Abstract
Frailty, one appealing target for improving successful aging of the elderly population, is a common clinical syndrome based on the accumulation of multisystemic function declines and the increase in susceptibility to stressors during biological aging. The age-dependent senescence, the frailty-related stem cell depletion, chronic inflammation, imbalance of immune homeostasis, and the reduction of multipotent stem cells collectively suggest the rational hypothesis that it is possible to (partially) cure frailty with stem cells. This systematic review has included all of the human trials of stem cell therapy for frailty from the main electronic databases and printed materials and screened the closely related reviews themed on the mechanisms of aging, frailty, and stem cells, to provide more insights in stem cell strategies for frailty, one promising method to recover health from a frail status. To date, a total of four trials about this subject have been registered on clinicaltrials.gov. The use of mesenchymal stem cells (MSCs), doses of 100 million cells, single peripheral intravenous infusion, follow-up periods of 6–12 months, and a focus primarily on safety and secondarily on efficacy are common characteristics of these studies. We conclude that intravenous infusion of allogenic MSCs is safe, well tolerated, and preliminarily effective clinically. More preclinical experiments and clinical trials are warranted to precisely elucidate the mechanism, safety, and efficacy of frailty stem cell therapy.
Keywords: aging, frailty, stem cells, cell therapy, systematic review
Introduction
According to the 2018 Aging and Health report by the World Health Organization,1 by 2050, there will be an estimated population aged 60 years or more of 2 billion people, accounting for 22% of the whole population, nearly double that in 2015 (900 million, 9%). A longer life brings with it opportunities for society as a whole in many ways, which are heavily dependent on the person's health and successful aging. However, the cumulative decline in physical and mental capacity currently disturbing many old people brings negative implications to the added years.1 Hence, almost all countries are forced to face the challenge of ensuring that their social system and health system are ready for this demographic change. Under these circumstances, frailty, the most problematic manifestation of aging2 and one of the major parts of Comprehensive Geriatric Syndrome, is intensively associated with physical and mental function declines and deserves public attention.
An appropriate target to promote successful aging: frailty
Frailty is an independent clinical definition, different from both comorbidity (one of its etiology factors) and disability (one of its adverse outcomes).3 Frailty has recently been defined by a professional global task force as a progressive systematic decline of physiological reserves and an increase of vulnerability to minor stressors.4,5 The main clinical manifestations of frailty present as unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity. These symptoms, which are named Fried phenotypes,3 are more comprehensively presented as the Frailty Index (FI),6 taking into account multidimensional cumulative deficits, which are closely related with adverse clinical events, such as falls, fractures, disability, and mortality3,4,6
Several major tools are used to assess frailty5: (1) the Fried Phenotype Criteria and its rapid screen form7; (2) the FI and the Clinical Frailty Scale of Rockwood and Mitnitski, which is concerned with poly-morbidities; and (3) mixed models, such as the Frailty Criteria of the International Nutrition Society, the Study of the Osteoporotic Fractures Index, the Tilburg Frailty Indicator, and the Edmonton Frailty Scale. Among a world of measurements, currently, the most internationally well-accepted assessments are Fried's criteria and the FI. The FI and the Edmonton Frailty Scale are superior to other methods when predicting death.2,8,9
Based on a representative review (n = 61,500) and a cohort study (n = 16019),10,11 the general prevalence of frailty, which increases with age and is higher in women than in men, is 11.0%–14.9% among the population over 65 years and 40% among people over 80. According to the consensus, the prevalence of frailty in community dwellings was ∼3.5%–27% in the Asia-Pacific region, which was comparable to that in Europe and America.5 The steadily aging population base and a series of interlinked clinical frailty-related events collectively impose a heavy burden on the public health cost worldwide. Frail participants had an average total health cost of €2,476/year and prefrail participants of €2,056/year, which is approximately twice as high as that of the nonfrail (€1,217/year) in Spain.12 This situation received particular attention in Asia, where the elderly who urgently need health care are often unable to access enough publicly funded health care services.13
To decrease the cumulative vulnerability and dependence of the older population, which cause complicated demographic, health-related, and social problems, frailty can thus be selected as an appropriate target that we must urgently deal with. Therefore, our aim is to develop a good understanding of the potential mechanisms and the efficacy of matching therapy. Nevertheless, the optimal preventions and treatments are still poorly explored, and there are no specific, effective, and pathophysiology reversing strategies for the treatment of frailty.4,14
Biological aging combined with stressors: the driving force of frailty
Biological aging is natural and involves a gradual decline of physiological reserves; nevertheless, in frailty, this process is accelerated and concomitant with falling homeostasis.2,12 Among all of the aging symptoms elucidated by López-Otín et al.,15,16 stem cell exhaustion and altered intercellular communication are likely the ultimate characteristics contributing to the clinical manifestations of aging-related frailty.17–19 However, there is uncertainty regarding the precise level and the kind of these aging characteristics15,16 as they integrate with the accelerating cumulative decline of physiological reserves observed in aging-related frailty.2 Simply put, the age-related pathophysiology mechanism, combined with inner and outer stressors, which drive frailty, calls for a convenient regenerative strategy that is more effective than current therapeutic methods.8,20–23 Therefore, much attention has recently been focused on stem cell strategies, which possess promising potential.
Matched therapy strategy of frailty: stem cells
Embryonic stem cells (ESCs) and adult stem cells are two main categories of stem cells, along with the embryonic-like inducible pluripotent stem cells (iPSCs) derived from different somatic cells by activating the “Yamanaka factors” Oct4, Sox2, Klf4, and Myc (“OSKM”).24,25 Mesenchymal stem cells (MSCs), one subset of adult stem cells, have several advantages in frailty therapy: the wide autologous or allogenic sources of acquisition (bone marrow, adipose tissue, umbilical cord or cord blood, placenta, and peripheral blood)19,26 and the therapeutic properties of migration to inflammation and injury sites, differentiation into various tissue-specific precursor cells, secretion of trophic bioactive compounds, and mediation of immunomodulatory effects.24,27 There is, clearly, an opportunity to now apply stem cell strategies for the age-related and stressor-involved clinical condition of frailty for the aging population.
Although the subject of stem cell therapy for frailty has been considered by some leading research teams globally,19,24,28 few human trials are registered at clinicaltrials.gov at present,29,30 which act as the potential new landmarks of frailty therapy. To date, there is little agreement on frailty stem cell therapy,19,24,28 thus calling for more insights into this promising approach. The question how the aging process and relatively minor stressor events combine to build the foundation for frailty and why stem cell therapy is the favorable approach for treating aging-related frailty are the issues addressed in this systematic review.
Methods
Given the contradiction between the significance of frailty stem cell therapy and the limited numbers of human trials directly adopting stem cells as intervention to treat frailty, the search strategy was not just rigorously confined to randomized clinical trials of stem cell-based frailty therapy, but also included leading reviews elaborating on the stem cell function decline in frailty during biological aging and the promising potentials of stem cells in frailty treatment.
Search strategy
With inclusion and exclusion criteria prespecified as below, we identified recent publications reporting the advance of frailty, mainly addressing the pathophysiological mechanism and potential targets for stem cells, and all of the publications on stem cell treatments for frailty, by searching several main electronic databases (EMBASE, All EMBASE REVIEWS, MEDLINE, and Cochrane CENTRAL from Ovid SP; PUBMED; OpenGrey; CBM) and clinicaltrials.gov (November 22, 2018; in English and Chinese), using the key search strategy “stem cell AND frail,” with a series of Boolean operators. Two individuals carried out the database searches and screened abstracts or full texts independently; a third author resolved the disagreements. The relevant bibliographies were screened to further identify valuable publications.
Inclusion and exclusion criteria
Mainly, we included the studies in which the frailty patients were directly treated with allogeneic or autologous stem cells of different sources and in which the safety and efficacy were compared with the control counterparts treated with placebo (or not). Due to the low number of frailty stem cell trials completed presently and the high significance of this promising novel strategy of stem cells to treat frailty, some other types of important literature were also searched and screened as independent parts (not shown). We excluded studies that did not directly use stem cells as an intervention to treat frailty, such as the transplantation of hematopoietic stem cells for diseases that include leukemia.
Data extraction
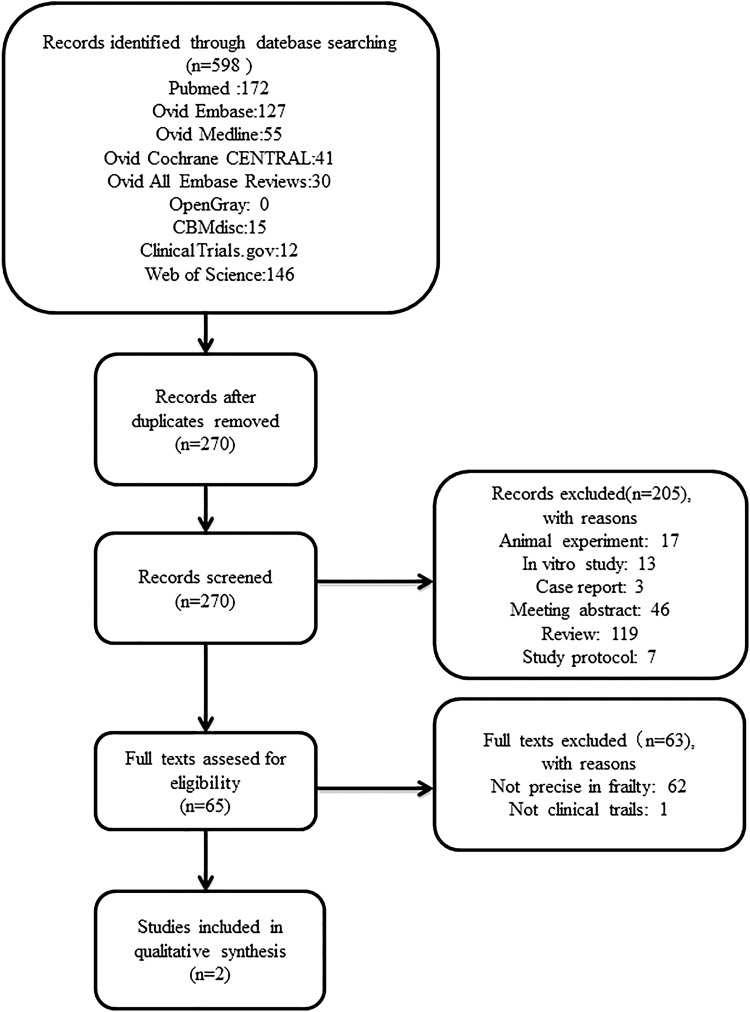
The following items were extracted by two individuals independently from each included study and registered clinical trial: reference details (title and date); condition and interventions (stem cell type, dose, delivery route, and frequency); aims and characteristics (study type, phase, and study design); recipients (age and sex); and main outcome measures. When safety and efficacy tests were performed serially, we schemed to extract the data at the different time points in the safety part but only extracted data for the final time point in the efficacy part, for acute and chronic adverse reactions were both indispensable for the safety assessment. For missing or incomplete data, we requested them from the authors or else estimated numerical values by digital ruler software. The flowchart is shown in Figure 1.
FIG. 1.
Flowchart of the literature retrieving and screening.
Results
The design characteristics of the included allogenic bone marrow derived MSC studies for frailty
Regarding the one human trial of the only two original articles included so far, this project was launched as a phase I/II, randomized, blinded, and placebo-controlled clinical trial (No. NCT02065245) and was named as CRATUS (the Greek god symbolizing power and strength)24,29–31 in 2014. It was estimated to be completed in 2020. The project is under the charge of the team of Joshua M. Hare of the Interdisciplinary Stem Cell Institute at the University of Miami Miller School of Medicine and their commercial collaborator EMMES Corporation. The primary objective is to determine the safety of different doses of allogenic bone marrow mesenchymal stem cells (Allo-BMMSCs) and the tolerability of cell infusion; the secondary objective is to explore the potential treatment efficacy in improving frailty.
The Allo-BMMSCs, a U.S. FDA-regulated drug product, were derived from bone marrow of eligible male or female donors aged 20–45 years, cultured and amplified in vitro, and then identified by measuring the gene expression of white blood cell RNA.31 Patients of both sexes, aged 60 to 95 years, with a score of 4–7 on the Canadian Clinical Frailty Scale (apparently vulnerable to severely frail) and a score of less than or equal to 24 on the Mini Mental State Examination (MMSE) were taken as eligible subjects. In total, 65 participants were enrolled. Groups treated with 20, 100, or 200 million cells (5 patients per group) and groups treated with 100 or 200 million cells or placebo (10 patients per group) were formed for the pilot safety phase and for randomized phase trials. All of the cell intervention subjects received single peripheral intravenous infusion of Allo-BMMSCs with a total volume of 80 mL at an average speed of 2 mL/min, so the total infusion time was 40 minutes.14,29–31 Within the 12-month follow-up period, primary outcomes include any incidence, mainly in the first 30 days postinfusion, expressed as treatment-emergent serious adverse events (TE-SAEs), such as death, stroke, hospitalization for worsening dyspnea, nonfatal pulmonary embolism, and clinically significant serum chemistry and hematology test abnormalities. Secondary outcomes include indicators for physical function, quality of life, exercise, change in ejection fraction, and inflammatory markers, assessed at 3 and 6 months postinfusion.29–31 The details of the study are shown in Table 1.
Table 1.
Characteristics of Included Studies of Human Frailty Stem Cell Therapy
| References | Phase | Frailty measurement | Enrollment | Sex | Age years | Stem cell type | Dose/patient | Delivery | Follow-up objectives | Positive outcomes | Variable indicators | Clinical trials ID/PMID/DOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bryon A. Tompkins et al.29 | Phase I (open-labeled) | Canadian Clinical Frailty Scale (4–7 scores) | 15 | All | 60–95 (78.4 ± 4.7) | Allo-BMMSCs | 2 × 107 (n = 5) 1 × 108 (n = 5) 2 × 108 (n = 5) | Single peripheral intravenous infusion | Safety and potential efficacy (12 months) | 1. Safety (no TE-SAEs) 2. Tolerated immunomodulation (no activation of CD69 T cells and CD25 T cells) 3. Inflammation (TNF-α↓) |
1. Function (FEV1; MMSE; SF-36 MCS; SF-36 PCS; EQ-5D) 2. Inflammation (CRP; IL-6; fibrinogen; D-dimer; WBCs) |
NCT02065245; 28444181; 10.1093/gerona/glx056 |
| Bryon A. Tompkins et al.28 | Phase I/II (randomized, double-blinded) | Canadian Clinical Frailty Scale (4–7 scores) | 30 | All | 60–95 (75.5 ± 7.3) | Allo-BMMSCs | 1 × 108 (n = 10) 2 × 108 (n = 10) Placebo (n = 10) |
Single peripheral intravenous infusion | Safety and potential efficacy (12 months) | 1. Safety (no TE-SAEs, related LAEs, and hospitalizations) 2. Immuno-inflammatory improvement (CD25 T cell activation↓; B cell intracellular TNF-α↓) |
1. Function: (6MWT; FEV1 4MGST; SPPB score; activity of CHAMPS questionnaire; MFI; handgrip strength; EF) 2. Immune biomarkers (CD8 T cells; CD4 cells; CD4/CD8 ratio; Serum TNF-α; IL-6, CRP; D-dimer; CBC; fibrinogen) 3. Quality of life (SQOL-F; IIEF) |
NCT02065245; 28977399; 10.1093/gerona/glx137 |
Activity CHAMPS questionnaire, reduced activity Community Healthy Activities Model Program for Seniors questionnaire; Allo-BMMSCs, allogenic bone marrow mesenchymal stem cells of 20–45 year donors; CBC, complete blood cell count; CRP, C-reactive protein; EF, ejection fraction; EQ-5D, EuroQol five dimensions questionnaire; FEV1, forced expiratory volume in 1 second; IIEF, International Index of Erectile Dysfunction; IL-6, interleukin 6; LAEs, related long-term adverse events; 4MGST, 4-m gait speed test; 6MWT, 6-minute walk distance test; MCS, Mental Component Score; MFI, exhaustion-multidimensional fatigue inventory; MMSE, Mini-Mental State Examination; PCS, Physical Component Score; SF-36, 36-Item Short Form Health Survey; SPPB score, short physical performance battery score; SQOL-F, Sexual Quality of Life-Female Questionnaires; TE-SAEs, treatment emergent-serious adverse events, defined as the composite of death, nonfatal pulmonary embolism, stroke, hospitalization for worsening dyspnea; TNF-α, tumor necrosis factor-α; WBCs, white blood cells.
The comprehensive analysis of the results of enrolled phase I/II clinical trials
According to outcomes from the only two published studies of stem cell trials for frailty,29,30 all 15 patients of the pilot phase and 30 patients of the randomized phase actually had scores of 4–6 on the Clinical Frailty Scale, so the basal degree of frailty ranged between “moderate” and “vulnerable,” and no severely frail patients were enrolled. The average age of subjects was 78.4 ± 4.7 in the pilot study, 75.5 ± 7.3 in the randomized phase, and 76.0 ± 6.7 in the whole study. Among the 45 subjects, nearly all were of the Caucasian race, and no participants of Hispanic or Latino ethnicity were included.
Comparing all 45 patients who underwent cell infusion and the control counterparts for the main safety evaluation, 2 patients died 8 months postinfusion and in 4 patients donor-specific reactions occurred, as observed by calculated panel reactive antibodies, which, however, were unrelated events or had no clinical significance. Notably, no patients demonstrated adverse signs of cardiopulmonary reactions after the infusion, and the basic clinical hematology and chemistry tests were stable during the entire study period.
As regards the efficacy, remarkably, 100-million cell doses exhibited a more effective reaction than the 20- and 200 cell doses, and the level of tumor necrosis factor-α (TNF-α), an important biomarker closely associated with inflammation and immunity, had significantly decreased at 6 months in all cell treatment groups. However, examination of other physical indices, cardiopulmonary function, quality of life, and biomarker levels, such as the 6-minute walk distance test (6MWT), exhaustion-multidimensional fatigue inventory (MFI), and C-reactive protein (CRP) levels, did not give consistent results and/or did not show statistical significance between groups.
In summary, the allogenic MSC intervention for frailty is safe and well tolerated with no TE-SAEs and no significant immune reactions throughout the whole duration of the study. In addition, single peripheral infusion of allogenic MSCs preliminarily proved efficacy.
Overview of worldwide ongoing human trials of stem cell therapy for frailty
Systematic analysis of all the ongoing and completed clinical trials applying stem cells of multiple types to treat frailty provides an overview of the progress in this novel field.
A total of four human trials, Nos. NCT01501461, NCT02065245, NCT02982915, and NCT03169231, were registered on clinicaltrials.gov between 2011 and 2017. The first one, No. NCT01501461, was registered in 2011 by Zuniga et al. of the Instituto de Medicina Regenerativa and the Ageless Regenerative Institute in Mexico, but it was withdrawn in 2018 because the company was dissolved. The other three trials, including No. NCT02065245 (analyzed above), are ongoing (Table 2).
Table 2.
Other Registered Human Trails on Stem Cell Therapy for Frailty
| Trials ID | Start year | Status | Phase | Condition or disease | Age/sex | Enrollment number | Intervention | Cells dose/patient | Delivery | Follow-up time frame | Primary outcome indicators | Secondary outcome indicators |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT01501461 | 2011 | Withdrawn | Not applicable (open-label, nonrandomized) | Frailty syndrome | 55–90 years/all | 0 | Auto-ADSCs | Not applicable | Single intravenous injection | 1. 3 months 2. 6 months |
1. Improvement in PPT 2. Number of Aes 3. Improved BC/BD 4. Quality of life |
Exercise capacity |
| NCT02982915 | 2016 | Recruiting | Phase I/II (double blind, randomized) | Aging frailty | 65–90 years/all | 43 | Allo-BMMSCs; co-injecting with fluzone high dose vaccine | 2 × 107; 1 × 108 | Single peripheral intravenous infusion | 1. 1 and 4 weeks postvaccination 2. 30 days 3. 6 months 4. 12 months |
1. Incidence of TE-SAEs 2. Adaptive immunity 3. Primary B cell response |
1. Postvaccination changes 2. Changes of biomarkers 3. Different decline rate from aging frailty 4. Death from any cause |
| NCT03169231 | 2017 | Recruiting | Phase IIb (double blind, randomized, parallel assignment) | Aging frailty | 70–85 years/all | 120 | Allo-BMMSCs | 2.5 × 107; 5 × 107; 1 × 108; Placebo. | Single peripheral intravenous infusion | 6 months | 6MWT | 1. Physical function capacity using PROMIS-PF-SF 20a 2. Serum TNF-α |
AEs, adverse events; Auto-ADSCs, autologous adipose-derived stem cells; BC/BD, body composition/bone density; PROMIS-PF-SF 20a, Patient-Reported Outcome Measurement-Physical Function-Short Form 20a; TE-SAEs, treatment-emergent serious adverse events.
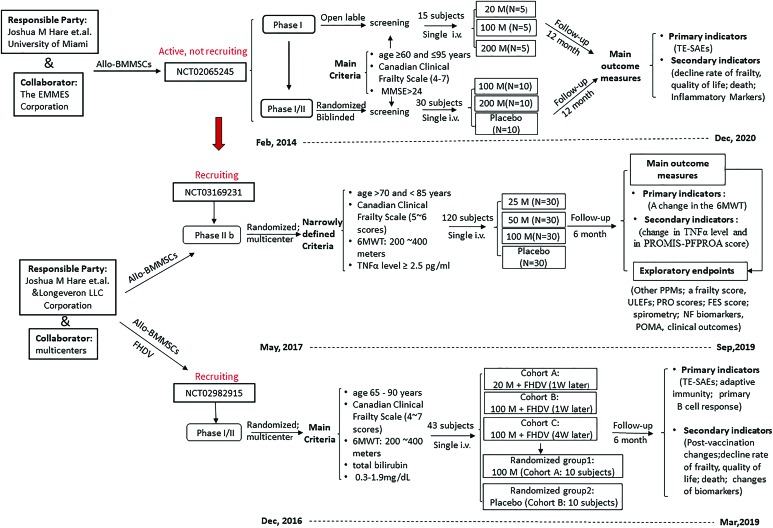
The trial Nos. NCT02982915 and NCT03169231 are both multicenter, randomized, blinded, and placebo-controlled clinical studies launched by the company Longeveron LLC in 2016 and 2017, respectively, which are also the trials of Joshua Hare. Both studies adopt the same cell product, derived from allogenic human bone marrow and named Longeveron MSCs (LMSCs), as co-treatment or independent treatment strategies. The study No. NCT02982915 is a phase I/II trial to test the safety and efficacy of LMSCs for improving the vaccine immune response. A total of 43 subjects, of both sexes, aged 65 to 90 years, and having scores of 4 to 7 on the Canadian Frailty Scale and a distance of >200 and <400 m on the 6MWT, were enrolled. In the pilot phase, three cohort groups, A, B, and C, were arranged to receive an infusion of 20–100 million LMSCs, followed by an intramuscular injection of Fluzone High Dose Vaccine at 1–4 weeks postinfusion. Groups A and B corresponded to the patients who had received LMSCs in the pilot phase. In the randomized phase, two groups (10 patients each) received a single infusion of 100 million LMSCs or placebo. The trial No. NCT03169231, a phase IIb study conducted in 11 medical centers in California and Florida, includes 120 subjects and is a follow-up study on that of Hare et al. in Miami (No. NCT02065245). The objective is to assess the safety of LMSC intervention and its efficacy in improving physical function (mobility and tolerance) and TNF-α levels. The enrollment criteria are more narrowly defined,4 for example, age of 70 to 85 years, a score of 5 (mildly frail) or 6 (moderately frail) on the Clinical Frailty Scale, a distance of >200 and <400 m on the 6MWT, and a serum TNF-α level >2.5 pg/mL. Three treatment groups (doses of 25, 100, and 200 million LMSCs) and one placebo group were arranged in parallel and followed up for 180 days postinfusion. The details and a comparison of ongoing trials are illustrated in Figure 2.
FIG. 2.
Current worldwide ongoing clinical trials of stem cells for frailty. This flowchart shows the main schemed work of the three total ongoing clinical trials (NCT01501461withdrawn); the NCT03169231 is the next-step trial of NCT02065245, mainly to assess the safety and efficacy of Longeveron Mesenchymal Stem Cells, with more narrowly defined criteria. Allo-BMMSCs, allogenic bone marrow mesenchymal stem cells; FES, falls efficacy scale score; FHDV, fluzone high dose vaccine; M, million cells; 6MWT, 6-minute walk distance test; MMSE, Mini-Mental State Examination; N, number of patient; NF, neuroinflammatory biomarkers; POMA, Performance Oriented Mobility Assessment; PPMs, physical performance measures; PROMIS-PFPROA score, PROMIS-Physical Function Patient Reported Outcome Assessment; PRO scores, patient-report outcome scores; Single i.v., single peripheral intravenous infusion; TNF-α, tumor necrosis factor-α; ULEFs, upper and lower extremity function; W, week. It is originally produced on basis of data from clinicaltrials.gov. Color images are available online.
Discussion
People are living longer. Frailty has become a public priority, as the global population is aging at an accelerating speed,4 and it is a major contributor to disability, dependence, and death, and it reduces health and successful aging.1
Complicated mechanism of frailty
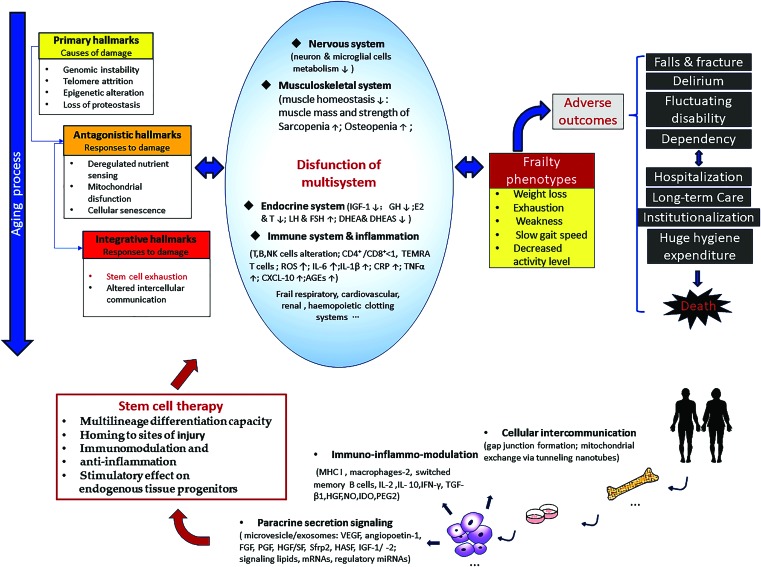
For aging-related frailty, the complicated underlying mechanism, involving genetic, epigenetic, and environmental factors,32–34 has not been clearly elucidated yet.4,28,35 Nevertheless, it is generally agreed upon that the underlying mechanism of frailty intertwines with an accelerated aging process2,6,16 and is influenced by stressors, such as damaged cells, pro-inflammatory macromolecules, toxic metabolites, pathogenic microbes, and social dysfunction (Fig. 3).
FIG. 3.
Main relationships among aging frailty and stem cell therapy. This figure illustrates the underlying mechanism, main clinical phenotypes and adverse outcomes of frailty which intertwined with biological aging, and the appealing potentials of stem cells to treating frailty. AGEs, advanced glycation end products; CRP, C-reactive peptide; CXCL-10, CXC chemokine ligand-10; DHEA & DHEAS, dehydroepiandrosterone and DHEA sulfate; E2 & T, estradiol and testosterone; FGF, fibroblast growth factor; GH, growth hormone; HASF, hypoxic-induced Akt-regulated stem cell factor; HGF, hepatocyte growth factor; HGF/SF, hepatocyte growth factor/scatter factor; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-gamma; IGF-1, insulin-like growth factor-1; IGF-1/-2, insulin-like growth factor-1/-2; IL-6, interleukin 6; IL-1β, interleukin beta-1; IL-2, interleukin-2; IL-10, interleukin-10; LH & FSH, luteinizing hormone and follicle stimulating hormone; MHC I, major histocompatibility complex class I; NK cells, natural killer cells; NO, nitric oxide; PEG2, prostaglandin E2; PGF, placental growth factor; ROS, reactive oxygen species; Sfrp2, secreted frizzled-related protein 2; TEMRA T cells, antigen experienced CD8+ T cells re-expressing the naive marker CD45RA; TGF-β1, transforming growth factor-beta1; VEGF, vascular endothelial growth factor. It is based on the literature of López-Otín et al.; Clegg et al.; Fried et al.; Schulman et al.; Tompkins et al.; Larrick et al.2,3,14,16,19,28 Color images are available online.
Stem cell depletion and exhaustion is one of the ultimate culprits in aging and frailty,16,30,36 as it compromises endogenous rejuvenation of the physiological reserve in aging-related frailty.14,16,28 All adult stem cells lose function over time, for instance, those in stem cell compartments of hematopoietic tissue,37 forebrain, bone, and muscle fibers.38 Satellite cells, or skeletal muscle stem cells, are impaired and lost in aging muscle, causing the main frailty phenotypes of losing muscle mass and strength.39 Circulating osteogenic progenitor cells, surrogates of the mesenchymal repository in the body, decrease with age, and the stem cell properties also decrease, both facilitating frailty.40 Besides, BMMSCs from old animals show decreased expression levels of multiple genes related to cellular maturation and migration. Further proof obtained from experiments with interleukin-10 (IL-10), IL-1 receptor antagonist (IL-1RN), inducible nitric oxide synthase (iNOS), transforming growth factor (TGF)-β3, matrix metalloproteinase-9 (MMP9) (after blocking TNF receptor 2 [TNFR2]), and interferon gamma receptor 1 (IFNGR1) in BMMSCs suggests that the downregulation of special receptors in BMMSCs compromises their protective properties and contributes to the functional attrition of these cells.41
Inflammation is also a core mechanism behind frailty.30,42,43 Changes in several kinds of inflammasome or pro-inflammatory pathways are highly important. The overactivation of the NF-κB pathway and the NLRP3 inflammasome leads to an increased production and release of inflammatory cytokines, such as IL-1β, TNF-α, and interferons.44,45 The activation of NF-κB in the microenvironment of the hypothalamus triggered by inflammatory and stress responses results in a reduced production of gonadotropin releasing hormone (GnRH), which facilitates frailty-associated changes, such as muscle weakness, osteopenia and bone fragility, and reduction of neurogenesis.46 Besides, the sirtuin pathway can modulate inflammatory responses. SIRT1, SIRT2, and SIRT6 downregulate the inflammatory activation by deacetylating NF-κB subunits and repressing the transcription of inflammation-related genes.47 Many inflammatory mediators are independently correlated with frailty, such as CRP, IL-6, TNF-α, and CXC chemokine ligand-10 (CXCL-10).48 The increased IL-6 and TNF-α levels can individually or collectively decrease muscle mass and strength, facilitating the development of sarcopenia.49 High levels of CRP, IL-6, and TNF-α are even independent predictors of mortality.14,50 Noteworthily, some anti-inflammatory factors were reported to be reduced in frailty, such as vitamin C, E, α-tocopherol, and total thiol levels.51,52
Except the two core observations mentioned above, the declining physiology reserve and compromised capacity of rejuvenation in frailty also unfolds as a consequence of changes in other aspects. The frailty-associated functional impairment of the immune system has been well documented.53,54 Immunosenescence manifests as a decline in the clearing of infectious agents, senescent cells, and infected or even malignant cells,16 which aggravates the aging and frailty phenotypes. The activity of T cells is impaired, as indicated by the decrease in the CD4:CD8 ratio,55 an indicator for infection.56 In a microenvironment with high TNF-α levels, the function of B cells is compromised, which leads to a shift to subsets of dysfunctional and exhausted B cells rather than memory B cells.57,58 Moreover, the association of oxidative stress biomarkers, including malondialdehyde (MDA), paraoxonase-1 (PON-1), lipoprotein phospholipase A2 (LpPLA2), 4-hydroxy-2,3-nonenal (HNE), derivate of reactive oxygen metabolites (d-ROM), oxidized glutathione/glutathione (GSSG/GSH), isoprostanes, protein carbonylation, and 8-hydroxy-20-deoxyguanosine, with frailty was assayed.51,52,59 Higher levels of hematological fibrinogen VIII and D-dimer lead to fatigue and increase the risk of venous thromboembolism compared to nonfrail people, even after adjusting for cardiovascular diseases (CVDs) and diabetes.60,61 In addition, in the endocrine system, serum hormones, such as testosterone and dehydroepiandrosterone (DHEA), 25(OH) vitamin D, growth hormone, insulin-like growth factor-1 (IGF-1), and ghrelin, are closely related with frailty.62–64 The main effect of testosterone is in activating protein synthesis. The testosterone and its higher affinity form, dihydrotestosterone, can upregulate the expression of muscle-specific genes and increase muscle strength through the Wnt/β-catenin signaling pathway.60,61 The situation of frailty becomes worse when more than two synthetic hormones are lacking, especially when coupled with vitamin B12 deficiency and/or celiac disease.65
At the organismal level, the multidimensional and multifactorial mechanism that causes frailty phenotypes or syndromes manifests as unintentional weight loss (especially the lean body mass), declining strength and endurance, slower gait speeds, reduced balance, less activity, and impaired cognition and social function.14,24,33 Among them, the loss of muscle mass and strength, sarcopenia, and cognition impairment play large roles in frailty syndrome.66,67 Interestingly, body weight can sometimes increase in frailty. This is because fat mass increases and muscle mass decreases with aging, leading to sarcopenic obesity.68 Clinically, the lower skeletal muscle index, lower hip bone mineral density, and larger waist circumference can raise the risk of osteoporosis, fall, and fracture in frail people.69 Timed walk and grip strength can act as predictors of mild cognitive impairment, as cognition impairment disturbs both gait speed and grip strength.33 Frailty and delirium seem distinct geriatric syndromes, with frailty being a chronic condition and delirium an acute change of cognition. Frailty may predispose patients to delirium, and delirium disturbs the recovery of frailty from stressors, predicting a negative prognosis.70 Besides, an independent impact of depression on frailty has been proposed.71
Promising stem cell strategy for frailty
As no standard and effective treatment for frailty patients exists, the repletion of multipotent stem cells is an appealing strategy to rejuvenate the multifactorial dysfunction in frailty. As an important step in conducting any stem cell therapy is an appropriate choice of cell sources, various types of stem cells are exploited, such as ESCs72 (highly undifferentiated and pluripotent), MSCs73,74 (easily available and low immunogenicity), and iPSCs75,76 (possessing pluripotency to differentiation). Meanwhile, it is considered that limbal stem cells are basically matured and endothelial progenitor cells are favored for their special properties of perivascular reparation, which are needed in regenerative medicine.77 Among them, the distinctive advantages of MSCs of low immunogenicity, relatively abundant sources, easy isolation and expansion,72 multilineage differentiation, secretion of immunomodulatory and anti-inflammatory factors, and stimulation of endogenous progenitors4,14,19,28 make MSCs an attractive candidate strategy for frailty treatment.28
MSCs secrete a variety of factors and this can be regulated by the microenvironment.78 TGF-β and IL-10 are relatively well studied.16 MSCs modulate TGF-β to activate the STAT6 pathway in response to IL-4 signaling.79 TGF can regulate immunity by facilitating the increase of T regulatory cells (Treg) and the decrease of CD4+ and CD8+ T cells and T helper 1 (Th1) cells.80 MSCs secrete IL-10 by directly interacting with T cells to inhibit the production of pro-inflammatory cytokines by macrophages, which modulates anti-inflammatory and immunoregulatory actions. In addition, MSCs can release extracellular vesicles (exosomes or microvesicles), which contain cytokines and growth factors, including vascular endothelial growth factor, hepatocyte growth factor/scatter factor,81 fibroblast growth factor, IGF-1 and IGF-2, and placental growth factor,14 and some other signaling lipids, mRNAs, and miRNAs.82
In recent years, clinical and preclinical investigations applying stem cells have made considerable progress in the treatment of a wide spectrum of diseases of the elderly population, most of which are closely interrelated with frailty and contribute to adverse outcomes. MSCs secrete paracrine factors, exosomes, and small extracellular vesicles, reduce inflammatory factors, and activate the resident cells after injury.14,19,24,28 It has been shown that MSCs promote the proliferation, differentiation, and migration of resident stem cells to prevent cardiomyocyte apoptosis, reducing fibrosis after myocardial infarction by modulating secreted frizzled-related protein 2, IGF-1 hypoxia-induced Akt-regulated stem cell factor,83,84 and the proteins, peptides, and miRNAs secreted in/on exosomes and extracellular vesicles. The outcomes of many CVDs were improved by MSCs, for example, myocardial infarction85 and nonischemic86 and ischemic cardiomyopathy.81 It is likely that these beneficial effects are mainly mediated by the secreting function, especially the paracrine system,87 and secondarily by the direct cellular contact, such as the formation of gap junctions through tunneling nanotubes.28,88 These hypotheses, however, remain to be verified. As the 6MWT, an important physical function assessment tool that was originally developed for assessing cardiac and pulmonary disorders,29,30 it can support the proof for the potential benefits of MSCs in the treatment of frailty.14 Therapeutic effects of stem cells have also been shown in Parkinson's disease,89 amyotrophic lateral sclerosis,73 chronic obstructive pulmonary disease,90 idiopathic pulmonary interstitial fibrosis,91 diabetes,73 lupus,92 traumatic brain and spinal cord injury,74 stroke,93 and atherosclerosis.94,95 These indirectly suggest the feasibility of the application of stem cells in frailty treatment.
Current challenges in stem cell therapy for frailty
For frailty, the paucity of relevant acknowledged animal models and the lack of clinical standard diagnosis, outcome measures, and reliable, validated, and sensitive biomarkers pose barriers to the preclinical and clinical research.4 Therapeutic interventions to ameliorate the signs and symptoms of aging-related frailty mainly focus on resistance exercise regimes, the Mediterranean diet,96 and protein, caloric, vitamin D,5,97 and hormonal supplementation,97–99 which, independently or in combination, have made some progress.100 However, there are no effective and special treatment strategies for frailty so far.4,14
Challenges exist, although preclinical and clinical evidence collectively predict a promising future of the stem cell approach for frailty. Current human trials show preliminary efficacy, but many outcome items are variable.29,30 Inspiringly, a phase IIb human trial, including 120 subjects, is ongoing to compensate this (No. NCT03169231). However, there are no solid data that provide evidence that sarcopenia or osteoporosis could be reversed by stem cell therapy, which are both closely related with frailty. In osteoporosis, the number of BMMSCs declines. It is uncertain whether infused stem cells differentiate into osteoblasts and induce bone formation, for that the transplanted stem cells do not migrate to bone surfaces, do not show long-term engraftment, and disproportionally facilitate adipogenesis instead of osteogenesis.101,102 Thus, genetically modified stem cells, for example, iPSCs, were proposed as an alternative approach.102 For sarcopenia, exogenous stem cells, such as satellite cells,103 muscle-derived stem cells,104 perivascular stem cells,105 ESCs, and iPSCs,75 were used to promote the regeneration of skeletal myofiber. However, limited success has been reached so far. There may be reasons like that the satellite cells are generally quiescent in adult skeletal muscle106,107 and a small contribution is made by them even in a circumstance of a large hypertrophy of the skeletal muscle.108 Besides, cell deliverability and in vitro expansion are issues that require attention.109 Interestingly, a cohort study,110 in which 998 hematopoietic cell transplantation (HCT) survivors and 297 matched siblings were examined, found that frailty increased the risk of mortality by 2.76 times, even after adjusting for predictors, but the young adult HCT survivors were 8.4-fold more likely to be frail at old age than their siblings. These findings appear to suggest that hematopoietic cell therapy can not only not ameliorate frailty but also exacerbate the situation. Reasons may include the following.110 First, this study was not an interventional trial directly investigating the efficacy of stem cell therapy on frailty. The included subjects should be comparable between intervention and control groups, that is, the participants receiving HCT and controls should have the same underlying disease. Second, HCT injured normal tissues, which intensified the susceptibility of the ill fragile body and eased the development of frailty when confronted with harmful factors compared with their siblings. Third, hematopoietic cells mainly differentiate into blood cells, including red blood cells, white cells, and platelets, but the comparatively limited potency compared to other types of stem cells compromises their application when applied to cure illnesses other than diseases of the blood system, such as frailty.
We would like to mention some limitations of our review. There are few studies about stem cell therapy for frailty, both in animal models and clinical trials, so in this systematic review we could not conduct a deep meta-analysis. With the aging global population and the promising exploration of stem cells, numerous studies about this interesting subject are expected to follow. In addition, stem cells are a biological therapeutic strategy, but the stability and oncogenicity require consistent long-term verification. Third, our main focus was aging-related frailty, and the prevalence of frailty in young adults was not taken into account. Younger people increasingly tend to suffer from frailty, and future investigations should take this into account as well.
The major strength of the present systematic review is that it elaborates on the relationships of aging-related frailty and stem cell therapy from a holistic and logistic perspective.
Conclusion
Frailty urgently requires attention. Stem cell therapy for frailty possesses great potential. Currently, although there still are challenges, single peripheral intravenous infusion of allogenic MSCs is proved to be safe, well tolerated, and effective in modulating immunity and inflammation, and it preliminarily shows a tendency to improve physical functions and quality of life. Finally, many other human trials on this subject will explore the depth and breadth of this novel cell-based frailty treatment.
Acknowledgments
Supported by National Key Research and Development Program of China, No. 2017YFC0840100; Building World-Class Universities (Disciplines) and Guiding Special Funds, No. 2040204401004; Sichuan Science and Technology Infrastructure Platform Construction Special Funds, No. 2018TJPT0015; Science and Technology Project of Sichuan Province, No. 0040205302202; and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, No. ZY2017201. The sponsors played no role in any part of this study or its presentation and publication.
Availability of Data and Materials
The human clinical trial data included in this review are available at https://clinicaltrials.gov.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. World Health Organisation. Ageing and health in 2018. www.who.int/mediacentre/factsheets/fs404/en (accessed November22, 2018)
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 4. Pahor M, Kritchevsky SB, Waters DL, et al. Designing Drug Trials for Frailty: ICFSR Task Force 2018. J Frailty Aging 2018;7:150–154 [DOI] [PubMed] [Google Scholar]
- 5. Dent E, Lien C, Lim WS, et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J Am Med Dir Assoc 2017;18:564–575 [DOI] [PubMed] [Google Scholar]
- 6. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012;16:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: A call to action. J Am Med Dir Assoc 2013;14:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017;152:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly S OBI, Smuts K, O'Sullivan M, Warters A. Prevalence of frailty among community dwelling older adults in receipt of low level home support: A cross-sectional analysis of the North Dublin Cohort. BMC Geriatr 2017;17:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verlaan SL-MG, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults—A systematic review and meta-analysis. J Am Med Dir Assoc 2017;18:374–382 [DOI] [PubMed] [Google Scholar]
- 12. Ferrucci L, Cavazzini C, Corsi A, et al. Biomarkers of frailty in older persons. J Endocrinol Invest 2002;25(10 Suppl):10–15 [PubMed] [Google Scholar]
- 13. Reddy SR, Ross-Degnan D, Zaslavsky AM, Soumerai SB, Wagner AK. Health care payments in the Asia Pacific: Validation of five survey measures of economic burden. Int J Equity Health 2013;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulman IH, Balkan W, Hare JM. Mesenchymal stem cell therapy for aging frailty. Front Nutr 2018;5:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aunan JR, Watson MM, Hagland HR, Soreide K. Molecular and biological hallmarks of ageing. Br J Surg 2016;103:e29–e46 [DOI] [PubMed] [Google Scholar]
- 16. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sousa-Victor P, Munoz-Canoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 2016;50:109–117 [DOI] [PubMed] [Google Scholar]
- 18. Gonen O, Toledano H. Why adult stem cell functionality declines with age? Studies from the fruit fly Drosophila melanogaster model organism. Curr Genomics 2014;15:231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larrick JW, Mendelsohn AR. Mesenchymal stem cells for frailty? Rejuvenation Res 2017;20:525–529 [DOI] [PubMed] [Google Scholar]
- 20. Silva RB, Aldoradin-Cabeza H, Eslick GD, Phu S, Duque G. The effect of physical exercise on frail older persons: A systematic review. J Frailty Aging 2017;6:91–96 [DOI] [PubMed] [Google Scholar]
- 21. Guigoz Y. Frailty and nutrition: What we have learned from research and clinical practice on the Mini Nutritional Assessment. J Frailty Aging 2012;1:52–55 [DOI] [PubMed] [Google Scholar]
- 22. Goisser S, Guyonnet S, Volkert D. The role of nutrition in frailty: An Overview. J Frailty Aging 2016;5:74–77 [DOI] [PubMed] [Google Scholar]
- 23. Fielding RA, Travison TG, Kirn DR, et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: Results from the VIVE2 Randomized Trial. J Nutr Health Aging 2017;21:936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Couteur DG, Anderson RM, Newman AB, de Cabo R. Stem cell transplantation for frailty. J Gerontol A Biol Sci Med Sci 2017;72:1503–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676 [DOI] [PubMed] [Google Scholar]
- 26. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147 [DOI] [PubMed] [Google Scholar]
- 27. Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tompkins BA, Landin AM, Florea V, Natsumeda M, Rieger AC, Balkan W, Schulman IH, Hare JM. Allogeneic mesenchymal stem cells as a treatment for aging frailty. In: Frailty and Sarcopenia-Onset, Development and Clinical Challenges. InTech, 2017, pp. 221–243 [Google Scholar]
- 29. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo controlled clinical trial. J Gerontol A Biol Sci Med Sci 2017;72:1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: A phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci 2017;72:1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golpanian S, DiFede DL, Pujol MV, et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 2016;7:11899–11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vina J, Tarazona-Santabalbina FJ, Perez-Ros P, et al. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med 2016;50:88–108 [DOI] [PubMed] [Google Scholar]
- 33. Robertsona DA, Savvaa GM, Kennya RA. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev 2013;12:840–851 [DOI] [PubMed] [Google Scholar]
- 34. McGowan PO, Szyf M. Environmental epigenomics: Understanding the effects of parental care on the epigenome. Essays Biochem 2010;48:275–287 [DOI] [PubMed] [Google Scholar]
- 35. Gomes MJ, Martinez PF, Pagan LU, et al. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017;8:20428–20440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peffers MJ, Collins J, Loughlin J, Proctor C, Clegg PD. A proteomic analysis of chondrogenic, osteogenic and tenogenic constructs from ageing mesenchymal stem cells. Stem Cell Res Ther 2016;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010;22:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature 2012;490:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia-Prat L, Sousa-Victor P, Munoz-Canoves P. Functional dysregulation of stem cells during aging: A focus on skeletal muscle stem cells. FEBS J 2013;280:4051–4062 [DOI] [PubMed] [Google Scholar]
- 40. Gunawardene P, Bermeo S, Vidal C, et al. Association between circulating osteogenic progenitor cells and disability and frailty in older persons: The Nepean Osteoporosis and Frailty Study. J Gerontol A Biol Sci Med Sci 2016;71:1124–1130 [DOI] [PubMed] [Google Scholar]
- 41. Bustos ML, Huleihel L, Kapetanaki MG, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Resp Crit Care Med 2014;189:787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonzalez RWD, Geffner L. Stem cells targeting inflammation as potential anti-aging strategies and therapies. Cell Tissue Transplant Ther 2015;1:1–8 [Google Scholar]
- 43. Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011;333:1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee JS, Ward WO, Ren H, et al. Meta-analysis of gene expression in the mouse liver reveals biomarkers associated with inflammation increased early during aging. Mech Ageing Dev 2012;133:467–478 [DOI] [PubMed] [Google Scholar]
- 46. Zhang G, Li J, Purkayastha S. Hypothalamic programming of systemic ageing involving IKK-b, NF-kB and GnRH. Nature 2013;497:211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rothgiesser KM, Erener S, Waibel S, Lüscher B, Hottiger MO. SIRT2 regulates NF-kB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 2010;123:4251–4258 [DOI] [PubMed] [Google Scholar]
- 48. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8 [DOI] [PubMed] [Google Scholar]
- 49. Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64:1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003;23:15–39 [DOI] [PubMed] [Google Scholar]
- 51. Saum KU, Dieffenbach AK, Jansen EH, et al. Association between oxidative stress and frailty in an elderly German population: Results from the ESTHER Cohort Study. Gerontology 2015;61:407–415 [DOI] [PubMed] [Google Scholar]
- 52. Namioka N, Hanyu H, Hirose D, Hatanaka H, Sato T, Shimizu S. Oxidative stress and inflammation are associated with physical frailty in patients with Alzheimer's disease. Geriatr Gerontol Int 2017;17:913–918 [DOI] [PubMed] [Google Scholar]
- 53. Ghatreh-Samani M, Esmaeili N, Soleimani M, Asadi-Samani M, Ghatreh-Samani K, Shirzad H. Oxidative stress and age-related changes in T cells: Is thalassemia a model of accelerated immune system aging?. Cent Eur J Immunol 2016;41:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qin L, Jing X, Qiu Z, et al. Aging of immune system: Immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging (Albany NY) 2016;8:848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strindhall J, Skog M, Ernerudh J, et al. The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: The Swedish HEXA immune study. Age (Dordr) 2013;35:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McElhaney JE, Effros RB. Immunosenescence: What does it mean to health outcomes in older adults? Curr Opin Immunol 2009;21:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016;17:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev 2011;10:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ingles M, Gambini J, Carnicero JA, et al. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: Lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc 2014;62:1324–1328 [DOI] [PubMed] [Google Scholar]
- 60. Spillane M, Schwarz N, Willoughby DS. Upper-body resistance exercise augments vastus lateralis androgen receptor-DNA binding and canonical Wnt/beta-catenin signaling compared to lower-body resistance exercise in resistance-trained men without an acute increase in serum testosterone. Steroids 2015;98:63–71 [DOI] [PubMed] [Google Scholar]
- 61. Haren MT, Siddiqui AM, Armbrecht HJ, et al. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl 2011;34:55–68 [DOI] [PubMed] [Google Scholar]
- 62. Eichholzer M, Barbir A, Basaria S, et al. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III). Aging Male 2012;15:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ensrud KE, Blackwell TL, Cauley JA, et al. ; Osteoporotic Fractures in Men Study Group. Circulating 25-hydroxyvitamin D levels and frailty in older men: The Osteoporotic Fractures in Men study. J Am Geriatr Soc 2011;59:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yeap BB, Paul Chubb SA, Lopez D, Ho KK, Hankey GJ, Flicker L. Associations of insulin-like growth factor-1 and its binding proteins, and testosterone, with frailty in older men. Clin Endocrinol (Oxf) 2013;78:752–759 [DOI] [PubMed] [Google Scholar]
- 65. Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinol Metab Clin North Am 2013;42:391–405 [DOI] [PubMed] [Google Scholar]
- 66. Zaslavsky O, Cochrane BB, Thompson HJ, Woods NF, Herting JR, LaCroix A. Frailty: A review of the first decade of research. Biol Res Nurs 2013;15:422–432 [DOI] [PubMed] [Google Scholar]
- 67. Furtado GE, Caldo A, Rieping T, et al. Physical frailty and cognitive status over-60 age populations: A systematic review with meta-analysis. Arch Gerontol Geriatr 2018;78:240–248 [DOI] [PubMed] [Google Scholar]
- 68. Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci 2011;66:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu LK, Lee WJ, Chen LY, et al. Association between frailty, osteoporosis, falls and hip fractures among community-dwelling people aged 50 years and older in Taiwan: Results from I-Lan Longitudinal Aging Study. PLoS One 2015;10:e0136968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: The crossroads of frailty and delirium. J Am Geriatr Soc 2011;59 Suppl 2:S262–S268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc 2008;56:2211–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sanina C, Hare JM. Mesenchymal stem cells as a biological drug for heart disease: Where are we with cardiac cell-based therapy? Circ Res 2015;117:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rajan TS, Scionti D, Diomede F, et al. Gingival stromal cells as an in vitro model: Cannabidiol modulates genes linked with amyotrophic lateral sclerosis. J Cell Biochem 2017;118:819–828 [DOI] [PubMed] [Google Scholar]
- 74. Schira J, Gasis M, Estrada V, et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain 2012;135(Pt 2):431–446 [DOI] [PubMed] [Google Scholar]
- 75. Mizuno Y, Chang H, Umeda K, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J 2010;24:2245–2253 [DOI] [PubMed] [Google Scholar]
- 76. Duncan T, Valenzuela M. Alzheimer's disease, dementia, and stem cell therapy. Stem Cell Res Ther 2017;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell stem cell 2015;17:11–22 [DOI] [PubMed] [Google Scholar]
- 78. Fontaine MJ, Shih H, Schafer R, Pittenger MF. Unraveling the mesenchymal stromal cells' paracrine immunomodulatory effects. Transfus Med Rev 2016;30:37–43 [DOI] [PubMed] [Google Scholar]
- 79. Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature 1996;380:627–630 [DOI] [PubMed] [Google Scholar]
- 80. Nasef A, Chapel A, Mazurier C, et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr 2007;13:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012;308:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017;35:851–858 [DOI] [PubMed] [Google Scholar]
- 83. Engels MC, Rajarajan K, Feistritzer R, et al. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells 2014;32:1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schmeckpeper J, Verma A, Yin L, et al. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. J Mol Cell Cardiol 2015;85:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hare JM, DiFede DL, Rieger AC, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol 2017;69:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res 2016;118:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol 2008;51:933–943 [DOI] [PubMed] [Google Scholar]
- 89. Singh Dolt K, Hammachi F, Kunath T. Modeling Parkinson's disease with induced pluripotent stem cells harboring alpha-synuclein mutations. Brain Pathol (Zurich, Switzerland) 2017;27:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koppitz M, Eschenburg C, Salzmann E, Rosewich M, Schubert R, Zielen S. Mucolytic effectiveness of tyloxapol in chronic obstructive pulmonary disease—A double-blind, randomized controlled trial. PLoS One 2016;11:e0156999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chambers DC, Enever D, Ilic N, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology (Carlton, Vic) 2014;19:1013–1018 [DOI] [PubMed] [Google Scholar]
- 92. Thiel A, Yavanian G, Nastke MD, et al. Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci Rep 2015;5:17685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moshayedi P, Nih LR, Llorente IL, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016;105:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003;108:457–463 [DOI] [PubMed] [Google Scholar]
- 95. Goldschmidt-Clermont PJ. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. Am Heart J 2003;146(4 Suppl):S5–S12 [DOI] [PubMed] [Google Scholar]
- 96. Voelker R. The Mediterranean diet's fight against frailty. JAMA 2018;319:1971–1972 [DOI] [PubMed] [Google Scholar]
- 97. O'Connell MD, Wu FC. Androgen effects on skeletal muscle: Implications for the development and management of frailty. Asian J Androl 2014;16:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fairhall N, Sherrington C, Kurrle SE, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc 2015;16:41–48 [DOI] [PubMed] [Google Scholar]
- 99. Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling Japanese older adults: The INE (Intervention by Nutrition and Exercise) Study. J Am Med Dir Assoc 2015;16:654–660 [DOI] [PubMed] [Google Scholar]
- 100. Sacha JSM, Sobon J, Borysiuk Z, Feusette P. Is it time to begin a public campaign concerning frailty and pre-frailty? A review article. Front Physiol 2017;8:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009;27:1887–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep 2014;12:41–47 [DOI] [PubMed] [Google Scholar]
- 103. Montarras D, Morgan J, Collins C, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005;309:2064–2067 [DOI] [PubMed] [Google Scholar]
- 104. Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J Cell Biol 2002;157:851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2011;2:499. [DOI] [PubMed] [Google Scholar]
- 106. Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J Exp Zool 1978;206:451–456 [DOI] [PubMed] [Google Scholar]
- 107. Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 1997;191:270–283 [DOI] [PubMed] [Google Scholar]
- 108. Fukada S, Uezumi A, Ikemoto M, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 2007;25:2448–2459 [DOI] [PubMed] [Google Scholar]
- 109. Naranjo JD, Dziki JL, Badylak SF. Regenerative medicine approaches for age-related muscle loss and sarcopenia: A mini-review. Gerontology 2017;63:580–589 [DOI] [PubMed] [Google Scholar]
- 110. Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: Results from the bone marrow transplant survivor study. JAMA Oncol 2016;2:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The human clinical trial data included in this review are available at https://clinicaltrials.gov.