Abstract
Vaccines offer a promising therapeutic strategy to treat substance use disorders (SUD). Vaccines have shown extensive preclinical proof of selectivity, safety, and efficacy against opioids, nicotine, cocaine, methamphetamine, and designer drugs. Despite clinical evaluation of vaccines targeting nicotine and cocaine showing proof of concept for this approach, no vaccine for SUD has yet reached the market. This review first discusses how vaccines for treatment of opioid use disorder (OUD) and reduction of opioid-induced fatal overdoses fit within the current medication assisted treatment (MAT) portfolio, and then summarizes ongoing efforts toward translation of vaccines targeting heroin, oxycodone, fentanyl, and other opioids.
Keywords: vaccine, antibody, opioid, GMP, translation, opioid epidemic
1. Opioid use disorders and overdose statistics
a. World.
Opioid use disorders (OUD) and opioid-induced fatal overdoses are a world-wide public health concern [1]. Each country or world region has different patterns of misuse as well as opioid of choice [2]. In recent years, the United States of America, Canada, and Europe have reported an increase in the incidence of opioid-related fatal overdoses [1, 3, 4]. Beyond Europe and North America, other countries have registered an overall upward trend in OUD. For instance, several African countries have seen an increase in OUD involving tramadol [5]. Although OUD is present globally, it is often challenging to provide a comprehensive worldwide snapshot of the incidence and consequences of OUD as statistics reflect the interplay of multiple factors including geographical access to specific opioid products (e.g., heroin vs prescription opioids), country-specific prescription opioid or treatment guidelines, and availability of approved treatments for OUD.
b. United States.
Results from the 2016 National Survey on Drug Use and Health revealed that 11.8 million people ages 12 and older reported opioid misuse in the past year and 948,000 used heroin (Substance Abuse and Mental Health Services Administration (SAMHSA, 2017)). Furthermore, 1.8 million people had a pain reliever use disorder and 626,000 had a heroin use disorder (SAMHSA, 2017). But most alarmingly, of the 63,362 deaths due to drug overdoses in 2016, 42,249 were attributed to prescription and illicit opioids (https://www.cdc.gov/drugoverdose/data/statedeaths.html, [6]). The drug overdose death rate more than tripled between 1999 and 2016 and is continuing to increase for opioids. The increase is most steep for synthetic opioids other than methadone, a category that includes fentanyl and its analogs [7], which are highly potent opioids. The rates of synthetic opioid-related deaths initially began to increase in 2013, doubled between 2015 and 2016 (from 3.1 to 6.2 deaths per 100,000), and continued to increase sharply in 2017 (CDCHAN-00413, July 11, 2018). Projections predict that the annual number of opioid fatal overdoses will increase to 81,700 by 2025 [8].
Compared to other SUDs, OUD presents a complex clinical scenario due to the availability of multiple opioid compounds. Fluctuations in drug price and availability often influence a patient's transition between several opioids of choice, including from prescription to illicit opioids. Consequently, prescription opioids such as oxycodone often act as gateways to heroin use [9, 10], although more recent data suggest that heroin is increasingly the first opioid used among those with OUD [11]. In addition to heroin and prescription opioids such as oxycodone and hydrocodone, synthetic opioids are playing a role in the increasing incidence of opioid-related fatal overdoses. Fatal overdoses often result from use of heroin or counterfeit prescription opioids containing fentanyl or its structural analogs, such as carfentanil, acetylfentanil, and alfentanil [12]. Fentanyl was also involved in fatal overdoses related to cocaine, benzodiazepines, antidepressants, and other counterfeit or illicit drugs [6]. In addition to the role of fentanyl and fentanyl analogs in the epidemiology of OUD, the public health threat of these compounds may go beyond OUD and SUD. For instance, the Russian military employed aerosolized carfentanil and remifentanil to incapacitate terrorists holding hostages in a Moscow theater in 2002 [13]. The fentanyl chemical family has been recently included in the Chemical Threat Risk Assessment list of the Department of Homeland Security in light of the possibility that overdoses due to fentanyl and its analogs may occur because of accidental exposure, industrial accidents, chemical warfare or terrorism attacks. Hence, current public and private efforts for development of medications to reduce OUD and opioid-related fatalities should take in account that the target population may include patients with OUD and other SUDs, as well as those individuals in professions at risk of exposure such as military and law enforcement personnel, first responders, airport or custom officials, and workers in chemical plants.
In response to the OUD crisis, the NIH leadership has launched an “all-hands-on-deck” initiative called HEAL (Helping to End Addiction Long-term) to end the opioid epidemic, advocating a comprehensive approach to target OUD including development of novel treatment strategies [14, 15]. In this context, our team and others have focused on development of vaccines to treat OUD and reduce overdoses, which will be reviewed in the next sections.
2. Current approved medications and their limitations: small molecule pharmacotherapeutics.
While several effective medications are currently available for treating OUD, including methadone, buprenorphine, and naltrexone, they all have advantages and disadvantages. For example, methadone has been used for decades to treat opioid dependence and its clinical utility is clear [16]. However, the stigma associated with methadone maintenance and the inconvenience of daily visits to methadone clinics make this an unattractive option for many patients, particularly those who may have recently started using illicit opioids. Buprenorphine, a partial mu opioid agonist and kappa opioid antagonist, also effectively treats OUD [17]. It is a safer medication than methadone in that it is much less likely to cause clinically significant respiratory depression [18]. The ability to obtain buprenorphine from physicians’ offices also makes it an attractive treatment option. However, special training and licenses are needed in order to prescribe this medication, and induction onto buprenorphine treatment is sometimes difficult because it can precipitate withdrawal symptoms in individuals who are physically dependent on opioids [19, 20]. Buprenorphine itself also has abuse potential and, in some countries, has largely replaced heroin as the opioid of choice among intravenous drug users [21-23]. While the combination of buprenorphine with naloxone appears to have lower abuse potential than buprenorphine alone, it also has some abuse liability (e.g., [24]). New sustained-release formulations of buprenorphine that mitigate some of the concerns with sublingual buprenorphine have been approved recently by the FDA and other regulatory bodies throughout the world and are now available for treating OUD. Naltrexone, an opioid antagonist, is effective in virtually eliminating the agonist effects of heroin and other opioids [25-28]. However, compliance with medication ingestion of oral formulations is a problem clinically, leading to low treatment success. Sustained-release formulations of naltrexone are potential solutions to this problem and have comparable efficacy to agonist maintenance therapy in the management of OUD, but detoxification is required before opioid-dependent patients can be transitioned onto naltrexone, which is a difficult hurdle for those who may be interested in this treatment approach. While the available agonist and antagonist medications are effective clinically, the difficulties associated with their use highlight the need for novel medication approaches for the treatment of OUD. Desirable characteristics of an alternative medication for OUD include: 1) lack of abuse liability, 2) does not require detoxification, 3) long duration of therapeutic action, 4) good safety profile, 5) free of drug-drug interactions, and 6) compatibility with opioid agonist or antagonist therapy to allow its combined use with these medications to enhance overall efficacy. Opioid vaccines, which have been well characterized in animal models but have not yet been tested in humans in the U.S., offer these characteristics.
3. Brief historical perspective of vaccines for SUD.
Immunotherapies or immunopharmacotherapies have been proposed as a treatment strategy for SUD as early as the 1970’s [29, 30]. Both active immunization (vaccination) and passive immunization (transfer of pre-made antibodies) strategies against drugs of abuse rely on the presence of anti-drug antibodies to bind drugs of abuse in serum and extracellular fluids and prevent their entry across the blood brain barrier. By restricting the distribution of the antibody-bound drug, antibody-based therapies reduce the amount of unbound (free) drug that reaches the brain and suppress drug-induced pharmacological, physiological and behavioral effects. In 1974, Bonese et al. demonstrated that active immunization with a vaccine consisting of a morphine-based hapten conjugated to bovine serum albumin (BSA) stimulated antibodies that reduced intravenous heroin self-administration in non-human primates [29]. Since this first milestone, the field of vaccines for SUD has evolved and generated extensive preclinical proof of efficacy and selectivity, as well as clinical proof of concept, for this strategy against nicotine, cocaine, methamphetamine, opioids, and designer drugs such as synthetic cathinones [31].
4. Vaccines for OUD: mechanism of action.
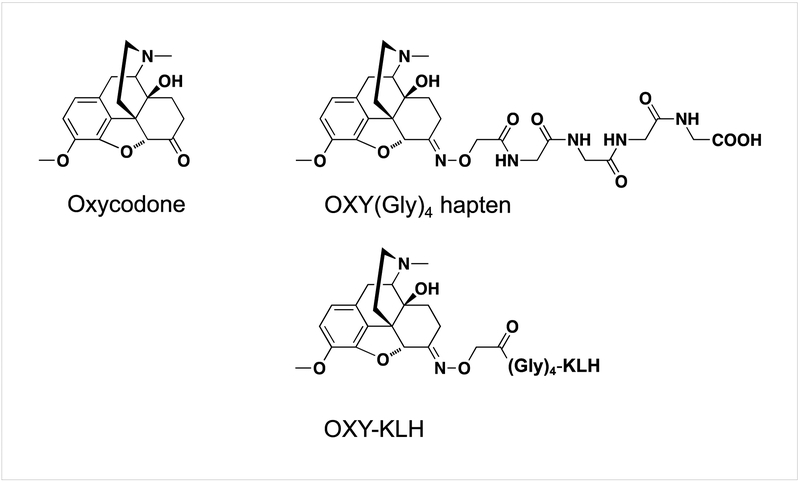
Vaccines for OUD consist of small molecule opioid-based haptens chemically bound to a larger immunogenic carrier, which is usually a protein of bacterial, viral or other foreign origin. Figure 1 provides an example of a candidate vaccine for oxycodone. Conjugates are mixed or adsorbed on adjuvants or other particle-based delivery systems and then injected into the animal or human subject preferably via the intramuscular or subcutaneous route of administration. Once injected, the vaccine will stimulate the innate and adaptive immune system to generate opioid-specific antibodies that will selectively bind the target opioid and effectively reduce opioid distribution to the brain. Because antibody-bound opioids are no longer able to cross the blood-brain barrier, vaccine-induced antibodies reduce the concentration of unbound opioid (free drug) that reaches the brain, thus attenuating or diminishing opioid-induced behavior and opioid-induced physiological effects associated with overdose. Compared to FDA-approved medications consisting of pharmacological agonists and antagonists of the opioid receptors (e.g., methadone or naltrexone), vaccines operate through a pharmacokinetic mechanism of action by reducing or slowing drug entry to the brain. Because of their selectivity for the target opioid, vaccines are not expected to interfere with endogenous neuropeptides nor with medications used for treatment of OUD or reversal of overdose. Indeed, it is expected that anti-opioid vaccines can be combined with methadone, buprenorphine, naltrexone and naloxone. Because of the antibody’s long half-life, vaccines will provide longer-lasting protection compared to small molecule-based pharmacotherapies. Preclinical data in mice, rats, dogs, and non-human primates showed that vaccines can reduce opioid distribution to the brain in acute and chronic challenge models, opioid intravenous self-administration, opioid-induced motor activity, analgesia, respiratory depression, bradycardia, and lethality. These studies support the efficacy and safety of this approach and its translation. Studies in humans will determine whether this is a promising approach for treatment of OUD and reduction of opioid-related overdoses.
Figure 1.
A candidate vaccine targeting oxycodone and hydrocodone use disorders. The oxycodone-based hapten [OXY(Gly)4] is conjugated to the keyhole limpet hemocyanin (KLH) carrier protein and adsorbed on adjuvant. Immunization with OXY-KLH reduced oxycodone-induced behavioral, pharmacological and toxic effects in mice and rats.
5. Clinical status of vaccines for SUD and OUD.
Nicotine and cocaine vaccines have been evaluated in humans, including Phase III clinical trials [32-36]. Most of these clinical studies were conducted with vaccines consisting of nicotine- or cocaine-based haptens conjugated to carrier proteins such as recombinant Exotoxin A from Pseudomonas Aeruginosa [32], Cholera Toxin B subunit protein [36], and virus-like particles (VLP) from the bacteriophage Qβ [37]. Most of these studies involved formulation of vaccines in the FDA-approved alum adjuvant. Results from these clinical trials are reviewed elsewhere [31, 38-40]. In these initial studies, high individual variability and insufficient antibody levels resulted in limited clinical efficacy. However, clinical data showed that a subset of immunized subjects achieved clinically effective antibodies indicating that this approach is viable as long as more effective vaccines are developed and that treatment will be tailored to the target population clinical profile. More recent clinical trials involved a nicotine vaccine (SEL 068) containing a novel particle-based platform developed by Selecta Biosciences [41], Pfizer’s nicotine vaccine containing cross-reactive material-197 (CRM197) and formulated in alum mixed with the TLR9 agonist CpG adjuvant [42], and a cocaine vaccine containing a carrier derived from a disrupted adeno-associated virus (AAV, [43]). No data are available for these latest clinical trials. To date, one clinical study conducted in Iran showed safety of an opioid vaccine consisting of a morphine-BSA conjugate, but no efficacy data are available [44, 45]. As several promising candidate vaccines for OUD have entered late-stage preclinical development, it will be important to test their safety and efficacy in humans. Clinical studies of opioid vaccines will provide the opportunity to test whether specific immunological mechanisms underlie or predict vaccine efficacy against opioids in humans.
6. Vaccines for OUD: pros and cons vs current therapies
a. What can vaccines offer?
Vaccines can offer a long-lasting, safe, and cost-effective alternative for treatment of OUD and reduce risk of overdose. Given the half-life of polyclonal antibodies, vaccines are expected to provide longer-lasting protection. Based on clinical data from first-generation nicotine and cocaine vaccines, vaccines may provide antibodies for at least 6 months. Vaccines are not opioid receptor antagonists, and so detoxification prior to initiating the vaccination protocol would not be necessary. Vaccine immunogenicity should not be affected by ongoing opioid use. It has been shown that influenza and hepatitis B vaccines retain immunogenicity in methadone-treated patients or opioid users [46, 47]. Similarly, in mice and rats, chronic infusion of morphine via osmotic minipumps did not affect immunogenicity of an oxycodone vaccine [48]. Preclinical studies showed that vaccines can be combined with existing opioid medication-assisted treatments (MAT). For instance, an oxycodone vaccine has been combined with extended-release naltrexone and naloxone in mice and rats [48]. Standard MAT’s may be restricted by admission to a methadone program or, in the case of buprenorphine, by regulations for outpatient prescription, while a vaccine may offer a broader population coverage and appeal to a wider patient population. Since no vaccine or other biologic for SUD or OUD has yet reached the market, it is too early to estimate the cost of a vaccination regimen and the regulatory pathway for patients with OUD. Vaccines are expected to provide longer-lasting protection and will require less frequent dosing than small molecules. Once antibodies are at peak levels after a primary immunization regimen, 6-month or yearly boosts are expected to maintain effective antibody levels. In comparison, extended-release naltrexone or buprenorphine is given monthly. The cost of a new product will be determined by many factors including the manufacturer, reimbursement strategy, and the market size.
b. Limitations of vaccines.
Preclinical and clinical data have consistently shown that vaccine efficacy in reducing the effects of clinically relevant doses of drugs of abuse depends upon the quantity and quality of the antibody response. Clinical studies of vaccines targeting either nicotine or cocaine showed that only a subset of immunized subjects (~30% or top 30th percentile) achieved sufficient drug-specific antibody levels, measured as titers or concentrations of at least >40μg/ml, but preferably >100μg/ml. Although it is well established that drug-specific antibody titers correlate with vaccine efficacy in animal and human subjects, the immunological underpinnings of vaccines for SUD are still poorly understood compared to vaccines for infectious diseases. Preclinical studies have shown that other features of polyclonal antibodies are important in achieving vaccine efficacy against drugs of abuse, such as selectivity and affinity (e.g., Kd) for the target drug, and the antibody class (e.g., IgG) or subclass (i.e., IgG1-3). Hence, individual variability in post-vaccination polyclonal antibody responses plays a critical role in determining vaccine efficacy. The source of individual variability in vaccine efficacy against OUD or SUD is not fully understood, and it is likely the result of several variables: 1) vaccine design, composition, and immunization regimen; 2) individual variability in the pre- and post-immunization status of the host’s immune system; 3) sex and age; 4) genetic make-up of the host’s immune system; and 5) individual drug intake patterns. Additionally, patients with OUD may prefer a single opioid product (e.g., heroin vs oxycodone), engage in use of multiple opioids simultaneously (e.g., heroin and fentanyl), or transition between different opioids, all of which may be critical factors for vaccine efficacy. Furthermore, because vaccine efficacy depends upon the ability of drug-specific antibodies to quickly and selectively bind the target opioid, the stoichiometric ratio between opioid molecules and the antibody opioid-binding sites is important. It has been shown that vaccines may be more effective in blocking high doses of oxycodone delivered s.c., rather than heroin doses delivered i.v. in rats [49], suggesting that the type of opioid product as well as its dose, route of administration, volume of distribution and pharmacokinetic profile will have an impact on vaccine efficacy. These data further suggest that vaccines will likely be most effective in clinical scenarios where patients are using smaller doses or intermittent dosing patterns that do not saturate the antibody binding capability, or in abstinent patients at risk of relapse. In the case of heroin, oxycodone, and other prescription opioids, it may be that vaccines will be most effective when combined with standard MAT, and in those patients at risk of relapse to avoid opioid-induced fatal overdoses or in those individuals at earlier stages of OUD.
Fentanyl and its analogs present a unique clinical scenario as these compounds are several orders of magnitude more potent than heroin and oxycodone, and therefore smaller doses are sufficient for inducing fatal overdose. This suggests that clinical efficacy of fentanyl vaccines will benefit from favorable stoichiometry of available antibody vs. low molar drug doses. Because vaccine efficacy may be limited by poly-opioid misuse, multivalent immunization strategies involving co-administration of multiple vaccines may provide broad protection against multiple opioids. The concept of multivalent vaccine formulation has first been shown to increase efficacy against nicotine [50, 51], and then extended to bivalent vaccines targeting oxycodone, 6-acetylmorphine and morphine [52], and heroin/fentanyl mixtures [53, 54].
In summary, vaccines will likely offer a safe, versatile and cost-effective intervention that can be co-administered in combination with other FDA-approved treatments. Because of individual variability and other factors affecting vaccine efficacy, clinical implementation of immunopharmacotherapies will benefit from identifying patients most likely to respond to vaccines, perhaps through use of diagnostic assays or predictive biomarkers.
7. Vaccines for OUD: preclinical development.
Several vaccine candidates have shown efficacy against heroin, 6-acetyl-morphine, morphine, oxycodone, hydrocodone, fentanyl and fentanyl like-compounds in mice, rats, and non-human primates. This section will review our current understanding of the immunological mechanisms underlying vaccine efficacy against opioids and other drugs of abuse, current vaccine candidates in the preclinical pipeline, and strategies to improve vaccine efficacy.
a. Immunology of vaccines for OUD.
After immunization, vaccines are processed by professional antigen presenting cells (APC) displaying Major Histocompatibility Complex (MHC) II receptors or simply passively transported to the nearest draining lymph node. In secondary lymphoid organs, such as spleen and lymph nodes, APC will present vaccine fragments through MHC II receptors to B and T cell lymphocytes, or alternatively vaccines will directly stimulate B cells in the presence of cognate CD4+ T cells. In general, CD4+ T cell-dependent B cell activation occurs in germinal centers (GC) formed in lymph nodes and spleen after vaccination [55-58]. The GC is a transient hot spot where B cell clonal expansion and maturation into specialized B cell subsets occur in the presence of CD4+ T follicular helper cells (Tfh), or their specialized subset CD4+ GC Tfh cells (GC-Tfh) primarily responsible for supporting GC B cells and their differentiation into long-lived plasma cells or memory B cells [59, 60]. Because vaccines for OUD or SUD consist of drug-based haptens conjugated to foreign immunogenic carriers (e.g., Fig. 1), the next section reviews hapten-specific B cells, carrier-specific T cells, and immunological pathways associated with vaccine efficacy against drugs of abuse.
While investigating potential sources of individual variability in response to vaccines for SUD, our group has found that the pre-immunization frequency of hapten-specific B cells correlates with vaccine efficacy against oxycodone in individual mice [61]. This finding was supported by evidence that the frequency of early-activated B cell subsets correlated with vaccine efficacy against oxycodone and nicotine [61, 62]. These data suggest that the pre-immunization B cell repertoire may be a source of individual variability and that certain individuals may respond better to a specific vaccine formulation. Indeed, when comparing two formulations of oxycodone vaccines containing structurally related opioid haptens, a higher frequency of B cells specific for the most immunogenic hapten was found prior to vaccination [63]. Analysis of the post-vaccination B cell repertoire indicated that the magnitude and quality of GC formation correlated with the magnitude of individual variability in vaccine efficacy against opioids [61]. This finding is consistent with vaccines against other targets and indicates that vaccine development should focus on vaccine components that favor activation of the GC reaction.
As B cell activation is supported by cognate CD4+ T cells, we further demonstrated that the frequency of carrier-specific MHCII-restricted CD4+ T cells prior to vaccination correlates with vaccine efficacy against oxycodone, and that ablation of CD4+ T cells prevents B cell activation and vaccine efficacy in mice [61]. Further, immunization with OXY-KLH of a mouse genetic model missing a functional T cell receptor (TCR alpha knockout mice) or immunization of wild-type mice with the OXY hapten conjugate to the T cell-independent model carriers ficoll and dextran demonstrated that vaccine efficacy requires an intact TCR and T cell epitopes such as peptides or proteins [64]. The role of carrier proteins is highlighted by studies showing that the same hapten conjugated to different carrier proteins or peptides may have different abilities to activate hapten-specific B cells and induce antibodies with efficacy against the target opioid [64, 65]. Novel carriers such as the Toll-like receptor 5 (TLR5) agonist flagellin [66], the heat shock protein 70 [67], and nanoparticles [68, 69] can be used to improve vaccine efficacy against drugs of abuse. These studies suggest that a better understanding of the contribution of T cell activation to vaccine efficacy against OUD or SUD will guide development of more effective vaccine components.
It is well-established that adjuvants play a critical role in achieving vaccine efficacy against drugs of abuse or other targets [70-73]. In the context of vaccines for OUD, several adjuvant or particle platforms have been tested [74-77] suggesting that each individual vaccine candidate will need to be individually evaluated and formulated based on the components of choice. In our experience, an oxycodone vaccine was more effective in alum than in the recently FDA-approved squalene-based MF59 [78]. Compared to MF59, alum was more effective in inducing early, but not late, T cell differentiation toward the GC-Tfh subset [78], and these data were consistent with the hypothesis that early events (including GC formation) shortly after vaccination are critical to vaccine efficacy against OUD or other drugs of abuse. Furthermore, early patterns of CD4+ T cell polarization (e.g., ratio of Th1 vs Tfh and GC Tfh subsets, [79]) may be shaping vaccine efficacy against drugs of abuse. Future studies may benefit from high throughput screening of vaccine formulations paired with analysis of adaptive immune responses to identify biomarkers of vaccine efficacy against drugs of abuse.
Molecular or cellular pathways and immunological mechanisms underlying vaccine efficacy against OUD or other SUD targets are still relatively poorly understood. Several studies have shown the role of TLR in inducing effective antibodies against opioids or other drugs of abuse, as several vaccine candidates have been improved by incorporation of the TLR4 agonist MPLA or its derivatives [75], the TLR9 agonist CpG [77, 80], a TLR3 agonist derived from viral genomic double-stranded RNA (dsRNA, [81]), and the TLR5 agonist flagellin. In our experience, MPLA was not effective in improving efficacy of an oxycodone vaccine, but immunization of TLR4−/− mice yielded reduced oxycodone-specific IgG titers and antibody-secreting B cells [65]. Choice of a specific TLR agonist may depend on each individual vaccine formulation and their suitability for either pre-clinical or clinical immunization strategies.
Identification of other pharmacological targets for vaccine rational design or predictive biomarkers may involve more extensive investigations. Our group has recently tested the hypothesis that vaccine efficacy could be improved by modulation of key cytokines or co-stimulatory pathways often involved in T cell-dependent B cell activation [79]. Using this approach, we found that neutralization of IL-4 by means of an anti-IL-4 mAb (αIL-4) and immunization of IL-4−/− mice increased vaccine efficacy against oxycodone in mice [79]. This finding suggested that small molecule- or antibody-based inhibitors of the IL-4 signaling can be exploited for enhancement of vaccine efficacy against OUD or other target SUD and that IL-4 could be a biomarker of vaccine efficacy. Finally, this study found that increased levels of IgG2a and IgG3 correlated with increased efficacy against oxycodone in mice suggesting that IgG subclasses may play a role in vaccine efficacy against drugs of abuse [79].
Although these studies further our understanding of the immunological mechanisms underlying vaccine efficacy against OUD, their implications should be assessed carefully given that the majority of studies were conducted in rodents. Ultimately, only immunization of human subjects will provide information about the immunological responses to vaccines for OUD. Upon initiation of clinical trials of opioid vaccines, it will be possible to analyze plasma opioid-specific IgG antibody titers, concentrations, subclasses, and affinity. Additionally, it will be possible to study opioid-specific B cell lymphocytes to investigate whether specific polymorphisms are associated with vaccine efficacy or to test whether specific binding patterns between B cells and vaccines are critical for triggering an effective immune response against opioids. Alternatively, it will be possible to isolate opioid-specific mAb from the B cell repertoire, which is a routine approach in vaccinology to better understand the functional capabilities of B cells after vaccination. To date, there has been only one report of nicotine-specific mAb isolated from B cells of individuals immunized with the Nic-Qβ vaccine [82], and these mAb have been used in crystallography studies to characterize mAb binding to nicotine or the nicotine-based hapten [83]. Future availability of human samples from immunized subjects will provide an invaluable resource to study the human immunology of vaccines against OUD.
b. Heroin and morphine.
As mentioned above, reports of vaccines or antibodies targeting heroin or morphine could be found as early as the 1970’s [29, 30, 84-88]. Because effective pharmacotherapies for OUD were available (e.g., methadone, buprenorphine, naltrexone), advances in immunotherapies focused more on nicotine, cocaine, methamphetamine, and psychostimulants such as phencyclidine. The field of anti-opioid vaccines was renewed following a study from Anton and Leff reporting a vaccine targeting heroin, and its active metabolites 6-acetylmorphine (6-AM) and morphine, which prevented the behavioral effects of heroin, including heroin-induced reinstatement [89]. Several candidates have shown preclinical efficacy against heroin and its metabolites in mice, rats, and non-human primates (e.g., [90-95]). Overall, these vaccines are effective in reducing drug distribution to the brain in mice or rats challenged with intravenous or subcutaneous doses of heroin, 6-AM, and morphine. Furthermore, these vaccines reduce behavioral effects of heroin including antinociception in the hotplate and tail flick tests, locomotor activity, and intravenous heroin self-administration. Current candidate heroin vaccine formulations contain structurally different haptens, protein, adjuvant components, and have employed either maleimide or carbodiimide coupling chemistry.
Dr. Gary Matyas and his group at the Walter Reed Army Institute of Research has developed a series of vaccines targeting heroin, which identified a lead candidate consisting of a morphine-based hapten (MorHap) containing a PEGylated linker [(PEG)2-maleimide crosslinker] at the C3 position and conjugated to tetanus toxoid and admixed with an adjuvant consisting of liposomes containing monophosphoryl lipid A (MPLA), described as the Army Liposome Formulation (ALF) [76, 94, 96, 97]. The MorHap-TT admixed on ALF reduced tail-flick analgesia and motor activity in mice and rats challenged with s.c. and i.v. heroin doses [76, 94]. Interestingly, the MorHap-TT increased efficacy when ALF was adsorbed on aluminum hydroxide [74], which highlights how important it is to carefully titrate vaccine components. The MorHap-TT/ALF also retained efficacy in mice when co-administered with a candidate HIV vaccine consisting of synthetic peptides derived from the V2 loop of one of the HIV-1 envelope glycoproteins [76], further supporting that vaccines for SUD could be combined with other lines of treatment which may benefit subsets of the SUD patient population and their potential comorbidities.
Dr. Kim Janda and his group at The Scripps Research Institute (TSRI) has developed a series of vaccines containing heroin or morphine-based haptens modified at the nitrogen bridgehead, which have showed pre-clinical efficacy against heroin, 6-AM and morphine in various pre-clinical models [81, 92, 93, 98]. One recent study compared the benefit of incorporating either the TLR9 CpG or the TLR3 dsRNA adjuvants delivered in either alum or the VesiVax CALV liposome-based system [81]. In this study, the heroin-TT conjugate formulated in alum/CpG showed stability over time under different storage conditions, and effectively increased survival rates in mice challenged with 160mg/kg heroin [81]. Delivered in alum/CpG, heroin-TT conjugated through carbodiimide chemistry was more effective than an analogous maleimide-based conjugate in reducing heroin-induced antinociceptive effects in mice [92]. This lead vaccine formulation effectively decreased schedule-controlled responding in non-human primates challenged with 0.01-1.0 mg/kg heroin [92]. Vaccine efficacy was comparable to 3.2 μg/kg naltrexone, but not as effective as 32 μg/kg naltrexone [92], suggesting that vaccine efficacy against heroin will depend upon the dose and patterns of administration in patients with OUD. Because of the longer half-life of vaccine-induced antibodies, vaccines may provide extended protection compared to small molecule-based medications.
Our team has also developed a vaccine consisting of a morphine-based hapten (M) containing a tetraglycine linker at the C6 position on morphine and conjugated to either native KLH or a GMP-grade subunit KLH [49, 95, 99]. M-KLH is effective in reducing heroin, 6-AM, and morphine distribution to the brain in rats challenged with either i.v. or s.c. doses of either heroin or 6-AM. M-KLH is also effective in reducing heroin-induced hotplate analgesia and locomotor activity, as well as reinstatement of intravenous heroin self-administration in rats [49, 95, 99]. Active immunization with M-KLH reduced heroin-induced respiratory depression in rats challenged i.v. with 0.26 mg/kg heroin [49]. Co-administration of M-KLH with an analogous oxycodone vaccine was effective in blocking both 6-AM and oxycodone distribution to the brain in rats [52]. The M hapten was equally effective when conjugated to either sKLH or E. coli-expressed CRM (EcoCRM) in reducing heroin-induced hot plate analgesia and distribution of heroin, 6-AM, and morphine to the brain in mice [64].
c. Oxycodone and hydrocodone.
In contrast to heroin, fewer candidate vaccines against oxycodone and hydrocodone have emerged from the preclinical pipeline. Our group has identified a candidate vaccine targeting oxycodone and hydrocodone consisting of an oxycodone-based hapten modified at the C6 position (Fig. 1) with a tetraglycine linker and conjugated to the keyhole limpet hemocyanin (KLH) carrier protein and adsorbed on alum adjuvant. The Oxy(Gly)4-KLH vaccine generates antibodies specific for oxycodone and hydrocodone, which do not bind off-target opioids such as methadone, buprenorphine, naltrexone and naloxone as well as endogenous opioids [100]. Immunization with Oxy(Gly)4-KLH reduces oxycodone and hydrocodone distribution to the brain in mice and rats, and reduces their analgesic effects in the hot plate test [101]. The Oxy(Gly)4-KLH reduces acquisition of intravenous oxycodone self-administration in rats [102], oxycodone-induced motor activity in mice [78], and respiratory depression in both mice and rats [48, 79]. Immunization with Oxy(Gly)4-KLH does not interfere with the ability of naloxone to reverse oxycodone-induced respiratory depression and bradycardia in both mice and rats [48, 79]. Finally, the immunogenicity of Oxy(Gly)4-KLH was not affected by concurrent administration of either chronic morphine or extended-release naltrexone [48], indicating that it is possible to vaccinate patients currently using either opioid agonists or antagonists.
During preclinical development, the Oxy(Gly)4-KLH was identified by systematically testing hapten design and coupling chemistry, carrier proteins, and adjuvant. The lead Oxy(Gly)4 hapten conjugated to KLH was selected within a series of structurally similar conjugates containing oxycodone- and hydrocodone-based haptens modified at either the C6 or C8 position of the morphinan structure and conjugated by either carbodiimide or maleimide coupling chemistry [101]. Additional permutations involved comparing the effect of switching the terminal carboxyl group on Oxy(Gly)4 hapten with a thiol group ready for thioether linkage, or comparing the Oxy(Gly)4 hapten to an equivalent oxycodone-based hapten containing a PEGylated linker in lieu of the tetraglycine linker [64]. Because it is well established that carrier proteins play a critical role in vaccine efficacy and help with formulation optimization, the Oxy(Gly)4 hapten was shown to be effective when conjugated to native KLH, monomer or dimer subunit KLH, tetanus toxoid (TT), CRM197, and E. coli-expressed CRM (EcoCRM) and recombinant tetanus toxoid fragment (rTThc), but not when conjugated to a TT-derived small peptide [64, 65]. When possible, the Oxy(Gly)4-TT, Oxy(Gly)4-sKLH, Oxy(Gly)4-CRM197, Oxy(Gly)4-EcoCRM and Oxy(Gly)4-rTThc conjugates were characterized for size and aggregation status by mass spectrometry (MALDI-TOF), size exclusion chromatography (SEC) HPLC, dynamic light scattering (DLS) and SDS-PAGE [64]. MALDI-TOF and DLS were the most suitable assays for characterization of opioid-based conjugates [64]. Contrary to expectations, the Oxy(Gly)4-KLH was effective in Freund’s adjuvant and alum, but not in alum/MPLA, MPLA alone, or MF59 [65, 78]. Our experience with Oxy(Gly)4-KLH suggests that each individual vaccine component needs to be optimized for a specific formulation and tested under various conditions involving models of opioid exposures. Ultimately, steps toward product development and manufacturing will depend upon access to pharmaceutical grade components and the ability to fully characterize the vaccine formulation.
Another promising vaccine targeting oxycodone was identified by Dr. Janda and Dr. Michael Taffe at TSRI within a series of oxycodone- and hydrocodone-based haptens, and consists of an oxycodone-based hapten containing a succinic anhydride-derived linker attached at the N-methyl group of the morphinan structure and conjugated to TT by carbodiimide chemistry [103]. The Oxy-TT effectively reduced oxycodone-induced analgesia, oxycodone distribution to the brain, fatal overdose, and maintenance of intravenous oxycodone self-administration under different schedules of reinforcement in rats [103, 104]. These studies show that vaccines have the potential to reduce behavioral effects of clinically relevant doses of oxycodone and hydrocodone as well as their lethality.
d. Fentanyl and fentanyl analogs.
One of the critical challenges to developing a vaccine targeting the fentanyl chemical family will be to determine whether it is possible to identify a single vaccine that targets fentanyl and its analogs, or whether it will be necessary to develop multiple individual vaccines targeting different compounds within the fentanyl-like family. That question remains unanswered. In 1975, three studies have shown preclinical efficacy of immunotherapies against fentanyl [105-107]. One of these studies demonstrated that passive transfer of fentanyl-specific antibodies prevented fentanyl-induced respiratory depression in a dog model [107]. More recently, the Janda group has developed a vaccine that reduces antinociception induced by fentanyl as well as α-methylfentanyl, and cis-3-methylfentanyl, and protects mice from lethal doses of fentanyl [108]. Follow-up studies further demonstrated that vaccines are effective in reducing pharmacological and behavioral effects of heroin contaminated with fentanyl [53, 54].
Our team has also identified a candidate vaccine targeting fentanyl, which consists of a fentanyl-based hapten containing a tetraglycine linker conjugated to either native KLH or GMP-grade subunit KLH (sKLH) carrier proteins by EDAC coupling chemistry [109]. The resulting F(Gly)4-KLH was effective in mice and rats in acutely reducing fentanyl distribution to the brain and reducing fentanyl-induced antinociception [109]. Additionally, the F(Gly)4-sKLH was effective in reducing respiratory depression in rats challenged with increasing cumulative doses of fentanyl up to 0.1mg/kg [109]. Finally, immunization with F(Gly)4-sKLH did not interfere with the ability of the opioid antagonist naloxone to reverse fentanyl-induced respiratory depression [109], indicating that fentanyl vaccines can be combined with naloxone in reversing opioid overdose. Overall these studies support further development of vaccines targeting the fentanyl chemical family to reduce the incidence of fatal overdoses.
8. Translation of vaccines for OUD: overview of manufacturing, regulatory and clinical challenges.
a. Manufacturing.
Vaccine candidates showing promising preclinical proof of efficacy and safety will be identified for further development. The transition between early- and late-stage development presents unique challenges including vaccine formulation optimization, access to vaccine components suitable for pharmaceutical manufacturing and human testing, development of qualifying assays and release criteria to characterize the product, demonstration of scalability, technology transfer to third parties such as Contract Manufacturing Organizations (CMOs) and Contract Research Organizations (CROs) to synthesize the vaccine under Good Manufacturing Practices (GMP) and formally test its safety in toxicology studies under Good Laboratory Practices (GLP).
b. Regulatory.
Opioids are controlled substances regulated by the Drug Enforcement Agency. Accordingly, preclinical and clinical research involving anti-opioid vaccines will require appropriate DEA licensing to conduct studies with these compounds. The DEA groups opioids under either Schedule I (e.g., heroin) or Schedule II-V (e.g., oxycodone, morphine) licenses. Most academic research activities will require a Research License, which is routinely obtained through institutional applications. However, private sector efforts or collaborations with a CMO to generate GMP-grade materials may require acquisition of a DEA Manufacturing License.
Because opioid vaccines consist of multiple discrete or hybrid components (small molecule hapten, carrier protein, adjuvant), research teams seeking clinical testing of a new opioid vaccine in the United States will require FDA approval for initiating human testing through an Investigational New Drug application, which will be reviewed by the Center for Biologics Evaluation and Research (CBER). The IND package generally includes information about the vaccine: 1) preclinical efficacy and safety data, 2) release criteria that meet FDA guidelines, 3) details about the vaccine formulation and qualifying assays used to characterize the vaccine formulation, 4) details about the GMP-grade batch of vaccine used in GLP toxicology studies and in subsequent Phase I clinical trials, and 5) a detailed clinical protocol for testing of vaccines in human subjects.
c. Clinical: patient population.
Administration of vaccines targeting different opioids requires careful consideration of clinical study design. Although most Phase 1 studies are conducted in normal healthy volunteers with no history of substance use disorders, the potential for unintended vaccine-induced adverse effects related to endogenous opioids or off-target systems may make the risk/benefit ratio unfavorable in this population. Therefore, studies may need to be conducted in participants with OUD. However, testing an opioid vaccine in individuals with OUD raises its own set of practical and ethical concerns. For example, testing a heroin vaccine in a participant who predominantly uses heroin might drive him or her to seek out another more toxic opioid, such as fentanyl, if the vaccine successfully blocks the effects of heroin. Another potential concern is that the vaccine might be less effective in generating antibody responses in participants with active diseases, such as HIV infection, AIDS, or hepatitis, all of which are relatively common in users who inject drugs. If the vaccine is tested in participants who are physically dependent on opioids, an additional consideration relates to the most appropriate maintenance medication to use in order to prevent the emergence of withdrawal symptoms during the study while simultaneously avoiding potential cross-reactivity with the antibodies generated by the vaccine. Because multiple vaccinations may be required over several months, another potential concern to implement anti-opioid vaccines may include the challenge of conducting clinical trials and ensure optimal clinical management of patients with OUD. It may be that a transition period involving agonist maintenance (with either methadone or buprenorphine), along with initiation of vaccine administration, may be needed to minimize drop out. These and other ethical and scientific issues require extensive discussions with the FDA and Institutional Review Boards.
9. Conclusions.
Vaccines offer a promising strategy to treat OUD and potentially reduce the likelihood of fatal overdoses. Because of their selectivity and long-lasting efficacy, vaccines will provide an additional therapeutic strategy complementary to current behavioral and pharmacological therapies for OUD. As vaccine candidates move into late-stage development, it will be important to close the gap between preclinical and clinical research. As discussed in this review, it will also be important to leverage cutting-edge immunological tools to further understand the basis of individual variability to vaccines for OUD. These studies will provide a blueprint to develop more effective vaccines and to identify predictive biomarkers of vaccine efficacy that will enable individualized therapy for OUD.
HIGHLIGHTS.
Vaccines are a promising strategy to treat opioid use disorders (OUD) and prevent overdose
Vaccines offer benefits complementary to approved Medication Assisted Treatment (MAT)
Pre-clinical studies provide proof of efficacy, safety and selectivity for vaccines for OUD
Translation of vaccines for OUD is challenging, but necessary to validate this approach
Acknowledgements:
The authors thank the National Institute of Health for support under DA038876. The authors thank Dr. Carly Baehr for comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Dr. Pravetoni is the inventor of “Cytokine Signaling Immunomodulators and Methods”. U.S. Provisional Patent Application No. 62/334, 167 filed on May 10, 2016. International Application No. PCT/US2017/031907 filed on May 10, 2017. In the past 36 months, Dr. Comer has served on advisory boards, received grant funding from, collaborated with, and/or consulted for: Alkermes, Braeburn, Cerecor, Charleston Labs, Clinilabs, Collegium, Corbus, Daiichi Sankyo, Depomed, Egalet, Endo, Epiodyne, Indivior, Go Medical, Inspirion Delivery Sciences, Intracellular Therapies, Janssen, KemPharm, Kures, Lyndra, Mallinckrodt, Nektar, Neurolixis, Newron, Opiant, Otsuka, Pfizer, Shin Nippon Biomedical Laboratories, and Sun Pharma. She also has received honoraria from the World Health Organization (WHO).
REFERENCES
- 1.UNOCD, World Drug Report 2018. 2018.
- 2.The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry, 2018. 5(12): p. 987–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam T, Commentary - Building the evidence base for sustained public health response to the opioid epidemic in Canada. Health Promot Chronic Dis Prev Can, 2018. 38(6): p. 221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helander A, et al. , Intoxications involving acrylfentanyl and other novel designer fentanyls - results from the Swedish STRIDA project. Clin Toxicol (Phila), 2017. 55(6): p. 589–599. [DOI] [PubMed] [Google Scholar]
- 5.Salm-Reifferscheidt L, Tramadol: Africa's opioid crisis. Lancet, 2018. 391(10134): p. 1982–1983. [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, Einstein EB, and Compton WM, Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010-2016. Jama, 2018. 319(17): p. 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedegaard H, Warner M, and Minino AM, Drug Overdose Deaths in the United States, 1999-2016. NCHS Data Brief, 2017(294): p. 1–8. [PubMed] [Google Scholar]
- 8.Chen Q, et al. , Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States. JAMA Netw Open, 2019. 2(2): p. e187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfield MC, et al. , Prescription opioid use among patients seeking treatment for opioid dependence. J Addict Med, 2010. 4(2): p. 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cicero TJ and Kuehn BM, Driven by prescription drug abuse, heroin use increases among suburban and rural whites. Jama, 2014. 312(2): p. 118–9. [DOI] [PubMed] [Google Scholar]
- 11.Cicero TJ, Kasper ZA, and Ellis MS, Increased use of heroin as an initiating opioid of abuse: Further considerations and policy implications. Addict Behav, 2018. 87: p. 267–271. [DOI] [PubMed] [Google Scholar]
- 12.Armenian P, et al. , Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Riches JR, et al. , Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J Anal Toxicol, 2012. 36(9): p. 647–56. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND and Collins FS, The Role of Science in Addressing the Opioid Crisis. N Engl J Med, 2017. 377(4): p. 391–394. [DOI] [PubMed] [Google Scholar]
- 15.Collins FS, Koroshetz WJ, and Volkow ND, Helping to End Addiction Over the Long-term: The Research Plan for the NIH HEAL Initiative. Jama, 2018. 320(2): p. 129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strain EC and Stitzer ML, The Treatment of Opioid Dependence, ed. J.H.U. Press; 2005. [Google Scholar]
- 17.Carrieri MP, et al. , Buprenorphine use: the international experience. Clin Infect Dis, 2006. 43 Suppl 4: p. S197–215. [DOI] [PubMed] [Google Scholar]
- 18.Walsh SL, et al. , Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther, 1994. 55(5): p. 569–80. [DOI] [PubMed] [Google Scholar]
- 19.Walsh SL, et al. , Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl), 1995. 119(3): p. 268–76. [DOI] [PubMed] [Google Scholar]
- 20.Levin FR, et al. , A protocol to switch high-dose, methadone-maintained subjects to buprenorphine. Am J Addict, 1997. 6(2): p. 105–16. [DOI] [PubMed] [Google Scholar]
- 21.Obadia Y, et al. , Injecting misuse of buprenorphine among French drug users. Addiction, 2001. 96(2): p. 267–72. [DOI] [PubMed] [Google Scholar]
- 22.Lee CE, Tackling Subutex abuse in Singapore. Singapore Med J, 2006. 47(11): p. 919–21. [PubMed] [Google Scholar]
- 23.Alho H, et al. , Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend, 2007. 88(1): p. 75–8. [DOI] [PubMed] [Google Scholar]
- 24.Comer SD and Collins ED, Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther, 2002. 303(2): p. 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comer SD, et al. , Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology (Berl), 2002. 159(4): p. 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navaratnam V, et al. , Determination of naltrexone dosage for narcotic agonist blockade in detoxified Asian addicts. Drug Alcohol Depend, 1994. 34(3): p. 231–6. [DOI] [PubMed] [Google Scholar]
- 27.Schuh KJ, Walsh SL, and Stitzer ML, Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology (Berl), 1999. 145(2): p. 162–74. [DOI] [PubMed] [Google Scholar]
- 28.Verebey K, et al. , Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther, 1976. 20(3): p. 315–28. [DOI] [PubMed] [Google Scholar]
- 29.Bonese KF, et al. , Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature, 1974. 252(5485): p. 708–10. [DOI] [PubMed] [Google Scholar]
- 30.Killian A, et al. , Effects of passive immunization against morphine on heroin self-administration. Pharmacol Biochem Behav, 1978. 9: p. 347–352. [DOI] [PubMed] [Google Scholar]
- 31.Pravetoni M, Biologics to treat substance use disorders: Current status and new directions. Hum Vaccin Immunother, 2016: p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatsukami DK, et al. , Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clinical pharmacology and therapeutics, 2011. 89(3): p. 392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatsukami DK, et al. , Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical pharmacology and therapeutics, 2005. 78(5): p. 456–67. [DOI] [PubMed] [Google Scholar]
- 34.Kosten TR, et al. , Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend, 2014. 140: p. 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosten TR, et al. , Phase II human study of cocaine vaccine TA-CD. CPDD Annual Meeting, Quebec City, 2002. [Google Scholar]
- 36.Martell BA, et al. , Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Archives of general psychiatry, 2009. 66(10): p. 1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer P, et al. , A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. European journal of immunology, 2005. 35(7): p. 2031–40. [DOI] [PubMed] [Google Scholar]
- 38.Pentel PR and LeSage MG, New directions in nicotine vaccine design and use. Adv Pharmacol, 2014. 69: p. 553–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahim RE, Kessler PD, and Kalnik MW, Therapeutic vaccines against tobacco addiction. Expert review of vaccines, 2013. 12(3): p. 333–42. [DOI] [PubMed] [Google Scholar]
- 40.Kosten T, et al. , Vaccines against stimulants: Cocaine and Methamphetamine. British journal of clinical pharmacology, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser CC, et al. , Generation of a universal CD4 memory T cell recall peptide effective in humans, mice and non-human primates. Vaccine, 2014. 32(24): p. 2896–903. [DOI] [PubMed] [Google Scholar]
- 42.McCluskie MJ, et al. , Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int Immunopharmacol, 2015. 25(2): p. 518–27. [DOI] [PubMed] [Google Scholar]
- 43.Wee S, et al. , Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2012. 37(5): p. 1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akbarzadeh A, et al. , Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnology and applied biochemistry, 1999. 30 ( Pt 2): p. 139–46. [PubMed] [Google Scholar]
- 45.Akbarzadeh A, et al. , Immunotherapy of 347 volunteer outpatient morphine addicts by human therapeutic morphine vaccine in Kermanshah province of Iran. J Pharm Toxicol, 2009. 4(1): p. 30–35. [Google Scholar]
- 46.Moroz E, et al. , Active opioid use does not attenuate the humoral responses to inactivated influenza vaccine. Vaccine, 2016. 34(11): p. 1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borg L, et al. , Methadone-maintained former heroin addicts, including those who are anti-HIV-1 seropositive, comply with and respond to hepatitis B vaccination. Addiction, 1999. 94(4): p. 489–93. [DOI] [PubMed] [Google Scholar]
- 48.Raleigh MD, et al. , Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS One, 2017. 12(12): p. e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raleigh MD, et al. , Opioid dose- and route-dependent efficacy of oxycodone and heroin vaccines in rats. J Pharmacol Exp Ther, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornish KE, et al. , Immunogenicity of individual vaccine components in a bivalent nicotine vaccine differ according to vaccine formulation and administration conditions. PLoS ONE, 2013. 8(12): p. e82557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Villiers SH, et al. , Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pravetoni M, et al. , Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine, 2012. 30(31): p. 4617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang CS, et al. , Improved Admixture Vaccine of Fentanyl and Heroin Hapten Immunoconjugates: Antinociceptive Evaluation of Fentanyl-Contaminated Heroin. ACS Omega, 2018. 3(9): p. 11537–11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang CS, et al. , Efficacious Vaccine against Heroin Contaminated with Fentanyl. ACS Chem Neurosci, 2018. 9(6): p. 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McHeyzer-Williams M, et al. , Molecular programming of B cell memory. Nature reviews. Immunology, 2012. 12(1): p. 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarlinton DM, Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunology and cell biology, 2008. 86(2): p. 133–8. [DOI] [PubMed] [Google Scholar]
- 57.Victora GD and Mesin L, Clonal and cellular dynamics in germinal centers. Current opinion in immunology, 2014. 28: p. 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Victora GD and Nussenzweig MC, Germinal centers. Annual review of immunology, 2012. 30: p. 429–57. [DOI] [PubMed] [Google Scholar]
- 59.Crotty S, Follicular helper CD4 T cells (TFH). Annual review of immunology, 2011. 29: p. 621–63. [DOI] [PubMed] [Google Scholar]
- 60.Crotty S, A brief history of T cell help to B cells. Nature reviews. Immunology, 2015. 15(3): p. 185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laudenbach M, et al. , The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J Immunol, 2015. 194(12): p. 5926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laudenbach M, et al. , The frequency of early-activated hapten-specific B cell subsets predicts the efficacy of vaccines for nicotine dependence. Vaccine, 2015. 33(46): p. 6332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor JJ, et al. , Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. Journal of immunological methods, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baruffaldi F, et al. , Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol Pharm, 2018. 15(11): p. 4947–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pravetoni M, et al. , Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One, 2014. 9(5): p. e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lockner JW, et al. , Flagellin as carrier and adjuvant in cocaine vaccine development. Mol Pharm, 2015. 12(2): p. 653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang CS, et al. , Heat shock proteins: A dual carrier-adjuvant for an anti-drug vaccine against heroin. Bioorg Med Chem, 2019. 27(1): p. 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z, et al. , A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomedicine, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng H, et al. , Negatively Charged Carbon Nanohorn Supported Cationic Liposome Nanoparticles: A Novel Delivery Vehicle for Anti-Nicotine Vaccine. J Biomed Nanotechnol, 2015. 11(12): p. 2197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alving CR, Design and selection of vaccine adjuvants: animal models and human trials. Vaccine, 2002. 20 Suppl 3: p. S56–64. [DOI] [PubMed] [Google Scholar]
- 71.Alving CR, et al. , Adjuvants for vaccines to drugs of abuse and addiction. Vaccine, 2014. 32(42): p. 5382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Awate S, Babiuk LA, and Mutwiri G, Mechanisms of action of adjuvants. Frontiers in immunology, 2013. 4: p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson-Welder JH, et al. , Vaccine adjuvants: current challenges and future approaches. Journal of pharmaceutical sciences, 2009. 98(4): p. 1278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck Z, et al. , Immune response to antigen adsorbed to aluminum hydroxide particles: Effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. J Control Release, 2018. 275: p. 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matyas GR, et al. , Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine, 2013. 31(26): p. 2804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torres OB, et al. , Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. NPJ Vaccines, 2017. 2: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bremer PT, et al. , Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Molecular pharmaceutics, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson C, et al. , Alum adjuvant is more effective than MF59 at prompting early germinal center formation in response to peptide-protein conjugates and enhancing efficacy of a vaccine against opioid use disorders. Hum Vaccin Immunother, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laudenbach M, et al. , Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci Rep, 2018. 8(1): p. 5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bremer PT and Janda KD, Investigating the effects of a hydrolytically stable hapten and a Th1 adjuvant on heroin vaccine performance. Journal of medicinal chemistry, 2012. 55(23): p. 10776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hwang CS, et al. , Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol Pharm, 2018. 15(3): p. 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beerli RR, et al. , Isolation of human monoclonal antibodies by mammalian cell display. Proc Natl Acad Sci U S A, 2008. 105(38): p. 14336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tars K, et al. , Different binding modes of free and carrier-protein-coupled nicotine in a human monoclonal antibody. J Mol Biol, 2012. 415(1): p. 118–27. [DOI] [PubMed] [Google Scholar]
- 84.Hill JH, et al. , The interaction of 14C-morphine with sera from immunized rabbits and from patients addicted to heroin. Clin Exp Immunol, 1973. 15(2): p. 213–24. [PMC free article] [PubMed] [Google Scholar]
- 85.Hill JH, et al. , Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J Immunol, 1975. 114(4): p. 1363–8. [PubMed] [Google Scholar]
- 86.Wainer BH, et al. , A measurement of the specificities of antibodies to morphine-6-succinyl-BSA by competitive inhibition of 14 C-morphine binding. J Immunol, 1973. 110(3): p. 667–73. [PubMed] [Google Scholar]
- 87.Wainer BH, et al. , Morphine-3-succinyl--bovine serum albumin: an immunogenic hapten-protein conjugate. Science, 1972. 176(4039): p. 1143–5. [DOI] [PubMed] [Google Scholar]
- 88.Wainer BH, et al. , In vitro morphine antagonism by antibodies. Nature, 1973. 241(5391): p. 537–8. [DOI] [PubMed] [Google Scholar]
- 89.Anton B, et al. , Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin, 2009. 5(4): p. 214–29. [DOI] [PubMed] [Google Scholar]
- 90.Kosten TA, et al. , A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Progress in neuro-psychopharmacology & biological psychiatry, 2013. 45: p. 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, et al. , A conjugate vaccine attenuates morphine- and heroin-induced behavior in rats. Int J Neuropsychopharmacol, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bremer PT, et al. , Development of a Clinically Viable Heroin Vaccine. J Am Chem Soc, 2017. 139(25): p. 8601–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schlosburg JE, et al. , Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A, 2013. 110(22): p. 9036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sulima A, et al. , A Stable Heroin Analogue That Can Serve as a Vaccine Hapten to Induce Antibodies That Block the Effects of Heroin and Its Metabolites in Rodents and That Cross-React Immunologically with Related Drugs of Abuse. J Med Chem, 2018. 61(1): p. 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raleigh MD, et al. , Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. The Journal of pharmacology and experimental therapeutics, 2013. 344(2): p. 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jalah R, et al. , Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjug Chem, 2015. 26(6): p. 1041–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matyas GR, et al. , Facial recognition of heroin vaccine opiates: Type 1 cross-reactivities of antibodies induced by hydrolytically stable haptenic surrogates of heroin, 6-acetylmorphine, and morphine. Vaccine, 2014. 32(13): p. 1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stowe GN, et al. , A vaccine strategy that induces protective immunity against heroin. J Med Chem, 2011. 54(14): p. 5195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raleigh MD, Pentel PR, and LeSage MG, Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS One, 2014. 9(12): p. e115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pravetoni M, et al. , An oxycodone conjugate vaccine elicits oxycodone-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. The Journal of pharmacology and experimental therapeutics, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pravetoni M, et al. , Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. Journal of medicinal chemistry, 2013. 56(3): p. 915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pravetoni M, et al. , Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One, 2014. 9(7): p. e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimishima A, et al. , An Advance in Prescription Opioid Vaccines: Overdose Mortality Reduction and Extraordinary Alteration of Drug Half-Life. ACS Chem Biol, 2017. 12(1): p. 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen JD, et al. , Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology, 2018. 138: p. 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henderson GL, et al. , Antibodies to fentanyl. J Pharmacol Exp Ther, 1975. 192(2): p. 489–96. [PubMed] [Google Scholar]
- 106.Torten M, et al. , Proceedings: Immunopharmacology of a potent opiate, fentanyl. Development of radioimmunoassay and prevention of effects by immunologic means. Isr J Med Sci, 1975. 11(12): p. 1395. [PubMed] [Google Scholar]
- 107.Torten M, et al. , Prevention of the effects of fentanyl by immunological means. Nature, 1975. 253(5492): p. 565–6. [DOI] [PubMed] [Google Scholar]
- 108.Bremer PT, et al. , Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem Int Ed Engl, 2016. 55(11): p. 3772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raleigh MD, et al. , A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J Pharmacol Exp Ther, 2019. 368(2): p. 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]