Supplemental Digital Content is available in the text
Keywords: Bayesian statistics, HIV/AIDS, knowledge of HIV status, mathematical modeling, population health, self-report, surveillance, treatment and care cascade
Abstract
Objective:
HIV testing services (HTS) are a crucial component of national HIV responses. Learning one's HIV diagnosis is the entry point to accessing life-saving antiretroviral treatment and care. Recognizing the critical role of HTS, the Joint United Nations Programme on HIV/AIDS (UNAIDS) launched the 90-90-90 targets stipulating that by 2020, 90% of people living with HIV know their status, 90% of those who know their status receive antiretroviral therapy, and 90% of those on treatment have a suppressed viral load. Countries will need to regularly monitor progress on these three indicators. Estimating the proportion of people living with HIV who know their status (i.e. the ‘first 90’), however, is difficult.
Methods:
We developed a mathematical model (henceforth referred to as ‘Shiny90’) that formally synthesizes population-based survey and HTS program data to estimate HIV status awareness over time. The proposed model uses country-specific HIV epidemic parameters from the standard UNAIDS Spectrum model to produce outputs that are consistent with other national HIV estimates. Shiny90 provides estimates of HIV testing history, diagnosis rates, and knowledge of HIV status by age and sex. We validate Shiny90 using both in-sample comparisons and out-of-sample predictions using data from three countries: Côte d’Ivoire, Malawi, and Mozambique.
Results:
In-sample comparisons suggest that Shiny90 can accurately reproduce longitudinal sex-specific trends in HIV testing. Out-of-sample predictions of the fraction of people living with HIV ever tested over a 4-to-6-year time horizon are also in good agreement with empirical survey estimates. Importantly, out-of-sample predictions of HIV knowledge of status are consistent (i.e. within 4% points) with those of the fully calibrated model in the three countries when HTS program data are included. The model's predictions of knowledge of status are higher than available self-reported HIV awareness estimates, however, suggesting – in line with previous studies – that these self-reports could be affected by nondisclosure of HIV status awareness.
Conclusion:
Knowledge of HIV status is a key indicator to monitor progress, identify bottlenecks, and target HIV responses. Shiny90 can help countries track progress towards their ‘first 90’ by leveraging surveys of HIV testing behaviors and annual HTS program data.
Introduction
HIV testing services (HTS) are the entry point for diagnosis and access to life-saving antiretroviral therapy (ART) [1]. Early diagnosis and initiation of ART have been shown to drastically decrease viral load, which reduces individual morbidity and mortality, and limits onward HIV transmission [2]. HTS can also offer a pathway for primary prevention interventions, including programs that deliver pre-exposure prophylaxis, voluntary medical male circumcision, and prevention of mother-to-child transmission.
Recognizing the critical role of HTS in a country's national response, the Joint United Nations Programme on HIV/AIDS (UNAIDS) launched in 2014 the 90-90-90 targets stipulating that by 2020, 90% of people living with HIV (PLHIV) know their status, 90% of PLHIV who know their status receive ART, and 90% of those on treatment have a suppressed viral load [3–5]. To reach those targets, countries need to monitor progress on these three indicators, identify bottlenecks, and implement or adapt targeted testing and treatment services in a timely manner.
As of 2017, UNAIDS estimates that the biggest bottleneck globally in achieving the 90-90-90 targets is access to HIV testing, with about 25% of PLHIV not knowing their HIV status [6]. Estimating the proportion of PLHIV who know their status (i.e. the ‘first 90’), however, is difficult. For countries with robust and comprehensive HIV case surveillance systems, the proportion of diagnosed PLHIV can be estimated by triangulating HIV incidence and mortality with the cumulative number of new HIV diagnoses annually. In sub-Saharan Africa (SSA), where more than two-thirds of PLHIV reside [7], surveillance systems are not yet sufficiently developed. Many countries estimate the proportion of PLHIV who know their status primarily from nationally representative household surveys.
Most Demographic and Health Surveys (DHS) and AIDS Indicator Surveys (AIS) in SSA include HIV serology, with respondents self-reporting whether they have ever been tested for HIV, but are rarely being asked directly if they are aware of their HIV status. The proportion of HIV-positive respondents who report ever having been tested for HIV serves as an upper bound for the level of HIV awareness, because the last HIV test might have been HIV-negative (i.e. occurring before the person seroconverted). In recent years, Population-based HIV Impact Assessment (PHIA) surveys and a few other surveys conducted in SSA countries have collected information on both HIV seroprevalence and self-reported awareness status. These data have been used directly to estimate the ‘first 90’ [8,9]. However, comparison of self-reported awareness of HIV status with biomarker measurements of antiretroviral usage and viral load suppression reveals sometimes substantial nondisclosure of awareness of HIV status for persons who are on ART [9,10].
The infrequency of large population-based seroprevalence surveys, which are typically conducted every 5 years, also hampers regular monitoring of HIV awareness [11]. UNAIDS has previously estimated the change in knowledge of status over time in countries with survey data by applying additional increases in knowledge of status proportional to the scale-up in ART coverage between the current reporting year and the year of the last survey [12]. However, there is a need to better estimate progress towards the ‘first 90’ in relation to changes in ART coverage and HTS program efforts [13]. For example, the relationship between ART coverage and knowledge of status has likely changed as a function of eligibility for treatment initiation. Further, programmatic data of the numbers of people tested and those testing HIV-positive could help inform changes in testing levels.
To address these challenges, we developed a mathematical model – henceforth referred to as “Shiny90” – that formally synthesizes population-based surveys and HTS program data within a Bayesian framework to annually estimate knowledge of status among people (≥15 years) in SSA. Shiny90 estimates HIV testing and diagnosis rates over time by age, sex, and previous HIV testing history, to generate estimates of the ‘first 90’ and other indicators of interest such as positivity among HIV testers and yield of new HIV diagnoses. Key features of this new model are the following:
-
(1)
Shiny90 takes as inputs – and therefore is fully consistent with – national modeled estimates of HIV prevalence, incidence, mortality, and ART coverage derived using the UNAIDS-supported Spectrum modeling software.
-
(2)
uses data about self-reported HIV testing history from surveys that both include and do not include HIV serology.
-
(3)
incorporates programmatic data on the annual numbers of HIV tests administered (testing volume) and number of positive HIV tests (positivity), where available.
Methods
Modeling framework
HIV testing uptake is dynamically modeled using a deterministic framework based on a system of ordinary differential equations adapted from a well-established South African model [14]. Shiny90 stratifies a country/region's population by HIV testing history and, additionally among PLHIV, by knowledge of HIV status and ART status. This results in the following six main stages: HIV-susceptible who have never been tested; HIV-susceptible ever tested; PLHIV who have never been tested; PLHIV unaware who have ever been tested; PLHIV aware (untreated); and PLHIV on ART. A schematic of the compartmental flows between these different stages is presented in Fig. 1.
Fig. 1.
Intercompartmental flow describing HIV testing uptake as a function of HIV status (susceptible versus living with HIV), testing history (never versus ever tested), HIV awareness status, and antiretroviral treatment (ART) status.
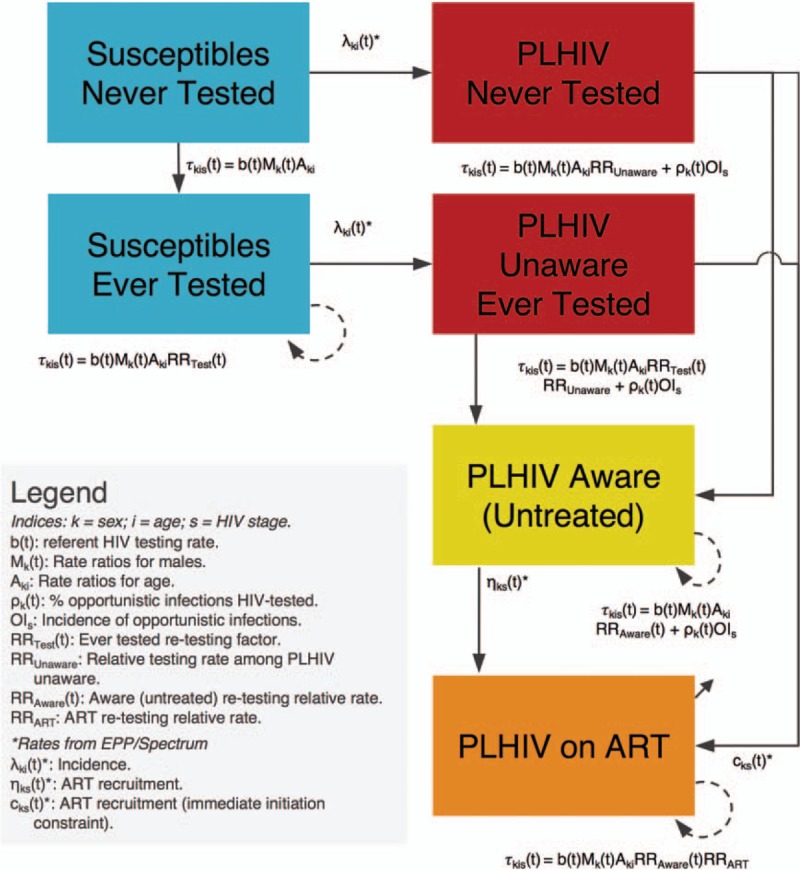
All parameters related to HIV testing  are estimated by the model. Other rates, such as HIV incidence, and ART recruitment (adjusted for ART discontinuation), are informed by Spectrum/EPP, and also demographic parameters governing entry in the model at 15 years of age and both natural and HIV-related mortality (not depicted on the figure for ease of visual interpretation).
are estimated by the model. Other rates, such as HIV incidence, and ART recruitment (adjusted for ART discontinuation), are informed by Spectrum/EPP, and also demographic parameters governing entry in the model at 15 years of age and both natural and HIV-related mortality (not depicted on the figure for ease of visual interpretation).
Individuals enter the population at age 15 years and are assumed to have never been tested for HIV (unless already living with HIV and on ART). Shiny90 has been developed to use as inputs annual estimates of HIV incidence, mortality, and ART coverage produced by countries and published annually by UNAIDS [15]. At the core of this estimation process is Spectrum's AIDS Impact Module and its Estimation and Projection Package (EPP) [16]. The Spectrum model, its assumptions, data requirements, and software are described in detail elsewhere [17]. Importantly for the new Shiny90 model, Spectrum produces epidemic statistics stratified by age and sex, CD4+ cell count category, and ART status.
The transition rates between HIV-susceptible individuals and PLHIV are informed by point estimates of the sex and age-specific incidence rates estimated by Spectrum/EPP, and also the transition rates from the three PLHIV untreated stages to the PLHIV on ART one (and the ART discontinuation rate) [17]. Spectrum/EPP also informs demographic rates and HIV disease progression and mortality [17]. Shiny90 is used to estimate all HIV testing rates, as further described in the next sections.
Model specification for HIV testing
The per capita rate τkius(t) at which individuals are tested for HIV varies by calendar time (t), sex (k), age (i), HIV testing history and awareness status (u), and, for PLHIV, CD4+ cell count (s). Specifically, it takes the following form:
Here, b(t) is the testing rate for the referent group of women in the 15–24-year age category for calendar year t, which is assumed to be negligible in SSA before 1995 (see Text S1 for full details). From 2000 onwards, b(t) is modeled as a first-order random walk with annual time steps. Mk(t) represents the HIV testing rate ratio for men (k = 1) aged 15–24 years relative to women aged 15–24 at time t (equal to one for this referent group). We allow changes in this ratio from 2005 and 2010 to account for potential scaling up of prevention of mother-to-child transmission programs in SSA countries [18,19], which could have influenced sex differences in HIV testing uptake. The term Aki contains the age and sex-specific HIV testing rate ratios for ages 15–24 (i = 1), 25–34 (i = 2), 34–49 (i = 3), and 50+ (i = 4) age groups, which are assumed to be time-invariant [8,14,20,21].
The term Fu(t) allows potential differences in HIV testing rates according to prior HIV testing history and HIV status between HIV-susceptible who have never been tested (u = 1), HIV-susceptible previously tested (u = 2), PLHIV who have never been tested (u = 3), PLHIV unaware who have ever been tested (u = 4), PLHIV aware not on treatment (u = 5), and PLHIV on ART (u = 6), as displayed in Fig. 1 and further described below.
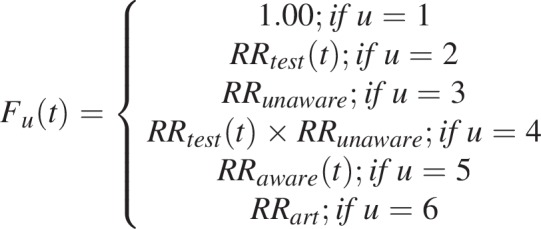 |
In many SSA countries, a substantial fraction of the population is tested every year, but the proportion of people reporting having ever been tested remains lower than what would be expected if everyone in the population had tested at an equal rate. Empirical evidence also suggests that rates of HIV testing are higher among people who have previously been tested for HIV [22–26]. The RRtest(t) rate ratio is introduced to take this potentially higher re-testing rate into account. PLHIV who are unaware of their status could also test at higher or lower rates than individuals who are HIV-susceptible. Hence, potential differential testing rates in this group are accounted for with the RRunaware rate ratio. Further, the number of positive tests is often very large, such that the cumulative number of positive HIV tests reported by HTS programs substantially outstrips the number of PLHIV who could have been newly diagnosed. This points to a non-negligible fraction of PLHIV aware of their status and PLHIV receiving ART that may also be re-tested for HIV each year [27,28]. For example, in many countries (e.g. Côte d’Ivoire [29]; Mozambique, F. Mbofana, pers. comm.; Senegal [30]; Sierra Leone [31]; Uganda [32]), the annual numbers of positive tests reported can represent up to 25–30% of the whole estimated PLHIV population, which is inconsistent with survey data on the proportion of PLHIV ever tested. To reproduce the number of positive tests, and in line with empirical evidence, we allowed re-testing of diagnosed PLHIV using the time-varying RRaware(t) rate ratio. Finally, PLHIV on treatment could also be re-tested for HIV, albeit at lower rate, through the RRart rate ratio. The main studies informing differential testing rates are summarized in supplementary material (Tables S1–S2).
Lastly, we consider that HTS uptake will depend on the proportion of untreated PLHIV experiencing HIV/AIDS-related symptoms who are not on ART. OIs is the time-invariant incidence of opportunistic infection by CD4+ cell count category s[33,34] (as tracked in Spectrum) and ρk(t) is the sex-specific proportion of these infections that are tested for HIV at time t (see Text S1 for full details on the model).
Data sources, likelihood function, and model calibration
Two main data sources are used for model calibration: household survey data about the proportion of adults who self-report having ever been tested for HIV; and HTS program data about the total number of HIV tests conducted each year and number of HIV-positive tests. For national surveys, we used the proportion of respondents reporting having ‘ever been tested and received the result of the last HIV test’, stratified by sex, age (15–24, 25–34, and 35–49 years), and, if available, HIV serostatus from nationally representative household surveys, including DHS, AIS, Multiple Indicator Cluster Surveys (MICS), PHIA surveys, and relevant country-specific surveys (e.g. South African National HIV Prevalence, Incidence, Behavior and Communication Survey; Kenya AIDS Indicator Survey). For surveys that do not include HIV serostatus (e.g. some MICS and DHS), data on ever testing by age and sex, irrespective of HIV status, are used in model calibrations. We assume that self-reports of ‘ever having been tested and receiving the result of the last HIV test’ are unbiased estimates of HIV testing history.
For HTS program data, Shiny90 can also be calibrated to the annual number of HIV tests performed in the population (≥15 years) and, if available, the number of positive tests (≥15 years; stratified by sex or overall). Such data may be useful to inform testing trends after the last population-based survey has been performed. Details of the likelihood specification can be found in the supplemental material (Text S2).
Notably, we purposely excluded two commonly referenced data types from surveys as potential inputs into Shiny90. First, information on HIV testing in the past year is not used in model calibration due to evidence that this likely overstates the true annual testing rate [14,35], perhaps due to ‘telescoping bias’ in which respondents may inadvertently recall testing that occurred beyond the last 12 months [36] (see supplemental materials, Text S3). Second, information on self-reported awareness of HIV-positive status, even when partially adjusted for detection of ART among PLHIV who report not knowing their status, is not incorporated due to evidence of systematic nondisclosure of knowledge of status [9,10,37–44]. In particular, nondisclosure of HIV status was found to be 1.4 times higher among individuals not on ART in Mozambique [38] compared to those on ART. This implies that adjustments for presence of ART metabolites may be insufficient, especially when ART coverage is low.
Model parameters were estimated using a Bayesian framework. To constrain the parameters space to plausible values in data-limited settings, we elicited prior distributions following a review of the literature (Tables S1–S2; prior distributions are described in Text S1). Posterior modes of model parameters were obtained via nonlinear optimization using the Broyden-Fletcher-Goldfarb-Shanno algorithm [45]. The joint posterior distribution was estimated using a Laplace approximation [46,47] and 95% credible intervals (CrIs) for quantities of interest were obtained by sampling 3000 parameter sets from this approximated joint posterior distribution. The posterior distributions of relevant outputs were summarized using their median, and 2.5th and 97.5th percentiles. This calibration method was chosen for its computational efficiency. Table S3 presents comparisons of summary statistics of the posterior distributions of selected model outputs using the Laplace approximation, Sampling Importance Resampling (SIR), and the Incremental Mixture Importance Sampling (IMIS) [48] algorithms. These suggest good performance of the Laplace approximation in our settings.
All analyses were conducted in the R statistical software [49]. The system of ordinary differential equations was solved using a Euler algorithm with a time step of 0.1 years. All functions are available for download from a Github repository (https://github.com/mrc-ide/first90release).
Model outputs (estimates)
Shiny90 generates outputs for comparisons to input data and several indicators of interest. It estimates the total number of HIV tests (negatives and positives), tests among first-time testers, positivity (the percentage of positive tests among all tests), yield (the percentage of new HIV diagnoses among all tests), the proportion of the population ever tested for HIV, the proportion of PLHIV who know their HIV status, and other indicators (Table S4).
Model validation
There are few empirical estimates of knowledge of status among PLHIV, and, as described earlier, these self-reported estimates are likely to reflect substantial under-reporting of HIV awareness [9,10,37–44]. We therefore validated Shiny90 by performing both in-sample comparisons (A) and out-of-sample predictions (B and C) of the proportion of the population ever tested for HIV (stratified by sex and HIV status). We focused our analyses on three countries with multiple surveys and availability of HTS program data: Côte d’Ivoire, Malawi, and Mozambique. For the out-of-sample predictions, we first excluded all surveys conducted after 2012 and all HTS program data after the last available pre-2012 survey (B). This was performed to examine the model's ability to predict testing histories over a time horizon of approximately 5 years (the time interval often observed between two population-based surveys). We then re-calibrated the model, this time incorporating the post-2012 HTS program data. To appreciate the added value of the HTS program data sources, we re-calibrated our model both on the sex-combined (C1) and sex-disaggregated HTS data (C2). In the case of Mozambique, available HTS program data were not stratified by sex and we instead used the fully calibrated model (A) to predict sex-stratified HTS program data (2009–2017) which was then used for the out-of-sample validation (C2). The data sources used for model calibration are presented in Table 1.
Table 1.
List of surveys of with information on the proportion of respondents having ever been tested for HIV (2000–2017) and HIV testing services program data used to calibrate Shiny90 in Côte d’Ivoire, Malawi, and Mozambique.
| Data types | Côte d’Ivoire | Malawi | Mozambique |
| Surveys | MICS 2000a (women only) | DHS 2004 | DHS 2003 |
| AIS 2005 | MICS 2006a (women only) | MICS 2008* (women only) | |
| DHS 2012 | DHS 2010 | AIS 2009 | |
| MICS 2016a (E) | MICS 2014a (E) | DHS 2011* | |
| PHIA 2017b (E) | DHS 2015 (E) | AIS 2015 (E) | |
| PHIA 2016 (E) | |||
| HIV testing services program data | Direction de l’information, de la planification et de l’évaluation (total tests and number positives for 2010–2017; sex-disaggregated for 2014–2017) | Malawi Integrated HIV Program Report (total tests and number positives 2003–2017; sex-disaggregated for 2013–2017c) | National HIV/AIDS Control Program (F. Mbofana, personal communication). (2013–2017; total tests and number positives) |
E: Indicates that the survey was excluded in the out-of-sample validation analyses.
AIS, AIDS Indicator Survey; DHS, Demographic and Health Survey; MICS, Multiple Indicator Cluster Survey; PHIA, Population-based HIV Impact Assessment.
aSurvey does not include serology and estimates of ‘ever tested for HIV’ cannot be stratified by HIV status.
bThe 2017 PHIA survey in Côte d’Ivoire has yet to be released in the public domain. Preliminary results from this survey were used in the model calibration and validation but the relevant point estimates cannot be presented at this time.
cOnly the total number of sex-disaggregated tests is available and the number of positive tests is for both sex combined.
Ethics
All analyses were performed on anonymized and de-identified data. Further, all DHS/AIS survey protocols have been approved by the Internal Review Board of ICF International in Calverton (USA) and by the relevant country authorities for other surveys (MICS and PHIA). Further information on the ethics approval can be found in the individual country reports. Ethics approval for secondary data analyses was obtained from McGill University's Faculty of Medicine Institutional Review Board (A10-E72-17B).
Results
Description of survey data on HIV testing history and HTS programs data
In each of the three countries, the survey data for the proportion of the population reporting having ever been tested for HIV and receiving the last test's result increased from under 15% at the beginning of the 2000 to 50% in Côte d’Ivoire (2016), 75% in Malawi (2016), and 51% in Mozambique (2015) (Fig. 2). Women are more likely to report having ever been tested than men. As for age, the highest proportions of participants reporting a history of HIV testing is consistently found in the 25–34-year-old age group in all three countries. Testing among PLHIV is higher than in the general population, with survey estimates indicating that 68% and 93% of PLHIV in Malawi (in 2016) and Mozambique (in 2015), respectively, report a history of HIV testing (Fig. 2).
Fig. 2.
Comparison of calibrated Shiny90 model fits with programmatic and survey data for Côte d’Ivoire (first column), Malawi (second column), and Mozambique (third column) over 2000–2017.
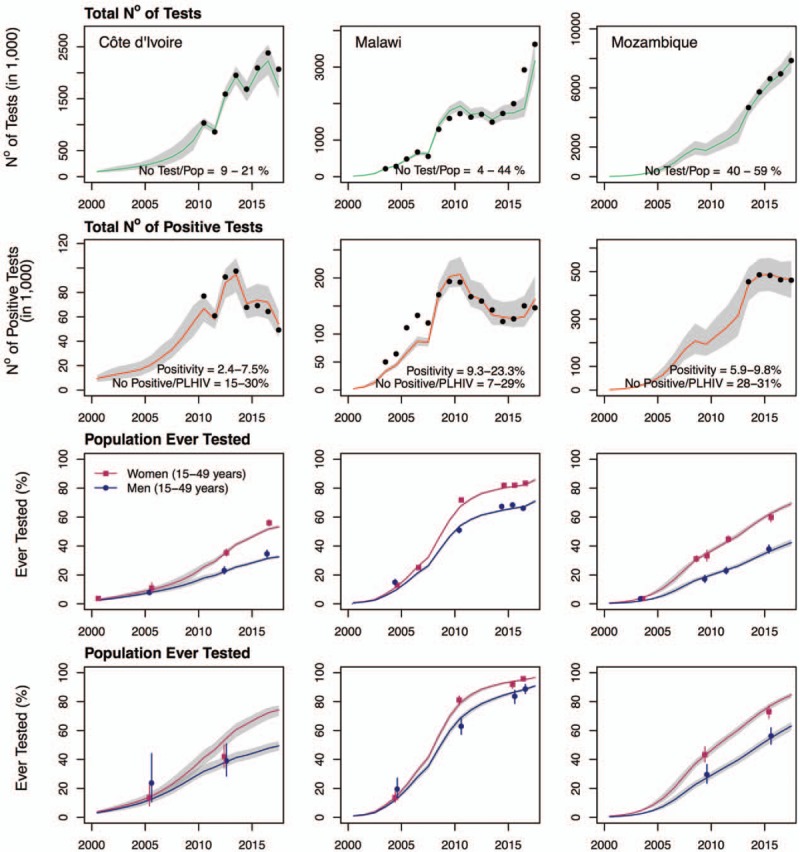
The shaded areas on all graphs correspond to the 95% credible intervals of the posterior estimates, with the lines corresponding to the median. Black dots on the first and second row of graphs correspond to the reported HIV testing services program data for the overall number of tests (top row) and number of positive tests (second row; see Table 1 for details). The points on the third and fourth rows of graphs are the survey estimates of the proportion ever tested among women (squares) and men (circles) among the overall population (third row) and people living with HIV (PLHIV; bottom row). The lines crossing the points are the 95% confidence intervals of the survey estimates.
The HTS program data suggest that a substantial number of HIV tests are administered annually. For example, the reported maximum annual number of tests performed corresponds to 21% of the population aged 15–49 years old in Côte d’Ivoire, 49% in Malawi, and 59% in Mozambique (Fig. 2). Concomitant with important increases in total testing volume, the number of positive tests has decreased in all three countries, resulting in downward trends in positivity rates. In addition, the number of positive tests reported in HTS program data suggests that a substantial fraction of diagnosed PLHIV could be re-tested every year. For example, the volume of positive tests corresponds to the equivalent of up to 30% of the total PLHIV population aged 15–49 years in Côte d’Ivoire, 29% in Malawi, and 31% in Mozambique. If these were all new diagnoses, we would expect that close to all PLHIV should be aware of their status within a few years.
In-sample comparisons: calibration on all available survey and HTS program data (A)
The calibrated Shiny90 models for Côte d’Ivoire, Malawi, and Mozambique can accurately reproduce annual HTS program data both for the total number of HIV tests performed and the number of positive tests (Fig. 2). In addition, the model adequately reflects sex-specific survey estimates of the proportion of respondents ever tested for HIV. In 2017, these were estimated by Shiny90 to be 33%, 71%, and 42% among men in Côte d’Ivoire, Malawi, and Mozambique, respectively. Testing was notably higher among women, with 53% (Côte d’Ivoire), 86% (Malawi), and 69% (Mozambique) of women reporting having ever been tested for HIV. Overall, for 2017, average testing rates were estimated to be 0.17 per year in Côte d’Ivoire, 0.21 per year in Malawi, and 0.52 per year in Mozambique.
Shiny90 is also able to replicate longitudinal trends in the proportion of PLHIV ever tested. It estimates that 66%, 95%, and 76% of PLHIV in Côte d’Ivoire, Malawi, and Mozambique, respectively, have ever been tested for HIV in 2017. In turn, knowledge of HIV status is estimated by Shiny90 at 58% in Côte d’Ivoire, 84% in Malawi, and 72% in Mozambique. These numbers are within the range of values obtained from the previous UNAIDS methodology for 2017 in Côte d’Ivoire (54%; uncertainty range 38–75%) and Malawi (90%; 84 to >95%), but the previous estimate was lower in Mozambique (59%; 49–70%) [6].
Shiny90 also suggests important differences in knowledge of status by sex, with higher proportions of women being aware of their status than men. Knowledge of HIV status is greatest in older age groups in all three countries. It increases from 41% among 15–24-year-olds to 65% among 35–49-years-olds in Côte d’Ivoire, from 69% (15–24 years) to 89% (35–49 years) in Malawi, and from 57% (15–24 years) to 74% (35–49 years) in Mozambique for the year 2017. Consistent with previous literature suggesting nondisclosure of awareness of HIV status, the proportions of PLHIV aware of their status are higher than survey estimates of self-reported awareness, when these are available (Table 2 and Fig. 3). In Malawi, estimates of knowledge of status are generally between estimates of ART coverage and PLHIV ever tested. In Côte d’Ivoire and Mozambique, the ‘first 90’ is closer to the proportion of PLHIV ever tested because survey and HTS program data suggest high rates of re-testing. For example, the rate ratios for re-testing were estimated to be 3.7 (95% CrI 3.2–4.3) in Côte d’Ivoire and 7.2 (95% CrI 6.4–7.7) in Mozambique, as compared to 1.2 (95% CrI 1.1–1.4) in Malawi. Posterior estimates for the main model parameters are reported in supplemental materials (Table S5).
Table 2.
Comparisons of empirical survey estimates of the proportion of individuals aged 15–49 years ever tested for HIV (by sex and HIV status) and self-reported awareness status among PLHIV with Shiny90 model predictions from (a) the fully calibrated model and from out-of-sample predictions that (b) excluded all post-2012 survey and HIV testing services (HTS) program data, (c1) excluded all post-2012 survey data (included sex-combined HTS program data), and (c2) excluded all post-2012 survey data, but included sex-disaggregated HTS data.
| Comparisons | Predictions (95% CrI) | |||||
| Country/outcome | Survey and year | Survey estimates (95% CI) | (a) Full data calibration | (b) Excluding survey and HTS data | (c1) Excluding survey data only (HTS data sex-combined) | (c2) Excluding survey data only (HTS data sex-disaggregated) |
| Côte d’Ivoire | ||||||
| Women ever tested | MICS 2016 | 56% (54–58%) | 52% (51–53%) | 51% (43–65%) | 53% (49–58%) | 53% (51–55%) |
| Men ever tested | MICS 2016 | 35% (32–37%) | 32% (30–33%) | 35% (28–46%) | 36% (31–41%) | 29% (28–31%) |
| WLHIV ever tested | PHIA 2017 | NPD | 74% (70–77%) | 72% (64–83%) | 74% (69–79%) | 74% (73–76%) |
| MLHIV ever tested | PHIA 2017 | NPD | 49% (46–52%) | 52% (44–66%) | 54% (48–61%) | 47% (45–49%) |
| PLHIV aware (’first 90’) | PHIA 2017 | 37%a | 58% (53–61%) | 56% (47–70%) | 59% (53–64%) | 57% (55–58%) |
| Malawi | ||||||
| Women ever tested | PHIA 2016 | 83% (82–84%) | 82% (81–83%) | 88% (77–96%) | 91% (90–92%) | 89% (87–90%) |
| Men ever tested | PHIA 2016 | 66% (65–68%) | 67% (66–68%) | 61% (51–76%) | 64% (60–68%) | 68% (66–70%) |
| WLHIV ever tested | PHIA 2016 | 96% (94–97%) | 95% (94–96%) | 98% (93–100%) | 98% (98–99%) | 98% (97–98%) |
| MLHIV ever tested | PHIA 2016 | 89% (86–92%) | 88% (87–89%) | 86% (79–93%) | 87% (84–89%) | 90% (88–91%) |
| PLHIV aware (’first 90’) | PHIA 2016 | 76% (73–78%) | 81% (79–82%) | 81% (73–89%) | 83% (82–85%) | 84% (82–85%) |
| Mozambique | ||||||
| Women ever tested | AIS 2015 | 60% (57–63%) | 62% (61–64%) | 56% (48–67%) | 70% (64–77%) | 65% (62–68%) |
| Men ever tested | AIS 2015 | 38% (35–41%) | 36% (35–38%) | 28% (24–37%) | 38% (32–46%) | 38% (36–40%) |
| WLHIV ever tested | AIS 2015 | 73% (68–77%) | 77% (75–79%) | 74% (67–83%) | 84% (78–89%) | 80% (76–83%) |
| MLHIV ever tested | AIS 2015 | 56% (51–62%) | 55% (51–57%) | 48% (42–56%) | 57% (50–64%) | 57% (53–60%) |
| PLHIV aware (’first 90’) | AIS 2015 | 40% (37–44%) | 63% (60–65%) | 52% (44–63%) | 68% (61–72%) | 66% (61–69%) |
95% CrI, 95% credible interval; 95% CI, 95% confidence interval; HTS, HIV testing services; MICS, multiple indicators cluster survey; MLHIV, men living with HIV; NA, not available; NPD, not in public domain (but included in the calibration); PHIA, population-based HIV impact assessment; WLHIV, women living with HIV.
aAge group is 15–64 years and estimate is not adjusted for presence of antiretroviral metabolites.
Fig. 3.
Comparisons of calibrated Shiny90 model fits with survey data on proportion of people living with HIV (PLHIV) aged 15–49 years ever tested, model-predicted proportion of PLHIV aware of their status (’first 90’), and survey estimates of awareness status and Spectrum/EPP's antiretroviral therapy (ART) coverage estimates.
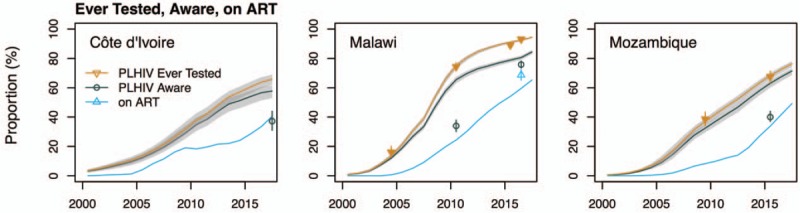
The shaded areas correspond to the 95% credible intervals of the posterior estimates. Estimates used for cross-validation are shown as empty symbols. (The self-reported estimate of awareness in Côte d’Ivoire corresponds to the 15–64-year age group.).
Out-of-sample predictions: removing both recent survey and HTS program data (B)
When excluding all data from surveys conducted after 2012 and HTS program data after the last survey, Shiny90's predictions underestimate by 6% points the 2016 survey estimate of the proportion of the population ever tested for HIV in Côte d’Ivoire (susceptible and PLHIV combined). In Malawi, the model's out-of-sample predictions for 2016 are higher than the survey estimates among women by 5% points and underestimate the same proportion among men by 5% points, but both are included within the predicted uncertainty intervals (Table 2 and Fig. 4). Finally, in Mozambique, the proportion of women ever tested is overestimated by 4% in 2015, but, for men, the underestimation is 10% points for that same year.
Fig. 4.
Out-of-sample predictions (solid lines) of models calibrated to survey data from 2000 to 2012, excluding all program data, for Côte d’Ivoire, Malawi, and Mozambique, and model predictions for the 2013–2017 period.
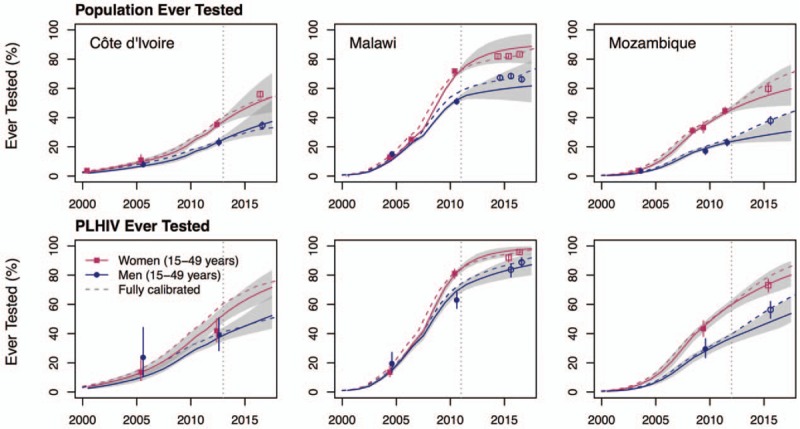
Dashed lines represent predictions from the fully calibrated models. The vertical lines indicate the date of the last survey data estimates included in the fitting (to the right of the lines are the predictions). The shaded areas correspond to the 95% credible intervals of the posterior estimates. Points represent the survey estimates (Table 1) and empty symbols indicate that these survey outcomes were not included in the likelihood but are shown for cross-validation purposes. The vertical solid lines crossing these points correspond to the 95% confidence intervals of the survey estimates. PLHIV, people living with HIV.
Estimates of ever testing among PLHIV are, arguably, a more relevant outcome to the ‘first 90’ than corresponding estimates among the overall population. For PLHIV, out-of-sample predictions are quite accurate, even over a full 5-year time horizon for the three countries (Fig. 4). In Côte d’Ivoire, the difference between the 2017 model prediction of the fraction of PLHIV ever tested and the empirical estimates is less than 3% points (for both sexes combined; empirical estimates from the Côte d’Ivoire PHIA are not yet in public domain and are not shown). A similar pattern is observed for Malawi with differences of less than 1 and 3% points between predictions and empirical estimates for women and men, respectively. In Mozambique, there are differences of 4 and 10% points of the proportion ever tested among men and women, respectively. As for the proportion of PLHIV aware of their status in 2017, the out-of-sample predictions are within 2% points of the ones obtained using full data calibration in Côte d’Ivoire and Malawi. In Mozambique, the difference is 13% points, but the uncertainty intervals are very wide and include the estimate from the full data calibration.
Out-of-sample predictions: added value of HTS program data (C)
Adding the post-2012 HTS program data (sex-combined; C1) yields mixed results with respect to improving estimates (Fig. 5, a). It adds little to the already accurate predictions in Côte d’Ivoire. In Malawi, however, it improves estimates for women but magnifies the underestimation in men (Table 2, Fig. 5). For Mozambique, HTS program data increase the accuracy of predictions for men, but result in overestimating the proportion of women ever tested. Predictions of knowledge of HIV status are nevertheless within 4% points of the ones obtained using the full data calibration, and have overlapping uncertainty intervals (Table 2).
Fig. 5.
Out-of-sample predictions of Shiny90 models calibrated to survey data from 2000 to 2012, including all available program data, for Côte d’Ivoire, Malawi, and Mozambique, and model predictions.
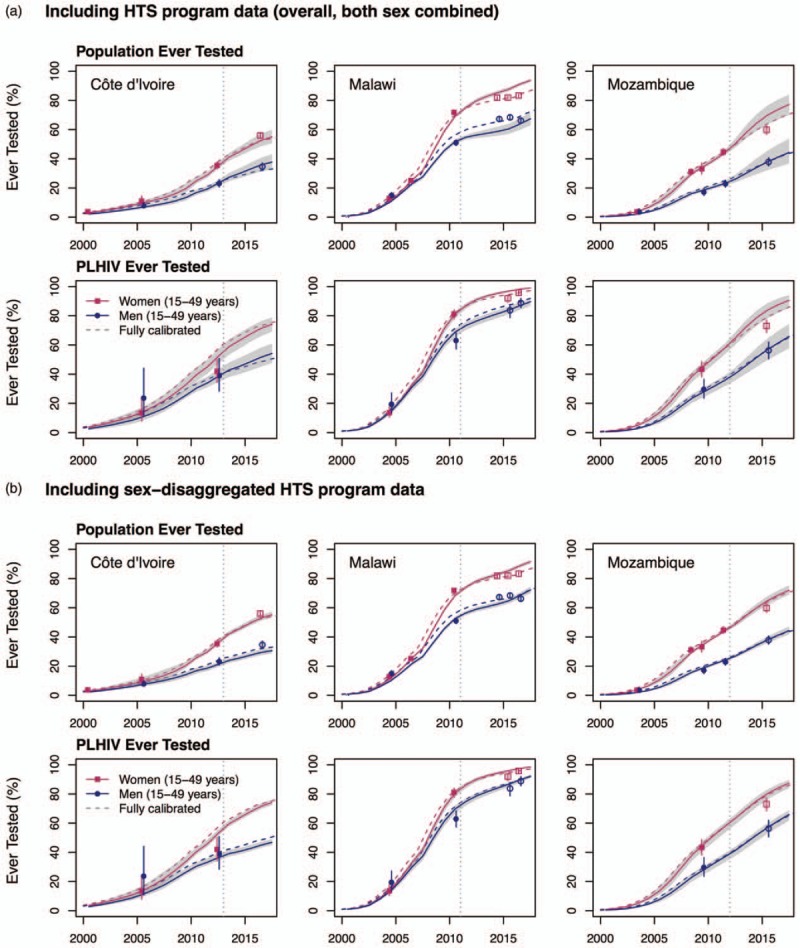
The top panel (a) uses the overall (both sex combined) HIV Testing Services (HTS) program data, whereas the bottom panel (b) uses the sex-disaggregated HTS program data. Dashed lines represent predictions from the fully calibrated models. The vertical lines indicate the date of the last survey data estimates included in the fitting (to the right of the lines are the predictions). The shaded areas correspond to the 95% credible intervals of the posterior estimates. Points represent the survey estimates (Table 1) and empty symbols indicate that these outcomes were not included in the likelihood but are shown for cross-validation purposes. The vertical solid lines crossing these points correspond to the 95% confidence intervals of the survey estimates. PLHIV, people living with HIV.
On the contrary, the sex-disaggregated HTS program data (C2) generally increase the accuracy of the predictions, in both the overall population and among PLHIV (Fig. 5, b). In all countries, the model's predictions for the proportion of the population ever tested for HIV were 6% or less points different from the predictions obtained using the full data calibration (Table 2). Among PLHIV, all predictions had overlapping uncertainty intervals with those of the empirical survey estimates, and differences were always less than 7% points. Predictions of the proportion of PLHIV aware of their status were also in very good agreement with those of the full data calibration, with differences of 3% points or less in all three countries.
Discussion
Knowledge of HIV status is a key indicator to monitor progress, identify bottlenecks, and ultimately implement effective HIV responses. In this study, we describe a new model that combines survey and HTS program data to estimate the ‘first 90’ in SSA. We validated Shiny90 through in-sample comparisons, and our results demonstrate that it can accurately reproduce longitudinal sex-specific trends in HIV testing among the overall population, and, more importantly, among PLHIV. Out-of-sample predictions of the fraction of individuals ever tested over a 4-to-6-year time horizon are also in good agreement with empirical survey estimates for PLHIV. When recent population-based surveys are not available, the accuracy of Shiny90's predictions for the proportion of the population ever tested for HIV is improved by the addition of sex-disaggregated HTS program data. Importantly, our out-of-sample validations provided estimates of the fraction of PLHIV aware of their status that are consistent with the ones obtained using full data calibration and their uncertainty intervals overlap.
We compared our results to empirical estimates of knowledge of status among PLHIV. As expected, Shiny90's predictions are higher than self-reported awareness status – even if adjusted for presence of antiretroviral metabolites. For example, our model-based estimates of knowledge of HIV status are 58% (95% CrI 53–61%) in Côte d’Ivoire, 81% (95% CrI 79–82%) in Malawi, and 63% (95% CrI 60–65%) in Mozambique, compared to 37% (not antiretroviral metabolites-adjusted), 76% (antiretroviral metabolites-adjusted), and 40% (antiretroviral metabolites-adjusted) of PLHIV who reported being aware of their HIV status in the 2017 Ivoirian, 2016 Malawian, and 2015 Mozambican surveys, respectively. The fundamental reason for the higher estimates of HIV status awareness is that the gap between the proportion of PLHIV ever tested, which are well reproduced by the model, and the proportion with knowledge of their status, is constrained by the high rate of testing (i.e. any PLHIV who have ever been tested, but are not aware, must have been infected since the most recent HIV test). For the model's predictions to be consistent with survey-based estimates of self-reported awareness, country-specific HIV incidence rates would need to be several-fold higher than the ones estimated by Spectrum/EPP and PHIA surveys, and/or re-testing rates among PLHIV ever tested would have to be much lower than suggested by the survey and HTS program data. Other possible explanations include over-reporting of HIV testing history by survey respondents who were not aware, or very substantial levels of return of false-negative HIV test results, though such levels would have to be extremely high considering the high levels of re-testing.
The Shiny90 model can be applied to countries with at least two population-based surveys that collected information on both HIV testing history and HIV prevalence. Because model predictions are expected to be more accurate over the short term, it is advisable to interpret with caution estimates produced for countries where the last population-based survey was conducted more than 5 years in the past. The HTS program data on the number of tests performed should be carefully assessed to ensure that it accurately represents annual national testing volume among the population aged 15 years and over, and that it includes information from both private and public sectors. Sex-disaggregated HTS program data should be especially useful for countries without recent survey estimates of HIV testing histories. However, model predictions should be reasonably accurate, even if sex-disaggregated HTS data are unavailable, if the male/female testing ratios have remained relatively constant in recent years. Finally, it is advised to ensure some temporal degree of overlap between survey and program data to facilitate estimation of re-testing parameters. The latter is especially important if countries wish to examine additional model outputs of interest. For example, the model can provide information on the distribution of negative tests among first-time and repeat testers, and the distribution of positive tests among new diagnoses, retests among PLHIV aware of their status (untreated), and re-tests among PLHIV on ART (Fig. 6).
Fig. 6.
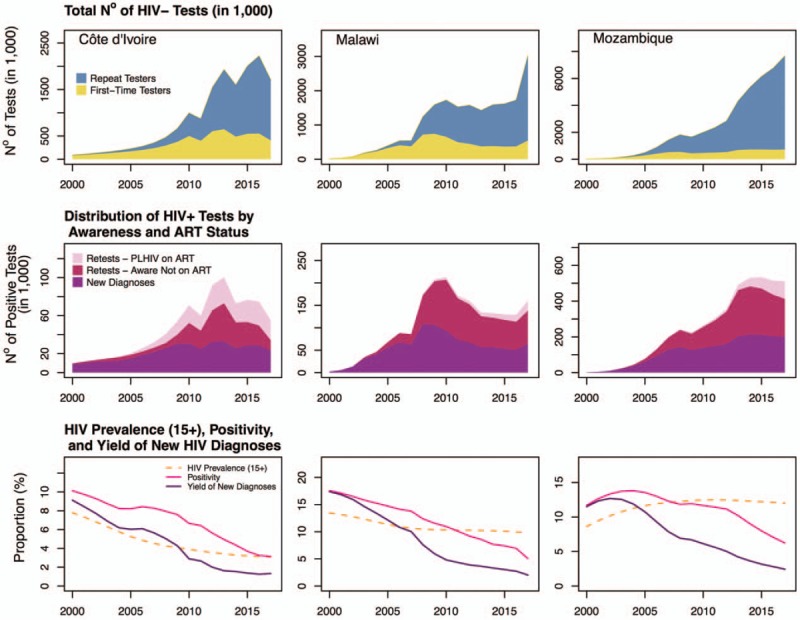
Model predictions of the distribution of the annual total number of HIV-negative tests performed among first-time testers versus repeat testers (top row), distribution of HIV positive tests by awareness status and antiretroviral treatment (ART) status (middle row), and longitudinal trends in HIV testing positivity, yield of new diagnoses, and Spectrum/EPP's estimates of HIV prevalence (aged 15+ years; bottom row); in Côte d’Ivoire (first column), Malawi (second column), and Mozambique (third column).
To facilitate model use, an online version was developed using the RShiny framework. Users can freely access the web-app (https://shiny.dide.imperial.ac.uk/shiny90/), review data sources, edit information, add new data, and run the model. It requires the users to provide a Spectrum/EPP projection file to use as input. Users can save their current analyses, perform sensitivity analyses, and export their results.
Model limitations
Our proposed approach to estimate the ‘first 90’ has several limitations, mainly due to data considerations. First, we assumed that self-reports of ever testing are accurate. Quantifying the sensitivity and specificity of those self-reports is difficult and their accuracy could differ by HIV status [14]. However, limited evidence suggests that testing histories are probably better reported than other potential indicators [50], but incorrect reports of HIV testing history could result in underestimation of the ‘first 90’ [51]. As evidence accrues on the sensitivity and specificity of those self-reports, adjustments for potential misclassification, if warranted, could be incorporated into the model. An additional source of uncertainty lies in the accuracy of HIV tests results provided back to HTS users. In the model, we assume that national HIV testing algorithms are accurate, but some programs have reported suboptimal field sensitivity and specificity [52–54]. Second, published HTS program statistics usually relate to public sector programs and do not necessarily reflect private sector testing, NGO testing programs, and self-testing. The latter poses additional challenges to the correct estimation of the number of HIV tests performed annually and difficulty in assessing trends over time in terms of positivity and yield of new diagnoses. We recommend sensitivity analyses to explore model robustness to assumptions regarding completeness of HTS program data. Thirdly, some national programs may have difficulties differentiating between tests performed on children aged less than 15 years from those in the modeled population. Data from Malawi suggest that children (<15 years) can comprise a small but nonnegligible fraction (∼16%) of overall testing volume [55], though pediatric tests account for a substantially lower fraction of HIV-positive tests due to the low HIV prevalence in children, the result of effective prevention of mother-to-child transmission programs.
Regarding model structure and assumptions, a fourth limitation is that the present model implementation does not incorporate uncertainty in both the denominator of the ‘first 90’ and the estimated ART coverage. This may result in an underestimation of uncertainty. Finally, the model does not, at present, disaggregate indicators by members of key populations (e.g. MSM, female sex workers, clients) or produce estimates of HIV diagnosis among children. Key populations are important to overall transmission dynamics in several countries [56–59], and the sustainable control of HIV epidemics also hinges on also achieving the 90–90–90 targets in these groups [60]. The general framework outlined above could in theory be used to monitor awareness status for key populations, but additional challenges related to representativeness of key population surveys, among others, are expected [13].
Model strengths
Our proposed approach to estimate the proportion of PLHIV who know their status has several strengths. First, our model uses Spectrum outputs and is therefore fully consistent with other epidemiological data (e.g. sex and age-specific HIV incidence, prevalence, mortality) and programmatic outcomes (ART coverage). Second, it integrates routinely collected HTS program data with population-based surveys. These data triangulation enables monitoring of HTS’ effectiveness by providing estimates of annual new HIV diagnoses. Third, our approach attempts to overcome the limitations of self-reported knowledge of HIV status by pooling information on ART coverage, HIV re-testing rates, and HIV incidence to estimate how many PLHIV acquired their infection after their last HIV-negative test. Finally, the present framework enables us to further refine the Shiny90 model and its assumptions as more granular program data become available (e.g. age-stratified HIV testing program data) and provides a foundational framework for future work to incorporate data about HIV testing and diagnosis into estimates of HIV incidence trends.
Conclusions
Identifying the proportion of PLHIV who know their status is challenging and the aim of our model is to triangulate different data sources to improve the accuracy of the ‘first 90’ indicator. Beyond the estimation of HIV knowledge of status, the model also produces estimates of annual number of new HIV diagnoses. Such information can help countries improve the effectiveness of their HIV testing programs and assist them in reaching the ‘first 90’ target by 2020.
Acknowledgements
We are grateful to Shelley Clark for her comments and suggestions and to Alexandra Hill and Martin Eden for developing the Shinny 90 app. In addition, we would like to thank Roma Bhatkoti, Drew Voetsch, Joshua Salomon, John Stover, Ray Shiraishi and other participants of the UNAIDS Reference Group on Modelling, Estimates, and Projections for useful feedback on an earlier version of the proposed modeling framework.
We acknowledge funding from the Steinberg Fund for Interdisciplinary Global Health Research (McGill University) and the Bill and Melinda Gates Foundation. MMG's research program is funded by a career award from the Fonds de recherche du Québec – Santé. JWE was supported by UNAIDS and the Bill and Melinda Gates Foundation.
Authors’ contribution: C.H., D.B., J.W.E., K.M., M.M.G., and R.B. conceived and designed the model. A.G., A.J., A.K., C.D., C.L.D., F.M., J.W.E., K.M., and M.M.G. obtained, administered, and processed the different databases. A.G., A.J., C.D., J.E., J.L., K.M., M.C.B., M.E., and M.M.G. contributed to model development and/or revisions. A.G., C.D., C.L.D., J.W.E., and M.M.G. performed the analyses and all authors contributed to results interpretation. M.M.G. drafted the manuscript and all authors critically reviewed it for important intellectual content. All authors approved the final version.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015; 528:S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, et al. Defeating AIDS: advancing global health. Lancet 2015; 386:171–218. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. 90-90-90: an Ambitious Treatment Target to Help End the AIDS Epidemic. 2014; Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 33. [Google Scholar]
- 5.UNAIDS. Fast-Track: Ending the AIDS Epidemic by 2030. 2014; Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 36. [Google Scholar]
- 6.UNAIDS. UNAIDS Data 2018. 2018; Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS), 370. [Google Scholar]
- 7.UNAIDS. Global AIDS Response Progress Reporting 2016 - Construction of core indicators for monitoring the 2011 United Nations Political Declaration on HIV and AIDS. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2016. [Google Scholar]
- 8.Staveteig S, Wang S, Head S, Bradley S, Nybro E. Demographic patterns of HIV testing uptake in sub-Saharan Africa. In: DHS Comparative Reports No 30. Calverton, MD: ICF International; 2013. pp. 81. [Google Scholar]
- 9.Kim AA, Mukui I, Young PW, Mirjahangir J, Mwanyumba S, Wamicwe J, et al. Undisclosed HIV infection and antiretroviral therapy use in the Kenya AIDS indicator survey 2012: relevance to national targets for HIV diagnosis and treatment. AIDS 2016; 30:2685–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishel J, Barrère B, Ksihor S. Validity of self-reported HIV status and implications for measurements of ARV coverage in Malawi. Calverton, MD: DHS Working Papers No. 81. ICF International; 2012. [Google Scholar]
- 11.Staveteig S, Croft TN, Kampa KT, Head SK. Reaching the ’first 90’: gaps in coverage of HIV testing among people living with HIV in 16 African countries. PLoS One 2017; 12:e0186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS,. Global AIDS Response Progress Reporting. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2016. [Google Scholar]
- 13.Hakim AJ, MacDonald V, Hladik W, Zhao J, Burnett J, Sabin K, et al. Gaps and opportunities: measuring the key population cascade through surveys and services to guide the HIV response. J Int AIDS Soc 2018; 21: Suppl 5: e25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS 2015; 29:1401–1409. [DOI] [PubMed] [Google Scholar]
- 15.UNAIDS. UNAIDS Data 2017. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2017. [Google Scholar]
- 16.Avenir Health. Spectrum software. Spectrum v. 5.72Glastonbury, CT: Avenir Health; 2017. [Google Scholar]
- 17.Stover J, Brown T, Puckett R, Peerapatanapokin W. Updates to the Spectrum/Estimations and Projections Package model for estimating trends and current values for key HIV indicators. AIDS 2017; 31: Suppl 1: S5–S11. [DOI] [PubMed] [Google Scholar]
- 18.Paintsil E, Andiman WA. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr 2009; 21:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Prevention of Mother-to-Child Transmission (PMTCT): Briefing Note. 2007; Geneva, Switzerland: World Health Organization, 18. [Google Scholar]
- 20.Kirakoya-Samadoulougou F, Jean K, Maheu-Giroux M. Uptake of HIV testing in Burkina Faso: an assessment of individual and community-level determinants. BMC Public Health 2017; 17:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean K, Anglaret X, Moh R, Lert F, Dray-Spira R. Barriers to HIV testing in Côte d’Ivoire: the role of individual characteristics and testing modalities. PLoS One 2012; 7:e41353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettifor A, MacPhail C, Suchindran S, Delany-Moretlwe S. Factors associated with HIV testing among public sector clinic attendees in Johannesburg, South Africa. AIDS Behav 2010; 14:913–921. [DOI] [PubMed] [Google Scholar]
- 23.Dalal S, Lee CW, Farirai T, Schilsky A, Goldman T, Moore J, et al. Provider-initiated HIV testing and counseling: increased uptake in two public community health centers in South Africa and implications for scale-up. PLoS One 2011; 6:e27293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luseno WK, Wechsberg WM. Correlates of HIV testing among South African women with high sexual and substance-use risk behaviours. AIDS Care 2009; 21:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isingo R, Wringe A, Todd J, Urassa M, Mbata D, Maiseli G, et al. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Trop Med Int Health 2012; 17:e15–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.South A, Wringe A, Kumogola Y, Isingo R, Manyalla R, Cawley C, et al. Do accurate HIV and antiretroviral therapy knowledge, and previous testing experiences increase the uptake of HIV voluntary counselling and testing? Results from a cohort study in rural Tanzania. BMC Public Health 2013; 13:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakim AJ, Mukasa B, Hundley L, Odiit M, Ogwal M, Sendagala S, et al. Correlates of undiagnosed HIV infection and retesting among voluntary HIV testing clients at Mildmay Clinic, Uganda. AIDS Behav 2019; 23:820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DIPE. Rapport annuel des indicateurs VIH du secteur santé en Côte d’Ivoire 2014 - Non consolidé. Abidjan, Côte d’Ivoire: Direction de l’Information, de la Planification et de l’Évaluation. Ministère de la santé et de la lutte contre le sida; 2015. [Google Scholar]
- 30.CNLS. Rapport Annuel 2015 du Conseil de Lutte contre le Sida du Sénégal. 2016; Dakar, République du Sénégal: Conseil National de Lutte contre le Sida - Sécrétariat Éxecutif, 26. [Google Scholar]
- 31.NAS. Sierra Leone National AIDS Response Progress Report 2014. 2014; Freetown, Sierra Leone: National AIDS Secretariat, 25. [Google Scholar]
- 32.UAC. Uganda HIV/AIDS Country Progress Report, July 2016-June 2017. 2017; Kampala, Uganda: Uganda AIDS Commission, 97. [Google Scholar]
- 33.Anglaret X, Minga A, Gabillard D, Ouassa T, Messou E, Morris B, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 2012; 54:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr 2006; 42:464–469. [DOI] [PubMed] [Google Scholar]
- 35.Karon JM, Song R, Brookmeyer R, Kaplan EH, Hall HI. Estimating HIV incidence in the United States from HIV/AIDS surveillance data and biomarker HIV test results. Stat Med 2008; 27:4617–4633. [DOI] [PubMed] [Google Scholar]
- 36.Croyle R, Loftus E. Ostrow D, Kessler R. Recollection in the kingdom of AIDS. Springer, Methodological Issues in AIDS Behavioral Research. Boston, MA: 2002. [Google Scholar]
- 37.Rohr JK, Xavier Gomez-Olive F, Rosenberg M, Manne-Goehler J, Geldsetzer P, Wagner RG, et al. Performance of self-reported HIV status in determining true HIV status among older adults in rural South Africa: a validation study. J Int AIDS Soc 2017; 20:21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuente-Soro L, Lopez-Varela E, Augusto O, Sacoor C, Nhacolo A, Honwana N, et al. Monitoring progress towards the first UNAIDS target: understanding the impact of people living with HIV who re-test during HIV-testing campaigns in rural Mozambique. J Int AIDS Soc 2018; 21:e25095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCusker J, Stoddard AM, McCarthy E. The validity of self-reported HIV antibody test results. Am J Public Health 1992; 82:567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher DG, Reynolds GL, Jaffe A, Johnson ME. Reliability, sensitivity and specificity of self-report of HIV test results. AIDS Care 2007; 19:692–696. [DOI] [PubMed] [Google Scholar]
- 41.Marzinke MA, Clarke W, Wang L, Cummings V, Liu TY, Piwowar-Manning E, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis 2014; 58:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan AK, Savage EJ, Lowndes CM, Paul G, Murphy G, Carne S, et al. Nondisclosure of HIV status in UK sexual health clinics: a pilot study to identify nondisclosure within a national unlinked anonymous seroprevalence survey. Sex Transm Infect 2013; 89:120–121. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez TH, Kelley CF, Rosenberg E, Luisi N, O’Hara B, Lambert R, et al. Lack of awareness of human immunodeficiency virus (HIV) infection: problems and solutions with self-reported HIV serostatus of men who have sex with men. Open Forum Infect Dis 2014; 1:ofu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mooney AC, Campbell CK, Ratlhagana MJ, Grignon JS, Mazibuko S, Agnew E, et al. Beyond social desirability bias: investigating inconsistencies in self-reported HIV testing and treatment behaviors among HIV-positive adults in North West Province, South Africa. AIDS Behav 2018; 22:2368–2379. [DOI] [PubMed] [Google Scholar]
- 45.Nash J. Compact Numerical Methods for Computers: Linear Algebra and Function Minimisation. 2nd edBristol, England: Adam Hilger; 1990. [Google Scholar]
- 46.Gelman A, Carlin J, Stern H, Dunson D, Vehtari A, Rubin D. Bayesian Data Analysis. 3rd edBoca Raton, FL: CRC Press; 2014. [Google Scholar]
- 47.Tierney L, Kadane JB. Accurate approximations for posterior moments and marginal densities. J Am Stat Assoc 1986; 81:82–86. [Google Scholar]
- 48.Raftery AE, Bao L. Estimating and projecting trends in HIV/AIDS generalized epidemics using Incremental Mixture Importance Sampling. Biometrics 2010; 66:1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team. R: A Language and Environment for Statistical Computing. R version 3.5.1Vienna, Austrial: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 50.An Q, Chronister K, Song R, Pearson M, Pan Y, Yang B, et al. Comparison of self-reported HIV testing data with medical records data in Houston, TX 2012-2013. Ann Epidemiol 2016; doi: 10.1016/j.annepidem.2016.02.013 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Rentsch C, Reniers G, Machemba R, Slaymaker E, Martson M, Wringe A, et al. Nondisclosure of HIV testing history in population-based surveys: implications for estimating a UNAIDS 90-90-90 target. Global Health Action 2018; 11:1553470. [Google Scholar]
- 52.Bock P, Phiri C, Piwowar-Manning E, Kosloff B, Mandla N, Young A, et al. Understanding low sensitivity of community-based HIV rapid testing: experiences from the HPTN 071 (PopART) trial in Zambia and South Africa. J Int AIDS Soc 2017; 20: Suppl 6: 21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kravitz Del Solar AS, Parekh B, Douglas MO, Edgil D, Kuritsky J, Nkengasong J. A commitment to HIV diagnostic accuracy: a comment on ‘Towards more accurate HIV testing in sub-Saharan Africa: a multisite evaluation of HIV RDTs and risk factors for false positives ’and’ HIV misdiagnosis in sub-Saharan Africa: a performance of diagnostic algorithms at six testing sites’. J Int AIDS Soc 2018; 21:e25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kosack CS, Page AL, Beelaert G, Benson T, Savane A, Ng’ang’a A, et al. Towards more accurate HIV testing in sub-Saharan Africa: a multisite evaluation of HIV RDTs and risk factors for false positives. J Int AIDS Soc 2017; 19:21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MoH. Integrated HIV Program Report, January-March 2016. 2016; Lilongwe, Malawi: Ministry of Health, Government of Malawi, 68. [Google Scholar]
- 56.Maheu-Giroux M, Vesga JF, Diabate S, Alary M, Baral S, Diouf D, et al. Changing dynamics of HIV transmission in Côte d’Ivoire: modeling who acquired and transmitted infections and estimating the impact of past HIV interventions (1976-2015). J Acquir Immune Defic Syndr 2017; 75:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maheu-Giroux M, Vesga JF, Diabate S, Alary M, Baral S, Diouf D, et al. Population-level impact of an accelerated HIV response plan to reach the UNAIDS 90-90-90 target in Cote d’Ivoire: Insights from mathematical modeling. PLoS Med 2017; 14:e1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukandavire C, Walker J, Schwartz S, Boily MC, Danon L, Lyons C, et al. Estimating the contribution of key populations towards the spread of HIV in Dakar, Senegal. J Int AIDS Soc 2018; 21: Suppl 5:: e25126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanser F, de Oliveira T, Maheu-Giroux M, Bärnighausen T. Concentrated HIV subepidemics in generalized epidemic settings. Curr Opin HIV AIDS 2014; 9:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maheu-Giroux M, Diabate S, Boily MC, Jean-Paul N, Vesga JF, Baral S, et al. Cost-effectiveness of accelerated HIV response scenarios in Côte d’Ivoire. J Acquir Immune Defic Syndr 2019; 80:503–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


