Supplemental Digital Content is available in the text
Keywords: AIDS, highly active antiretroviral therapy, HIV, mortality
Abstract
Background:
UNAIDS models use data from the International epidemiology Databases to Evaluate AIDS (IeDEA) collaboration in setting assumptions about mortality rates after antiretroviral treatment (ART) initiation. This study aims to update these assumptions with new data, to quantify the extent of regional variation in ART mortality and to assess trends in ART mortality.
Methods:
Adult ART patients from Africa, Asia and the Americas were included if they had a known date of ART initiation during 2001–2017 and a baseline CD4+ cell count. In cohorts that relied only on passive follow-up (no patient tracing or linkage to vital registration systems), mortality outcomes were imputed in patients lost to follow-up based on a meta-analysis of tracing study data. Poisson regression models were fitted to the mortality data.
Results:
464 048 ART patients were included. In multivariable analysis, mortality rates were lowest in Asia and highest in Africa, with no significant differences between African regions. Adjusted mortality rates varied significantly between programmes within regions. Mortality rates in the first 12 months after ART initiation were significantly higher during 2001–2006 than during 2010–2014, although the difference was more substantial in Asia and the Americas [adjusted incidence rate ratio (aIRR) 1.43, 95% CI: 1.22–1.66] than in Africa (aIRR 1.07, 95% CI: 1.04–1.11).
Conclusion:
There is substantial variation in ART mortality between and within regions, even after controlling for differences in mortality by age, sex, baseline CD4 category and calendar period. ART mortality rates have declined substantially over time, although declines have been slower in Africa.
Introduction
Antiretroviral treatment (ART) is estimated to have reduced the number of AIDS deaths globally by 6.6 million in the period up to 2013 [1], and in many of the countries most severely affected by the HIV epidemic, ART has had a profound demographic impact [2,3]. However, HIV case surveillance and vital registration systems are often weak or absent in the most severely affected countries, necessitating mathematical models to estimate AIDS mortality and the impact of ART. The Joint United Nations Programme on HIV/AIDS (UNAIDS) supports the development and application of the Spectrum tool for this purpose [4]; this tool is also widely used to estimate other HIV indicators and to evaluate the impact and cost-effectiveness of different interventions [5].
AIDS mortality estimates produced by Spectrum and other mathematical models depend on assumptions about mortality rates after ART initiation. UNAIDS has previously relied on the International epidemiology Databases to Evaluate AIDS (IeDEA) Collaboration to provide estimates of mortality of patients receiving ART in different regions to parameterize the Spectrum model [6,7]. Previous IeDEA analyses to estimate Spectrum parameters incorporated mortality data from patients starting ART in the pre-2011 [7] and 2009–2014 [6] periods. These analyses were limited in their ability to estimate mortality of patients starting ART at higher CD4 cell counts, as such patients only became eligible for ART in low-income and middle-income countries after the WHO recommended universal ART eligibility in 2015 [8]. The analyses conducted for UNAIDS have also not assessed whether there have been temporal changes in ART mortality (after controlling for differences in baseline CD4 cell count and ART duration), although some studies suggest that ART mortality rates have declined significantly [9–11]. A further concern is that previous analyses applied multiplicative ‘correction factors’ to observed mortality rates to correct for under-ascertainment of mortality in patients lost to follow-up (LTFU), but these correction factors might not have adequately reflected heterogeneity in ascertainment of mortality across settings, as they were based only on data from South Africa and Kenya.
This study aims to update adult ART mortality estimates for the Spectrum model, incorporating more recent IeDEA data (up to 2017) and advancing the mortality estimation methods. This study also aims to assess trends in adult ART mortality, to quantify mortality levels since introduction of universal ART eligibility, to evaluate the extent of inter-regional variation in mortality, and to assess whether inter-regional differences are explained by known predictors of mortality.
Methods
IeDEA is an international collaboration of ART programmes, divided into seven regions: Asia-Pacific; Central America, South America and the Caribbean (hereafter ‘Latin America’); North America; Central Africa; East Africa; Southern Africa and West Africa [12,13]. Participating programmes are embedded in routine care services, with frequencies of scheduled clinic visits varying across programmes, in line with local guidelines. Each region has an independent data centre that pools, de-identifies and analyses the data. Each programme participating in this study obtained ethical approval from relevant local institutions to collect and share patient data; in addition, each regional data centre obtained ethical approval to collate and analyse the de-identified data.
The analysis included adults (aged 15 years and older) who started ART between 2001 and 2017 and were ART-naïve at enrolment (or who had a date of first ART initiation if not ART-naïve), and who had a recorded CD4 cell count in the six months prior to starting ART or two weeks after starting ART. ART was defined as a combination of at least three antiretroviral drugs. Consistent with previous UNAIDS analyses [6,7], we defined baseline CD4 cell count as the CD4 cell count closest to the time of ART initiation and we defined patients as LTFU if their last visit date was more than six months prior to the database closure without any record of other outcomes (death or transfer). To estimate parameters in the Spectrum model, we used the same covariate categories as the Spectrum model: baseline CD4 cell count was categorized as 0–49, 50–99, 100–199, 200–249, 250–349, 350–499 or ≥500 cells/μl; time since ART initiation (‘duration’) was categorized as 0–5 months, 6–11 months or ≥12 months; and age was categorized as 15–24, 25–34, 35–44 or ≥45 years. We conducted sensitivity analyses to assess the effect of including patients with missing baseline CD4 values, using multiple imputation to assign baseline CD4 cell counts [14], and to assess the effect of modelling age effects using restricted cubic splines rather than a categorical approach [15]. Except in the case of sub-Saharan Africa (discussed below), follow-up time was defined from the date of ART start (or date of enrolment if later) to the date of death or the last date that the patient was known to be alive. The resulting mortality estimates are referred to as ‘ART mortality rates’ although some patients might not have been on ART at the time of death.
Because most patients starting ART at higher CD4 cell counts prior to 2015 were doing so because they met clinical rather than immunological ART eligibility criteria, and because such patients are not representative of asymptomatic individuals with higher CD4 cell counts, we excluded individuals who qualified for ART based solely on clinical criteria. In the Latin American and North American programmes, as well as in some Asian programmes, this meant excluding patients who had a clinical diagnosis of AIDS prior to starting ART, if they started ART with a CD4 cell count above the CD4 eligibility threshold that applied at the time. In the sub-Saharan African programmes and some Asian programmes, in which the recording of baseline clinical stage was incomplete, patients were excluded if their baseline CD4 cell count was above the CD4 eligibility threshold at the time of starting ART (on the assumption that they qualified for ART based only on clinical eligibility criteria). Details of the eligibility criteria that applied in each country in each year were obtained from previous reviews of ART eligibility criteria [16–19] and from consultation with local experts; full details are provided in Supplementary Table S1. A sensitivity analysis was conducted to assess the effect of not applying these exclusion criteria.
Programmes differed in their approach to ascertaining mortality. In the South African programmes and most North and Latin American programmes, patient records were regularly linked to vital registration systems, which were judged to have high completeness [20–22]. In some North and Latin American programmes, and most Asian programmes, attempts were made to contact patients who missed visits, either by telephone or by home visits (‘active follow-up’) [9,23,24], and this was also judged to yield reasonably accurate estimates of mortality. However, in most sub-Saharan African programmes, mortality was ascertained passively (no active follow-up or linkage to vital registration systems), and the high proportion of patients LTFU suggested substantial unrecorded mortality [25]. To address this problem, we used an imputation model to simulate mortality outcomes in the six months after LTFU in sub-Saharan African programmes (excluding South Africa) [26]. Suppose Ti is the time to death (in days after the last visit) for patient i who is classified LTFU. We assume Ti is Weibull-distributed with parameters λi and ϕ, where λi = xiβ (xi representing the covariate information for individual i and β representing the covariate effects on the mortality rate). We chose the Weibull distribution over the more flexible generalized gamma distribution as the Weibull distribution is easier to invert, which is important for simulation purposes. We estimated the ϕ and β coefficients by fitting the Weibull model to mortality data from an individual patient data meta-analysis of studies that traced LTFU patients in African ART programmes [27], augmented by more recent data from a large Zambian tracing study [28]. For each individual who was LTFU, we sampled a value ti from the Weibull distribution for Ti. If ti was 180 days or more, follow-up was censored at the last visit date + 180 days; otherwise the date of death was set to the last visit date + ti. For each LTFU patient, we simulated five outcomes, thus generating five datasets for the sub-Saharan African cohorts that we combined using Rubin's rules [14]. We limited the simulation of mortality outcomes to the first six months after last contact due to the limited numbers of deaths at longer tracing durations and the resulting uncertainty in the extrapolation of the Weibull hazard to longer durations.
Consistent with previous UNAIDS analyses [6,7], we used Poisson regression models to estimate average mortality rates for each covariate category in Spectrum. We fitted separate regression models for the first 12 months after ART initiation and for durations more than 12 months after ART initiation, to account for non-proportional hazards. We also ran separate regression models for the African and non-African regions, due to differences between generalized and concentrated HIV epidemic settings in both overall levels of mortality as well as sociodemographic determinants of mortality. We included region-specific effects in each model, and used random effect models (with random intercepts) to account for variation across programmes within each region. The ratio of mortality at the 90th percentile of random effects to that at the 10th percentile of random effects was calculated to demonstrate the extent of inter-programme variation in mortality within regions, assuming that random effects were normally distributed [29]. We treated South Africa as a separate ‘region’ because of the difference in mortality ascertainment in this country (relative to the rest of sub-Saharan Africa) and because of the large number of ART patients in this country. The West and Central African regions were combined due to the small numbers of patients in Central Africa, but in a sensitivity analysis we included separate effects for Central Africa. All statistical analyses were performed using STATA 15.1 (StataCorp, College Station, TX, USA).
In the Spectrum model, IeDEA estimates of all-cause mortality in ART patients were converted into estimates of HIV-related mortality by subtracting United Nations Population Division estimates of non-HIV mortality in each region [30] (more detail is provided in Supplementary material Section 2). In the results that follow, we present only the IeDEA estimates of all-cause mortality.
Results
846 418 adults started ART in 2001–2017, of whom we excluded 271 959 (32%) due to missing baseline CD4 values, 12 541 (1%) due to missing or invalid outcome dates, and 97 870 (12%) who qualified for ART solely on clinical criteria. Our main analysis therefore included 464 048 ART patients, from 72 different IeDEA programmes, the majority from sub-Saharan Africa (408 131, 88%). Demographic characteristics differed substantially across regions: the proportion of patients who were female ranged from 16% in North America to 65% in West and Central Africa, while the proportion of patients aged 45 years or older at the time of ART initiation ranged between 17% in Southern Africa and 41% in North America (Table 1). Baseline immunological status also differed substantially across regions, with the percentage starting ART at a CD4 cell count <200 cells/μl ranging between 33% in North America and 78% in South Africa. In the North American cohort a substantial fraction of patients (12%) acquired HIV following needle sharing.
Table 1.
Patient characteristics at antiretroviral treatment initiation, 2001–2017.
| Asia-Pacific | Latin America | North America | East Africa | South Africa (RSA) | Southern Africa (excluding RSA) | West and Central Africa | |
| No. of patients | 16 097 | 15 760 | 24 060 | 108 936 | 72 997 | 183 675 | 42 523 |
| Sex | |||||||
| Male | 11 376 (70.7%) | 9404 (59.7%) | 20 274 (84.3%) | 41 138 (37.8%) | 26 169 (35.9%) | 73 794 (40.2%) | 14 871 (35.0%) |
| Female | 4721 (29.3%) | 6356 (40.3%) | 3786 (15.7%) | 67 798 (62.2%) | 46 828 (64.2%) | 109 881 (59.8%) | 27 652 (65.0%) |
| Age (years) | |||||||
| 15–24 | 966 (6%) | 1975 (12.5%) | 2067 (8.6%) | 10 995 (10.1%) | 6227 (8.5%) | 19 639 (10.7%) | 2707 (6.4%) |
| 25–34 | 5821 (36.2%) | 5701 (36.2%) | 5800 (24.1%) | 40 135 (36.8%) | 29 961 (41.0%) | 73 007 (39.8%) | 14 877 (35.0%) |
| 35–44 | 5786 (35.9%) | 4525 (28.7%) | 6352 (26.4%) | 35 740 (32.8%) | 23 327 (32.0%) | 59 721 (32.5%) | 15 107 (35.5%) |
| 45+ | 3524 (21.9%) | 3559 (22.6%) | 9841 (40.9%) | 22 066 (20.3%) | 13 482 (18.5%) | 31 308 (17.1%) | 9832 (23.1%) |
| Period of ART start | |||||||
| 2001–2007 | 4196 (26.1%) | 2215 (14.1%) | 5244 (21.8%) | 24 310 (22.3%) | 21 203 (29.1%) | 23 874 (13%) | 11 319 (26.6%) |
| 2008–2010 | 4144 (25.7%) | 4502 (28.6%) | 8464 (35.2%) | 22 806 (20.9%) | 19 522 (26.8%) | 46 920 (25.5%) | 12 323 (29.0%) |
| 2011–2013 | 4270 (26.5%) | 5625 (35.7%) | 8017 (33.3%) | 36 006 (33.1%) | 19 869 (27.2%) | 54 477 (29.7%) | 12 486 (29.4%) |
| 2014–2017 | 3487 (21.7%) | 3418 (21.7%) | 2335 (9.7%) | 25 814 (23.7%) | 12 403 (17.0%) | 58 404 (31.8%) | 6395 (15.0%) |
| Baseline CD4+ cell count (cells/μl) | |||||||
| 0–49 | 4106 (25.5%) | 2889 (18.3%) | 2852 (11.9%) | 22 565 (20.7%) | 15 603 (21.4%) | 23 927 (13.0%) | 9817 (23.1%) |
| 50–99 | 2844 (17.7%) | 2016 (12.8%) | 1665 (6.9%) | 17 504 (16.1%) | 13 196 (18.1%) | 28 720 (15.6%) | 7562 (17.8%) |
| 100–199 | 4623 (28.7%) | 3431 (21.8%) | 3407 (14.2%) | 35 500 (32.6%) | 28 292 (38.8%) | 61 059 (33.2%) | 15 303 (36.0%) |
| 200–249 | 1070 (6.7%) | 1474 (9.4%) | 2068 (8.6%) | 7177 (6.6%) | 4306 (5.9%) | 17 244 (9.4%) | 3137 (7.4%) |
| 250–349 | 2190 (13.6%) | 3069 (19.5%) | 4701 (19.5%) | 15 796 (14.5%) | 8231 (11.3%) | 32 622 (17.8%) | 5495 (12.9%) |
| 350–499 | 984 (6.1%) | 1700 (10.8%) | 4657 (19.4%) | 8398 (7.7%) | 2436 (3.3%) | 15 637 (8.5%) | 865 (2.0%) |
| 500+ | 280 (1.7%) | 1181 (7.5%) | 4710 (19.6%) | 1996 (1.8%) | 933 (1.3%) | 4466 (2.4%) | 344 (0.8%) |
| Mode of infection | |||||||
| Needle sharing | 452 (2.8%) | 22 (0.1%) | 2946 (12.2%) | – | – | – | – |
| Other | 15 645 (97.2%) | 15 738 (99.9%) | 21 114 (87.8%) | – | – | – | – |
ART, antiretroviral treatment; RSA, Republic of South Africa.
The imputation model of mortality after LTFU in African tracing studies was based on 4751 adult patients for whom a vital status could be established (characteristics of the tracing studies and traced patients are included in Supplementary Tables S10 and S11, respectively). Of these, 77% were from Southern Africa, 18% were from East Africa and 5% were from Central Africa. The results of the Weibull regression model are shown in Table 2. Mortality risk among patients LTFU was significantly associated with older age, lower baseline CD4 cell count and shorter ART duration, but the effects of year and sex were not statistically significant. The Weibull shape parameter was 0.60 (95% CI: 0.57–0.63), indicating a strong negative relationship between time since LTFU and the mortality rate. The imputation model estimated that 57% of all deaths on ART in sub-Saharan Africa (excluding South Africa) occurred after LTFU, with this proportion increasing from 41% in 2001–2006 to 48% in 2007–2010, 58% in 2011–2014 and 77% in 2015–2017.
Table 2.
Imputation model: predictors of mortality in first 6 months after loss to follow-up.
| aHR (95% CI) | |
| Sex | |
| Male | 1.00 |
| Female | 1.05 (0.93–1.19) |
| Age at ART initiation (years) | |
| 15–24 | 1.00 |
| 25–34 | 1.23 (0.96–1.57) |
| 35–44 | 1.53 (1.19–1.96) |
| 45+ | 1.91 (1.48–2.48) |
| Time since ART initiation at LTFU (months) | |
| 0–5 | 1.00 |
| 6–11 | 0.51 (0.43–0.60) |
| 12–23 | 0.36 (0.29–0.43) |
| 24–35 | 0.39 (0.29–0.52) |
| 36+ | 0.34 (0.24–0.49) |
| CD4+ cell count at ART initiation (cells/μl) | |
| 0–49 | 1.00 |
| 50–99 | 0.71 (0.61–0.84) |
| 100–199 | 0.47 (0.40–0.55) |
| 200–249 | 0.39 (0.31–0.49) |
| 250–349 | 0.35 (0.28–0.44) |
| 350–499 | 0.33 (0.23–0.46) |
| 500+ | 0.22 (0.14–0.35) |
| Per year after 2000 (at ART initiation) | 0.97 (0.91–1.02) |
| Parameter (95% CI) | |
| Weibull scale parameter | 0.054 |
| Weibull shape parameter (ϕ) | 0.60 (0.57–0.63) |
The model was fitted to data from 4751 adult patients traced after LTFU, in whom a vital status could be established. The model controlled for differences in mortality rates between programmes (results not shown). The Weibull scale parameter is calculated as the average of the coefficients across all programmes. ART, antiretroviral treatment; CI, confidence interval; LTFU, lost to follow-up.
Figure 1 shows the cumulative all-cause mortality estimates, after imputing mortality outcomes for the African cohorts in which there was no active follow-up or vital registration linkage. In the non-African cohorts, mortality rates appeared relatively low in North America at shorter ART durations, but at longer durations cumulative mortality rates were higher than those in Latin America and Asia-Pacific. Cumulative mortality rates were consistently higher in the sub-Saharan African cohorts, with mortality rates in the African region being lowest in South Africa and highest in West and Central Africa.
Fig. 1.
Cumulative mortality hazard, by region.
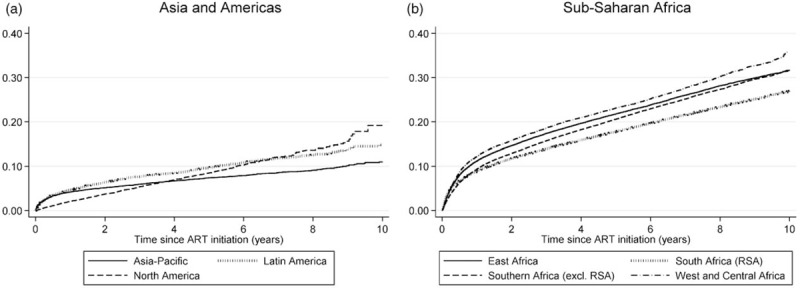
Sub-Saharan African results are obtained by imputing mortality outcomes in patients who were lost to follow-up.
In the multivariable analysis, mortality differences between regions remained significant (Table 3). Mortality in Latin America was roughly double that in Asia-Pacific, and although mortality in North America was not significantly different from that in Asia-Pacific during the first 12 months of ART, it was more consistent with that observed in Latin America at longer ART durations. Mortality rates did not differ significantly between African regions in the multivariable analysis, although in a sensitivity analysis in which Central and West Africa were considered separately, mortality appeared significantly lower in Central Africa than in the other African regions, during the first 12 months of ART (Table S12). There was substantial variation in mortality between programmes within each region. In the non-African regions, after controlling for region effects and other predictors, the mortality rate at the 90th percentile of random effects was 5.1 times that at the 10th percentile (exp(0.64 × 2 × 1.28), where 1.28 is the 90th percentile of the standard normal distribution). Heterogeneity was lower in the African cohorts, with the 90–10th percentile ratio being 2.9 in the first 12 months of ART and 3.2 at longer ART durations.
Table 3.
Multivariable analysis of mortality after antiretroviral treatment initiation.
| Asia and Americas | Sub-Saharan Africa | |||
| 1st 12 Months | >12 Months | 1st 12 Months | >12 Months | |
| Sexa | ||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 0.86 (0.76–0.97) | 0.96 (0.85–1.08) | 0.81 (0.79–0.83) | 0.73 (0.71–0.76) |
| Age group (years)a | ||||
| 15–24 | 1.00 | 1.00 | 1.00 | 1.00 |
| 25–34 | 1.22 (0.93–1.61) | 1.04 (0.69–1.57) | 0.98 (0.92–1.03) | 0.78 (0.72–0.84) |
| 35–44 | 1.39 (1.06–1.82) | 1.09 (0.72–1.63) | 0.99 (0.94–1.04) | 0.69 (0.64–0.74) |
| 45+ | 2.17 (1.66–2.84) | 1.94 (1.30–2.90) | 1.21 (1.13–1.29) | 0.82 (0.76–0.88) |
| Follow-up perioda | ||||
| 2001–2006 | 1.43 (1.22–1.66) | 1.68 (1.23–2.28) | 1.07 (1.04–1.11) | 2.05 (1.84–2.28) |
| 2007–2010 | 1.20 (1.07–1.36) | 1.26 (1.13–1.40) | 1.03 (1.00–1.06) | 1.22 (1.18–1.27) |
| 2011–2014 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2015–2017 | 1.01 (0.81–1.25) | 0.84 (0.71–0.98) | 1.00 (0.94–1.05) | 0.91 (0.88–0.94) |
| Baseline CD4+ cell count (cells/μl)a | ||||
| 0–49 | 1.00 | 1.00 | 1.00 | 1.00 |
| 50–99 | 0.71 (0.62–0.80) | 1.03 (0.90–1.18) | 0.58 (0.56–0.60) | 0.78 (0.74–0.82) |
| 100–199 | 0.36 (0.31–0.41) | 0.71 (0.63–0.81) | 0.34 (0.33–0.34) | 0.55 (0.53–0.57) |
| 200–249 | 0.22 (0.18–0.29) | 0.59 (0.49–0.72) | 0.23 (0.22–0.25) | 0.48 (0.44–0.52) |
| 250–349 | 0.16 (0.13–0.19) | 0.39 (0.33–0.47) | 0.18 (0.17–0.19) | 0.41 (0.39–0.44) |
| 350–499 | 0.16 (0.12–0.20) | 0.39 (0.31–0.49) | 0.16 (0.14–0.19) | 0.42 (0.36–0.49) |
| 500+ | 0.10 (0.07–0.15) | 0.33 (0.25–0.43) | 0.13 (0.10–0.17) | 0.24 (0.08–0.77) |
| Time since ART starta | ||||
| 0–5 Months | 1.00 | – | 1.00 | – |
| 6–11 Months | 0.43 (0.39–0.48) | – | 0.43 (0.42–0.44) | – |
| Injecting drug use historya | 1.56 (1.28–1.90) | 1.67 (1.43–1.95) | – | – |
| Regiona | ||||
| Asia-Pacific | 1.00 | 1.00 | – | – |
| Latin America | 1.98 (1.10–3.57) | 2.18 (1.23–3.83) | – | – |
| North Americab | 0.75 (0.46–1.23) | 2.52 (1.58–4.01) | – | – |
| East Africa | – | – | 1.00 | 1.00 |
| South Africa | – | – | 0.85 (0.55–1.32) | 0.97 (0.61–1.54) |
| Southern Africa (excl. RSA) | – | – | 1.09 (0.70–1.68) | 1.34 (0.84–2.15) |
| West and Central Africa | – | – | 0.97 (0.67–1.40) | 1.28 (0.87–1.89) |
| Baseline mortalityc | 4.5 (2.9–6.8) | 0.5 (0.3–0.9) | 33.1 (24.0–45.6) | 4.9 (3.5–6.9) |
| SD of random effects | 0.64 (0.48–0.86) | 0.60 (0.44–0.84) | 0.42 (0.31–0.51) | 0.45 (0.33–0.54) |
ART, antiretroviral treatment; RSA, Republic of South Africa; SD, standard deviation.
aIncidence rate ratio (adjusted) with 95% confidence interval in brackets.
bEstimates for North America in the first year of ART could be underestimated, as patients must have two HIV visits within 12 months to be enrolled in the cohort.
cPer 100 person-years, in individuals with baseline covariate pattern (males aged 15–24 followed up in the 2011–2014 period, with baseline CD4+ cell count <50 cells/μl).
Mortality rates in the first 12 months after ART initiation appeared to decline over time in the non-African cohorts, but changed relatively little in the African cohorts (Table 3). However, there was more substantial evidence of declines in mortality over time at longer durations, both in the African and non-African cohorts, with mortality rates in the 2015–2017 period being half of those in the 2001–2006 period in both analyses. Because this reduction may be due to residual confounding (with follow-up in more recent periods representing longer ART durations than follow-up in the 2001–2006 period), a sensitivity analysis was conducted in which additional duration categories were included in the model (12–23 months, 24–35 months and ≥36 months). In this sensitivity analysis, the decline in mortality over time remained significant, but was not as substantial as in the main analysis, and in the African regions mortality appeared to be higher only in the 2001–2006 period (Fig. 2). The ratio of the mortality rate at 24–35 months to that at 12–23 months was 0.85 (95% CI: 0.75–0.96) in the non-African regions and 0.78 (95% CI: 0.75–0.81) in the African regions, whereas the ratio of the mortality rate at least 36 months to that at 12–23 months was 0.78 (95% CI: 0.70–0.87) in the non-African regions and 0.62 (95% CI: 0.60–0.64) in the African regions (Table S13).
Fig. 2.
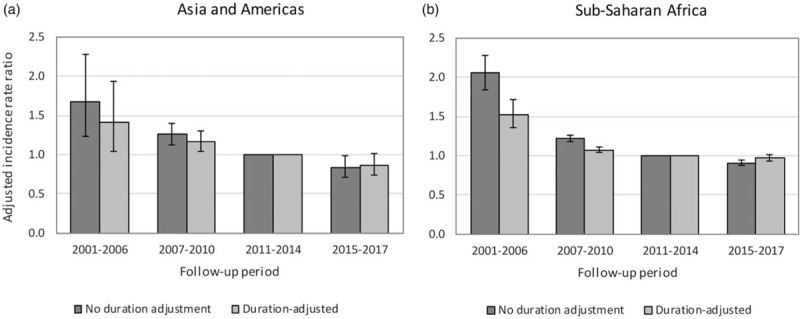
Effect of calendar period on mortality rates more than 12 months after antiretroviral treatment initiation, before and after controlling for differences in mortality by duration.
The effect of baseline CD4 cell count on mortality was more significant during the first 12 months after ART initiation than at longer ART durations, and mortality risks decreased as baseline CD4 cell counts increased (Table 3). However, in the sensitivity analysis in which patients were not excluded if their CD4 cell count was above the ART eligibility threshold at the time of ART initiation, the incidence rate ratios were in most cases closer to 1, suggesting a weaker negative relationship between baseline CD4 cell count and mortality at higher CD4 cell counts (Table 4). Results did not change substantially when CD4 cells counts were imputed for those individuals with missing baseline CD4 values, although in African cohorts the imputed CD4 cell counts appeared less strongly predictive of mortality during the first 12 months after ART initiation (Tables S15 and S16). Results also remained largely unchanged when alternative models of the age effect were considered (Tables S17 and S18), and when ART eligibility criteria were controlled for (Tables S19 and S20).
Table 4.
Effects of baseline CD4+ cell count (cells/μl) on mortality before and after excluding patients who did not meet antiretroviral treatment eligibility criteria at the time of antiretroviral treatment initiationa.
| Asia and Americas | Sub-Saharan Africa | |||
| Before exclusions | After exclusions | Before exclusions | After exclusions | |
| Effect of baseline CD4+ cell count (cells/μl) on mortality in 1st 12 months | ||||
| 0–49 | 1.00 | 1.00 | 1.00 | 1.00 |
| 50–99 | 0.70 (0.62–0.80) | 0.71 (0.62–0.80) | 0.58 (0.56–0.60) | 0.58 (0.56–0.60) |
| 100–199 | 0.36 (0.31–0.41) | 0.36 (0.31–0.41) | 0.34 (0.33–0.35) | 0.34 (0.33–0.34) |
| 200–249 | 0.25 (0.20–0.30) | 0.22 (0.18–0.29) | 0.25 (0.24–0.26) | 0.23 (0.22–0.25) |
| 250–349 | 0.16 (0.13–0.19) | 0.16 (0.13–0.19) | 0.21 (0.20–0.22) | 0.18 (0.17–0.19) |
| 350–499 | 0.16 (0.13–0.20) | 0.16 (0.12–0.20) | 0.21 (0.20–0.22) | 0.16 (0.14–0.19) |
| 500+ | 0.15 (0.11–0.19) | 0.10 (0.07–0.15) | 0.17 (0.16–0.19) | 0.13 (0.10–0.17) |
| Effect of baseline CD4+ cell count (cells/μl) on mortality after 1st 12 months | ||||
| 0–49 | 1.00 | 1.00 | 1.00 | 1.00 |
| 50–99 | 1.03 (0.90–1.18) | 1.03 (0.90–1.18) | 0.78 (0.75–0.81) | 0.78 (0.74–0.82) |
| 100–199 | 0.72 (0.63–0.82) | 0.71 (0.63–0.81) | 0.55 (0.53–0.56) | 0.55 (0.53–0.57) |
| 200–249 | 0.62 (0.52–0.73) | 0.59 (0.49–0.72) | 0.45 (0.43–0.48) | 0.48 (0.44–0.52) |
| 250–349 | 0.42 (0.36–0.50) | 0.39 (0.33–0.47) | 0.43 (0.41–0.45) | 0.41 (0.39–0.44) |
| 350–499 | 0.43 (0.36–0.53) | 0.39 (0.31–0.49) | 0.44 (0.41–0.48) | 0.42 (0.36–0.49) |
| 500+ | 0.45 (0.37–0.56) | 0.33 (0.25–0.43) | 0.36 (0.33–0.40) | 0.24 (0.08–0.77) |
ART, antiretroviral treatment.
aIn Asia and the Americas, ART eligibility was assessed based on clinical and immunological criteria, depending on the published ART eligibility criteria for the country in the relevant year. In sub-Saharan Africa, ART eligibility was assessed based only on immunological criteria, due to incomplete recording of clinical stage. The results in the ‘After exclusions’ analysis are the same as those shown in Table 3.
Discussion
The current study shows evidence of substantial variation in mortality in treated HIV-positive adults, both across global regions and within regions. Consistent with previous studies of intercohort variation in mortality [29,31], we show that this variation cannot be explained only in terms of factors such as differences in baseline CD4 cell count, injecting drug use or completeness of mortality ascertainment, although differences observed between sub-Saharan African regions (Fig. 1b) ceased to be significant in the multivariable analysis. Possible explanations for residual variation in mortality include differences across programmes in the socioeconomic profiles of their patient populations, differences in facility type (e.g. in some settings tertiary centres may tend to treat sicker patients [32]), differences across regions in ART monitoring strategies (with virological monitoring generally yielding better mortality outcomes than clinical or immunological monitoring alone [33,34]), differences in the effectiveness and tolerability of the locally recommended drug regimens, differences in levels of antiretroviral drug resistance [35,36] and differences in resources for patient tracking and the diagnosis and management of comorbidities.
The unusually high estimates of mortality in sub-Saharan Africa (Figure 1 and Table 3) could be attributed to a number of factors. Tuberculosis accounts for almost half of all deaths in HIV-positive individuals in Africa [37], but is markedly less prevalent in high-income settings. Poor access to cotrimoxazole and isoniazid prophylaxis in resource-limited settings could also contribute to excess mortality associated with tuberculosis and other HIV-related diseases [38]. Non-HIV mortality, which accounts for much mortality in ART patients, is also substantially higher in the sub-Saharan African region than in other regions [30].
The unusual mortality pattern in North America (lower than in other concentrated epidemic settings at early ART durations, but higher at later durations) is consistent with previous inter-regional comparisons [31,39], and could be partly because patients are only included in the North American database if they have at least two visits in the year of enrolment [40], implying a selection bias due to the exclusion of individuals who die or transfer care before their second visit. The higher rates of mortality in North American patients compared with Asian patients, at longer ART durations, is consistent with a recent analysis that found significantly lower viral suppression in North American patients compared with Asian patients [41]. This may be due to the nature of the American healthcare system, with patients frequently transferring between public and private care, and the resulting instability in care contributing to higher long-term mortality [42]. The North American patients are also older on average than those in other regions, although controlling for age in different ways does not appear to change the relative difference between North America and other regions (Tables S17 and S18).
Even within regions, substantial variation in mortality is observed, with the ratio of the adjusted mortality at the 90th percentile of random effects to that at the 10th percentile being roughly 5 in concentrated epidemic settings and roughly 3 in sub-Saharan African settings. The slightly lower heterogeneity in the sub-Saharan African regions is probably due to the same model being used to impute mortality outcomes across all cohorts, which may lead to the true extent of the heterogeneity being understated. It is important that mathematical models of the impact of ART reflect the uncertainty regarding the ART mortality rates that apply locally. Ideally models should be calibrated to local mortality data to reduce this uncertainty [3,43], but when such data are not available and these IeDEA estimates are used, confidence intervals around model outputs should reflect the standard deviations of the random effects terms estimated here. Future analyses should aim to reduce the variation of these random effects, for example by regressing on country GDP per capita and on rates of viral suppression, factors that are likely to account for much of the variation in mortality within regions.
This study suggests that mortality rates in ART patients have declined over time in Asia and the Americas, even after controlling for improvements in baseline characteristics and changes in treatment duration. This is consistent with declines previously observed in concentrated epidemic settings [9–11]. However, in sub-Saharan African cohorts the reductions in mortality over time are only noticeable when considering follow-up more than 12 months after ART initiation, and when considering a more detailed model of duration effects, even these declines appear questionable after 2006 (Fig. 2b). South African data, which are based on more robust mortality ascertainment, also suggest little or no decline in treated mortality over time [44,45]. Declines in mortality might be expected, given innovations in service delivery (such as adherence clubs [46]), the introduction of newer drugs that are better tolerated and more effective in suppressing HIV [47], and the increasing use of fixed-dose combination ART [48]. Declines may also be due to improvements in baseline clinical stage, which we did not control for in this analysis because the information was missing in many cohorts, and because the Spectrum model (in common with most other mathematical models [49]) does not include a separate stratification by clinical stage. The absence of a strong decline in mortality in African cohorts may be related to declining visit frequencies [50], or increasing rates of treatment interruption [51], which may have offset the expected gains from therapeutic improvements. It is also possible that the relatively rapid growth in drug resistance in East and Southern Africa [35] and the relatively high prevalence of tenofovir drug resistance in sub-Saharan Africa [36] may be part of the reason why mortality in sub-Saharan Africa has not declined to the same extent as in other regions. Alternatively, the slow mortality decline may be due to the parameterization of the imputation model, which suggested only a modest decline over time in mortality in LTFU patients (Table 2), in contrast to the significant reduction in the original meta-analysis on which the imputation model was based [27]. Further research is required to determine the reasons for the relatively modest declines in treated mortality rates in the sub-Saharan African region.
This analysis advances previous parameterizations of the Spectrum model in a number of ways. In addition to incorporating more recent data and assessing time trends in ART mortality, this analysis adopts an innovative approach to correcting for unascertained mortality after LTFU, recognizing that the extent of the underascertainment of mortality is likely to differ substantially across settings. The previous analysis applied the same correction factors in all regions, leading to assumed mortality rates being roughly double those observed in IeDEA cohorts [6]. In the present analysis, no correction factors are applied in the North American, Latin American and Asia-Pacific datasets, with the result that mortality rates are substantially lower than estimated previously. Although the use of an imputation model (in place of correction factors) has not substantially changed the overall levels of mortality in the African regions, the major advantage of the imputation model is that adjustments are applied at an individual level rather than at an aggregated or cohort level, which makes it possible to estimate the effects of covariates on mortality with greater accuracy.
A further methodological innovation is that we have excluded patients who started ART if they qualified for ART based only on clinical criteria. Although this does not materially change estimates of mortality in patients with low baseline CD4+ cell counts (as such patients qualify for ART regardless of their clinical stage), it does lead to somewhat lower mortality rates in patients starting ART at higher CD4+ cell counts (Table 4) and thus more substantial modelled benefits from early ART initiation. This is consistent with evidence of the clinical benefits of early ART in randomized controlled trials [52,53]. The exclusion of individuals who qualified for ART based only on clinical criteria is important as these individuals are over-represented in high baseline CD4+ cell count categories, particularly in countries that have only recently moved to universal ART eligibility. These individuals also have relatively high mortality rates, making them unrepresentative of the general population of patients starting ART at higher CD4+ cell counts in the era of universal ART eligibility.
This analysis has some limitations. Although most of the programmes in the Asia-Pacific region relied on active follow-up to ascertain outcomes, it is unclear how consistently it was applied, with the result that there may be some underestimation of mortality rates. However, data from Thailand and Indonesia suggest that deaths after LTFU account for a relatively small fraction of total deaths in these settings [54,55], and other studies from North America and Latin America have found only modest differences in mortality between programmes that employ active follow-up and those that rely on linkage to vital registration systems [9,24]. Another limitation is that we have pooled data across IeDEA regions rather than fitting separate regression models for each region, which may have led to some inter-regional differences in covariate effects being obscured. Although initial attempts were made to fit the regression models separately for each region, this led in some cases to implausible point estimates with extremely wide CIs, which were judged to not be appropriate as inputs for Spectrum. A further limitation is that we lack information on causes of death in many IeDEA cohorts, and thus cannot assess the extent to which inter-regional differences in all-cause mortality might be driven by specific diseases such as tuberculosis, or non-HIV-related causes. The Poisson regression approach imposes an assumption of piecewise-constant mortality rates, which may be appropriate for the purpose of estimating parameters in the Spectrum model, but which may lead to biased estimates of the effects of covariates on mortality (as demonstrated in Figure 2, which compares the effects of using shorter duration intervals, and Tables S21 and S22, which compare the effects of fitting more flexible Cox proportional hazards models). Changes in mortality over the first 6 months of ART are particularly dramatic [56], but the Spectrum model requires a single parameter to represent average mortality over this treatment duration. Similarly, our analysis finds evidence that ART mortality continues to decline for durations greater than 24 months on ART (Table S13), but in the primary analysis we estimated a single mortality rate for more than 12 months after ART initiation, for consistency with the current Spectrum model structure.
Further limitations relate to the large fraction of deaths among persons LTFU that must be imputed in African settings, and the limited and imperfect data to inform the imputation model. The imputation model relied only on data from traced patients whose vital status could be established, but because a substantial proportion of LTFU patients could not be traced [27], and because the sampling of LTFU patients to be traced is not always random, mortality estimates may be biased. Due to the limited amount of tracing study data from outside Southern Africa, it was not feasible to include region in the imputation model, although this covariate was included in the main analysis. This might have led to some bias in Rubin's variance estimates, as our analysis and imputation models do not involve the same set of independent variables [57,58]. Despite these limitations, the estimate that 57% of all deaths were not recorded in patient record systems is similar to an estimate of 65% in South Africa, which was also found to increase over time, from around 40% of deaths before 2006 to 66% in 2011–2014 [20]. This increase over time in the fraction of deaths that are unrecorded, both in our analysis and the South African study, is a reflection of increasing LTFU in more recent periods [59].
Estimates of AIDS mortality globally have been contentious and have differed markedly across models [60,61]. The substantial heterogeneity in mortality rates shown in our analysis, between and within regions, suggests that more precise and accurate quantification of local ART programme impact will require the reconciliation of mortality data from research cohorts (such as presented in the current analysis) and mortality data from vital registration and case reporting systems. This analysis lays the foundation for an evidence synthesis approach that appropriately weights different mortality data sources when producing model estimates of AIDS mortality at a country level. These estimates are critical for evaluating the success of ART programmes and for identifying the subpopulations most affected by AIDS mortality.
Acknowledgements
We are grateful to Olga Tymejczyk, Tor Petersen and Ellen Brazier, who provided us with information on ART eligibility criteria in various countries. We thank the members of IeDEA and the IeDEA data centres for collecting and collating these data (for a full list of all IeDEA members, see www.iedea.org). We are also grateful to members of the UNAIDS Reference Group on Estimates, Modelling and Projections, who provided helpful input on an earlier version of this analysis.
L.F.J., J.W.E., N.A., C.Y. and M.E. conceived the study. P.F.R., G.C., D.N., M.Y., D.K.E., C.B.H., J.Y.C., A.J., K.N.A., A.H.S. and C.Y. contributed to the data collection. E.Z. and L.F.J. collated the data. L.F.J., J.W.E., N.A., C.Y., G.B., D.N. and P.F.R. contributed to the data analysis. L.F.J. drafted the article with input from all authors. All authors have read and approved the final article.
Asia-Pacific: The TREAT Asia HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health's National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health and the National Institute on Drug Abuse, as part of the International Epidemiology Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney.
Caribbean, Central America and South America: This work was supported by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH) and the Office of The Director, National Institutes of Health (OD). P.F.R. was supported by NIH Award Number K01AI131895 (The HIV Care Continuum and Health Policy: Changes through Context and Geography).
Central Africa: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI096299 (PI: Anastos and Nash).
East Africa: Research reported in this publication was supported by the National Institute Of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institute on Drug Abuse (NIDA), National Cancer Institute (NCI) and the National Institute of Mental Health (NIMH), in accordance with the regulatory requirements of the National Institutes of Health under Award Number U01AI069911 East Africa IeDEA Consortium.
North America: This work was supported by National Institutes of Health grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, M01RR000052, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R01AG053100, R24AI067039, U01AA013566, U01AA020790, U01AI031834, U01AI034989, U01AI034993, U01AI034994, U01AI035004, U01AI035039, U01AI035040, U01AI035041, U01AI035042, U01AI037613, U01AI037984, U01AI038855, U01AI038858, U01AI042590, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01AI103390, U01AI103397, U01AI103401, U01AI103408, U01DA03629, U01DA036935, U01HD032632, U10EY008057, U10EY008052, U10EY008067, U24AA020794, U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR000454, UM1AI035043, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Cancer Institute, National Institute for Mental Health and National Institute on Drug Abuse.
Southern Africa: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI069924.
West Africa: Research reported in this publication was supported by the US National Institutes of Health (NIAID, NICHD, NCI and NIMH) under Award Number U01AI069919 (PI: Dabis).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge funding from UNAIDS.
Conflicts of interest
The authors declare no conflicts of interest. This research was funded by the National Institutes of Health and UNAIDS (see Funding statement on title page).
Supplementary Material
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. 2013; Available: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Accessed 13 April 2014]. [Google Scholar]
- 2.Reniers G, Slaymaker E, Nakiyingi-Miiro J, Nyamukapa C, Crampin AC, Herbst K, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA). AIDS 2014; 28: Suppl 4: S533–S542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LF, May MT, Dorrington RE, Cornell M, Boulle A, Egger M, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: a mathematical modelling study. PLoS Med 2017; 14:e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stover J, Brown T, Puckett R, Peerapatanapokin W. Updates to the Spectrum/Estimations and Projections Package model for estimating trends and current values for key HIV indicators. AIDS 2017; 31: Suppl 1: S5–S11. [DOI] [PubMed] [Google Scholar]
- 5.Stover J, Bollinger L, Izazola JA, Loures L, DeLay P, Ghys PD. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the fast-track approach. PLoS One 2016; 11:e0154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderegg N, Johnson LF, Zaniewski E, Althoff KN, Balestre E, Law M, et al. All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS 2017; 31: Suppl 1: S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiannoutsos CT, Johnson LF, Boulle A, Musick BS, Gsponer T, Balestre E, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect 2012; 88: Suppl 2: i33–i43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Guideline on when to start antiretroviral therapy and on preexposure prophylaxis for HIV. 2015; Available: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. [Accessed 24 April 2016]. [PubMed] [Google Scholar]
- 9.Carriquiry G, Fink V, Koethe JR, Giganti MJ, Jayathilake K, Blevins M, et al. Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc 2015; 18:20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De La Mata NL, Kumarasamy N, Khol V, Ng OT, Van Nguyen K, Merati TP, et al. Improved survival in HIV treatment programmes in Asia. Antivir Ther 2016; 21:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaniewski E, Tymejczyk O, Kariminia A, Desmonde S, Leroy V, Ford N, et al. IeDEA–WHO Research-Policy Collaboration: contributing real-world evidence to HIV progress reporting and guideline development. J Virus Erad 2018; 4: Suppl 2: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molenberghs G, Kenward MG. Missing data in clinical studies. Chichester, UK: John Wiley & Sons; 2007. [Google Scholar]
- 15.Shepherd BE, Rebeiro PF. Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr 2017; 74:e60–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Granich R. When will sub-Saharan Africa adopt HIV treatment for all?. South Afr J HIV Med 2016; 17:a459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Williams B, Montaner J. Realizing the potential of treatment as prevention: global ART policy and treatment coverage. Curr HIV/AIDS Rep 2014; 11:479–486. [DOI] [PubMed] [Google Scholar]
- 18.Tymejczyk O, Brazier E, Yiannoutsos C, Wools-Kaloustian K, Althoff K, Crabtree-Ramirez B, et al. HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: a metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 15:e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Association of Providers of AIDS Care. Global HIV policy watch. 2018; Available: http://www.hivpolicywatch.org/. [Accessed 2 September 2018]. [Google Scholar]
- 20.Johnson LF, Dorrington RE, Laubscher R, Hoffmann CJ, Wood R, Fox MP, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc 2015; 18:20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adair T, Lopez AD. Estimating the completeness of death registration: an empirical method. PLoS One 2018; 13:e0197047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CJ, Rajaratnam JK, Marcus J, Laakso T, Lopez AD. What can we conclude from death registration? Improved methods for evaluating completeness. PLoS Med 2010; 7:e1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De La Mata NL, Ly PS, Nguyen KV, Merati TP, Pham TT, Lee MP, et al. Loss to follow-up trends in HIV-positive patients receiving antiretroviral treatment in Asia from 2003 to 2013. J Acquir Immune Defic Syndr 2017; 74:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas AD, Zaniewski E, Anderegg N, Ford N, Fox MP, Vinikoor M, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc 2018; 21:e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkhof MW, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Messou E, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One 2010; 5:e14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chammartin F, Zürcher K, Keiser O, Weigel R, Chu K, Kiragga AN, et al. Outcomes of patients lost to follow-up in African antiretroviral therapy programs: individual patient data meta-analysis. Clin Infect Dis 2018; 67:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes CB, Sikazwe I, Sikombe K, Eshun-Wilson I, Czaicki N, Beres LK, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: findings from a multistage sampling-based survey. PLoS Med 2018; 15:e1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May MT, Hogg RS, Justice AC, Shepherd BE, Costagliola D, Ledergerber B, et al. Heterogeneity in outcomes of treated HIV-positive patients in Europe and North America: relation with patient and cohort characteristics. Int J Epidemiol 2012; 41:1807–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United Nations Department of Economic and Social Affairs Population Division. World population prospects: the 2017 revision, key findings and advance tables. 2017; Available: https://population.un.org/wpp/Publications/Files/WPP2017_KeyFindings.pdf. [Accessed 29 November 2018]. [Google Scholar]
- 31.Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med 2014; 11:e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One 2010; 5:e12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keiser O, Chi BH, Gsponer T, Boulle A, Orrell C, Phiri S, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS 2011; 25:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estill J, Egger M, Johnson LF, Gsponer T, Wandeler G, Davies MA, et al. Monitoring of antiretroviral therapy and mortality in HIV programmes in Malawi, South Africa and Zambia: mathematical modelling study. PLoS One 2013; 8:e57611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Date AA, Vitoria M, Granich R, Banda M, Fox MY, Gilks C. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull WHO 2010; 88:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarrin I, Global Mortality Disparities in Women Working Group for IeDEA, EuroSIDA, CASCADE and COHERE in EuroCoord. Mortality differences after ART initiation in HIV-positive women from Europe, the Americas and sub-Saharan Africa; 2000-2014 [Abstract TUPDC0101]. International AIDS Conference. Amsterdam, Netherlands; 2018. [Google Scholar]
- 40.Rebeiro PF, Althoff KN, Lau B, Gill J, Abraham AG, Horberg MA, et al. Laboratory measures as proxies for primary care encounters: implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol 2015; 182:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiamsakul A, Kariminia A, Althoff KN, Cesar C, Cortes CP, Davies MA, et al. HIV viral load suppression in adults and children receiving antiretroviral therapy - results from the IeDEA collaboration. Journal of Acquired Immune Deficiency Syndrome 2017; 76:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebeiro PF, Abraham AG, Horberg MA, Althoff KN, Yehia BR, Buchacz K, et al. Sex, race, and HIV risk disparities in discontinuity of HIV care after antiretroviral therapy initiation in the United States and Canada. AIDS Patient Care STDs 2017; 31:129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahiane SG, Marsh K, Grantham K, Crichlow S, Caceres K, Stover J. Improvements in Spectrum's fit to program data tool. AIDS 2017; 31: Suppl 1: S23–S30. [DOI] [PubMed] [Google Scholar]
- 44.Cornell M, Johnson LF, Wood R, Tanser F, Fox MP, Prozesky H, et al. Twelve-year mortality in adults initiating antiretroviral therapy in South Africa. J Int AIDS Soc 2017; 20:21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson LF, Keiser O, Fox MP, Tanser F, Cornell M, Hoffmann CJ, et al. Life expectancy trends in adults on antiretroviral treatment in South Africa. AIDS 2016; 30:2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One 2013; 8:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 2016; 3:e510–e520. [DOI] [PubMed] [Google Scholar]
- 48.Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health 2014; 19:501–513. [DOI] [PubMed] [Google Scholar]
- 49.Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 2012; 9:e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haas AD, Johnson LF, Grimsrud A, Ford N, Mugglin C, Fox MP, et al. Extending visit intervals for clinically stable patients on antiretroviral therapy: multicohort analysis of HIV programs in Southern Africa. Journal of Acquired Immune Deficiency Syndrome 2019; 81:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, Lawn SD, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. J Acquir Immun Defic Syndr 2010; 55:e17–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 53.INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teeraananchai S, Chaivooth S, Kerr SJ, Bhakeecheep S, Avihingsanon A, Teeraratkul A, et al. Life expectancy after initiation of combination antiretroviral therapy in Thailand. Antivir Ther 2017; 22:393–402. [DOI] [PubMed] [Google Scholar]
- 55.Kusuma Dewi P, Widiarta G. Predictors of mortality among patients lost to follow up antiretroviral therapy. Jurnal Ners 2018; 13:114–121. [Google Scholar]
- 56.Yiannoutsos CT. Modeling AIDS survival after initiation of antiretroviral treatment by Weibull models with changepoints. J Int AIDS Soc 2009; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robins JM, Wang N. Inference for imputation estimators. Biometrika 2000; 87:113–124. [Google Scholar]
- 58.Meng XL. Multiple-imputation inferences with uncongenial sources of input. Stat Sci 1994; 9:538–573. [Google Scholar]
- 59.Grimsrud A, Balkan S, Casas EC, Lujan J, Van Cutsem G, Poulet E, et al. Outcomes of antiretroviral therapy over a 10-year period of expansion: a multicohort analysis of African and Asian HIV programs. J Acquir Immun Defic Syndr 2014; 67:e55–e66. [DOI] [PubMed] [Google Scholar]
- 60.Hallett TB, Zaba B, Stover J, Brown T, Slaymaker E, Gregson S, et al. Embracing different approaches to estimating HIV incidence, prevalence and mortality. AIDS 2014; 28: Suppl 4: S523–S532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eaton JW, Bacaër N, Bershteyn A, Cambiano V, Cori A, Dorrington RE, et al. Assessment of epidemic projections using recent HIV survey data in South Africa: a validation analysis of ten mathematical models of HIV epidemiology in the antiretroviral therapy era. Lancet Glob Health 2015; 3:e598–e608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


