011.213.0020.Analysis from three databases. The SEER registry was used for incidence, the Healthcare Costs and Utilization Project for hospitalizations, and the Compressed Mortality File for mortality showing that older age, male gender, non-White race, and residence in the Northeast region were associated with increased risk of non-cardia gastric cancer.
- 45.Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18(7):1945–52. doi: 10.1158/1055-9965.epi-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut. 2014;63(1):64–71. doi: 10.1136/gutjnl-2013-305033. [DOI] [PubMed] [Google Scholar]
- 47.Group USCSW. United States Cancer Statistics. Incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 1999–2013. www.cdc.gov/uscs [Google Scholar]
- 48.••.Dong E, Duan L, Wu BU. Racial and ethnic minorities at increased risk for gastric cancer in a regional US population study. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2017;15(4):511–7. doi: 10.1016/j.cgh.2016.11.033.Recent study highlighting the racial/ethnic differences in the USA with respect to gastric cancer.
- 49.•.SEER. SEER*Explorer: an interactive website for SEER cancer statistics [Internet]. Beta version. Surveillance Research Program, National Cancer Institute; https://seer.cancer.gov/explorer/. Accessed April 14 2014.Link to the SEER cancer statistics which includes - Recent Trends of cancers, 2000–2014, Long Term Trends, 1975–2014, Rates by Age, Stage Distribution (incidence only), 5-year Relative Survival 2007–2013, and Relative Survival Rates by Time Since Diagnosis.
- 50.••.Uthman OA, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health. 2013;67(10):854–60. doi: 10.1136/jech-2012-201108.A meta-analysis of 36 studies from 1966 to 2013 showing the role of socioeconomic status and gastric cancer risk.
- 51.Malaty HM, Evans DG, Evans DJ Jr, Graham DY. Helicobacter pylori in Hispanics: comparison with blacks and whites of similar age and socioeconomic class. Gastroenterology. 1992;103(3):813–6. [DOI] [PubMed] [Google Scholar]
- 52.•.Nguyen T, Ramsey D, Graham D, Shaib Y, Shiota S, Velez M, et al. The prevalence of Helicobacter pylori remains high in African American and Hispanic veterans. Helicobacter. 2015;20(4):305–15. doi: 10.1111/hel.12199.The prevalence of H pylori is surrogate marker of incidence of gastric cancer in a population. This veterans based study concluded that veterans have a 30% H pylori prevalence in the US, of which African American (50–59 yr - 53.3%) and Hispanic (60–60 yr - 48.1%) men had the highest prevalence, thereby highlighting the racial/ethnic differences between H pylori prevalence in the US.
- 53.••.Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. Jama. 2010;303(17):1723–8. doi: 10.1001/jama.2010.496.This article suggests that between 1977 and 2006, the rates of non-cardia gastric cancer increased in Whites 25–39 years of age, the reason for which were unclear.
- 54.•.Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60(12):1644–9. doi: 10.1136/gut.2010.236737.The paper discusses key findings regarding epidemiology of non cardia gastric cancer by anatomical site, race and age group. In this data analysis between 1976–2007, non cardia gastric cancer decreased in both blacks and whites, and for all anatomical subsites except increased incidence in corpus cancer in younger and middle age white men as well as blacks.
- 55.••.Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59(8):1698–709. doi: 10.1007/s10620-014-3063-0.A systematic review of 37 studies through September, 2013 from 22 countries summarizing global trends in H. pylori prevalence.
- 56.•.Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20(Suppl 1):3–7. doi: 10.1007/s10120-016-0658-5.This paper highlights the concept birth cohort effect of H pylori prevalence in Japan which is directly related to its rapid economic development. Less than 2% of subjects born after 2000 have H pylori infection in Japan. This has been the key reason for decline in gastric cancer in Japan, in addition to their primary and secondary prevention strategies.
- 57.••.Porras C, Nodora J, Sexton R, Ferreccio C, Jimenez S, Dominguez RL, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer causes & control: CCC. 2013;24(2):209–15. doi: 10.1007/s10552-012-0117-5.Data from major Latin American countries showing a high H. pylori prevalence with no significant difference in prevalence between young and old.
- 58.Shu L, Wang XQ, Wang SF, Wang S, Mu M, Zhao Y, et al. Dietary patterns and stomach cancer: a meta-analysis. Nutr Cancer. 2013;65(8):1105–15. doi: 10.1080/01635581.2013.828086. [DOI] [PubMed] [Google Scholar]
- 59.••.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP). 2007;16(4):312–27. doi: 10.1097/01.cej.0000236255.95769.22.Case control and meta-analysis confirming fruit or vegetable intake was associated with a decreased risk of gastric cancer regardless of the anatomical location and the histological type.
- 60.••.Kamada T, Haruma K, Ito M, Inoue K, Manabe N, Matsumoto H, et al. Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter. 2015;20(3):192–8. doi: 10.1111/hel.12193.A 40-year study showing that the precursor lesions for gastric cancer declined markedly despite no change in H. pylori prevalence but associated with westernization of the diet and reduced salt intake and smoking.
- 61.Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23(6 Pt B): 492–501. doi: 10.1016/j.semcancer.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 62.••.Wang C, Weber A, Graham DY. Age, period, and cohort effects on gastric cancer mortality. Dig Dis Sci. 2015;60(2):514–23. doi: 10.1007/s10620-014-3359-0.First study examining the combined effects of age, period, and birth cohort on the incidence of gastric cancer in a defined population.
- 63.••.Peleteiro B, Castro C, Morais S, Ferro A, Lunet N. Worldwide burden of gastric cancer attributable to tobacco smoking in 2012 and predictions for 2020. Dig Dis Sci. 2015;60(8):2470–6. doi: 10.1007/s10620-015-3624-x.This study analyzed population attributable fractions with respect to smoking for 118 countries and gender and regional differences in gastric cancer incidence attributable to smoking
- 64.••.Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(10002):1457–64. doi: 10.1016/s0140-6736(15)60310-5.RCT from China showing that an oral recombinant H. pylori vaccine provided significant protection from H. pylori in children.
- 65.•.Yeh JM, Hur C, Schrag D, Kuntz KM, Ezzati M, Stout N, et al. Contribution of H. pylori and smoking trends to US incidence of intestinal-type noncardia gastric adenocarcinoma: a microsimulation model. PLoS medicine. 2013;10(5):e1001451. doi: 10.1371/journal.pmed.1001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.••.Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150(5):1113–24.e5. doi: 10.1053/j.gastro.2016.01.028.Meta-analysis of 24 studies through May, 2015 confirming a 47% reduction in gastric cancer with H. pylori eradication. Benefits accrued in all populations studied.
- 67.Lee YC, Chiang TH, Liou JM, Chen HH, Wu MS, Graham DY Mass eradication of Helicobacter pylori to prevent gastric cancer: theoretical and practical considerations. Gut and Liver. 2016;10(1):12–26. doi: 10.5009/gnl15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham DY, Malaty HM, Evans DG, Evans DJ Jr, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100(6):1495–501. [DOI] [PubMed] [Google Scholar]