Abstract
Females are at higher risk for certain opioid addictive behaviors, but the influence of ovarian hormones is unknown. In our rat model of heroin self-administration, females exhibited higher relapse rates that correlated with rates of heroin seeking on the first extinction session. We administered estradiol alone, or in combination with progesterone, 30 min prior to the first extinction session in freely cycling, heroin-seeking female rats. Although neither treatment produced long-term effects on relapse, each treatment regulated distinct aspects of heroin seeking. Estradiol treatment enhanced extinction memory retention, whereas the combination treatment acutely reduced expression of heroin seeking.
Epidemiological data indicate that women may be more vulnerable to opioid addiction than men, as they meet dependence criteria faster after first-time use, enter treatment earlier (Anglin et al. 1987; Hser et al. 1987), and report higher craving for opioids once dependent (Back et al. 2011). This is consistent with reports from preclinical models of opioid self-administration in rats, where females have been shown to acquire heroin self-administration faster than males and to self-administer heroin over a wider range of intravenous doses (Lynch and Carroll 1999; Carroll et al. 2002). Additionally, females work harder for heroin on progressive ratio schedules of reinforcement, and consume more heroin on a mg/kg basis (Lynch et al. 2002; Cicero et al. 2003; Lacy et al. 2016). No prior studies, however, have reported sex differences in cued reinstatement, or relapse, in these opioid models.
The influence of ovarian hormones on opioid seeking in these reported sex differences is not well understood. Females self-administer less heroin during the high-estrogen state of proestrus (Lacy et al. 2016), suggesting that higher circulating estradiol levels may be therapeutic in freely cycling females. Progesterone also peaks during proestrus (Becker et al. 2005; Lebron-Milad and Milad 2012), but no effects of progesterone in preclinical opioid self-administration models have been reported (Sedki et al. 2015), underscoring the need for further research in this area. For psychostimulants like cocaine, research suggests that estrogen promotes cocaine seeking and/or taking (Lynch et al. 2002; Perry et al. 2013). This may occur, at least in part, due to estradiol's ability to enhance striatal dopamine release (Becker and Rudick 1999), thereby acting to “prime” drug seeking. Opiate seeking, in contrast, may be less reliant on striatal dopamine release (Pettit et al. 1984; Hnasko et al. 2005), highlighting the potential for differential estrogen effects on opiate seeking compared to psychostimulants.
Whereas most preclinical studies including females have focused on heroin-taking behaviors, heroin-seeking behavior under conditions of drug unavailability (i.e., extinction) provides an indicator of relapse propensity. Extinction training, wherein the animal learns to stop seeking drug when it is no longer available, can recruit distinct neural circuits to reduce drug seeking (Peters and De Vries 2014; Augur et al. 2016). Evidence from rodent and human studies of fear learning indicates that estradiol promotes extinction learning and/or memory retention (Milad et al. 2009, 2010; Zeidan et al. 2011; Graham and Milad 2012; Maeng et al. 2017; Graham and Scott 2018), and similar effects have been reported for cocaine conditioned place preference (Twining et al. 2013). While the neural circuits controlling extinction of fear and cocaine seeking are similar (Peters et al. 2009), the extent to which these circuits are shared for opioid seeking is unknown (Peters et al. 2013). No prior studies have reported effects of estradiol treatment (in freely cycling rodents) on conditioned opioid behaviors.
We first characterized heroin-taking and -seeking behaviors in female rats in our heroin self-administration model. Identical heroin self-administration procedures were applied to age-matched (P50–75 on arrival) female (n = 40) and male (n = 8) Wistar rats. Rats were moderately food-restricted (15–17 g/d females; 20–22 g/d males) in order to promote acquisition. Intravenous catheters were surgically implanted as previously described (Augur et al. 2016), and each infusion contained 0.04 mg heroin (NIDA Drug Supply Program) in 50 µL saline. Lever presses over the course of behavioral training for each experiment were first analyzed using a three-way RM ANOVA, followed by two-way ANOVAs where a three-way interaction was observed (see Supplemental Table 1 for statistical details). Heroin-taking occurred over 12 daily sessions, progressing from an FR1 schedule of reinforcement (7 d) to an FR2 (2 d), and concluding on an FR4 (3 d). Successful acquisition was gauged by active versus inactive lever discrimination, which emerged on day 8–10 of self-administration (Fig. 1A). The rates of responding during acquisition of heroin taking were similar between sexes, as was the number of heroin infusions self-administered (two-way ANOVA: Time F(11,132) = 11.66, P < 0.0001) (Fig. 1B). Importantly, each heroin infusion was signaled by a compound tone (4 kHz, 78 dB) and light stimulus (5 sec), and a 20 sec time-out period followed each lever press (house light off, heroin unavailable). These conditioned cues are effective at triggering cued reinstatement after extinction.
Figure 1.
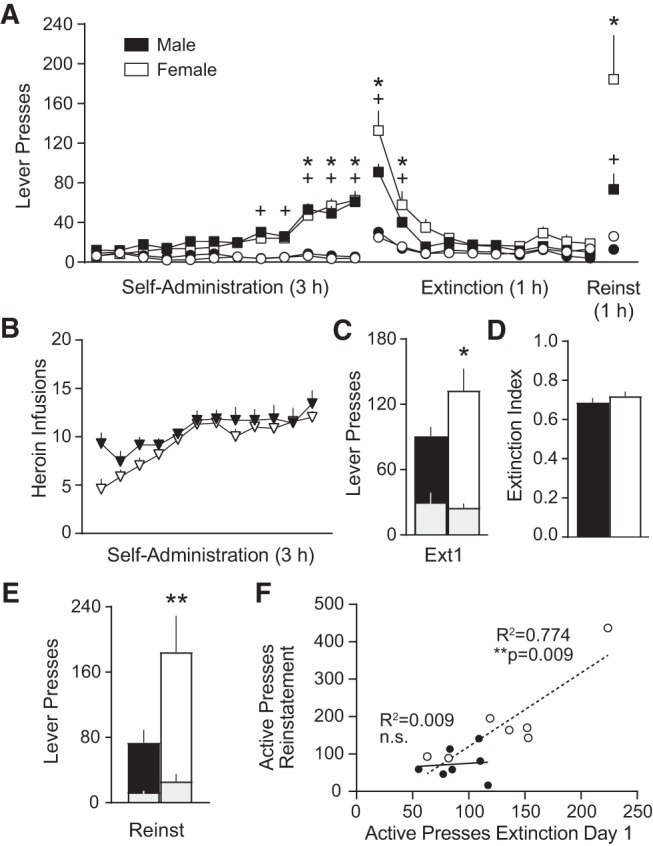
Heroin seeking in females versus males. (A) Heroin seeking over the course of heroin self-administration, extinction, and cued reinstatement in female and male rats (Charles River Laboratories). n = 7/group (n = 1 catheter failure/group eliminated). Sidak's post-hoc active versus inactive lever (*) P < 0.05 females, (+) P < 0.05 males. (B) Number of heroin infusions consumed in females and males over the course of self-administration. (C) Heroin seeking on the first day of extinction was higher in females. Sidak's post-hoc males versus females (*) P < 0.05. (D) The extinction index did not differ between sexes. (E) Females relapsed at higher rates on the cued reinstatement test. Sidak's post-hoc males versus females (**) P < 0.0001. (F) Heroin seeking on extinction day 1 correlated with cued relapse rates in females, but not males. All data are mean ± SEM, except data from individual rats shown in (F). Gray bars = inactive presses nested within active presses.
Extinction training, wherein responding on both levers was without consequence (no heroin, no cues), commenced 5 d after the last heroin self-administration session. Notably, the first extinction training provides an opportunity to measure heroin seeking when heroin is no longer available. Responding on this session can thus be viewed as the first “lapse” in drug seeking, reflecting motivation to seek heroin. Females had significantly higher rates of heroin seeking on extinction day 1 than males (Fig. 1C). Lever discrimination disappeared by extinction day 3, and the sexes exhibited similar rates of extinction over the remaining sessions (10 total). We also examined the extinction index, as a measure of extinction memory retention, using the formula: 1 − [(active lever presses on extinction day 2)/(sum of active lever presses on extinction days 1 and 2)]. This results in a value between 0 and 1, where higher values indicate better extinction memory. This within-subject measure of the decrease in heroin seeking from extinction day 1 to extinction day 2 was analyzed using an unpaired, two-tailed t-test and was similar between the sexes (Fig. 1D). Lever discrimination reemerged on the cued reinstatement test, wherein the heroin light-tone cue (FR2, 20 sec time-out) became available again on the active lever, to trigger a relapse. Females relapsed at a higher rate than males on this test (Fig. 1E). Interestingly, extinction day 1 response rates on the active lever correlated with relapse rates only in females (Pearson's r = 0.88, P = 0.009) (Fig. 1F).
With regards to extinction, female rats in the low-estrogen phase of metestrus exhibit poor fear extinction memory, similar to women in the early follicular phase, also marked by low estrogen. However, extinction is more likely to be successful if extinction training is conducted during proestrus (in female rats) or the late follicular/mid-luteal phase (in women), when estradiol is high (Milad et al. 2009; Zeidan et al. 2011; Graham and Daher 2016). We were unable to assess the role of the estrous cycle phase on extinction success in this experiment, as all but one female rat was in metestrus or diestrus on extinction day 1; one was in estrus. Interestingly, Graham and Scott (2018) recently assessed the interaction between estradiol dose and estrous cycle phase in freely cycling female rats undergoing fear conditioning and extinction procedures. They found that a low dose of β-estradiol (15 µg/kg, s.c.) improved extinction memory retrieval across all estrous cycle phases except proestrus, where it had no effect. Thus, if females are in a low-estrogen state, this low dose of β-estradiol enhances extinction memory, but if they are already in a high-estrogen state, they do not reap additional benefits. In fact, a higher dose of β-estradiol (100 µg/kg, s.c.) impaired extinction memory retrieval in rats that were in proestrus (but not other phases) at the time of administration (on extinction day 1). These results are consistent with a proposed inverted U-shaped dose-response curve for estradiol on drug seeking (Hu and Becker 2008).
We next assessed the role of acute, low-dose estradiol administration in freely cycling, heroin-seeking female rats on extinction memory retention, as well as any long-term effects this treatment might have on relapse. Estradiol (15 µg/kg, s.c.) or vehicle (sesame oil) was administered 30 min prior to extinction day 1 training. This dose and treatment interval has been shown to enhance fear extinction memory retention (Milad et al. 2009; Zeidan et al. 2011; Graham and Daher 2016; Maeng et al. 2017; Graham and Scott 2018) and increase activity acutely in extinction neural circuits in both female rats and humans (Zeidan et al. 2011; Maeng et al. 2017). Notably, these rapid effects of estradiol are consistent with nongenomic actions of estradiol (Luine and Frankfurt 2012; Srivastava et al. 2013; Kow and Pfaff 2018). For this experiment (and the next), where treatments were administered prior to extinction day 1, we conducted two-tailed, unpaired planned comparison t-tests for key variables. These included active and inactive lever responding on extinction day 1, extinction day 2, and the cued reinstatement test. Lever presses over the entire course of each experiment were also analyzed using three-way ANOVAs, and two-way ANOVAs where appropriate (Supplemental Table 1). Rats acquired heroin self-administration, indicated by successful lever discrimination at the end of self-administration (Fig. 2A), and there were no group differences in the number of heroin infusions self-administered (two-way ANOVA: Time F(11,143) = 11.26, P < 0.0001) (Fig. 2B), indicating that the groups were appropriately balanced before treatments were administered.
Figure 2.
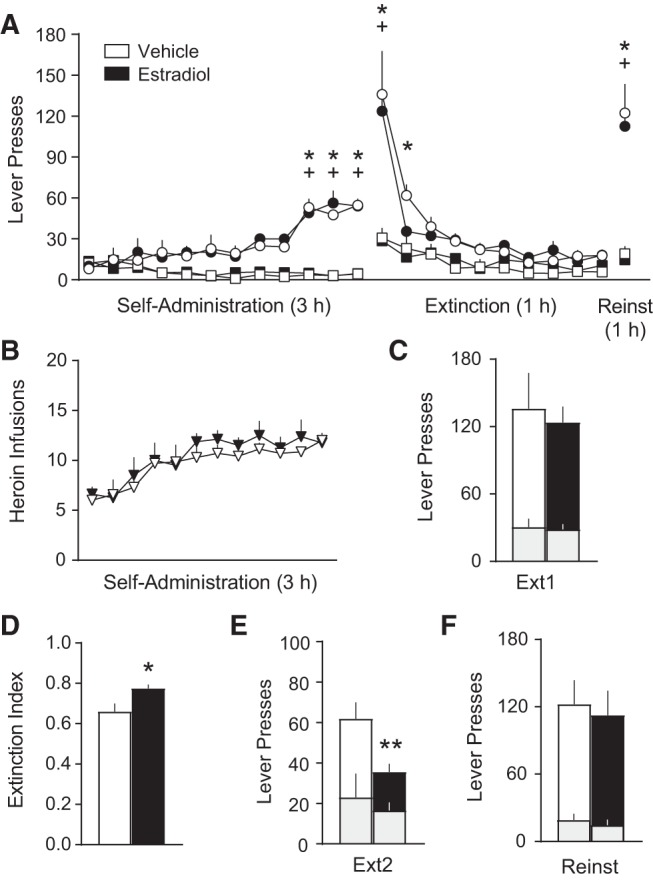
Estradiol selectively enhances extinction memory retention without altering heroin seeking. (A) Heroin seeking over the course of heroin self-administration, extinction, and cued reinstatement did not differ between groups prior to treatment. n = 7 vehicle (n = 1 statistical outlier excluded, >2 standard deviations from mean); n = 8 estradiol. Sidak's post-hoc active versus inactive lever (*) P < 0.01 vehicle, (+) P < 0.0001 estradiol. (B) Number of heroin infusions over the course of self-administration also did not differ. (C) Heroin seeking on the first day of extinction was not altered by estradiol treatment (15 µg/kg, s.c.; Sigma-Aldrich, E8875) 30 min prior. (D) The extinction index was significantly higher (indicating better extinction) in the estradiol group. Unpaired, two-tailed t-test (*) P < 0.05. (E) Heroin seeking on the second day of extinction was reduced by estradiol treatment the previous day. Unpaired, two-tailed t-test (**) P < 0.01. (F) Estradiol treatment on extinction day 1 did not alter relapse rates on the cued reinstatement test. All data are mean ± SEM. Gray bars = inactive presses nested within active presses.
No effects of treatment were observed acutely on the first extinction session (Fig. 2C). However, the estradiol group had a higher extinction index than the vehicle group (t(13) = 2.729, P = 0.0172) (Fig. 2D) and had lower rates of heroin seeking on extinction day 2 (t(13) = 3.324, P = 0.0098) (Fig. 2E), indicating that estradiol treatment selectively enhanced extinction memory retention. Lever discrimination disappeared by extinction day 3 in the vehicle group, and extinction day 2 in the estradiol group. The groups responded similarly over the remaining nine daily extinction sessions, and they were then subjected to the cued reinstatement test. No effects of treatment were observed on this test (Fig. 2F), and lever discrimination reemerged in both groups (Fig. 2A). Thus, while estradiol effectively enhanced extinction memory retention, it did not alter long-term propensity to relapse.
Since the peak in estradiol during proestrus is rapidly followed by a peak in progesterone levels (Becker et al. 2005; Lebron-Milad and Milad 2012), and some evidence suggests progesterone may be capable of enhancing estradiol effects on extinction (Graham and Daher 2016) and/or reducing drug seeking (Feltenstein and See 2007; Feltenstein et al. 2008), we next examined whether a combination treatment of estradiol + progesterone prior to extinction training might confer additional therapeutic effects on heroin seeking. Estradiol or estradiol + progesterone (2 mg/kg, s.c.) was administered 30 min prior to extinction day 1 training. The estradiol dose was held constant with the previous experiment, and the progesterone dose was chosen based on relevant literature (Feltenstein et al. 2008; Graham and Daher 2016). Both groups acquired heroin self-administration, indicated by successful lever discrimination at the end of self-administration (Fig. 3A), and there were no group differences in the number of heroin infusions self-administered (two-way ANOVA: Time F(11,143) = 13.64, P < 0.0001; Time × Treatment F(11,143) = 2.119, P = 0.0225, no Sidak's post-hoc comparisons were significant) (Fig. 3B), indicative of balanced groups prior to treatment.
Figure 3.
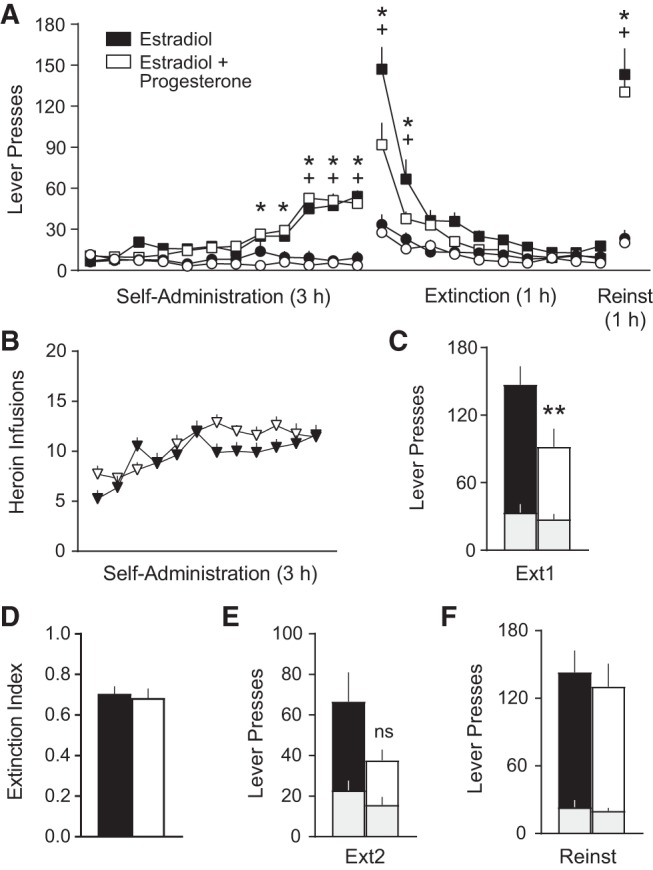
Combination treatment with estradiol + progesterone acutely reduces heroin seeking. (A) Heroin seeking over the course of heroin self-administration, extinction, and cued reinstatement did not differ between groups prior to treatment. n = 8 estradiol; n = 7 estradiol + progesterone (n = 1 statistical outlier excluded, >2 standard deviations from mean). Sidak's post-hoc active versus inactive lever (+) P < 0.001 estradiol, (*) P < 0.05 estradiol + progesterone. (B) Number of heroin infusions over the course of self-administration also did not differ. (C) Heroin seeking on the first day of extinction was reduced by estradiol + progesterone treatment (15 µg/kg estradiol + 2 mg/kg progesterone, s.c.; Sigma-Aldrich, P0130) 30 min prior. Sidak's post-hoc estradiol versus estradiol + progesterone (**) P < 0.0001 (Unpaired, two-tailed t-test P < 0.05). (D) The extinction index did not differ between the combination treatment group versus estradiol alone. (E) Heroin seeking on the second day of extinction was not significantly different between treatments (P = 0.098). (F) Neither treatment on extinction day 1 altered relapse rates on the cued reinstatement test. All data are mean ± SEM. Gray bars = inactive presses nested within active presses.
Heroin seeking was acutely reduced by the combination treatment on extinction day 1 (t(13) = 2.404, P = 0.0319) (Fig. 3C). No differences were observed in the extinction index (Fig. 3D) or rates of heroin seeking on extinction day 2 (Fig. 3E), indicating that combination treatment does not further enhance extinction memory retention relative to estradiol alone. Lever discrimination disappeared by extinction day 3, and the groups responded similarly over the remaining nine daily extinction sessions. Furthermore, no effects of treatment were observed on the cued reinstatement test (Fig. 3F), and lever discrimination reemerged in both groups (Fig. 3A). Thus, combination treatment with estradiol + progesterone selectively reduced acute heroin seeking on extinction day 1, but provided no additional therapeutic benefit on extinction retention, and produced no long-term effects on relapse.
Because we rarely sampled proestrus (the high-estrogen phase) in our female rats, groups were underpowered for cycle phase analyses. However, pooling the estradiol groups from the last two experiments provided sufficient power to compare the low-estrogen phases of metestrus/diestrus versus estrus (Supplemental Fig. 1). Since no studies have assessed the role of exogenously administered ovarian hormones on heroin seeking in freely cycling females, this study was designed to address this gap in knowledge by examining the effects of acutely administered exogenous estradiol and progesterone on the expression of heroin-seeking behavior versus extinction memory success. The hormones were administered 30 min prior to the first extinction training session to simulate conditions under which they might be used clinically to enhance extinction retention, and to maintain consistency with prior studies (Milad et al. 2009; Zeidan et al. 2011; Graham and Daher 2016; Maeng et al. 2017; Graham and Scott 2018). We chose this approach, as opposed to using ovariectomized (OVX) females, to preserve the clinical relevance of our findings and because ovariectomy alters heroin self-administration (Roth et al. 2002), which we wanted to avoid.
Consistent with a growing literature indicating that estradiol has beneficial effects on extinction memory (Wegerer et al. 2014; Graham and Daher 2016; Maeng et al. 2017; Graham and Scott 2018), we found that β-estradiol enhanced extinction memory retention in heroin-seeking female rats, measured by the extinction index. A proposed mechanism by which estradiol may enhance extinction is through increased production of endogenous brain-derived neurotrophic factor (BDNF) (Liu et al. 2001; Scharfman et al. 2003; Barker et al. 2015), as well as more rapid, synergistic signaling interactions that increase hippocampal spine density (Srivastava et al. 2013). Exogenously applied hippocampal BDNF induces extinction memory for conditioned fear (Peters et al. 2010). We have observed similar extinction-like reductions in heroin seeking with exogenous-hippocampal BDNF; however, these effects did not reach statistical significance (Barker et al. 2015). The extent to which such known mechanisms of fear extinction memory are shared for heroin seeking is an area that warrants further investigation (Peters et al. 2009, 2013). Additional studies are also needed to examine other acute doses of β-estradiol, as well as endogenous estradiol levels (e.g., in blood plasma), which may correlate with extinction memory success.
Because our final experiment compared the combination treatment of estradiol + progesterone to an estradiol-only treatment group, we cannot attribute the observed effects solely to progesterone, although it is possible progesterone may produce similar effects independently. One study found no effects of exogenously administered progesterone in a preclinical model of opioid seeking (on food restriction-induced enhancement of reinstatement in OVX rats) (Sedki et al. 2015). In freely cycling female rats, progesterone administration (at the same dose as the latter and present study) reduced the enhanced cocaine-primed reinstatement observed during estrus, but had no effect when females were in other cycle phases (Feltenstein et al. 2008). These results are consistent with findings that levels of progesterone in freely cycling female rats are inversely correlated with cocaine seeking (Feltenstein and See 2007), and are in line with our findings reported here on heroin seeking, with a combination progesterone + estradiol treatment. Future studies should examine the effects of progesterone alone, as well as chronic treatment with ovarian hormones, on heroin seeking.
One mechanism by which progesterone may decrease drug seeking is through its known anxiolytic effects mediated by its metabolite, allopregnanolone (Bitran et al. 1995). This nongenomic effect of progesterone occurs via allopregnanolone's ability to rapidly enhance GABAA-receptor mediated currents (Bitran et al. 1993, 1995). Opioid withdrawal is characterized by an aversive emotional and physical state entailing increased anxiety and hyperalgesia (Le Roy et al. 2013; Koob 2019). This withdrawal-associated anxiety may be more pronounced in female opiate abusers compared to males (Kosten et al. 1985), and may account, at least in part, for the additional therapeutic benefit conferred by gabapentin in conjunction with naltrexone to manage opiate withdrawal symptoms (Martínez-Raga et al. 2004). Interestingly, women with posttraumatic stress disorder (PTSD) exhibit a blunted metabolism of progesterone to allopregnanolone compared to trauma-exposed women without PTSD (Pineles et al. 2018), suggesting that this metabolic pathway may provide endogenous protection against multiple forms of anxiety, and perhaps anxiety-precipitated relapse.
We found that females had higher rates of heroin seeking than males on the first day of extinction, a form of postabstinence relapse (Fuchs et al. 2006; Reichel and Bevins 2009; Giannotti et al. 2018). Females also had higher rates of cued reinstatement of heroin seeking after extinction. Because both extinction day 1 responding and cued reinstatement responding have been shown to correlate with the variable α (a measure of demand elasticity) in behavioral economics models (Bentzley et al. 2014; Cox et al. 2017), both forms of relapse likely relate to the motivational drive to seek heroin (or inelastic demand). Interestingly, we observed a correlation between heroin seeking on extinction day 1 and cued relapse in females only. However, the latter studies, which were conducted using psychostimulants, indicate that α correlates with relapse rates in both sexes (Bentzley et al. 2014; Cox et al. 2017). The present study is the first to report a correlation between extinction day 1 and reinstatement for opioid seeking; thus the apparent lack of correlation in males may be unique to opioids. Since our hormone treatments were restricted to early extinction, and no long-term effects of this acute treatment on relapse were noted, future studies should examine the effects of ovarian hormone treatment on cued relapse rates in freely cycling females.
Supplementary Material
Acknowledgments
We thank Angela Kearns, Jasper Heinsbroek, Jordan Hopkins, and Alex Yue for their helpful technical support and/or feedback on the data. We also thank the NIDA Drug Supply Program for providing heroin. NIDA/NIH grants DA038235, DA044524, and DA045836 to J.P.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.050187.119.
References
- Anglin MD, Hser YI, McGlothlin WH. 1987. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse 13: 59–71. 10.3109/00952998709001500 [DOI] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J. 2016. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci 36: 10174–10180. 10.1523/JNEUROSCI.0773-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W. 2011. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 37: 313–323. 10.3109/00952990.2011.596982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, De Vries TJ, Peters J. 2015. Brain-derived neurotrophic factor and addiction: pathological versus therapeutic effects on drug seeking. Brain Res 1628: 68–81. 10.1016/j.brainres.2014.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. 1999. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64: 53–57. 10.1016/S0091-3057(99)00091-X [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, et al. 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673. 10.1210/en.2004-1142 [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. 2014. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci 111: 11822–11827. 10.1073/pnas.1406324111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Purdy RH, Kellogg CK. 1993. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav 45: 423–428. 10.1016/0091-3057(93)90260-Z [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. 1995. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol 7: 171–177. 10.1111/j.1365-2826.1995.tb00744.x [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. 2002. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology 161: 304–313. 10.1007/s00213-002-1030-5 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. 2003. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74: 541–549. 10.1016/S0091-3057(02)01039-0 [DOI] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G. 2017. Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol Psychiatry 81: 949–958. 10.1016/j.biopsych.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. 2007. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89: 183–189. 10.1016/j.drugalcdep.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. 2008. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology 34: 343–352. 10.1016/j.psyneuen.2008.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. 2006. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26: 3584–3588. 10.1523/JNEUROSCI.5146-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti G, Barry SM, Siemsen BM, Peters J, McGinty JF. 2018. Divergent prelimbic cortical pathways interact with BDNF to regulate cocaine-seeking. J Neurosci 38: 8956–8966. 10.1523/JNEUROSCI.1332-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Daher M. 2016. Estradiol and progesterone have opposing roles in the regulation of fear extinction in female rats. Neuropsychopharmacology 41: 774–780. 10.1038/npp.2015.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. 2012. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry 73: 371–378. 10.1016/j.biopsych.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Scott E. 2018. Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Horm Behav 97: 67–74. 10.1016/j.yhbeh.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. 2005. Morphine reward in dopamine-deficient mice. Nature 438: 854–857. 10.1038/nature04172 [DOI] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Booth MW. 1987. Sex differences in addict careers. 3. Addiction. Am J Drug Alcohol Abuse 13: 231–251. 10.3109/00952998709001512 [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. 2008. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend 94: 56–62. 10.1016/j.drugalcdep.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. 2019. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. 1985. Ethnic and gender differences among opiate addicts. Int J Addict 20: 1143–1162. 10.3109/10826088509056356 [DOI] [PubMed] [Google Scholar]
- Kow LM, Pfaff DW. 2018. Can distinctly different rapid estrogen actions share a common mechanistic step? Horm Behav 104: 156–164. 10.1016/j.yhbeh.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. 2016. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology 233: 3201–3210. 10.1007/s00213-016-4368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. 2012. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord 2: 3 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C, Laboureyras E, Laulin JP, Simonnet G. 2013. A polyamine-deficient diet opposes hyperalgesia, tolerance and the increased anxiety-like behaviour associated with heroin withdrawal in rats. Pharmacol Biochem Behav 103: 510–519. 10.1016/j.pbb.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. 2001. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol 433: 499–514. 10.1002/cne.1156 [DOI] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. 2012. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol 33: 388–402. 10.1016/j.yfrne.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144: 77–82. 10.1007/s002130050979 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164: 121–137. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebrón-Milad K. 2017. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res 95: 163–175. 10.1002/jnr.23826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Raga J, Sabater A, Perez-Galvez B, Castellano M, Cervera G. 2004. Add-on gabapentin in the treatment of opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 28: 599–601. 10.1016/j.pnpbp.2003.11.020 [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. 2009. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164: 887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. 2010. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168: 652–658. 10.1016/j.neuroscience.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. 2013. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav 64: 573–578. 10.1016/j.yhbeh.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, De Vries TJ. 2014. Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology 231: 447–453. 10.1007/s00213-013-3258-7 [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. 2009. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16: 279–288. 10.1101/lm.1041309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. 2010. Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328: 1288–1290. 10.1126/science.1186909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Pattij T, De Vries TJ. 2013. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci 34: 689–695. 10.1016/j.tips.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. 1984. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 84: 167–173. 10.1007/BF00427441 [DOI] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, Pinna G, Irvine J, Webb A, Arditte Hall KA, Hauger R, Miller MW, Resick PA, Orr SP, et al. 2018. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 93: 133–141. 10.1016/j.psyneuen.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. 2009. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev 2: 184–194. 10.2174/1874473710902020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, Carroll ME. 2002. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav 72: 313–318. 10.1016/S0091-3057(01)00777-8 [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. 2003. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 23: 11641–11652. 10.1523/JNEUROSCI.23-37-11641.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedki F, Gardner Gregory J, Luminare A, D'Cunha TM, Shalev U. 2015. Food restriction-induced augmentation of heroin seeking in female rats: manipulations of ovarian hormones. Psychopharmacology 232: 3773–3782. 10.1007/s00213-015-4037-4 [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD. 2013. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience 239: 17–33. 10.1016/j.neuroscience.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Twining RC, Tuscher JJ, Doncheck EM, Frick KM, Mueller D. 2013. 17β-estradiol is necessary for extinction of cocaine seeking in female rats. Learn Mem 20: 300–306. 10.1101/lm.030304.113 [DOI] [PubMed] [Google Scholar]
- Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. 2014. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem 116: 145–154. 10.1016/j.nlm.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927. 10.1016/j.biopsych.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


