Abstract
Burkitt’s Lymphoma (BL) has three peaks of occurrence, in children, adults and elderly, at 10, 40 and 70 years respectively. To the best of our knowledge, no study has been conducted to assess predictors of survival in the three age groups. We hypothesized that survival predictors may differ by age group. We, therefore, sought to determine survival predictors for BL in these three groups: children (<15 years of age), adults (40–70 years of age) and elderly (>70 years of age). Using the Surveillance, Epidemiology, and End Results (SEER) database covering the years 2000–2013, we identified 797 children, 1,994 adults and 757 elderly patients newly diagnosed with BL. We used adjusted Cox proportional hazards regression models to determine prognostic factors for survival for each age group. Five-year relative survival in BL for children, adults and elderly were 90.4, 47.8 and 28.9%, respectively. Having at least Stage II disease and multiple primaries were associated with higher mortality in the elderly group. In adults, multiple primaries, Stage III or IV disease, African American race and bone marrow primary were associated with increased mortality whereas Stage IV disease and multiple primaries were associated with worse outcome in children. These findings demonstrate commonalities and differences in predictors of survival that may have implications for management of BL patients.
Keywords: Burkitt’s Lymphoma, prognosis, survival, SEER
Sporadic Burkitt’s Lymphoma (BL) is the most common non-Hodgkin’s B-cell lymphoma in children.1,2 In adults, sporadic BL accounts for 1–2% of the non-Hodgkin’s Lymphomas (NHL).2,3 Burkitt’s Lymphoma is an aggressive tumor that arises in various parts of the lymphatic system.3 It is one of the fastest growing tumors, with a doubling time, (i.e., the time for a tumor to grow to twice its original size) of just 24 hrs.3
Burkitt’s Lymphoma has three clinical types, each of which has distinct characteristics. The endemic type of BL is found mainly in tropical climates where malaria is endemic3 and has a strong link to Epstein–Barr virus (EBV) infection.3 It is thought that Falciparum malaria promotes the infectivity of EBV through reactivating latent EBV, suppressing T-cell immunity against EBV and produces the myc translocation that is common in BL.3–5 The endemic type normally involves the jaws and has a favorable prognosis with appropriate treatment. The sporadic type of BL is more common in non-malaria endemic regions such as North America, East Asia and Eastern Europe3 and is less likely to be associated with EBV than the endemic type.6 The sporadic type is more widespread in the body, with most cases occurring in the abdomen, reproductive system and lymph nodes.2 The last type of BL is immunosuppression related BL, mainly found in those with immunosuppression from HIV/AIDS, post-transplant recipients and congenital immunodeficiency.3,7,8 All three forms of BL are responsive to treatment in children provided they have access to effective treatment.9,10 The use of brief intensive chemotherapy in adults is generally effective, however, BL is commonly misclassified as other forms of NHL especially diffuse large B cell lymphoma and hence may not be treated appropriately resulting in very poor prognosis.11–14
Studies of BL have largely focused on African/endemic disease because it is more common, easier to diagnose, and has a disfiguring nature and unique characteristics as aforementioned. The majority of published studies are from tropical areas where the endemic type is more common.15–18 Other studies have looked at BL as part of NHL in general and not as a distinct entity.9,19,20 Additionally, existing studies of BL have been limited by small sample sizes or only included subsets of ages, either adults or children.10,21
Burkitt’s Lymphoma has a tri-modal peak of occurrence in children, adults and elderly around the ages of 10, 40 and 70 years, respectively.22,23 Some studies have suggested that the different peaks have differences in etiology or clinical characteristics/presentation.24–26 For example, Luciano et al.27 found that survival significantly improved in cases treated from 2002 to 2008 compared to cases treated from 1973 to 2001, and attributed the improvement to improved chemotherapy (with Rituximab) and advances in HIV/AIDS treatment. In addition, they found that children have better survival compared to adult and elderly populations.27 However, differences in predictors of survival between the different age groups were not examined. Children and adults respond differently to treatment, and the ability to withstand chemotherapy differs across the age categories. Also, co-morbidities in elderly patients may affect their prognosis of the disease.28 Separate analyses of these age groups would, therefore, help to define differences in prognostic predictors by age group.
Our main hypothesis is that predictors of survival for BL are not the same for children, adults and elderly. Using data in the Surveillance, Epidemiology, and End Results Program (SEER) database, we examined this hypothesis by determining predictors of survival in children, adults and elderly patients.
Methods
Study population
Data for the current analysis were acquired from the SEER program of the National Cancer Institute. These data covered the period from 1973 to 2013.29 In this study, we restricted analyses to the period from 2000 to 2013, because the SEER program began to obtain information from all of its 18 registries in 2000, and the addition of Rituximab in 199730 to modern treatment has shown improved survival.14,28,31 Data collected in the 18 registries represents 30% of the US population. SEER data available to investigators contains demographics, incidence, mortality, survival and clinical information including treatment, without patient-identifying information.29
Selection of variables
The International Classification of Disease, ICD-O329 morphology codes 9687 and 9826 were used to identify Burkitt’s Lymphoma and Burkitt’s Leukemia cases, respectively, in the SEER data. The primary outcome of interest was disease-specific death from BL. Covariates used in the analysis included: age at diagnosis, gender, marital status, race and ethnicity, primary site of tumor, number of primaries, histologic behavior of the tumor, staging based on Ann Arbor classification, geographical region and year of diagnosis. ICD-O3 site code was used to categorize primary site into: lymph nodes, gastrointestinal tract, central nervous system, head and neck, bone marrow and others.32 The number of primaries were obtained from the variable “Sequence number” which codes for the number and sequence of all primary tumors reported during the lifetime of a patient.29 Tumor behavior was categorized into: metastatic (comprising both distant and regional), localized and unknown. Ann Arbor staging consists of four stage groups: (i) Stage I comprises disease involving single lymph nodes; (ii) Stage II comprises disease involving two or more lymph nodes on the same side of the diaphragm; (iii) Stage III, lymph nodes on both sides of the diaphragm and (iv) Stage IV represents disseminated disease.29 The SEER cancer registries were recoded into geographical regions including Northeast, West, South and Mid-west based on the census regions and divisions.33
Statistical analysis
In the present analysis, we excluded individuals without reported age information. Survival time was calculated from date of diagnosis to date last known to be alive, date of death or cut-off date of December 31, 2013. Those who were alive at the cut-off date or last follow-up date were considered censored observations. Age at diagnosis was defined in three categories as follows: (i) children 15 years or younger; (ii) adults aged 40 to 70 years and (iii) elderly are those who are older than 70 years. Separate analyses were carried out for children, adults and elderly patients. Individuals between the ages of 16 to 39 were excluded. Survival analysis was first done using Kaplan–Meier (product-limit) survival tables to calculate five-year survival. The analysis was also stratified by sex, primary site, geographical region and tumor stage. To determine if there was a trend in survival, 5-year rolling survival rates were calculated during the study period. Relative survival, defined as the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in the US population of all persons of the same sex, age and race as the patient cohort was also estimated. Survival times were compared using Z-test and its corresponding p values.34
Cox proportional hazard modeling was used to determine predictors of survival in each age category. Variables included in the multivariable regression models were sex, race/ethnicity, number of primaries, tumor stage, year of diagnosis and primary site. Because BL is associated with HIV/AIDS and we did not have information about HIV/AIDS status in the SEER data, we conducted a sensitivity analysis by excluding registries from the Northeast and West coasts that were more likely to contain more HIV/AIDS cases.35 Analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC) and SEER⋆stat 8.3.2. All reported p-values are two-sided and p≤0.05 was considered a significant level.
Results
Demographic characteristics
During 2000–2013, in total, 3,548 individuals were diagnosed with BL. The majority of these patients were white (81, 82 and 85% in children, adults and elderly, respectively).
The age and standard deviation for the children, adults and elderly were 8.21 ± 4.09 years, 53.63 ± 8.79 years and 78.51± 4.76 years, respectively. Among the 797 children with BL, approximately half had lymph-node disease and 20% were diagnosed with Stage IV disease.
Among adults, 1,994 BL diagnoses were found. More than half (51.7%) of these patients were diagnosed with Stage IV disease and the majority were male (74%). A lymph node was the primary diagnostic site (65%) followed by the GI tract (13%). In the elderly, 757 BL diagnoses were recorded in the SEER data. Forty percent were diagnosed with Stage IV disease, and a lymph node was the main primary site of diagnosis (57.5%) (Table 1).
Table 1.
Demographic and clinical characteristics of 3,548 identified cases of Burkitt’s Lymphoma from SEER during 2000–2013
| Children (n=797) | Adults (n=1,994) | Elderly (n=757) | ||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | |
| Age (Mean±SD) | 8.21±4.09 | 53.63±8.79 | 78.51±4.76 | |||
| Race | ||||||
| White | 644 | 80.8 | 1,643 | 82.4 | 645 | 85.2 |
| Black | 78 | 9.8 | 189 | 9.5 | 34 | 4.5 |
| Asian or Pacific Islander | 66 | 8.3 | 145 | 7.3 | 71 | 9.4 |
| Unknown | 9 | 1.2 | 17 | 0.9 | 7 | 0.9 |
| Marital status | ||||||
| Single | 795 | 99.8 | 897 | 45.0 | 311 | 41.1 |
| Married | 0 | 0.0 | 1,017 | 51.0 | 416 | 55.0 |
| Unknown | 2 | 0.3 | 80 | 4.0 | 30 | 4.0 |
| Sex | ||||||
| Female | 175 | 22.0 | 527 | 26.4 | 341 | 45.0 |
| Male | 622 | 78.0 | 1,467 | 73.6 | 416 | 55.0 |
| Primary site | ||||||
| Lymph nodes | 423 | 53.1 | 1,320 | 66.2 | 435 | 57.5 |
| Head and neck | 35 | 4.4 | 38 | 1.9 | 24 | 3.2 |
| GI tract | 112 | 14.1 | 260 | 13.0 | 123 | 16.3 |
| Bone marrow | 151 | 19.0 | 180 | 9.0 | 91 | 12.0 |
| CNS | 7 | 0.9 | 36 | 1.8 | 9 | 1.2 |
| Others | 69 | 8.7 | 160 | 8.0 | 75 | 9.9 |
| Number of primaries | ||||||
| One primary only | 783 | 98.2 | 1,667 | 83.6 | 511 | 67.5 |
| 2 Primaries | 14 | 1.76 | 305 | 15.8 | 206 | 27.2 |
| 3 primaries | 0 | 0.0 | 22 | 1.1 | 40 | 5.3 |
| Behavior | ||||||
| Localized | 166 | 20.8 | 303 | 15.2 | 140 | 18.5 |
| Metastatic | 463 | 58.1 | 1,469 | 73.7 | 478 | 63.1 |
| Unknown | 168 | 21.1 | 222 | 11.1 | 139 | 18.4 |
| Year of diagnosis | ||||||
| 2000–2004 | 259 | 32.5 | 640 | 32.1 | 263 | 34.7 |
| 2005–2009 | 288 | 36.1 | 758 | 38.0 | 283 | 37.4 |
| 2010–2013 | 250 | 31.4 | 596 | 29.9 | 211 | 27.9 |
| Stage | ||||||
| I | 165 | 20.7 | 303 | 15.2 | 137 | 18.1 |
| II | 138 | 17.3 | 260 | 13.0 | 104 | 13.7 |
| III | 106 | 13.3 | 176 | 8.8 | 72 | 9.5 |
| IV | 218 | 27.4 | 1,031 | 51.7 | 302 | 39.9 |
| Unknown | 170 | 21.3 | 224 | 11.2 | 142 | 18.8 |
Abbreviations: CNS: central nervous system; GI: gastrointestinal; SD: standard deviation.
Survival analysis
Five-year relative survival in BL was significantly different for children, adults and elderly (90.4, 47.8 and 28.9%, respectively; p < 0.01). The median absolute survival time was 30 months for adults but just 5 months for elderly, and the five-year absolute survival for the two groups was 46% and 22%, respectively (Table 2). There was no change in survival rates from 2000 to 2013 in children and elderly (Supporting Information Table 2 and Supporting Information Fig. 1). However, in adults survival significantly improved from 2003 to 2006 and has been stable since then. In stratified analyses by sex, no differences were observed in survival between males and females. Survival did not vary significantly by geographic region for children and elderly except in adults where survival was significantly poorer for the South (42.3%, p = 0.001) and the western region (45.0%, p = 0.007) compared to the northeast (53%).
Table 2.
Five-year absolute and relative survival in Burkitt′s Lymphoma by age group, gender, tumor site and tumor stage
| Children | Adults | Elderly | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OS | RS | p values | OS | RS | p values | OS | RS | p values | |
| Overall | 90.3 | 90.4 | 46.0 | 47.8 | 21.7 | 28.9 | |||
| Sex | |||||||||
| Female | 87.9 | 88.0 | Ref. | 46.7 | 48.2 | Ref. | 24.1 | 28.3 | Ref. |
| Male | 90.9 | 90.9 | 0.49 | 45.8 | 47.7 | 0.30 | 19.7 | 26.7 | 0.33 |
| Region | |||||||||
| Northeast | 92.1 | 92.2 | Ref. | 53.0 | 55.0 | Ref. | 21.5 | 25.6 | Ref. |
| Midwest | 90.5 | 90.5 | 0.64 | 46.7 | 48.6 | 0.17 | 20.5 | 26.1 | 0.47 |
| South | 90.2 | 90.3 | 0.54 | 42.3 | 44.1 | 0.001 | 17.4 | 22.5 | 0.17 |
| West | 89.8 | 89.9 | 0.43 | 45.0 | 46.7 | 0.007 | 23.1 | 30.8 | 0.93 |
| Primary site | |||||||||
| Lymph node | 89.8 | 89.8 | Ref. | 44.4 | 46.1 | Ref. | 20.1 | 26.2 | Ref. |
| Head and neck | 89.4 | 89.4 | 0.80 | 67.6 | 70.3 | 0.007 | 18.8 | 19.6 | 0.68 |
| GI tract | 95.2 | 95.2 | 0.08 | 55.0 | 57.0 | 0.05 | 25.5 | 34.9 | 0.23 |
| Bone marrow | 85.7 | 85.8 | 0.39 | 28.2 | 29.1 | 0.001 | 19.3 | 23.2 | 0.49 |
| CNS | 100 | 100 | 0.38 | 45.3 | 47.2 | 0.69 | 0.0 | 0.0 | – |
| Others | 92.2 | 92.2 | 0.59 | 57.2 | 59.3 | 0.004 | 29.1 | 36.1 | 0.05 |
| Stage | |||||||||
| I | 96.2 | 96.2 | Ref. | 66.1 | 68.9 | Ref. | 39.4 | 49.1 | Ref. |
| II | 94.6 | 94.7 | 0.55 | 61.3 | 63.5 | 0.31 | 20.4 | 27.5 | 0.01 |
| III | 92.0 | 92.0 | 0.17 | 49.2 | 51.1 | 0.0004 | 18.5 | 26.4 | 0.06 |
| IV | 84.7 | 84.8 | 0.001 | 38.2 | 39.7 | <0.00001 | 16.1 | 20.7 | 10−05 |
| Unknown | 86.4 | 86.5 | 0.005 | 32.1 | 33.2 | <0.00001 | 18.3 | 22.9 | 0.001 |
Abbreviation: CNS: central nervous system; GI: gastrointestinal; OS: observed survival; RS: relative survival.
Bold: Statistically significant at the 0.05 level.
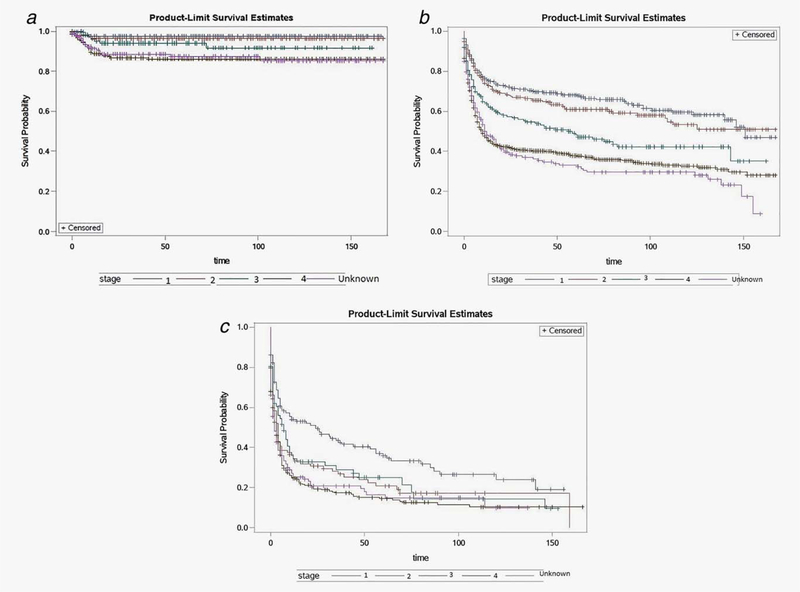
In children, BL patients with Stage IV disease had significantly worse five-year relative survival compared to patients with Stage I disease or with Stage II disease (Fig. 1a). In adults, patients with Stage IV disease had significantly worse five-year survival compared to patients with Stage III, II and I disease (Fig. 1b) (p = 10−3, p < 10−5 and p < 10−5 respectively). Elderly patients with Stage IV disease or with Stages II–III disease had significantly worse 5-year survival compared to patients with Stage I disease (Fig. 1c) (p = 10−5 and p = 0.0098, respectively).
Figure 1.
(a) Kaplan–Meier curves of survival in children diagnosed during 2000–2013 with Burkitt’s Lymphoma, stratified by disease stage. (b) Kaplan–Meier survival curves in adults diagnosed during 2000–2013 with Burkitt’s Lymphoma, stratified by disease stage. (c) Kaplan–Meier survival curves in elderly adults diagnosed during 2000–2013 with Burkitt’s Lymphoma, stratified by disease stage. [Color figure can be viewed at wileyonlinelibrary.com]
Children with tumors originating in the bone marrow had five-year survival of 86% while in those with tumors in lymph nodes it was 90% (p = 0.39). In adults, 5-year survival in BL in patients with bone marrow primaries (28%) was significantly worse than in patients with GI tract (55%), head and neck (64%) and lymph node primaries (44%). No differences were seen in 5-year survival by these sites in the elderly (Table 2).
Survival predictors
Multivariate analysis was used to evaluate associations of race, stage of tumor, multiple tumor involvement, number of primaries and time since diagnosis. In children, Stage IV disease was predictive of poor outcome (Table 3; p = 0.0012 and p = 0.0025, respectively). The presence of two primary tumors was also predictive of poor outcome in children (HR: 4.11, 95% CI: 1.57–10.76; p = 0.004, Table 3). In adults, multivariate analyses showed that Stage III, Stage IV, African–American race, earlier year of diagnosis from 2000–2004 and involvement of multiple primaries were significantly associated with poor prognosis. In the elderly, Stages II, III and IV disease, and having 3 or more primary tumors were associated with poor survival in multivariate models (Table 3).
Table 3.
Multivariable analysis1 of survival predictors for Burkitt’s Lymphoma in children, adults and elderly in current study
| Children | Adults | Elderly | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p values | HR (95% CI) | p values | HR (95% CI) | p values | |
| Race | ||||||
| White | Ref. | Ref. | Ref. | |||
| Black | 1.57 (0.75–3.30) | 0.23 | 1.49 (1.23–1.81) | <0.0001 | 1.02 (0.69–1.50) | 0.92 |
| Asian or Pacific Islander | 0.97 (0.38–2.46) | 0.95 | 1.02 (0.80–1.30) | 0.87 | 0.80 (0.59–1.08) | 0.14 |
| Others | – | 1.19 (0.60–2.39) | 0.63 | 1.27 (0.47–3.43) | 0.63 | |
| Primary site | ||||||
| Lymph node | Ref. | Ref. | Ref. | |||
| Head & neck | 0.89 (0.20–3.84) | 0.87 | 0.66 (0.37–1.17) | 0.15 | 1.35 (0.84–2.15) | 0.22 |
| GI tract | 0.65 (0.22–1.89) | 0.43 | 0.98 (0.80–1.19) | 0.82 | 0.94 (0.74–1.21) | 0.63 |
| Bone marrow | 1.89 (0.41–8.76) | 0.42 | 1.35 (0.99–1.86) | 0.06 | 1.12 (0.77–1.61) | 0.56 |
| CNS | – | 1.07 (0.66–1.75) | 0.16 | 1.15 (0.54–2.44) | 0.72 | |
| Others | 0.50 (0.17–1.46) | 0.21 | 0.94 (0.74–1.20) | 0.65 | 0.86 (0.63–1.17) | 0.33 |
| Number of primaries | ||||||
| 1 | Ref. | Ref. | Ref. | |||
| 2 | 4.11 (1.57–10.76) | 0.004 | 1.29 (1.11–1.49) | 0.001 | 1.10 (0.91–1.32) | 0.32 |
| >3 | – | 2.43 (1.59–3.72) | <0.0001 | 1.65 (1.18–2.29) | 0.003 | |
| Year of diagnosis | ||||||
| 2000–2004 | Ref. | Ref. | Ref. | |||
| 2005–2009 | 0.75 (0.42–1.33) | 0.32 | 0.85 (0.73–0.98) | 0.03 | 1.10 (0.91–1.34) | 0.32 |
| 2010–2013 | 0.59 (0.29–1.21) | 0.15 | 0.80 (0.68–0.94) | 0.01 | 1.10 (0.89–1.38) | 0.38 |
| Stage | ||||||
| I | Ref. | Ref. | Ref. | |||
| II | 1.44 (0.38–5.43) | 0.59 | 1.11 (0.85–1.47) | 0.45 | 1.53 (1.13–2.09) | 0.01 |
| III | 2.77 (0.80–9.63) | 0.11 | 1.67 (1.26–2.23) | 0.0004 | 1.45 (1.02–2.06) | 0.04 |
| IV | 5.15 (1.78–14.92) | 0.003 | 2.32 (1.88–2.87) | <0.0001 | 1.90 (1.45–2.46) | <0.0001 |
| Unknown | 2.42 (0.41–14.16) | 0.33 | 1.99 (1.40–2.81) | 0.0001 | 1.76 (1.22–2.54) | 0.002 |
Model adjusted for sex.
Abbreviation: CI: confidence interval; HR: hazard ratio; Ref: reference.
Bold: Statistically significant at the 0.05 level.
In sensitivity analysis we excluded registries in the Northeast and the West coasts that appears to have higher HIV/AIDS cases and found the results were not materially changed. Specifically, in children Stage IV disease was still significantly associated with poor outcome (HR: 6.94, 95% CI: 1.55–31.00) while multiple primaries was not significantly associated with poorer survival (HR: 8.09, 95% CI: 0.84–77.48). In adults, multiple primaries, Stage III and Stage IV disease, and black race remained significantly associated with poor outcome (Supporting Information Table 1). In addition, Asian or Pacific Islanders were found to have significantly poorer survival (HR: 5.12, 95% CI: 1.60–16.43; p = 0.006). Elderly with Stage IV disease remained significantly at risk of higher mortality while having multiple primaries was marginally associated with higher risk (Supporting Information Table 1).
Discussion
We found significant differences in BL survival between children, adults and elderly patients. Children had the best overall survival, followed by adults and then the elderly. Five-year survival proportions were dependent on tumor stage, with patients with advanced stage disease having the worst survival in all three age groups.
Our findings are consistent with results in a study by Castillo et al.32 who reported that elderly had poor survival (survival rate: 30%). Another study found that the 5-year relative survival for patients less than age 20 years was 87%, while adults >60 years of age had a 5-year relative survival of 33%.27 The differences in survival between our results and theirs 27 may be due to different age cut-offs used in the analyses.
The poorer survival of elderly adults with BL compared to younger patients may arise from a combination of factors, including co-morbidities, differences in response to or tolerance of chemotherapy, and differences in tumor etiology or tumor genetic variability across the three age groups.36,37 A study evaluating the benefit of intensive chemotherapy over conventional therapy found better survival in children <15 years of age compared to adults and better survival in patients on intensive chemotherapy compared to conventional treatment.38 Onciu et al.39 found no difference in outcome for adults on intensive chemotherapy with hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone 1 high-dose methotrexate and cytarabine) versus those on a Rituximab-containing regimen. Thus, additional studies looking at roles of the factors considered herein, especially in elderly adults, are warranted.
Our study also found that survival did not vary by gender in any age group. African American race was found to be a predictor of mortality only in adults, which is in line with prior studies.27,32 For example, Costa et al.27 found whites had better survival compared to African Americans (HR: 0.78, 95% CI: 0.64–0.95, p = 0.01). Similar observation was reported by Castillo et al.32 (HR: 1.60, 95% CI: 1.30–1.97, p < 0.001; whites as the reference group) and found no difference in risk even after adjusting for socioeconomic status. For each of the racial groups analyzed in our study, more than half presented with Stage IV disease (whites 5 51.3%, African Americans5 55.6%, American Indian= 50% and Pacific Islander5 50.3%). Stage IV disease was the most common presentation for all the three age categories analyzed regardless of race. The poor outcome for African Americans was, however, only found in adults. The high mortality for African Americans may be due to inequality in access to care40 that may be more of an issue in adults compared to children or elderly due to variation is socioeconomic status in the age groups and access to insurance or Medicare.41,42
No significant differences in survival were seen by site of tumor in children and in elderly, but in adults survival was found to be significantly lower in lymph node (44%) and bone marrow tumors (28%) compared to head and neck (68%) tumors. Our finding is consistent with results by Castillo et al.32 in which they reported better survival for BL patients older than 20 years of age with head and neck tumors compared to those with tumors located in a lymph node. This association difference disappeared upon adjustment for sex, race, stage and age.32 In the present study, we found that bone marrow tumor site was a significant predictor of mortality in the univariate model (HR= 1.43, 95% CI: 1.18–1.74) but this association was marginally significant after adjustment for confounders (HR = 1.35, 95% CI: 0.99–1.86). A prior study by Goldman et al.43 found 88% eventfree survival for children and adolescents with bone marrow BL treated with a Rituximab-containing regimen. In another study, Mead et al.44 found BL patients with bone marrow primary tumors had poorer prognoses (HR = 2.64, 95% CI: 0.94–7.43). The lack of significant mortality differences for children and elderly with bone marrow BL was unexpected since bone marrow primary disease is more likely to disseminate, and these age groups have relatively weaker immune systems compared to adults. Bone marrow BL may have unique cytogenetic abnormalities that make it more resistant to chemotherapy or more aggressive.
Overall our analysis showed some variation in the prognostic markers between the three age categories. Elderly patients with three or more primaries and at least Stage II disease had higher mortality. Adults with two or more primaries, Stages III and IV disease, cancer diagnosis in 2000 to 2004 period and African American race had greater mortality. Adults with bone marrow disease had poorer survival compared to tumors located in the lymph node. Children had the most favorable outcome with only Stage IV disease and the presence of 2 primaries associated with increased mortality. Our findings support our hypothesis that BL in the three different age groups have different survival rates and predictors of mortality, which may represent distinct disease entities in the three age groups. Studies have shown that there are multimodal peaks of occurrence of BL in different age groups.22,24,25 Genetic variations in different age groups have been reported in some studies.25,39 Chromosomal variations on chromosome 13 and 22 were found to be associated with poor survival in children while only chromosome 17 was associated with poor survival in adults.39 Few studies have looked at the prognosis of BL in the elderly group who has the worst outcome for the disease.45–47
We were also able to identify multiple primaries as an important predictor of mortality in all three age groups. We attempted to identify the secondary malignancies in children. The secondary malignancies included one osteosarcoma, one breast cancer, one case of acute myeloid leukemia, one case of myeloid/monocytic leukemia unspecified, and one case classified as miscellaneous lymphoreticular neoplasm not otherwise specified and the rest were acute lymphocytic leukemia. Acute leukemia can be treated with bone marrow transplant and this could subsequently predispose to immunosuppression related BL.48 It may be that these cases with secondary tumors represent a subset of cases with immunosuppression related BL and they may have higher risk of mortality compared to sporadic BL.
Because our analysis used secondary data from the SEER data set, there were some limitations. Several genetic markers and laboratory parameters have been identified as important prognostic markers but were not available for our analyses. For example, Huang et al.49 found low albumin and MUM1 expression to be highly correlated with increased mortality in BL. Another study found BCL-6 expression to be associated with better survival.50 Havelange et al.51 found differences in genetic markers for children and adults with BL, which may account for observed differences in survival between children and adults. Children had higher expression of 13q, 7q and 5q CN-LOH while adults had increased expression of 18q 21 CN-LOH.51 In addition, chemotherapeutic treatment information is unavailable in the public SEER data set. While SEER diagnoses are considered to be reasonably accurate, no central pathological confirmation of diagnosis was involved which might have reduced possible disease misclassification, especially with the recoding to the ICD-O3 coding system. The misclassification, however, is not the case for BL because, when the manual pathology reports for SEER cancer patients were compared for concordance with the computer entered data, agreement was 85% for BL.52 On the other hand, it is clinically difficult to distinguish between BL and other forms of Non-Hodgkin’s Lymphoma particularly Diffuse Large B Cell Lymphoma (DLBCL), and the two conditions have different treatment modalities.11,14 The use of treatment for BL to treat DLBCL and vice versa is associated with poor prognosis.11,14 Therefore, misclassification and use of inappropriate treatment may result in poor survival in patients with these conditions. Another limitation was absence of data on HIV/AIDS status in the SEER data set, and BL is commonly associated with HIV/AIDS especially in adults. However, in the sensitivity analysis by excluding cases from registries in the northeastern and western regions that report higher cases of HIV/AIDS, the results were not materially changed (e.g., relative survival5 90.3%, 45.4% and 24.6% in children, adults and elderly respectively).
In summary, we have shown that survival for BL in elderly adults remains dismal despite improvements in treatment for children. Our findings generally support our hypothesis that BL in children, adults and the elderly has different survival proportions and predictors of mortality. BL that originates from the bone marrow compared to other sites appears to have higher mortality for adults. Perhaps more aggressive therapy for adults with BL involving the bone marrow would be indicated, and effective treatment options for elderly adults with BL are needed.
Supplementary Material
What’s new?
Occurrences of Burkitt’s lymphoma peak around ages 10, 40 and 70. What factors predict survival at each age? These authors investigated, using data from the Surveillance, Epidemiology, and End Results (SEER) database in the United States. They determined five-year relative survival rates of 90, 48 and 29% in children, adults and elderly patients, respectively. Some predictors of poor outcome were shared among all age groups, such as multiple primary tumor sites and advanced stage disease. Only in non-elderly adults, African-American race and bone marrow site of origin contributed to poor outcomes.
Acknowledgement
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2013), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.
Grant sponsor: NIH/NCI; Grant number: 1P20CA210300–01 (PIs:X.-O.S. and T.V.T.); Grant sponsor: University of South Florida (Start-up grant; PI: H.N.L.)
Footnotes
Conflict of interest: No conflicts of interest to declare among any of the authors.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005.Bethesda (MD): National Cancer Institute; based on November 2007 SEER data submission, posted to the SEER; web site, 2008. [Google Scholar]
- 2.Mbulaiteye SM, Biggar RJ, Bhatia K, et al. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatric Blood Cancer 2009;53:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. The Lancet 2012;379: 1234–44. [DOI] [PubMed] [Google Scholar]
- 4.Ramiro AR, Jankovic M, Callen E, et al. Role of genomic instability and p53 in AID-induced c-myc–Igh translocations. Nature 2006;440:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donati D, Zhang LP, Chene A, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun 2004;72:5412–8. doi: 10.1128/IAI.72.9.5412-5418.2004 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A, Childhood and adolescent cancer statistics, 2014. Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 7.Ferry JA, Burkitt’s lymphoma: clinicopathologic features and differential diagnosis. Oncologist 2006;11:375–83. doi: 11/4/375 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Gong JZ, Stenzel TT, Bennett ER, et al. Burkitt lymphoma arising in organ transplant recipients: a clinicopathologic study of five cases. Am J Surg Pathol 2003;27:818–27. [DOI] [PubMed] [Google Scholar]
- 9.Yaniv I, Fischer S, Mor C, et al. Improved outcome in childhood B-cell lymphoma with the intensified French LMB protocol. Med Pediatr Oncol 2000;35:8–12. doi: [pii]. [DOI] [PubMed] [Google Scholar]
- 10.Rebelo-Pontes HA, Abreu MC, Guimaraes DM, et al. Burkitt’s lymphoma of the jaws in the Amazon region of Brazil. Med Oral Patol Oral Cir Bucal 2014;19:e32–8. doi: 18936 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aldoss IT, Weisenburger DD, Fu K, et al. Adult Burkitt lymphoma: advances in diagnosis and treatment. Oncology 2008;22:1508–17. [PubMed] [Google Scholar]
- 12.Magrath IT, Janus C, Edwards BK, et al. An effective therapy for both undifferentiated (including Burkitt’s) lymphomas and lymphoblastic lymphomas in children and young adults. Blood 1984;63:1102–11. [PubMed] [Google Scholar]
- 13.Ostronoff M, Soussain C, Zambon E, et al. Burkitt’s lymphoma in adults: a retrospective study of 46 cases. Nouv Rev Fr Hematol 1992;34: 389–97. [PubMed] [Google Scholar]
- 14.Corazzelli G, Frigeri F, Russo F, et al. RDCODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol 2012;156:234–44. doi: 10.1111/j.1365-2141.2011.08947.x [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Mutalima N, Molyneux E, Jaffe H, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PloS One 2008;3:e2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otieno MW, Remick SC, Whalen C, Adult Burkitt’s lymphoma in patients with and without human immunodeficiency virus infection in Kenya. Intl J Cancer 2001;92:687–91. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter LM, Newton R, Casabonne D, et al. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer 2008;1221319–23. [DOI] [PubMed] [Google Scholar]
- 18.Mava Y, Baba UA, Timothy SY, et al. Retrospective study of childhood burkitts lymphoma in north eastern Nigeria. West Afr J Med 2013;32: 297–301. [PubMed] [Google Scholar]
- 19.Han X, Kilfoy B, Zheng T, et al. Lymphoma survival patterns by WHO subtype in the United States, 1973–2003. Cancer Causes Control 2008; 19:841–58. [DOI] [PubMed] [Google Scholar]
- 20.Wrobel G, Kazanowska B, Chybicka A, et al. Progress in the treatment of non-Hodgkin’s lymphoma (NHL) in children. The report of Polish Pediatric Leukaemia/lymphoma Study Group (PPLLSG). Przegl Lek 2004;61:45–8. [PubMed] [Google Scholar]
- 21.Patton LL, McMillan CW, Webster WP, American Burkitt’s lymphoma: a 10-year review and case study. Oral Surg Oral Med Oral Pathol 1990; 69:307–16. [DOI] [PubMed] [Google Scholar]
- 22.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS, Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer 2010;126:1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010;116:5600–4. doi: 10.1182/blood-201003-275917 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbulaiteye SM, Anderson WF, Ferlay J, et al. Pediatric, elderly, and emerging adult-onset peaks in Burkitt’s lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol 2012;87:573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM AIDS -related BL and CD4 count: aclue? Blood 2010;116:5435–6. doi: 10.1182/blood-2010-09-306407 [doi]. [DOI] [PubMed] [Google Scholar]
- 26.Morton LM, Slager SL, Cerhan JR, et al. Etiologicheterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;2014:130–44. doi: 10.1093/jncimonographs/lgu013 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa LJ, Xavier AC, Wahlquist AE, Hill EG, Trends in survival of patients with Burkitt lymphoma/leukemia in the USA: an analysis of 3691 cases. Blood 2013;121:4861–6. doi: 10.1182/blood-2012-12-475558 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood 2008;112:2248–60. doi: 10.1182/blood-2008-03-145128 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute. Overview of the SEER Program. Available at: http://seer.cancer.gov/about/. Accessed on August 13, 2016.
- 30.Grillo-Lopez AJ, White CA, Varns C, et al. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol 1999; 26:66–73. [PubMed] [Google Scholar]
- 31.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol 2011;22:1859–64. doi: 10.1093/annonc/mdq677 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Castillo JJ, Winer ES, Olszewski AJ, Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the Surveillance, Epidemiology, and End Results database. Cancer 2013;119:3672–9. [DOI] [PubMed] [Google Scholar]
- 33.Census regions and division of the united states. Available at: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Accessed on August 13, 2016.
- 34.Brown CC, The statistical comparison of relative survival rates. Biometrics 1983;941–8. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention ( CDC). Epidemiology of HIV/AIDS–United States, 1981–2005. MMWR Morb Mortal Wkly Rep 2006;55:589–92. doi: mm5521a2 [pii]. [PubMed] [Google Scholar]
- 36.Hoelzer D, Thiel E, Loffler H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood 1988;71:123–31. [PubMed] [Google Scholar]
- 37.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a doseintensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000;18:547–61. [DOI] [PubMed] [Google Scholar]
- 38.Cairo MS, Sposto R, Perkins SL, et al. Burkitt’s and Burkitt-like lymphoma in children and adolescents: a review of the Children’s Cancer Group Experience. Br J Haematol 2003;120:660–70. [DOI] [PubMed] [Google Scholar]
- 39.Onciu M, Schlette E, Zhou Y, et al. Secondary chromosomal abnormalities predict outcome in pediatric and adult high-stage Burkitt lymphoma. Cancer 2006;107:1084–92. [DOI] [PubMed] [Google Scholar]
- 40.Richardson LD, Norris M, Access to health and health care: how race and ethnicity matter. Mount Sinai J Med 2010;77:166–77. [DOI] [PubMed] [Google Scholar]
- 41.House JS, Kessler RC, Herzog AR, Age, socioeconomic status, and health. Milbank Q 1990;383–411. [PubMed] [Google Scholar]
- 42.House JS, Lepkowski JM, Kinney AM, et al. The social stratification of aging and health. J Health Soc Behav 1994;213–234. [PubMed] [Google Scholar]
- 43.Goldman S, Smith L, Galardy P, et al. Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia: a Children’s Oncology Group Report. Br J Haematol 2014;167:394–401. doi: 10.1111/bjh.13040 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol 2002;131264–74. [DOI] [PubMed] [Google Scholar]
- 45.Poirel H, Cairo M, Heerema N, et al. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Leukemia 2009;23:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly JL, Toothaker SR, Ciminello L, et al. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma 2009; 9:307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lones MA, Sanger WG, Le Beau MM, et al. Chromosome abnormalities may correlate with prognosis in Burkitt/Burkitt-like lymphomas of children and adolescents: a report from Children’s Cancer Group Study CCG-E08. J Pediatr Hematol Oncol 2004;26:169–78. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro RS, McClain K, Frizzera G, et al. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 1988;71:1234–43. [PubMed] [Google Scholar]
- 49.Huang H, Liu ZL, Zeng H, et al. Clinicopathological study of sporadic Burkitt lymphoma in children. Chin Med J (Engl) 2015;128:510–4. doi: 10.4103/0366-6999.151106 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seegmiller AC, Garcia R, Huang R, et al. Simple karyotype and bcl-6 expression predict a diagnosis of Burkitt lymphoma and better survival in IG-MYC rearranged high-grade B-cell lymphomas. Mod Pathol 2010;23:909–20. [DOI] [PubMed] [Google Scholar]
- 51.Havelange V, Pepermans X, Ameye G, et al. Genetic differences between paediatric and adult Burkitt lymphomas. Br J Haematol 2016;173: 137–44. doi: 10.1111/bjh.13925 [doi]. [DOI] [PubMed] [Google Scholar]
- 52.Clarke CA, Undurraga DM, Harasty PJ, et al. Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer. Epidemiol Biomarkers Prev 2006;15:630–8. doi: 15/4/630 [pii]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.