Abstract
The study of human cardiomyopathies and the development and testing of new therapies has long been limited by the availability of appropriate in vitro model systems. Cardiomyocytes are highly specialized cells whose internal structure and contractile function are sensitive to the local microenvironment and the combination of mechanical and biochemical cues they receive. The complementary technologies of human induced pluripotent stem cell (hiPSC) derived cardiomyocytes (CMs) and microphysiological systems (MPS) allow for precise control of the genetics and microenvironment of human cells in in vitro contexts. These combined systems also enable quantitative measurement of mechanical function and intracellular organization. This review describes relevant factors in the myocardium microenvironment that affect CM structure and mechanical function and demonstrates the application of several engineered microphysiological systems for studying development, disease, and drug discovery.
Keywords: In vitro cardiac model, Human induced pluripotent stem cell, derived cardiomyocytes (hiPSC-CMs), Microphysiological systems (MPS), Heart-on-a-chip, Cardiac mechanobiology, Drug discovery
1. Introduction
Cardiovascular disease is the leading cause of death in the developed world. A large fraction of the morbidity and mortality is the result of cardiomyopathies that affect cardiomyocytes (CMs: specialized muscle cells of the heart) (Benjamin et al., 2018). CMs are arranged in vascularized anisotropic layers within the extra–cellular matrix (ECM) structure of the heart to allow for coordinated organ pumping (Riegler et al., 2015; Greenbaum et al., 1981; Arts et al., 2001 ). Sarcomeres, the fundamental contractile unit of cardiomyocytes are connected in series into cell–spanning myofibrils, which are anchored to the cells’ microenviroment (including ECM and neighboring cells) through specialized cellular adhesion complexes (Pardo et al., 1983; Clark et al., 2002). Subtle disruption of the internal structures within cardiomyocytes or the organization and adhesion of cells within their ECM scaffold can have profound effects on cellular function and cytoskeletal organization, which in turn leads to organ level remodeling and disease (Ferreira–Cornwell et al., 2002; Fatkin et al., 2014; Spudich, 2014; Davis et al., 2016).
Historically, animal models have been the primary system used to study cardiovascular diseases at the organ and organism level. Large animal models (pigs, sheep, monkeys) can provide a close match for the hemodynamic environment and protein expression found in humans, but their use in research is limited because of the cost to house and maintain them, as well as the difficulty of creating genetic models (Dixon and Spinale, 2009; Milani–Nejad and Janssen, 2014). Small rodents (mice and rats) have been used more extensively because of their fast life cycle and genetic tractability, but these animals are not ideal models for human physiology (Povsic et al., 2017; Camacho et al., 2016). Outside of the obvious differences in hemodynamics (rats ~350; mice ~650; humans 60 beats per min), variation in the expression of proteins that generate force and control the electrical activity in the heart have limited the accuracy of small animal models for disease studies and drug development (Denayer et al., 2014; Milani–Nejad and Janssen, 2014). For instance, beta cardiac myosin accounts for 95% of the sarcomeric myosin in mature human ventricular myocytes, and is the site of roughly 35% of the known mutations which cause hypertrophic cardiomyopathy, but is only expressed during development and disease in small rodent hearts (Weiss and Leinwand, 1996; Spudich, 2014). Several proposed and developing therapies for heart failure and cardiomyopathies (including omecamtiv mercabil) have been aimed at altering the kinetics of myosin motor function, but the known differences in myosin kinetics between species may limit the predictive power of mouse models (Planelles–Herrero et al., 2017; Spudich, 2014; Tardiff et al., 2015; Nagy et al., 2015; Malik et al., 2011). Furthermore, differences in ion channel expression (notably hERG channels) can complicate modeling long QT syndrome and the effect of anti–arythmagenic drugs that target these channels (Spencer et al., 2014; Salama and London, 2007; Sanguinetti and Mitcheson, 2005). Cardiotoxicity induced by cancer therapies including doxorubicin and sunitinib is variable in humans, and potential genetic factors that contribute to this heterogeneity are difficult to study in mouse models (Force and Kolaja, 2011; Cheng and Force, 2010; Kerkelä et al., 2006; Chu et al., 2007; Burke et al., 2016; Arad et al., 2002). Finally, the complexity of the in vivo environment – dynamically remodeling tissue properties (Weber et al., 1988), multicellular components (Pinto et al., 2016), and chemical signals from around the body (Ammarguellat et al., 2002; Ross, 2004) – complicates the measurement and interpretation of specific CM phenotypes.
A human–derived cell model system for studying cardiomyocyte function and dysfunction could address some of the shortcomings of animal models, but cell sources are limited (Pluess and Ehler, 2015). Primary human cardiomyocytes are difficult to obtain, maintain in culture, and manipulate using modern molecular biology tools like CRISPR/Cas9 mediated gene editing (Parameswaran et al., 2013). This limits their usefulness for studying disease mechanisms or adaptive cellular responses. There have been some studies using excised cardiac tissues from myectomy samples that can be cultured for up to 4 weeks, but viable human myectomy samples are quite scarce (Brandenburger et al., 2012; Kang et al., 2016). Embryonic or mesenchymal stem cell derived cardiomyocytes provided an alternative, scalable cell source, but acquisition and differentiation of these cells has historically been relatively expensive and inefficient (Laflamme et al., 2007; Fernandes et al., 2010; Rangappa et al., 2003).
Human induced pluripotent stem cell (hiPSC) derived CMs have gained traction as a scalable cell source derived from patients (or edited to match specific human mutations) (Zhang et al., 2009). These cells are a powerful tool for human disease modeling and drug screening with the potential to enable personalized precision medicine (Gowran et al., 2016; Sayed et al., 2016). The establishment of increasingly robust and cost–effective protocols for differentiating these cells into cardiomyocyte–like cells has greatly increased their use (Yang et al., 2014; Chen et al., 2015b; Burridge et al., 2015; Lian et al., 2013). More recent studies of human embryonic stem cells have similarly improved the efficiency of directed differentiation into cardiomyocytes and shown very similar molecular characterization to hiPSCs, but the acquisition and use of human embryonic stem cell lines has more regulatory barriers than hiPSCs (Mallon et al., 2014; Kobold et al., 2015). Many studies have reported observable phenotypes, including less robust or organized myofibrils, in hiPSC–CMs with mutations linked to human disease (Lan et al., 2013; Wang et al., 2014; Karakikes et al., 2014; Hinson et al., 2015; Gowran et al., 2016; Chopra et al., 2018). Some studies have used patch clamping and multielectrode arrays to study the effects of mutations in ion channels on iPSC–CM electrophysiology (Braam et al., 2013; Sala et al., 2016; Bellin et al., 2013; Davis et al., 2012). Several of mutations linked to cardiomyopathies also disrupt the calcium handling process, which can be visualized with live cell imaging of cells in 2D culture (Lan et al., 2013; Sun et al., 2012; Wyles et al., 2016). Live–cell imaging techniques have also been used to observe intracellular movement generated by the contractile structures within iPSC–CMs, which is affected by some disease causing mutations (Laurila et al., 2016; Lan et al., 2013; Sun et al., 2012). However, the constraint of adherence to a rigid substrate means that these measurements cannot accurately capture the forces generated by the cells or replicate fundamental responses to length change and pressure. The usefulness of these cells for studies of structure and mechanical function has traditionally been limited by cell population heterogeneity, immaturity, and a stiff 2D culture environment.
Currently, many hiPSCs are grown and differentiated on tissue culture plastic with physisorbed matrix proteins such as laminin, fibronectin, or Matrigel (Lam and Longaker, 2012; Kohen et al., 2009; Hughes et al., 2010). Tissue culture plastic has a stiffness of around 3 GPa, nearly a million times stiffer than the native environment of the myocardium (Acevedo–Acevedo et al., 2015). Additionally, whether the proteins/growth factors are physisorbed or chemisorbed to the substrate may change bioactivity of the proteins (Psarra et al., 2015). This differentiation protocol results in a spontaneously beating, multilayered sheet of cells characterized by high heterogeneity both within each batch, between different batches, and between different labs. Some groups have demonstrated that culturing the cells in a 3D spheroid culture or 3D adhered culture improves some functional measures of maturity (Kerscher et al., 2016; Nguyen et al., 2014). Others have shown that implanting iPSC–derived cells into a living heart greatly improves maturation (Kadota et al., 2017). Mimicking elements of the native microenvironment may be a key step to expand the utility of hiPSC–CMs for studying human cardiomyocyte structure and function in vitro.
Despite the potential of iPSC–CMs, immature, embryonic–like phenotype and large intrapopulation heterogeneity limit their ability to model adult cardiomyocyte physiology (Karakikes et al., 2015). Some of the key metrics of immaturity are the features of the contractile structures, myofibrils, which are less aligned and less densely packed in hiPSC–CMs compared to native CMs (Yang et al., 2014; Ruan et al., 2016). This lack of maturity is also reflected in the ion channel expression, calcium handing, t–tubule organization, and resultant electrophysiology of these cells (Ronaldson–Bouchard et al., 2018; Denning et al., 2016). Gene expression and protein analysis of receptors and sarcomeric proteins indicate that iPSC–CMs resemble early embryonic CMs and are lacking several important functional proteins expressed in mature human ventricular myocardium (eg. MYH7, TNNI3, and ADRB1) (Gowran et al., 2016; Leonard et al., 2018; Ronaldson–Bouchard et al., 2018). Modeling of specific cardiac cell responses with stem–cell derived cardiomyocytes has been limited by the absence of certain receptors (eg. Beta adrenergic receptor 1) until a more mature state is reached (Jung et al., 2016). Strategies of varying complexity have been proposed to increase the maturity of cells, including temporal maturation windows, specific media compositions, electrical pacing, cyclic stretch (Tandon et al., 2009; Yang et al., 2014; Richards et al., 2016). Many of these strategies aim to direct the cells down a mature lineage by shifting their metabolism and cell cycle state via addition of media components (Wheelwright et al., 2018; Correia et al., 2017). The lack of maturity in hiPSC–CMs has also been speculated to be due in part to the different environmental cues they receive in 2D cell culture compared to their native environment in vivo (Eschenhagen et al., 2015).
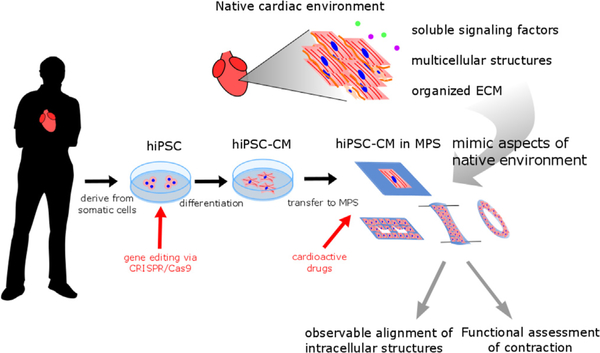
In recent years, different types of microphysiological systems (MPS) have been engineered to provide an in vitro environment more similar to the native in vivo environment, to improve the ability of hiPSC–CMs to match that of native, mature CMs (Fig. 1). MPS utilize bioengineering and biology to model native tissue functions in vitro (DARPA/DSO, 2011). Substrate stiffness, particular ECM availability, and interactions with neighboring cells have all been tuned in MPS and have been shown to modulate cell phenotypes, protein expression, and cytoskeletal organization (Tzatzalos et al., 2016; Mathur et al., 2015; Huebsch et al., 2016; Ronaldson–Bouchard et al., 2018; Ulmer et al., 2018). Additional controllable inputs, including specialized media components and electrical pacing (at both physiologic rates of 1 Hz and extraphysiologic rates of 6–10 Hz) have also been shown to promote maturity in these cell populations (Nunes et al., 2013; Hirt et al., 2014; Ronaldson–Bouchard et al., 2018; Mills et al., 2017). While the structural and functional maturity of the stem–cell derived cells in these constructs falls short of native cells in excised tissue, they are much improved over traditional hiPSC–CMs. Furthermore, many of these platforms are designed to specifically enable measurement of force generation by cardiomyocytes in more physiologically relevant mechanical contexts (Laurila et al., 2016; McCain et al., 2013; Sidorov et al., 2017). Some of these systems focus on single cells to allow for detailed observation of remodeling of intracellular structures including myofibrils, sarcomeres and adhesion complexes (Ribeiro et al., 2015; Aratyn–Schaus et al., 2016; Chopra et al., 2018). Single cell approaches also attempt to address the high degree of heterogeneity in the population of cells acquired during the differentiation process (Ribeiro et al., 2017). Other systems use multicellular constructs to form microtissues, allowing the observation of tissue level functions by incorporating more native–like cell–cell adhesion, but making the deconvolution of differences in heterogeneous cell populations more challenging (Sidorov et al., 2017; Ronaldson–Bouchard et al., 2018; Mathur et al., 2015; Mills et al., 2017).
Fig. 1.
Microphysiological systems (MPS) mimic aspects of native cardiac environment to enable disease modeling and drug discovery using human induced pluripotent stem cell derived cardiomyocytes (hiPSC–CMs).
While certain questions about cardiac function are testable in hiPSC–CMs grown in traditional 2D formats, MPS are better in vitro platforms to study structural and functional phenotypes of cardiac disease and drug responses because of two key features. First, MPS promote a more mature hiPSC–CM morphology (e.g. aligned sarcomere, pronounced T–tubules) and function (e.g. lower resting membrane potential, expression of adult CM proteins, contraction) by recapitulating aspects of the cardiac microenvironment. Second, many of these platforms are designed to allow for quantitative, physiologically relevant measurement of mechanical output. In the following sections, we describe the native environment of the heart and cardiac MPS which use hiPSC–CMs to model human cardiac function and disease and support drug discovery.
2. Microenvironment of the native heart
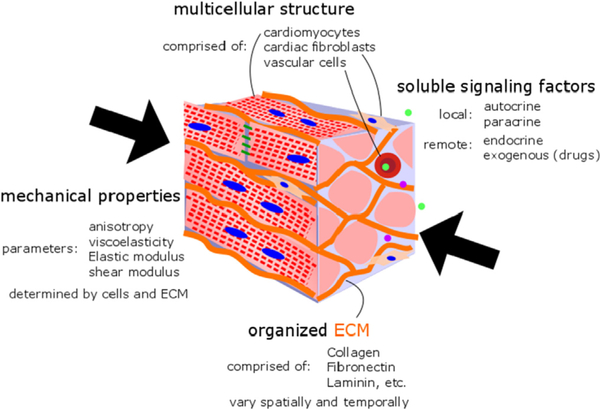
The microenvironment of CMs consists of various biophysical and biochemical cues that guide CM behavior and function (Fig. 2). Cells (including cardiomyocytes) can sense changes in their local microenvironment though mechanosensitive adhesion proteins and their associated protein complexes (Jacot et al., 2010; Majkut et al., 2014). These adhesión complexes form a mechanical link between the exterior and interior structures of cells and transmit intracellular signals to initiate changes in gene transcription and cellular morphology and function (Samarel, 2005; Pluess and Ehler, 2015).
Fig. 2.
Native myocardial microenvironment can be characterized by mechanical properties, cell populations, extracellular matrix components, and soluble signaling factors.
2.1. Multicellular structure
The heart wall is composed of CMs, endocardial cells, epicardial cells, cardiac fibroblasts, cardiac endothelial cells, leukocytes and macrophages (Rienks et al., 2014; Zhou and Pu, 2016). CMs comprise the largest fraction of the volume of the heart and are responsible for the contraction of the heart (Zak, 1973; Bayomy et al., 2012). Distinct subtypes including atrial and ventricular CMs and cardiac conduction cells are distributed throughout the different chambers of the heart and have unique functional characteristics including protein expression and conduction profiles (Später et al., 2014). This specialization and spatial arrangement enables coordinated contraction roughly once a second, generating strains ranging from −0.2 to 0.4 and tensile and compressive stresses throughout the myocardium (Huang et al., 2017b). The propagation of contraction though the tissue is mediated by mechanical and electrical cell–cell contacts with other cardiomyocytes and cardiac fibroblasts (Vasquez et al., 2011; Kohl and Gourdie, 2014). Cardiac fibroblasts are responsible for secreting and maintaining the ECM scaffold, a major determinant of the heart’s mechanical properties (Bayomy et al., 2012; Schroer and Merryman, 2015). Both CMs and cardiac fibroblasts are sensitive to cyclic stress and strain, which can activate intracellular signaling and remodeling (Jacot et al., 2010; Schroer and Merryman, 2015). Cardiac endothelial cells form the vasculature of the heart and influence cardiac morphogenesis and homeostasis by releasing paracrine signaling factors (Zhou and Pu, 2016; Brutsaert, 2003; Pinto et al., 2016). The vasculature allows for nutrient exchange and transport of soluble signaling factors and immune cells to the heart, which can have major effects on the development and remodeling after disease (Epelman et al., 2015; Ma et al., 2018). Recent studies have highlighted the roles of resident macrophages and recruited leukocytes in the maintenance of homeostasis and injury response (Chen and Frangogiannis, 2016; Sager et al., 2016). Finally, epicardial and endocardial cell layers on the external and internal surfaces of the heart play key roles in the heart’s development and regeneration, as they contribute to paracrine signaling and give rise to the progenitors of cardiac fibroblasts and endothelial cells in the myocardium (Masters and Riley, 2014; Luxàn et al., 2016; Haack and Abdelilah–Seyfried, 2016).
2.2. Mechanical properties
Biophysical properties including structural features, mechanical properties and electrical conductivity have significant effects on cardiac cell function (Atmanli et al., 2017). In healthy myocardial tissue, cells exist in an aligned (and therefore anisotropic) structure with cardiac fibroblasts maintaining ECM homeostasis and blood vessels interspersed throughout. The combination of cells and ECM contribute to bulk mechanical properties including elastic modulus and viscoelasticity (Wang et al., 2016). A variety of techniques, including assessment of echocardiogram/MRI imaging of contracting hearts, bulk tissue mechanical measurements, and microscale atomic force microscopy have all been used to measure the mechanical properties of myocardial tissue (Yao et al., 2012; Pislaru et al., 2014). Viscoelasticity has been measured based on high–frequency echocardiograms and is affected by cardiac conditions including myocardial infarction (Pislaru et al., 2014). The normal stiffness (elastic modulus, E) of the embryonic myocardium has been reported to be around 10 kPa. (Engler et al., 2008). Furthermore, embryonic and neonatal CMs cultured on hydrogels with a stiffness around 10 kPa have shown improved contractile function compared with their function in softer or stiffer environments (Engler et al., 2008; Bhana et al., 2010).
2.3. Organized ECM
Bulk tissue mechanical properties depend on the intrinsic material properties of ECM proteins and cells as well as the arrangement and linkages within these structures. The ECM is comprised of a variety of fibrillar proteins aligned circumferentially and anisotropically around the chambers (Greenbaum et al., 1981; Hanson et al., 2013). In healthy myocardium the ECM fibers orientation is longitudinally along CMs with few crosslinks (Bayomy et al., 2012). Collagen I is the most abundant ECM protein in the heart and together with Collagen III maintain structural integrity of the heart (Thimm et al., 2015; Singelyn and Christman, 2011). Collagen I provides tensile strength while collagen III provides distensibility to the structure of myocardium (Takawale et al., 2015; Segura et al., 2014). Basement membrane occupies the interstitial space between ECM and CMs and includes proteins such as laminin, fibrillin, fibronectin and collagen type IV, as well as proteoglycans which can bind soluble growth factors and cytokines (Takawale et al., 2015). In addition to their contribution to the mechanical properties of the myocardium, the specific composition of the ECM can trigger activation of biochemical signaling through the binding of specific integrin types to cell adhesion ligands found in many ECM proteins (Samarel, 2005; Okada et al., 2013). Integrin binding recruits a complex of proteins known as a focal adhesion which connect the cytoskeleton to the ECM and signal downstream to promote a range of cell survival, spreading, and differentiation (Huang et al., 2017a).
The cardiac microenvironment undergoes spatiotemporal changes during development and disease stages (Kapelko, 2001; Bowers et al., 2010; Jourdan–LeSaux et al., 2010; Lockhart et al., 2011; Bayomy et al., 2012; Jung et al., 2012; Hanson et al., 2013). During mouse heart development the ECM is an important component of the cardiovascular progenitor niche (Schenke–Layland et al., 2011). Thimm and colleagues showed elastin enriched regions overlapped with regions with mouse cardiomyocyte differentiation, and observed the inverse correlation with presence of collagen I (Thimm et al., 2015). During fetal–to–adult development, the ECM remodels, shifting from a composition rich in fibronectin to a composition richer in collagen I and laminin (Williams et al., 2014; Rienks et al., 2014; Hanson et al., 2013). Adult ECM contributes to signaling that maintains tissue homeostasis (Williams et al., 2014). However, the composition of the cardiac ECM shifts again during aging or disease with an accumulation in collagen I and decrease in the ratio of collagen III to collagen I (Weber et al., 1988; Kapelko, 2001; Atmanli et al., 2017). Hence, the myocardium stiffness also evolves from softer (few kPa) to stiffer (10 kPa) during development. (Engler et al., 2008, 2007). With age and the onset of cardiac diseases, including dilated and hypertrophic cardiomyopathies, the ECM fraction increases and crosslinks become more pronounced, which are characteristics of tissue fibrosis. Fibrosis changes the mechanical properties of the myocardium, which increased from around 10 kPa to >35 kPa (Bayomy et al., 2012; Wenket al., 2011; Ho et al., 2010; Conrad et al., 1995; Dean et al., 2005; Berry et al., 2006).
2.4. Soluble signaling factors contribute to remodeling and feedback
In addition to specific adhesion ligands, soluble signaling factors are an important biochemical component of the microenvironment (Euler, 2015; Bergmann, 2010). Many signaling factors (e.g. transforming growth factor, fibroblast growth factor, Wnt, interleukins) are released by cardiac cells and mediate autocrine and paracrine signaling (Melendez et al., 2010; Sakurai et al., 2013; Kong et al., 2014; Ozhan and Weidinger, 2015). Other factors, including both natural compounds (e.g. Angiotensin) and exogenous drugs (e.g. Verapamil) are carried into the heart by the vascular network (Tsutsui et al., 2007). There is significant crosstalk between mechanosensitive signaling through integrins and signaling from soluble factors that helps coordinate development and exacerbate disease (Ross, 2004; Banerjee et al., 2006; Schroer et al., 2014).
Periods of dynamic cardiac remodeling, including both development and disease are generally characterized by increases in soluble signals and dynamically changing mechanical properties. These changes can contribute to a positive feedback loop of adverse remodeling. Ischemia, pressure overload, aging and viral infections are key initiators of cardiac remodeling process (Rienks et al., 2014; Heymans, 2006). Remodeling leads to changes in size, mass, composition and stiffness of the left ventricle, which can in turn activate more mechanosensitive signaling and tissue remodeling (Kehat and Molkentin, 2010; Rienks et al., 2014). These complex and interacting inputs and responses are important features of cardiac disease in vivo, but make it difficult to decipher subtle effects within cells. The development of engineered environments can allow for precise control of different factors of this native environment and improve functional assessment of iPSC–CMs in vitro.
3. Microphysiological systems (MPS)
The term MPS encompasses microengineered devices at various hierarchical length scales, ranging from single cells microniches to complex engineered tissues with multiple cell types. Each type of MPS brings its own set of advantages and disadvantages, which we review below (Table 1 ). The engineering of MPS has provided new means to create in vitro model systems that better recapitulate specific properties of the native in vivo microenvironment of the heart (Kim et al., 2009; Benam et al., 2015). The higher level of control over cellular and microtissue spatial structures, mechanical properties, and biochemical cues has enabled enhancing the maturity of hiPSC–CMs, as well as reproducing more physiological biophysical behavior (Pasqualini et al., 2015; Sheehy et al., 2017).
Table 1.
Summary and comparison of MPS systems used for functional assessment of hiPSC-CMs. For each type of MPS systems, the controllable features and measurable output are described as reported (+) or not reported (−). The number of replicates possible to derive from 106 cells is a theoretical maximum based on the loading conditions described in the representative dted studies, though for many of the smaller scale constructs, the number of replicates is more limited by the later data-acquisition processes.
| Approach Type | MPS | Controllable features |
Measurable outputs |
Scalable for high throughput studies |
Ref. |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spatial structures |
Mechanical properties |
Biochemical cues |
Mechanical function |
Electrophysiology |
Visual observation |
number of replicates that can be made from 106 cells |
||||||
| Cell | Tissue | In situ | End-point analysis | |||||||||
| Top-Down | Single cell | Micropatterned substrates | + | − | + | + | + | − | + | + | + (5×103−5×105) | (McCain et al., 2012; Ribeiro et al., 2015; Chopra et al., 2018) |
| Microfluidic & microelectrodes | possible | − | possible | possible | possible | + | + | + | +(5×103−5×105) | Werdich et al., 2004; Cheng et al., 2006; Qian et al., 2017) | ||
| 2D Multicell | Micropatterned and microstructured substrates | + | + | + | + | + | + | + | + | +(1×104−1×105) | (Stancescu et al., 2015; Alford et al., 2010; Grosberg et al., 2011;Shim et al., 2012; Feinberg et al., 2012; Lind et al., 2017b; Kujala et al., 2016) | |
| Microfluidic heart-on-chip | possible | possible | + | + | + | − | + | + | +(2–20) | (Marsano et al., 2016; Mathur et al., 2015, 2016) | ||
| Bottom up | EHMs/EHTs | − | + | − | + | + | + | − | + | +(0.5–200) | (Mannhardt et al., 2017; Huebsch et al., 2016; Chen et al., 2015a;Tiburcy et al., 2017; Boudou et al., 2012; Mills et al., 2017) | |
| Hybrid | 3D | 3D bioprinting/ recellularized cardiac ECM scaffolds | − | + | + | + | + | + | + | + | −(0.5–2) | (Borovjagin et al., 2017; Schwan et al., 2016) |
Cardiac MPS have been developed and used for more than two decades (Eschenhagen et al., 1997) with other cell systems than iPSC–CMs, in other contexts and under other names, including organ–on–a–chip, heart–on–a–chip, cardiac microtissues, engineered cardiac tissues, engineered human myocardium or even bioMEMS (Huh et al., 2013; Stancescu et al., 2015; Zhang and Radisic, 2017; Conant et al., 2017). There is also a large overlap between MPS designed as in vitro cardiac models and other embodiments, such as engineered tissue patches, aimed towards regenerative cardiac medicine. The description of these systems and applications falls beyond the scope of this review and we refer the readers to the corresponding literature (Bouten et al., 2011; Zorlutuna et al., 2013; Capulli et al., 2016; Ogle et al., 2016; Song et al., 2018).
Similarly to Song et al., we distinguish MPS based on the type of approach used to control the cellular organization as: bottom–up, i.e., where the cellular organization is acquired intrinsically through maturation and self–organization of the cellular construct; or top–down, where organization is provided through engineering methods, such as scaffold, structures or patterns (Song et al., 2018). A combination of these approaches is sometimes also found.
In both cases, the engineering effort is set towards improving the physiological function, mostly through the enhancement of the structural and functional maturity of the cardiomyocytes by designing the mechanical properties and biochemical functions of the scaffolds, ligands, and multicellular environment.
3.1. Top-down MPS
Engineering cellular environments with specific spatial, mechanical, and biochemical cues can allow for detailed study of the mechanobiological effects of environment on cardiomyocyte function. However, many of these approaches rely on specialized fabrication equipment and techniques which might not be easily adaptable to every group.
Single cell MPS
In top–down approaches, where the organization is provided through engineering methods, we first consider MPS systems focused on single cells. Small islands of ECM proteins are patterned using engineering methods including microcontact printing or lithography techniques (Moeller et al., 2018; Ribeiro et al., 2015; Alom et al., 2007). These systems represent an ideal link between in silico and in vitro molecular model and the more complex multi–cell MPS and engineered tissues (Aratyn–Schaus et al., 2016; Lind et al., 2017b). Patterning ECM serves dual purposes of increasing uniformity within an initially heterogeneous population and, in the case of rectangular patterns, promoting increased alignment of intracellular structures (Ribeiro et al., 2015). Combining ECM patterning on materials with tunable stiffness such as polyacrylamide (Ribeiro et al., 2015; Chopra et al., 2018), PEG hydrogels (Lee et al., 2017), or micropillars (Rodriguez et al., 2014; Tan et al., 2003) provides physiologically relevant mechanical cues and allows for measurement of force generation by single cells. However, there are some notable limitation of these single cell systems, especially when used for relatively immature hiPSC–CMs. These cells lack cell–cell contacts that promote appropriate patterning of sodium channels and gap junctions (Geisler et al., 2010). Without these junctions, it is difficult to study the conduction of calcium currents and action potentials, which are both important features of cardiac electrophysiology. Some microfluidic/microelectrode systems have been used to measure the electrophysiology of isolated primary cardiomyocytes, but the differences in ion channel expression and cytoskeletal organization between primary cardiomyocytes and hiPSC–CMs should temper expectations for acquiring accurate electrophysiologic mechanisms with single cell systems (Werdich et al., 2004; Cheng et al., 2006).
Multiple cells in 2D/3D MPS
Similarly to single cell MPS, some 2D multicellular constructs use ligand micropatterning or surface microstructuring to form planar MPS (Feinberg et al., 2012; Wang et al., 2014; Ariyasinghe et al., 2017). Some MPS, including the muscular thin film, use a combination of micropatterning and lithography based microfabrication to create a series of flexible 2D microtissues whose mechanics are well characterized (Grosberg et al., 2012; McCain et al., 2013; Shim et al., 2012). Other microfabrication techniques including electro spinning have been used for the creation of scaffolds to support engineered tissues (Huang et al., 2017a; Liu et al., 2017; Schenke–Layland et al., 2011; Capulli et al., 2016; Wanjare et al., 2017). These systems allow for top–down engineering of the tissue morphology and extra–/intracellular organization, while simultaneously enabling the study of cell–cell interactions in a microtissue format. Many 2D systems are designed to allow for microscopy and electrophysiologic measurements of coordinated cardiomyocytes. However, cardiomyocytes in these constructs lack the 3D cell–cell interactions which they experience in the native myocardium, which may also limit the ability of these systems to model complex processes like action potential conduction (Lemoine et al., 2017). Methods for top–down engineering of 3D MPS have also been proposed, mostly hedging on 3D printing of scaffolds onto which chemical cues can be spatially patterned (DeForest et al., 2009; Greiner et al., 2012; Scheiwe et al., 2015).
3.2. Bottom-up MPS
Bottom–up MPS are commonly produced by casting of hiPSC–CMs with or without other cells such as fibroblast in a microfabricated mold. The microtissue is typically formed through the combined aggregation of a random hydrogel scaffold and cell–cell contact formation (Mannhardt et al., 2017; Tiburcy et al., 2017; Sidorov et al., 2017; Ronaldson–Bouchard et al., 2018; Boudou et al., 2012). In most cases, fibroblasts or other mesenchymal cells are added to the hiPSC–CM to form a functional tissue, eventually forming a co–culture. Different geometries have been used for these kinds of constructs, including rings (Tiburcy et al., 2017), sheets (Huebsch et al., 2015), and linear or dogbone shapes mounted on each end (Mannhardt et al., 2017; Sidorov et al., 2017; Ronaldson–Bouchard et al., 2018; Boudou et al., 2012; Leonard et al., 2018). While some have rigid boundaries, many include a flexible component with defined mechanical properties which allows for construct shortening and calculation of force. Many of these systems also include electrodes that allow for direct electrical stimulation of the cardiac construct (Ronaldson–Bouchard et al., 2018; Boudou et al., 2012; Sidorov et al., 2017). While such engineered myocardium tissue better replicate the complex cell–cell interactions found in vivo, deconvolving differences in heterogeneous cell populations is more challenging with a large number of cells. Some recent efforts have been made to miniaturize these constructs, reducing the number of required cells per construct from millions to thousands or hundreds (Boudou et al., 2012; Mills et al., 2017). These modifications may make these constructs more scalable for high throughput screening applications.
3.3. Hybrid MPS
Finally, hybrid MPS platform have been proposed that largely overlap with engineered cardiac tissue (Lind et al., 2017a). Most are based on 3D bioprinting, which offers the capability to precisely position single cell and/or scaffold components to form hierarchical tissues including vasculature. The description of such systems fall beyond the scope of our review and we refer reader to the recent literature (Fleischer et al., 2017; Borovjagin et al., 2017). Another strategy relies on laser cutting thin strips of decellularized cardiac tissue and adding hiPSC–CMs to an ECM scaffold with physiologic organization, which in turn promotes cell alignment and maturity (Schwan et al., 2016).
3.4. Designing MPS: choosing materials and ligands
For most MPS, achieving a physiologically–representative extracellular environment is critical, as the mechanical properties and spatial organization of biochemical cues directly impacts the hiPSC–CMs phenotype (Vining and Mooney, 2017). Polydimethylsiloxane (PDMS) is a flexible, bioinert polymer that can be formulated to span a large range of roughly physiological stiffnesses (5 kPa–3 MPa), and it can be easily molded into micro patterned structures (Palchesko et al., 2012). hiPSC–CMs cultured on PDMS grooves or cyclically stretched PDMS sheets demonstrate higher maturity markers and functional characteristics than 2D counterparts (Kroll et al., 2017; Jung et al., 2016). This material is also often used for micropillars, stamps for micropatterning, and as structural material for larger scale MPS, including the deformable anchoring posts of several bottom–up MPS (Boudou et al., 2012; Ronaldson–Bouchard et al., 2018; Ribeiro et al., 2015; Leonard et al., 2018; Rodriguez et al., 2014). Hydrogels are tunable in the range of physiologic cardiac tissue that includes stiffnesses matching early developmental time points and are cell substrate and tissue scaffolds. Various synthetic hydrogel systems have been proposed, based on polyacrylamide (Ribeiro et al., 2015; Chopra et al., 2018), polyethylene glycol (PEG) (Lee et al., 2017), hyaluronic acid (Kloxin et al., 2010), crosslinkable proteins, such as or biosynthesized materials, such as elastin protein (Chung et al., 2012).
While the mechanical properties are important, they alone fail to provide precise control over the tissue and intracellular organization and to spatially mimic the native extracellular matrix. Some MPS focus on coating different ECM proteins on substrate to observe relationship between cell–ECM interaction (Williams et al., 2014; Kong et al., 2013), Williams and colleagues coated a combination of proteins on tissue culture plastic and compared fetal, neonatal, and adult ECM composition. Neonatal ECM lead to greater expansion of neonatal rat ventricular CMs (Williams et al., 2014). Vitronectin–coated–synthetic fibers electrospun on glass coverslips have also shown different synthetic combinations leading to changes in contractility and mitochondrial function in iPSC–CMs (Chun et al., 2015). CMs have been shown to have different spread area, adherence properties and onset of beating depending composition of the ECM proteins (Simpson et al., 1994; Vanwinkle et al., 1996; Bick et al., 1998; Bullard et al., 2005; Deitch et al., 2012). Collagen IV and laminin have been shown to increase hiPSC–CM spread area and contraction velocity on micropillars compared to fibronectin, though there was no statistical difference in force generation between these three (Rodriguez et al., 2014).
Overall, MPS platforms are useful tools for modeling specific aspects of cardiac biology at a range of scales and levels of complexity. For applications where animal models or traditional cell culture approaches fail to replicate relevant features of human cardiac biology, MPS may be a viable alternative. The choice of MPS will be driven by the end application for in vitro disease modeling and drug testing.
4. Use of MPS for studying CM biology
A key advantage of many of these MPS over animal models is the ability to precisely tune the mechanical and biochemical environment experienced by the cells and observe the dynamic reorganization of structures and corresponding changing in function and gene transcription.
4.1. Changes in intracellular myofibril organization
Many of these systems are designed to be optically transparent and thus suitable for optical imaging of cells and their internal structures in situ (Ribeiro et al., 2015; McCain et al., 2013). Single cell micropatterning studies have discovered that myofibril organization is affected by pattern shape, and that these structures dynamically remodel to align in directions of principal stress associated with clustering of adhesion structures (Parker et al., 2008; Grosberg et al., 2011; Yuan et al., 2017). Alignment in a rectangular shape with an aspect ratio of 7:1 promotes alignment of myofibrils and increased force generation (Ribeiro et al., 2015). Increased resolution, alignment, and registration of myofibrils is a key feature of hiPSC maturation (Leonard et al., 2018). A recent study using tagged alpha–actinin has enabled visualization of centripetal motion of contractile proteins during the re–formation of sarcomeres and myofibrils after replating on patterned substrates (Chopra et al., 2018). In addition to patterning, other features of the engineered substrate can also direct intracellular organization. The available binding proteins and stiffness of the substrate both affect myofibril alignment and resultant force generation (Chopra et al., 2011). Many 3D, bottom up MPSs have been shown to promote better myofibril maturity and density, but visualization of these structures in live cells is more challenging (Huebsch et al., 2016; Sidorov et al., 2017).
4.2. Effects of cell-cell interactions
Another key feature of the cells microenvironment often mimicked in MPS is the populations of neighboring cells. Pairs of cardiomyocytes have been shown to form adhesions to each other and align their myofibril structure and are capable of generating more force than a single cell alone, according to traction force measurements (McCain et al., 2012). Larger multicellular constructs often include (and indeed require) a population of fibroblasts or other mesenchymal cells for remodeling and maintaining the ECM (Tiburcy et al., 2017; Liau et al., 2017). The ratio and specific types of stromal cell populations added to these constructs can have profound effects on construct properties and CM function. Some studies have relied on the fraction of non–CM cells present in most iPSC–CM cultures, though others have generated a purified culture of CMs and added mesenchymal stem cells or fibroblasts directly at the point of tissue assembly (Hudson et al., 2011; Tiburcy et al., 2017). Tiburcy et al. reported similar peak force production at a final cell ratio of 1:1 non–CM:CM with both mixed iPS derived cells and purified iPSC–CMs with added fibroblasts (Tiburcy et al., 2017). These mesenchymal cells affect CM function directly (through cell–cell contacts) and indirectly by modulating the ECM stiffness and releasing paracrine signaling factors (Vunjak–Novakovic, 2017; Ariyasinghe et al., 2017; Sidorov et al., 2017). Fetal mouse cardiac mesenchymal cells express more ECM genes and promote a faster beating rate than mesenchymal stem cell derived mesenchymal cells when cocultured with embryonic cardiomyocytes (Hudson et al., 2011). Fetal mouse cardiac fibroblasts promote a more mature phenotype in ESC–CMs than adult cardiac fibroblasts, in part by activating the ERK/MAPK pathway through paracrine signaling (Liau et al., 2017). Some systems have also included endothelial cells, a third major cellular fraction in native hearts. These cells are crucial for providing oxygenation and nutrients for native tissue while also providing important paracrine signaling (Chen et al., 2005; Wanjare et al., 2017). While most MPS are designed to avoid the need for vascularization, applications trending towards therapeutic tissue engineering or more complex organ–on–chip systems will seek to include a vascular bed (Ogle et al., 2016).
4.3. Modeling disease causing mutations
The combination of MPS and hiPSC–CMs allow for the study of effects of mutations which are known or suspected to lead to human cardiac disease in a model of human cardiac cells. This technique has tremendous potential to clarify the underlying etiology of genetic diseases, and to guide decision making about therapies for patients with a specific genetic makeup (precision medicine) (Eschenhagen et al., 2015; Sayed et al., 2016). Many mutations in sarcomeric or structural proteins have been linked to the development of cardiomyopathies, the two most common types of which are hypertrophic and dilated (Fatkin et al., 2014; Spudich, 2014). By promoting better alignment of myofibrils and maturation of contractile machinery, MPS can enable functional measurements of iPSC–CM whose structures are more similar to native CMs. Mutations in sarcomeric proteins, including cardiac troponin C, beta myosin heavy chain and myosin binding protein C change force generation by single hiPSC–CMs, and these data in conjunction with data from in vivo experiments support contractile tension as a predictive indicator of the development of dilated or hypertrophic cardiomyopathy (Davis et al., 2016). Separately, traction force measurements on a micropatterned platform showed reduced force production and increase spatial heterogeneity of contraction from single hiPSC–CMs with myosin binding protein C mutations (heterozygous and homozygous deletion) in a dose dependent way (Ribeiro et al., 2017). Single cell analysis of cells with mutation of BAG3, a chaperone protein associated with the development of DCM, also revealed reduced contractility (Judge et al., 2017). Muscular thin films have been used to describe functional differences in hiPSC–CM contraction and myofibril organization with a mutation for Barth Syndrome, a mitochondrial condition (Wang et al., 2014). A titin mutation reduced force generation and normal myofibril formation when compared to WT differences in both micropatterned single cell platforms and 3D engineered heart tissue constructs (Chopra et al., 2014; Hinson et al., 2015; Chopra et al., 2018). These studies can clarify the mechanisms of disease progression from specific genetic causes and may eventually inform treatment choices for specific patients.
5. Drug discovery
The use of hiPSC–CMs as models to develop drugs for therapy and to screen compounds for cardiotoxicity have been proposed since their advent over a decade ago, but progress has been limited by the relative immaturity of these cells (Zhang et al., 2009). Since MPS enhance the maturity of hiPSC–CM, their utilization for drug discovery has increased (Huebsch et al., 2016; Ewart et al., 2018). Increasing maturity of hiPSC–CMs is correlated with the expression of specific beta adrenergic receptors, which are necessary to reproduce physiologic responses to norepinephrine (Tiburcy et al., 2017). Culturing cells on micropatterned PDMS substrates accelerated maturation by more than 6 weeks and promoted expression of beta adrenergic receptor 1 that enabled the an adrenergic response (Jung et al., 2016). However, there are still limitations to using MPS such as engineered heart tissue (EHTs) or single cell systems for drug discovery. Throughput and scalability are critical for drug discovery, but most EHTs require a relatively high amount of cell (>1 million cells/EHT) for one EHT and at least a week to develop (Eder et al., 2016). There have been efforts to miniaturize these constructs, which can improve the scalability for drug discovery applications and multi–factorial analysis (Mills et al., 2017; Boudou et al., 2012). In contrast, single cell systems can provide high–throughput, but have yet to demonstrate the maturity seen in EHTs (Ribeiro et al., 2015). Despite this lack of maturity, micropatterned single hiPSC–CMs can recapitulate the effects of cardioactive compounds isoproterenol and caffeine on CM structure and function (Ribeiro et al., 2017). To date, most studies that have used hiPSC–CMs to screen for cardiotoxicity or drug development have examined the impact of these compounds on the electrophysiological properties of the cell (Sayed et al., 2016; Burridge et al., 2016; Sala et al., 2016). These examinations are fully warranted, as disruption in the electrical properties of CMs by several trail drugs have led to long QT syndrome (Gintant et al., 2017). However, examination of the impact of drugs on force production and contractility is also necessary, as drugs that are deleterious to either of these parameters may reduce cardiac function. Thus, the future of MPS for drug discovery will need platforms that can provide electrical and functional data in a high throughput platform.
6. Conclusión
Overall, this field has expanded with the growing accessibility of hiPSCs and low–cost differentiation strategies. However, the question remains open to what degree the immaturity of iPSC–CMs in traditional culture systems limits their utility for disease modeling or drug discovery. There are a range of MPS that are currently in use for studying hiPSC–CM maturation and recapitulation of both healthy and diseased myocardial tissue function.
Larger cardiac tissue constructs are also being investigated as development of directly therapeutic cardiac patches for personalized tissue engineering, though these currently focus on generic (not patient specific) stem cell derived CM cell sources (Chong et al., 2014). While questions of regulatory approval and fast, reliable iPSC cell creation and differentiation may slow the adoption of iPSC–CM use in direct therapeutic applications, a better understanding of iPSC–CM function and microenvironment may have many important implications for developing future therapies for heart disease.
Acknowledgments
This work was supported by the American Heart Association [AHA 17CSA33590101], the National Science Foundation [NSF CMMI 1662431; GRFP], the National Academies of Sciences, Engineering, and Medicine [Ford Foundation Fellowship] and the National Institutes of Health [NIH 1R21HL13099301; TIMBS T32] and SNSF Early Postdoc.Mobility fellowship grant: [P2SKP2_164954].
References
- Acevedo–Acevedo, Suehelay, Crone Wendy C., 2015. Substrate stiffness effect and chromosome missegregation in HIPS cells. J. Negat. Results Biomed 14 (December), 22 BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford Patrick W., Feinberg Adam W., Sheehy Sean P., Parker Kevin K., 2010. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials 31 (13). Elsevier:3613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alom Sami, Ruiz Ab, Chen Christopher S., 2007. Microcontact printing: a tool to pattern. Soft Matter 3, 168–177. [DOI] [PubMed] [Google Scholar]
- Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL, 2002. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA–salt hypertensive rats: role of ETA receptors. Hypertension 39 (2), 679–684. [DOI] [PubMed] [Google Scholar]
- Arad Michael, Seidman JG, Christine E Seidman, 2002. Phenotypic diversity in hypertrophic cardiomyopathy. Hum. Mol. Genet 11 (20), 2499–2506. [DOI] [PubMed] [Google Scholar]
- Aratyn–Schaus Yvonne, Pasqualini Francesco S., Yuan Hongyan, McCain Megan L., Ye George J.C., Sheehy Sean P., Campbell Patrick H., Parker Kevin Kit, 2016. Coupling primary and stem cell–derived cardiomyocytes in an in vitro model of cardiac cell therapy. J. Cell Biol 212 (4), 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyasinghe Nethika R., Reck Caitlin H., Viscio Alyssa A., Petersen Andrew P., Lyra–Leite Davi M., Cho Nathan, McCain Megan L., 2017. Engineering micromyocardium to delineate cellular and extracellular regulation of myocardial tissue contractility. Integrative Biol.: Quantitative Biosci. Nano to Macro 9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts T, Costa KD, Covell JW, McCulloch AD, 2001. Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am. J. Physiol. Heart Circ. Physiol 280 (5), H2222–H2229. [DOI] [PubMed] [Google Scholar]
- Atmanli Ayhan, John Domian Ibrahim, 2017. Recreating the cardiac microenvironment in pluripotent stem cell models of human physiology and disease. Trends Cell Biol. 27 (5), 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, ekkala KY, Borg TK, Baudino TA, 2006. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann. N. Y. Acad. Sci 1080, 76–84. [DOI] [PubMed] [Google Scholar]
- Bayomy Ahmad F., Bauer Michael, Qiu Yiling, Liao Ronglih, 2012. “Regeneration in heart disease–is ECM the key? Life Sci. 91 (17–18), 823–827. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin Milena, Casini Simona, Davis Richard P., D’Aniello Cristina, Haas Jessica, Oostwaard Ward–van, Dorien Tertoolen, Leon GJ, et al. , 2013. Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long–QT syndrome. EMBO J. 32 (24). EMBO Press:3161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam Kambez H., Dauth Stephanie, Hassell Bryan, Anna Herland, Jain Abhishek, Jang Kyung–Jin, Karalis Katia, et al. , 2015. Dx.Doi.Org. Engineered in Vitro Disease Models, vol. 10, pp. 195–262 (1). [DOI] [PubMed] [Google Scholar]
- Benjamin Emelia J., Virani Salim S., Callaway Clifton W., Chang Alexander R., Cheng Susan, Chiuve Stephanie E., Cushman Mary, et al. , 2018. Heart disease and stroke statistics–2018 update: a report from the American heart association. Circulation 137, e67–e492. January. [DOI] [PubMed] [Google Scholar]
- Bergmann MW, 2010. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ. Res 107 (10), 1198–1208. [DOI] [PubMed] [Google Scholar]
- Berry Mark F., Engler Adam J., Woo Y Joseph, Pirolli Timothy J., Bish Lawrence T., Jayasankar Vasant, Morine Kevin J., Gardner Timothy J., E Discher Dennis, Sweeney H Lee, 2006. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol.: Heart Circul Physiol June 290 (6), H2196–H2203. [DOI] [PubMed] [Google Scholar]
- Bhana Bashir, Iyer Rohin K., Chen Wen Li Kelly, Zhao Ruogang, Sider Krista L., Likhitpanichkul Morakot, Simmons Craig A., Radisic Milica, 2010. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng 105 (6), 1148–1160. [DOI] [PubMed] [Google Scholar]
- Bick RJ, Snuggs MB, Poindexter BJ, Buja LM, Van Winkle WB, 1998. Physical, contrattile and calcium handling properties of neonatal cardiac myocytes cultured on different matrices. Cell Adhes. Commun 6 (4), 301–310. [DOI] [PubMed] [Google Scholar]
- Borovjagin Anton V., Ogle Brenda M., Berry Joel L., Zhang Jianyi, 2017. From microscale devices to 3D printing. Circ. Res 120 (1), 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudou Thomas, Legant Wesley R., Mu Anbin, Borochin Michael A., Thavandiran Nimalan, Radisic Milica, Zandstra Peter W., Epstein Jonathan A., Margulies Kenneth B., Chen Christopher S., 2012. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. 18 (9–10), 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouten CVC, Dankers PYW, Driessen–Mol A, Pedron S, Brizard AMA, T Baaijens FP, 2011. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev 63 (4), 221–241. [DOI] [PubMed] [Google Scholar]
- Bowers Stephanie L.K., Banerjee Indroneal, Baudino Troy A., 2010. The extracellular matrix: at the center of it all. J. Mol. Cell. Cardiol. 48 (3), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam SR, Tertoolen L, Casini S, Matsa E, Lu HR, Teisman A, Passier R, et al. , 2013. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res. 10 (1), 48–56. [DOI] [PubMed] [Google Scholar]
- Brandenburger Matthias, Wenzel Jan, Roman Bogdan, Richardt Doreen, Nguemo Filomain, Reppel Michael, Hescheler Jürgen, Heinrich Terlau, Dendorfer Andreas, 2012. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc. Res 93 (1), 50–59. [DOI] [PubMed] [Google Scholar]
- Brutsaert Dirk L., 2003. Cardiac endothelial–myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev 83 (1), 59–115. [DOI] [PubMed] [Google Scholar]
- Bullard Tara A., Borg Thomas K., Price Robert L., 2005. The expression and role of protein kinase C in neonatal cardiac myocyte attachment, cell volume, and myofibril formation is dependent on the composition of the extracellular matrix. Microsc. Microanal 11 (03), 224–234. [DOI] [PubMed] [Google Scholar]
- Burke Michael A., Chang Stephen, Wakimoto Hiroko, Gorham Joshua M., Conner David A., Christodoulou Danos C., Parfenov Michael G., et al. , 2016. Molecular profiling of dilated cardiomyopathy that progresses to heart failure. Archiv. Cardiovas. Dis 104 (12), 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge Paul W., Holmstrom Alexandra, Wu Joseph C., 2015. Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. In Current Protocols in Human Genetics 87, 21, 3.1–21.3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge Paul W., Li Yong Fuga, Matsa Elena, Wu Haodi, Ong Sang–Ging, Sharma Arun, Holmstrom Alexandra, et al. , 2016. Human induced pluripotent stem cell–derived cardiomyocytes recapitulate the predilection ofbreastcancer patients to doxorubicin–induced cardiotoxicity. Nat. Med 22 (5), 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho Paula, Fan Huimin, Liu Zhongmin, Jia–Qiang He, 2016. Small mammalian animal models of heart disease. American Journal of Cardiovascular Disease 6 (3), 70–80. [PMC free article] [PubMed] [Google Scholar]
- Capulli AK, MacQueen LA, Sheehy SP, Parker KK, 2016. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev 96, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Frangogiannis NG, 2016. Macrophages in the remodeling failing heart. Circ. Res 119 (7), 776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Gaopeng, Sen Li, Karakikes Ioannis, Ren Lihuan, Chow Maggie Zi–Ying, Chopra Anant, Keung Wendy, et al. , 2015a. Phospholamban as a crucial determinant of the inotropic response of human pluripotent stem cell–derived ventricular cardiomyocytes and engineered 3–dimensional tissue constructs. circulation. Arrhythmia and Electrophysiology 8 (1), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Vincent C., Ye Jingjing, Shukla Praveen, Hua Giau, Chen Danlin, Lin Ziguang, Liu Jian–chang, et al. , 2015b. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 15 (2), 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, Pozzi A, 2005. An orthotopic model of lung cancer to analyze primary and metastatic NSCLC growth in integrin alpha1–null mice. Clin. Exp. Metastasis 22 (2), 185–193. [DOI] [PubMed] [Google Scholar]
- Cheng Hui, Force Thomas, 2010. Why do kinase inhibitors cause cardiotoxicity and what can Be done about it? Prog. Cardiovasc. Dis 53 (2), 114–120. [DOI] [PubMed] [Google Scholar]
- Cheng Wei, Klauke Norbert, Sedgwick Helen, Smith Godfrey L., Cooper Jonathan M., 2006. Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform. Lab a Chip 6 (11), 1424–1431. [DOI] [PubMed] [Google Scholar]
- Chong James J.H., Yang Xiulan, Don Creighton W., Minami Elina, Liu Yen–Wen, Weyers Jill J., Mahoney William M., et al. , 2014. Human embryonic–stem–cell–derived cardiomyocytes regenerate non–human primate hearts. Nature 510 (7504), 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, Raz–Ben Aroush D, et al. , 2014. Augmentation of integrin–mediated mechano–transduction by hyaluronic acid. Biomaterials 35 (1), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY, 2011. Cardiac myocyte remodeling mediated by N–Cadherin–Dependent mechanosensing. Am. J. Physiol. Heart Circ. Physiol 300 (4), H1252–H1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra Anant, Kutys Matthew L., Zhang Kehan, Polacheck William J., Sheng Calvin C., Luu Rebeccah J., Eyckmans Jeroen, et al. , 2018. Force generation via β–cardiac myosin, titin, and α–actinin drives cardiac sarcomere assembly from cell–matrix adhesions. Dev. Cell 44 (1), 87–96 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Tammy F., Rupnick Maria A., Kerkela Risto, Dallabrida Susan M., Zurakowski David, Nguyen Lisa, Woulfe Kathleen, et al. , 2007. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370 (9604), 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Young Wook, Voyles David E., Rath Rutwik, Hofmeister Lucas H., Boire Timothy C., Wilcox Henry, Lee Jae Han, Bellan Leon M., Hong Charles C., Sung Hak–Joon, 2015. Differential responses of induced pluripotent stem cell–derived cardiomyocytes to anisotropic strain depends on disease status. J. Biomech 48 (14), 3890–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Cindy, Anderson Erica, Pera Renee Reijo, Pruitt Beth L., Heilshorn Sarah C., 2012. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell–derived cardiomyocytes in 3D cultures. Soft Matter 8 (39), 10141–10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark Kathleen A., McElhinny Abigail S., Beckerle Mary C., Gregorio Carol C., 2002. Striated muscle cytoarchitecture: an intricate web of form and function. Annu. Rev. Cell Dev. Biol 18 (1), 637–706. [DOI] [PubMed] [Google Scholar]
- Conant Genevieve, Lai Benjamin Fook Lun, Lu Rick Xing Ze, Korolj Anastasia, Wang Erika Yan, Radisic Milica, 2017. High–content assessment of cardiac function using heart–on–a–chip devices as drug screening model. Stem Cell Rev. 13 (3), 335–346. [DOI] [PubMed] [Google Scholar]
- Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OHL, 1995. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91 (1), 161–170. [DOI] [PubMed] [Google Scholar]
- Correia Cláudia, Koshkin Alexey, Duarte Patrícia, Hu Dongjian, Teixeira Ana, Domian Ibrahim, Serra Margarida, Paula M, Alves, 2017. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep 7 (1), 8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARPA/DSO, 2011. Broad Agency Announcement Microphysiological Systems DSO DARPA–BAA–11–73.
- Davis Jennifer, Davis L Craig, Correll Robert N., Makarewich Catherine A., Schwanekamp Jennifer A., Moussavi–Harami Farid, Wang Dan, et al. , 2016. “A tension–based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165 (5), 1147–1159. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RP, Casini S, van den Berg CW, Hoekstra M, Remme CA, Dambrot C, Salvatori D, et al. , 2012. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 125 (25), 3079–309. [DOI] [PubMed] [Google Scholar]
- Dean Rachael G., Leanne C Balding, Candido Riccardo, Burns Wendy C., Cao Zemin, Twigg Stephen M., Burrell Louise M., 2005. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J. Histochem. Cytochem 53 (10), 1245–1256. SAGE PublicationsSage CA: Los Angeles, CA. [DOI] [PubMed] [Google Scholar]
- DeForest Cole A., Polizzotti Brian D., Anseth Kristi S., 2009. Sequential click reactions for synthesizing and patterning three–dimensional cell microenvironments. Nat. Mater 8 (8). Nature Publishing Group:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch Sandra, Gao Bruce Z., Dean Delphine, 2012. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Mol. Cell. BioMech 9 (3), 227–249. [PMC free article] [PubMed] [Google Scholar]
- Denayer Tinneke, Stoohr Thomas, Van Roy Maarten, 2014. Animal models in translational medicine: validation and prediction. New Horizons in Translational Medicine 2 (1), 5–11. [Google Scholar]
- Denning Chris, Borgdorff Viola, James Crutchley, Karl S, Firth A, George Vinoj, Kalra Spandan, Alexander Kondrashov, et al. , 2016. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta Mol. Cell Res 1863 (7 Pt B), 1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon Jennifer A., Spinale Francis G., 2009. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circulation. Heart Fail 2 (3), 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder Alexandra, Vollert Ingra, Hansen Arne, Eschenhagen Thomas, 2016. Human engineered heart tissue as a model system for drug testing. Adv. Drug Deliv. Rev 96 (January), 214–224. [DOI] [PubMed] [Google Scholar]
- Engler Adam J., Carag–Krieger Christine, Johnson Colin P., Raab Matthew, Tang Hsin–Yao, Speicher David W., Sanger Joseph W., Sanger Jean M., E Discher Dennis, 2008. Embryonic cardiomyocytes beat best on a matrix with heart–like elasticity: scar–like rigidity inhibits beating. J. Cell Sci 121 (Pt 22), 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler Adam J., Rehfeldt Florian, Sen Shamik, E Discher Dennis, 2007. Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation BT – cell mechanics. In Cell Mechanics 83, 521–545. [DOI] [PubMed] [Google Scholar]
- Epelman Slava, Liu Peter P., Mann Douglas L., 2015. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol 15 (2), 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, et al. , 1997. Three–dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb. J.: Off. Publ. Feder. Am. Soc. Exp. Biol 11 (8), 683–694. [DOI] [PubMed] [Google Scholar]
- Eschenhagen Thomas, Mummery Christine, Knollmann Bjorn C., 2015. Modelling sarcomeric cardiomyopathies in the dish: from human heart samples to IPSC cardiomyocytes. Cardiovasc. Res 105 (4), 424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler G, 2015. Good and bad sides of TGFbeta–signaling in myocardial infarction. Front. Physiol 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart Lorna, Dehne Eva–Maria, Fabre Kristin, Gibbs Susan, Hickman James, Hornberg Ellinor, et al. , 2018. Application of microphysiological systems to enhance safety assessment in drug discovery. Annu. Rev. Pharmacol. Toxicol 58 (1), 65–82. [DOI] [PubMed] [Google Scholar]
- Fatkin Diane, Seidman Christine E., Seidman Jonathan G., 2014. Genetics and disease of ventricular muscle. Cold Spring Harbor Perspectives Med. January 4(1), a021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg Adam W., Alford Patrick W., Jin Hongwei, Ripplinger Crystal M., Werdich Andreas A., Sheehy Sean P., Grosberg Anna, Parker Kevin Kit, 2012. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials 33 (23), 5732–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE, 2010. Human embryonic stem cell–derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol 49 (6), 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira–Cornwell MC, Luo Y, Narula N, Lenox JM, Lieberman M, Radice GL, 2002. Remodeling the intercalated disc leads to cardiomyopathy in mice misexpressing cadherins in the heart. J. Cell Sci 115 (Pt 8), 1623–1634. [DOI] [PubMed] [Google Scholar]
- Fleischer Sharon, Shapira Assaf, Feiner Ron, Dvir Tal, 2017. Modular assembly of thick multifunctional cardiac patches. Proc. Natl. Acad. Sci. U. S. A 114 (8), 1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force Thomas, Kolaja Kyle L., 2011. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov 10 (2), 111–126. [DOI] [PubMed] [Google Scholar]
- Geisler Sarah B., Green Kathleen J., Isom Lori L., Meshinchi Sasha, Martens Jeffrey R., Delmar Mario, Russell Mark W., 2010. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures ofadult rat cardiomyocytes. J. Biomed. Biotechnol 2010 (May), 624719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintant Gary, Bernard Fermini, Norman Stockbridge, Strauss David, 2017. The evolving roles of human IPSC–derived cardiomyocytes in drug safety and discovery. Cell Stem Cell 21 (1), 14–17. [DOI] [PubMed] [Google Scholar]
- Gowran Aoife, Rasponi Marco, Visone Roberta, Nigro Patrizia, Perrucci Gianluca L., Righetti Stefano, Zanobini Marco, Pompilio Giulio, 2016. Young at heart: pioneering approaches to model nonischaemic cardiomyopathy with induced pluripotent stem cells. Stem Cell. Int 2016, 4287158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH, 1981. Left ventricular fibre architecture in man. Br. Heart J 45 (3), 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner Alexandra M., Richter Benjamin, Bastmeyer Martin, 2012. “Micro–Engineered 3D scaffolds for cell culture studies. Macromol. Biosci 12 (10), 1301–1314. [DOI] [PubMed] [Google Scholar]
- Grosberg Anna, Alford Patrick W., McCain Megan L., Parker Kevin Kit, 2011. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab a Chip 11 (24), 4165–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosberg Anna, Nesmith Alexander P., Goss Josue A., Brigham Mark D., McCain Megan L., Parker Kevin Kit, 2012. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J. Pharmacol. Toxicol. Methods 65 (3), 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack T, Abdelilah–Seyfried S, 2016. The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development (Cambridge, England) 143 (3), 373–386. Oxford University Press for The Company of Biologists Limited. [DOI] [PubMed] [Google Scholar]
- Hanson Kevin P., Jung Jangwook P., Tran Quyen A., Hsu Shao–Pu P., Iida Rioko, Ajeti Visar, Campagnola Paul J., et al. , 2013. Spatial and temporal analysis of extracellular matrix proteins in the developing murine heart: a blueprint for regeneration. Tissue Eng. 19 (9–10), 1132–1143. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans S, 2006. Inflammation and cardiac remodeling during viral myocarditis. Ernst Schering Res. Found. Workshop 55, 197–218. [DOI] [PubMed] [Google Scholar]
- Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, et al. , 2015. Titin mutations in IPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349 (6251), 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt Marc N., Jasper Boeddinghaus, Mitchell Alice, Schaaf Sebastian, Bornchen Christian, Müller Christian, Schulz Herbert, et al. , 2014. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol 74 (September), 151–161. [DOI] [PubMed] [Google Scholar]
- Ho Carolyn Y., López Begona, Coelho–Filho Otavio R., Lakdawala Neal K., Cirino Allison L., Jarolim Petr, Kwong Raymond, et al. , 2010. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med 363 (6), 552–563. Massachusetts Medical Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Guoyou, Li Fei, Zhao Xin, Ma Yufei, Li Yuhui, Lin Min, Jin Guorui, Lu Tian Jian, Genin Guy M., Xu Feng, 2017a. Functional and biomimetic materials for engineering of the three–dimensional cell microenvironment. Chem. Rev. 117 (20), 12764–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Huimei, Ruan Qinyun, Lin Meiyan, Yan Lei, Huang Chunyan, Fu Liyun, 2017b. Investigation on left ventricular multi–directional deformation in patients of hypertension with different LVEF. Cardiovasc. Ultrasound 15 (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson James E., Brooke Gary, Blair Chris, Ernst Wolvetang, Cooper–White Justin John, 2011. Development of myocardial constructs using modulus–matched acrylated polypropylene glycol triol substrate and different nonmyocyte cell populations. Tissue Eng. 17 (17–18), 2279–2289. [DOI] [PubMed] [Google Scholar]
- Huebsch Nathaniel, Loskill Peter, Deveshwar Nikhil, Ian Spencer C, Judge Luke M., Mandegar Mohammad A., Fox Cade B., et al. , 2016. Miniaturized IPS–Cell–Derived cardiac muscles for physiologically relevant drug response analyses. Sci. Rep. 6, 24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch Nathaniel, Loskill Peter, Mandegar Mohammad A., Marks Natalie C., Alice S Sheehan, Ma Zhen, Mathur Anurag, et al. , 2015. Automated video–based analysis of contractility and calcium flux in human–induced pluripotent stem cell–derived cardiomyocytes cultured over different spatial scales. Tissue Eng. C Methods 21 (5), 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes Chris S., Postovit Lynne M., Lajoie Gilles A., 2010. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10 (9), 1886–1890. [DOI] [PubMed] [Google Scholar]
- Huh Dongeun, Kim Hyun Jung, Fraser Jacob P., Shea Daniel E., Khan Mohammed, Anthony Bahinski, Hamilton Geraldine A., Ingber Donald E., 2013. Microfabrication of human organs–on–chips. Nat. Protoc 8 (11), 2135–2157, 1. [DOI] [PubMed] [Google Scholar]
- Jacot JG, Martin JC, Hunt DL, 2010. Mechanobiology of cardiomyocyte development. J. Biomech 43 (1), 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan–LeSaux Claude, Zhang Jianhua, Lindsey Merry L., 2010. Extracellular matrix roles during cardiac repair. Life Sci. 87 (13–14), 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge Luke M., Perez–Bermejo Juan A., Truong Annie, Alexandre J, Ribeiro S, Yoo Jennie C., Jensen Christina L., Mandegar Mohammad A., et al. , 2017. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight 2 (14), 94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Gwanghyun, Fajardo Giovanni, Ribeiro Alexandre J.S., Kooiker Kristina Bezold, Coronado Michael, Zhao Mingming, Hu Dong–Qing, et al. , 2016. Time–dependent evolution of functional vs. Remodeling signaling in induced pluripotent stem cell–derived cardiomyocytes and induced maturation with biomechanical stimulation. Faseb. J 30 (4), 1464–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Jangwook P., Squirrell Jayne M., Lyons Gary E., Eliceiri Kevin W., Ogle Brenda M., 2012. Imaging cardiac extracellular matrices: a blueprint for regeneration. Trends Biotechnol. 30, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Shin, Pabon Lil, Reinecke Hans, Murry Charles E., 2017. In vivo maturation of human induced pluripotent stem cell–derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Reports 8 (2), 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Qiao Y, Li G, Baechle K, Camelliti P, Rentschler S, Efimov IR, 2016. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci. Rep 6 (1), 28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelko Valeri I., 2001. Extracellular matrix alterations in cardiomyopathy: the possible crucial role in the dilative form. Exp. Clin. Cardiol 6 (1), 41–49. [PMC free article] [PubMed] [Google Scholar]
- Karakikes Ioannis, Mohamed Ameen, Termglinchan Vittavat, Wu Joseph C., 2015. Human induced pluripotent stem cell–derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res 117 (1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakikes Ioannis, Termglinchan Vittavat, Wu Joseph C., 2014. Human–induced pluripotent stem cell models of inherited cardiomyopathies. Curr. Opin. Cardiol 29 (3), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Molkentin JD, 2010. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122 (25), 2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkelä Risto, Grazette Luanda, Yacobi Rinat, Iliescu Cezar, Patten Richard, Beahm Cara, Walters Brian, et al. , 2006. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med 12 (8), 908–916. [DOI] [PubMed] [Google Scholar]
- Kerscher Petra, Turnbull Irene C., Hodge Alexander J., Kim Joonyul, Dror Seliktar, Easley Christopher J., Costa Kevin D., Lipke Elizabeth A., 2016. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials 83 (March), 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Deok–Ho, Wong Pak Kin, Park Jungyul, Levchenko Andre, Sun Yu, 2009. Microengineered platforms for cell mechanobiology. Annu. Rev. Biomed. Eng 11, 203–233. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Benton JA, Anseth KS, 2010. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobold Sabine, Guhr Anke, Kurtz Andreas, Loser Peter, 2015. Human embryonic and induced pluripotent stem cell research trends: complementation and diversification of the field. Stem Cell Reports 4 (5), 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen Naomi, Little Lauren, Healy Kevin, 2009. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases 4 (4), 69–79. [DOI] [PubMed] [Google Scholar]
- Kohl P, Gourdie RG, 2014. Fibroblast–myocyte electrotonic coupling: does it occur in native cardiac tissue? J. Mol. Cell. Cardiol 70, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Ping, Christia Panagiota, Frangogiannis Nikolaos G., 2014. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci 71 (4), 549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Yen P., Carrion Bita, Singh Rahul K., Putnam AndrewJ., 2013. Matrix identity and tractional forces influence indirect cardiac reprogramming. Sci. Rep 3 (1), 3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll Katharina, Chabria Mamta, Wang Ken, Hoausermann Fabian, Schuler Franz, Polonchuk Liudmila, 2017. Electro–mechanical conditioning of human IPSC–derived cardiomyocytes for translational research. Prog. Biophys. Mol. Biol 130 (November), 212–222. [DOI] [PubMed] [Google Scholar]
- Kujala Ville J., Pasqualini Francesco Silvio, Goss Josue A., Nawroth Janna C., Parker Kevin Kit, 2016. Laminar ventricular myocardium on a microelectrode array–based chip. J. Mater. Chem. B 4 (20), 3534–3543. [DOI] [PubMed] [Google Scholar]
- Laflamme Michael A., Chen Kent Y., Naumova Anna V., Muskheli Veronica, Fugate James A., Dupras Sarah K., Reinecke Hans, et al. , 2007. Cardiomyocytes derived from human embryonic stem cells in pro–survival factors enhance function of infarcted rat hearts. Nat. Biotechnol 25 (9), 1015–1024. [DOI] [PubMed] [Google Scholar]
- Lam Mai T., Longaker Michael T., 2012. Comparison of several attachment methods for human IPS, embryonic and adipose–derived stem cells for tissue engineering. J. Tissue Eng. Regenerative Med 6 (S3) s80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Feng, Lee Andrew S., Liang Ping, Sanchez–Freire Veronica, Nguyen Patricia K., Wang Li, Han Leng, et al. , 2013. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient–specific induced pluripotent stem cells. Cell Stem Cell 12 (1), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila Eeva, Ahola Antti, Hyttinen Jari, Aalto–Setaoloa Katriina, 2016. “Methods for in vitro functional analysis of IPSC derived cardiomyocytes – special focus on analyzing the mechanical beating behavior. Biochim. Biophys. Acta Mol. Cell Res 1863 (7), 1864–1872. [DOI] [PubMed] [Google Scholar]
- Lee Soah, Serpooshan Vahid, Tong Xinming, Venkatraman Sneha, Lee Meelim, Lee Jaecheol, Orlando Chirikian, Wu Joseph C., Wu Sean M., Fan Yang, 2017. Contractile force generation by 3D HiPSC–derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle–mimicking stiffness. Biomaterials 131 (July), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine Marc D., Mannhardt Ingra, Breckwoldt Kaja, Prondzynski Maksymilian, Flenner Frederik, Ulmer Baorbel, Hirt Marc N., et al. , 2017. Human IPSC–derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci. Rep 7 (1), 5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard Andrea, Bertero Alessandro, Powers Joseph D., Beussman Kevin M., Bhandari Shiv, Regnier Michael, Murry Charles E., Sniadecki Nathan J., 2018. Afterload promotes maturation ofhuman induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J. Mol. Cell. Cardiol 118 (May), 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Xiaojun, Zhang Jianhua, Azarin Samira M., Zhu Kexian, Hazeltine Laurie B., Bao Xiaoping, Hsiao Cheston, Kamp TimothyJ., Palecek Sean P., 2013. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating wnt/β–catenin signaling under fully defined conditions. Nat. Protoc 8(1), 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau Brian, Jackman Christopher P., Li Yanzhen, Bursac Nenad, 2017. Developmental stage–dependent effects of cardiac fibroblasts on function of stem cell–derived engineered cardiac tissues. Sci. Rep 7 (1), 42290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind Johan U., Busbee Travis A., Valentine Alexander D., Pasqualini Francesco S., Yuan Hongyan, Moran Yadid, Park Sung–Jin, et al. , 2017a. Instrumented cardiac microphysiological devices via multimaterial three–dimensional printing. Nat. Mater 16 (3). Harvard University, Cambridge, United States: Nature Publishing Group:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind Johan U., Moran Yadid, Perkins Ian, O’Connor Blakely B., Eweje Feyisayo, Chantre Christophe O., Hemphill Matthew A., et al. , 2017b. Cardiac microphysiological devices with flexible thin–film sensors for higher–throughput drug screening. Lab a Chip 17 (21), 3692–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yaowen, Tian Xia, Wei Jiaojun, Liu Qingjie, Li Xiaohong, 2017. Micropatterned Co–culture of cardiac myocytes on fibrous scaffolds for predictive screening of drug cardiotoxicities. Nanoscale 9 (15), 4950–4962. Royal Society of Chemistry. [DOI] [PubMed] [Google Scholar]
- Lockhart Marie, Wirrig Elaine, Phelps Aimee, Wessels Andy, 2011. Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol 91 (6), 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxán G, et al. , 2016. Endocardial notch signaling in cardiac development and disease. Circ. Res 118 (1), e1–e18. [DOI] [PubMed] [Google Scholar]
- Ma Yonggang, Mouton Alan J., Lindsey Merry L., 2018. Cardiac macrophage biology in the steady–state heart, the aging heart, and following myocardial infarction Transl. Res 191,15–28 (January). Mosby. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut Stephanie, Dingal PC, Dave P, Discher Dennis E., 2014. Stress sensitivity and mechanotransduction during heart development. Curr. Biol 24 (10), R495–R501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, et al. , 2011. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 331 (6023), 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon Barbara S., Hamilton Rebecca S., Kozhich Olga A., Johnson Kory R., Fann Yang C., Rao Mahendra S., Robey Pamela G., 2014. Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res. 12 (2), 376–386. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhardt Ingra, Saleem Umber, Benzin Anika, Schulze Thomas, Klampe Birgit, Eschenhagen Thomas, Hansen Arne, 2017. Automated contraction analysis of human engineered heart tissue for cardiac drug safety screening. JoVE 122 (January) e55461–e55461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano Anna, Conficconi Chiara, Lemme Marta, Occhetta Paola, Gaudiello Emanuele, Votta Emiliano, Cerino Giulia, Redaelli Alberto, Rasponi Marco, 2016. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab a Chip 16 (3), 599–610. [DOI] [PubMed] [Google Scholar]
- Masters M, Riley PR, 2014. The epicardium signals the way towards heart regeneration. Stem Cell Res. 13 (3), 683–692. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur Anurag, Loskill Peter, Shao Kaifeng, Huebsch Nathaniel, Hong SoonGweon, Marcus Sivan G., Marks Natalie, et al. , 2015. Human IPSC–based cardiac microphysiological system for drug screening applications. Sci. Rep 5 (1), 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur Anurag, Ma Zhen, Loskill Peter, Jeeawoody Shaheen, Healy Kevin E., 2016. In vitro cardiac tissue models: current status and future prospects. Adv. Drug Deliv. Rev 96 (January), 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain ML, Lee H, Aratyn–Schaus Y, Kleber AG, Parker KK, 2012. Cooperative coupling of cell–matrix and cell–cell adhesions in cardiac muscle. Proc. Natl. Acad. Sci. Unit. States Am 109 (25), 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK, 2013. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl. Acad. Sci. U. S. A 110 (24), 9770–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL, 2010. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56 (2), 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani–Nejad Nima, Janssen Paul M.L., 2014. “Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther 141 (3), 235–249. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RichardJ., Titmarsh Drew M., Koenig Xaver, Parker Benjamin L., Ryall James G., Quaife–Ryan Gregory A., Voges Holly K., et al. , 2017. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. U. S. A 114 (40), E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller Jens, Denisin Aleksandra K., Sim Joo Yong, Wilson Robin E., Ribeiro Alexandre J.S., Pruitt Beth L., 2018. “Controlling cell shape on hydrogels using lift–off protein patterning.” edited by nic D. Leipzig. PLoS One 13 (1) e0189901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Kovács Á, Bódi B, Pásztor ET, Fülöp GÁ, Tóth A, Édes I, Papp Z, 2015. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br. J. Pharmacol 172 (18), 4506–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Doan C., Hookway Tracy A., Wu Qingling, Jha Rajneesh, Preininger Marcela K., Chen Xuemin, Easley Charles A., et al. , 2014. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Reports 3 (2), 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Sara S., Miklas Jason W., Liu Jie, Aschar–Sobbi Roozbeh, Xiao Yun, Zhang Boyang, Jiang Jiahua, et al. , 2013. “Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 10 (8), 781–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle Brenda M., Bursac Nenad, Domian Ibrahim, Huang Ngan F., Menascheo Philippe, Murry Charles E., Pruitt Beth, et al. , 2016. Distilling complexity to advance cardiac tissue engineering. Sci. Transl. Med 8 (342), 342ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Lai NC, Kawaraguchi Y, Liao P, Copps J, Sugano Y, Okada–Maeda S, et al. , 2013. Integrins protect cardiomyocytes from ischemia/reperfusion injury. J. Clin. Invest 123 (10), 4294–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozhan Gunes, Weidinger Gilbert, 2015. Wnt/β–Catenin signaling in heart regeneration. Cell Regen. 4 (1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchesko Rachelle N., Zhang Ling, Sun Yan, Feinberg Adam W., 2012. “Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve.” edited by nuno M neves. PLoS One 7 (12) e51499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran Sreejit, Kumar Sujeet, Verma Rama Shanker, Rajendra K, Sharma, 2013. “Cardiomyocyte culture – an update on the in vitro cardiovascular model and future challenges. Can. J. Physiol. Pharmacol 91 (12), 985–998. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW, 1983. Vinculin is a component of an extensive network of myofibril–sarcolemma attachment regions in cardiac muscle fibers. J. Cell Biol 97 (4), 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker Kevin Kit, Tan John, Chen Christopher S., Tung Leslie, 2008. Myofibrillar architecture in engineered cardiac myocytes. Circ. Res 103 (4), 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini Francesco Silvio, Sheehy Paul, Sean Agarwal, Ashutosh Aratyn–Schaus, Yvonne Parker, Kit Kevin, 2015. Structural phenotyping of stem cell–derived cardiomyocytes. Stem Cell Reports 4 (3), 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Alexander R., Ilinykh Alexei, Ivey Malina J., Kuwabara Jill T., D’antoni Michelle L., Ryan Debuque, Chandran Anjana, et al. , 2016. Revisiting cardiac cellular composition. Circ. Res 118 (3), 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pislaru Cristina, Urban Matthew W., Pislaru Sorin V., Kinnick Randall R., Greenleaf James F., 2014. Viscoelastic properties of normal and infarcted myocardium measured by a multifrequency shear wave method: comparison with pressure–segment length method. Ultrasound Med. Biol 40 (8), 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles–Herrero Vicente J., Hartman James J., Robert–Paganin Julien, Malik Fady I., Houdusse Anne, 2017. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat. Commun 8 (1), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess Marlene, Ehler Elisabeth, 2015. Cardiac cytoarchitecture in Health and disease. Cardiac Cytoarchitecture 1–14. [Google Scholar]
- Povsic Thomas J., Scott Rob, Mahaffey Kenneth W., Blaustein Robert, Edelberg Jay M., Lefkowitz Martin P., Solomon Scott D., et al. , 2017. Navigating the future of cardiovascular drug development–leveraging novel approaches to drive innovation and drug discovery: summary of findings from the novel cardiovascular therapeutics conference. Cardiovasc. Drugs Ther 31 (4), 445–458. [DOI] [PubMed] [Google Scholar]
- Psarra Evmorfia, Foster Elena, Konig Ulla, You Jungmok, Ueda Yuichiro, Eichhorn Klaus–J., Müller Martin, Stamm Manfred, Alexander Revzin, Uhlmann Petra, 2015. “Growth factor–bearing polymer brushes – versatile bioactive substrates influencing cell response. Biomacromolecules 16 (11), 3530–3542. American Chemical Society. [DOI] [PubMed] [Google Scholar]
- Qian Fang, Huang Chao, Lin Yi–Dong, Ivanovskaya Anna N., O’Hara Thomas J., Booth Ross H., Creek Cameron J., et al. , 2017. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab a Chip 17 (10), 1732–1739. [DOI] [PubMed] [Google Scholar]
- Rangappa Sunil, Fen Chen, Lee Eng Hin, Bongso Ariff, Wei Eugene Sim Kwang, 2003. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg 75 (3), 775–779. [DOI] [PubMed] [Google Scholar]
- Ribeiro Alexandre J.S., Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava Deepak, Pruitt Beth L., 2015. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. U. S. A 112 (41), 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Alexandre J.S., Schwab Olivier, Mandegar Mohammad A., Ang Yen–Sin, Conklin Bruce R., Srivastava Deepak, Pruitt Beth L., 2017. Multi–imaging method to assay the contractile mechanical output of micropatterned human IPSC–derived cardiac myocytes. Circ. Res 120 (10), 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Dylan J., Tan Yu, Coyle Robert, Yang Li, Xu Ruoyu, Yeung Nelson, Parker Arran, Menick Donald R., Tian Bozhi, Mei Ying, 2016. Nanowires and electrical stimulation synergistically improve functions of HiPSC cardiac spheroids. Nano Lett. 16 (7), 4670–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler Johannes, Tiburcy Malte, Ebert Antje, Tzatzalos Evangeline, Raaz Uwe, Abilez Oscar J., Shen Qi, et al. , 2015. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res 117 (8), 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienks Marieke, Papageorgiou Anna–Pia, Frangogiannis Nikolaos G., Heymans Stephane, 2014. Myocardial extracellular matrix: an ever–changing and diverse entity. Circ. Res 114 (5), 872–888. [DOI] [PubMed] [Google Scholar]
- Rodriguez Marita L., Graham Brandon T., Pabon Lil M., Han Sangyoon J., Murry Charles E., Sniadecki Nathan J., 2014. Measuring the contractile forces of human induced pluripotent stem cell–derived cardiomyocytes with arrays of microposts. J. Biomech. Eng 136, 051005–051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson–Bouchard Kacey, Ma Stephen P., Yeager Keith, Chen Timothy, Song LouJin, Sirabella Dario, Morikawa Kumi, Teles Diogo, Yazawa Masayuki, Vunjak–Novakovic Gordana, 2018. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556 (7700), 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, 2004. Molecular and mechanical synergy: cross–talk between integrins and growth factor receptors. Cardiovasc. Res 63 (3), 381–390. [DOI] [PubMed] [Google Scholar]
- Ruan Jia–Ling, Tulloch Nathaniel L., Razumova Maria V., Saiget Mark, Muskheli Veronica, Pabon Lil, Reinecke Hans, Regnier Michael, Murry Charles E., 2016. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell–derived human cardiac tissue. Circulation 134 (20), 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, et al. , 2016. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ. Res 119 (7), 853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Tsuchida M, Lampe PD, Murakami M, 2013. Cardiomyocyte FGF signaling is required for Cx43 phosphorylation and cardiac gap junction maintenance. Exp. Cell Res 319 (14), 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala Luca, Yu Zhiyi, Oostwaard Ward–van, van Dorien Veldhoven, Jacobus Pd, Moretti Alessandra, Laugwitz Karl–Ludwig, Mummery Christine L., Jzerman PI, Adriaan Bellin, Milena, 2016. A new HERG allosteric modulator rescues genetic and drug–induced long–QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol. Med 8 (9). EMBO Press:1065–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama Guy, London Barry, 2007. Mouse models of long QT syndrome. J. Physiol 578 (1), 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarel Allen M., 2005. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart Circ. Physiol 289 (6), H2291–H2301. [DOI] [PubMed] [Google Scholar]
- Sanguinetti Michael C., Mitcheson John S., 2005. “Predicting drug–hERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol. Sci 26 (3). Elsevier Current Trends:119–24. [DOI] [PubMed] [Google Scholar]
- Sayed Nazish, Liu Chun, Wu Joseph C., 2016. Translation of human–induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J. Am. Coll. Cardiol 67 (18), 2161–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiwe Andrea C., Frank Stephanie C., Autenrieth Tatjana J., Bastmeyer Martin, Wegener Martin, 2015. Subcellular stretch–induced cytoskeletal response of single fibroblasts within 3D designer scaffolds. Biomaterials 44 (March), 186–194. [DOI] [PubMed] [Google Scholar]
- Schenke–Layland Katja, Ali Nsair, Handel Ben Van, Angelis Ekaterini, M Gluck Jessica, Votteler Miriam, Goldhaber Joshua I., Mikkola Hanna K., Kahn Michael, MacLellan William R., 2011. Recapitulation of the embryonic cardiovascular progenitor cell niche. Biomaterials 32 (11), 2748–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer AK, Merryman WD, 2015. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J. Cell Sci 128, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer Alison K., Ryzhova LM, Merryman WD, 2014. Network modeling approach to predict myofibroblast differentiation. Cell. Mol. Bioeng 7 (3), 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan Jonas, Kwaczala Andrea T., Ryan Thomas J., Bartulos Oscar, Ren Yongming, Sewanan Lorenzo R., Morris Aaron H., Jacoby Daniel L., Qyang Yibing, Campbell Stuart G., 2016. Anisotropic engineered heart tissue made from laser–cut decellularized myocardium. Sci. Rep 6 (1), 32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura Ana Maria, Frazier OH, Maximilian Buja L, 2014. Fibrosis and heart failure. Heart Fail. Rev 19 (2), 173–185. [DOI] [PubMed] [Google Scholar]
- Sheehy Sean P., Grosberg Anna, Pu Qin, Behm David J., Ferrier John P., Eagleson Mackenzie A., Nesmith Alexander P., et al. , 2017. Toward improved myocardial maturity in an organ–on–chip platform with immature cardiac myocytes. Exp. Biol. Med 242 (17), 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Grosberg A, Nawroth JC, Parker KK, Bertoldi K, 2012. Modeling of cardiac muscle thin films: pre–stretch, passive and active behavior. J. Biomech 45 (5), 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov Veniamin Y., Samson Philip C., Sidorova Tatiana N., Davidson Jeffrey M., Lim Chee C., Wikswo John P., 2017. I–wire heart–on–a–chip I: three–dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. 48 (January), 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DG, Terracio L, Terracio M, Price RL, Turner DC, Borg TK, 1994. Modulation of cardiac myocyte phenotype in vitro by the composition and orientation of the extracellular matrix. J. Cell. Physiol 161 (1), 89–105. Wiley–Blackwell. [DOI] [PubMed] [Google Scholar]
- Singelyn Jennifer M., Christman Karen L., 2011. Modulation of material properties of a decellularized myocardial matrix scaffold. Macromol. Biosci 11 (6), 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H–H Greco, Rumma Rowza T., Keith Ozaki C, Edelman Elazer R., Chen Christopher S., 2018. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell 22 (3), 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Später Daniela, Hansson Emil M., Zangi Lior, Chien Kenneth R., 2014. How to make a cardiomyocyte. Development (Cambridge, England) 141 (23), 4418–4431. [DOI] [PubMed] [Google Scholar]
- Spencer C, Ian Baba, Shiro Nakamura, Kenta Hua, Ethan A, Sears arie A.F., Fu Chi–Cheng, Zhang Jianhua, et al. , 2014. Calcium transients closely reflect prolonged action potentials in IPSC models of inherited cardiac arrhythmia. Stem Cell Reports 3 (2), 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA, 2014. Hypertrophic and dilated cardiomyopathy: four decades ofbasic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys. J 106 (6), 1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancescu Maria, Molnar Peter, McAleer Christopher W., McLamb William, Long Christopher J., Oleaga Carlota, Prot Jean–Matthieu, Hickman James J., 2015. A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials 60 (August), 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yazawa M, Liu J, Han L, Sanchez–Freire V, Abilez OJ, Navarrete EG, et al. , 2012. Patient–specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med 4 (130), 130ra47–130ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takawale Abhijit, Siva S, Sakamuri VP, Kassiri Zamaneh, 2015. Extracellular matrix communication and turnover in cardiac physiology and pathology. Comprehensive Physiol. 5, 687–719. [DOI] [PubMed] [Google Scholar]
- Tan John L., Tien Joe, Pirone Dana M., Gray Darren S., Bhadriraju Kiran, Chen Christopher S., 2003. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. Unit. States Am 100 (4), 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon Nina, Cannizzaro Christopher, Chao Pen–Hsiu Grace, Maidhof Robert, Anna Marsano, Au Hoi Ting Heidi, Radisic Milica, Vunjak–Novakovic Gordana, 2009. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc 4 (2), 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff Jil C., Carrier Lucie, Bers Donald M., Poggesi Corrado, Ferrantini Cecilia, Coppini Raffaele, Maier Lars S., Ashrafian Houman, Huke Sabine, van der Velden Jolanda, 2015. Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc. Res 105 (4), 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm Terra N., Squirrell Jayne M., Liu Yuming, Eliceiri Kevin W., Ogle Brenda M., 2015. Endogenous optical signals reveal changes of elastin and collagen organization during differentiation of mouse embryonic stem cells. Tissue Eng. C Methods 21 (10), 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcy Malte, Hudson James E., Paul Balfanz, Schlick Susanne, Meyer Tim, Liao Mei–Ling Chang, Levent Elif, et al. , 2017. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and RepairClinical perspective. Circulation 135 (19), 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Matsushima S, Kinugawa S, Ide T, Inoue N, Ohta Y, Yokota T, Hamaguchi S, Sunagawa K, 2007. Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Hypertens. Res 30 (5), 439–449. [DOI] [PubMed] [Google Scholar]
- Tzatzalos Evangeline, Abilez Oscar J., Shukla Praveen, Wu Joseph C., 2016. Engineered heart tissues and induced pluripotent stem cells: macro– and microstructures for disease modeling, drug screening, and translational studies. Adv. Drug Deliv. Rev 96 (January), 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer Bärbel M., Stoehr Andrea, Schulze Mirja L., Patel Sajni, Gucek Marjan, Mannhardt Ingra, Funcke Sandra, Murphy Elizabeth, Eschenhagen Thomas, Hansen Arne, 2018. Stem cell reports article contractile work contributes to maturation of energy metabolism in HiPSC–derived cardiomyocytes. Stem Cell Reports 10 (3), 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanwinkle W, Barry Snuggs, Mark B, Maximilian Buja L, 1996. Cardiogel: a biosynthetic extracellular matrix for cardiomyocyte culture. Vitro Anim. Cell Dev. Biol. 32 (8), 478–485. [DOI] [PubMed] [Google Scholar]
- Vasquez C, Benamer N, Morley GE, 2011. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J. Cardiovasc. Pharmacol 57 (4), 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining Kyle H., Mooney David J., 2017. “Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol 18 (12), 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak–Novakovic Gordana, 2017. Tissue engineering of the heart: an evolving paradigm. J. Thorac. Cardiovasc. Surg 153 (3), 593–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Gang, McCain Megan L., Yang Luhan, He Aibin, Pasqualini Francesco Silvio, Agarwal Ashutosh, Yuan Hongyan, et al. , 2014. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart–on–chip technologies. Nat. Med 20 (6), 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Zhijie, Golob Mark J., Chesler Naomi C., 2016. Viscoelastic properties of cardiovascular tissues. Viscoelastic Viscoplastic Mater. 10.5772/64169. [DOI] [Google Scholar]
- Wanjare Maureen, Hou Luqia, Nakayama Karina H., Kim Joseph J., Mezak Nicholas P., Abilez Oscar J., Tzatzalos Evangeline, Wu Joseph C., Huang Ngan F., 2017. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci 5 (8), 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI, 1988. Collagen remodeling of the pressure–overloaded, hypertrophied nonhuman primate myocardium. Circ. Res 62 (4), 757–765. [DOI] [PubMed] [Google Scholar]
- Weiss Allison, Leinwand Leslie A., 1996. The mammalian myosin heavy chain gene family. Annu. Rev. Cell Dev. Biol 12 (1), 417–439. [DOI] [PubMed] [Google Scholar]
- Wenk JF, Sun K, Zhang ZH, Soleimani M, Ge LA, Saloner D, Wallace AW, Ratcliffe MB, Guccione JM, 2011. Regional left ventricular myocardial contractility and stress in a finite element model of posterobasal myocardial infarction. J. Biomech. Eng.–Trans. Asme 133 (4), 044501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdich Andreas A., Lima Eduardo A., Ivanov Borislav, Ges Igor, Anderson Mark E., Wikswo John P., Baudenbacher Franz J., 2004. A microfluidic device to confine a single cardiac myocyte in a sub–nanoliter volume on planar microelectrodes for extracellular potential recordings. Lab a Chip 4 (4), 357–362. [DOI] [PubMed] [Google Scholar]
- Wheelwright Matthew, Zaw Win, Mikkila Jennifer L., Amen Kamilah Y., Alford Patrick W., Metzger Joseph M., 2018. “Investigation of human IPSC–derived cardiac myocyte functional maturation by single cell traction force microscopy.” edited by katriina aalto–setala. PLoS One 13 (4) e0194909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Corin, Quinn Kyle P., Georgakoudi Irene, Iii Black, Lauren D, 2014. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 10 (1), 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyles Saranya P., Xing Li, Hrstka Sybil C., Reyes Santiago, Oommen Saji, Beraldi Rosanna, Edwards Jessica, Andre Terzic, Olson Timothy M., Nelson Timothy J., 2016. Modeling structural and functional deficiencies of RBM20 familial dilated cardiomyopathy using human induced pluripotent stem cells. Hum. Mol. Genet 25 (2), 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pabon L, Murry CE, 2014. Engineering adolescence: maturation of human pluripotent stem cell–derived cardiomyocytes. Circ. Res 114 (3), 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Jiang, Varner Victor D., Brilli Lauren L., Young Jonathan M., Taber Larry A., Perucchio Renato, 2012. Viscoelastic material properties of the myocardium and cardiac Jelly in the looping chick heart. J. Biomech. Eng 134 (2). American Society of Mechanical Engineers:024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Hongyan, Marzban Bahador, Parker Kevin Kit, 2017. Myofibrils in cardiomyocytes tend to assemble along the maximal principle stress directions. J. Biomech. Eng 139 (12), 121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak R, 1973. Cell proliferation during cardiac growth. Am. J. Cardiol 31 (2), 211–219. Excerpta Medica. [DOI] [PubMed] [Google Scholar]
- Zhang Boyang, Radisic Milica, 2017. Organ–on–a–Chip devices advance to market. Lab a Chip 17 (14), 2395–2420. [DOI] [PubMed] [Google Scholar]
- Zhang Jianhua, Wilson Gisela F., Soerens Andrew G., Koonce Chad H., Yu Junying, Palecek Sean P., Thomson James A., Kamp Timothy J., 2009. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res 104 (4) e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Pingzhu, Pu William T., 2016. Recounting cardiac cellular composition. Circ. Res 118 (3), 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlutuna Pinar, Vrana Nihal Engin, Khademhosseini Ali, 2013. The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs. IEEE Rev. Biomed. Eng 6 (January), 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]