ABSTRACT
The evolutionarily conserved Hippo signaling pathway is known to regulate cell proliferation and maintain tissue homeostasis during development. We found that activation of Yorkie (Yki), the effector of the Hippo signaling pathway, causes separable effects on growth and differentiation of the Drosophila eye. We present evidence supporting a role for Yki in suppressing eye fate by downregulation of the core retinal determination genes. Other upstream regulators of the Hippo pathway mediate this effect of Yki on retinal differentiation. Here, we show that, in the developing eye, Yki can prevent retinal differentiation by blocking morphogenetic furrow (MF) progression and R8 specification. The inhibition of MF progression is due to ectopic induction of Wingless (Wg) signaling and Homothorax (Hth), the negative regulators of eye development. Modulating Wg signaling can modify Yki-mediated suppression of eye fate. Furthermore, ectopic Hth induction due to Yki activation in the eye is dependent on Wg. Last, using Cut (Ct), a marker for the antennal fate, we show that suppression of eye fate by hyperactivation of yki does not change the cell fate (from eye to antenna-specific fate). In summary, we provide the genetic mechanism by which yki plays a role in cell fate specification and differentiation – a novel aspect of Yki function that is emerging from multiple model organisms.
Keywords: Drosophila eye, Hippo signaling, Wingless, Growth regulation, Patterning, Retinal differentiation
Highlighted article: Yorkie, best known for controlling organ growth downstream of Hippo signalling, plays a separable role in regulating cell fate specification in the fly eye.
INTRODUCTION
Growth regulatory pathways play an important role during organogenesis to regulate patterning, growth and differentiation (Raff, 1996; Baker, 2001; Tumaneng et al., 2012; Verghese et al., 2012, 2013; Pichaud, 2014). These pathways crosstalk with each other, and are re-utilized to generate complexity and diverse cell types in different organisms (Pires-daSilva and Sommer, 2003; Jukam and Desplan, 2011; Jukam et al., 2013). The Hippo signaling pathway, an evolutionarily conserved pathway, is involved in organ size regulation and regeneration, and in diseases like cancer (Kango-Singh and Singh, 2009; Zhao et al., 2011; Staley and Irvine, 2012; Halder and Camargo, 2013; Verghese et al., 2013). Initially identified in genetic screens in Drosophila, the pathway comprises a core kinase cascade and multiple upstream regulators that converge on the regulation of nuclear availability of the Yorkie (Yki) oncogene (the homolog of mammalian YAP/TAZ) (Huang et al., 2005; Edgar, 2006; Pan, 2010; Halder and Johnson, 2011; Verghese et al., 2013; Yu and Guan, 2013). The core kinase cascade comprises the two serine threonine kinases Hippo (Hpo, the Drosophila homolog of mammalian MST1/2) (Harvey et al., 2003; Jia et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003) and Warts (Wts, the Drosophila homolog of the mammalian NDR family proteins LATS1/2) (Justice et al., 1995; Xu et al., 1995), and their cognate adaptor proteins Salvador (Sav, the Drosophila homolog of mammalian WW45) (Kango-Singh et al., 2002; Tapon et al., 2002) and Mob as tumor suppressor (Mats, the Drosophila MOB homolog) (Lai et al., 2005). The upstream kinase Hpo binds Sav, and regulates the activity of the downstream Wts and Mats complex via phosphorylation. The Wts/Mats complex regulates Yki activity via phosphorylation, which leads to cytoplasmic sequestration and degradation of Yki. Yki is also regulated by phosphorylation-independent mechanisms where it binds Expanded (Ex), Hpo or Wts, and is localized to the plasma membrane, preventing its nuclear translocation (Oh and Irvine, 2010; Verghese et al., 2013; Yu and Guan, 2013).
Upstream of the kinase cascade, multiple genes impact the output of the Hippo pathway, e.g. genes regulating extracellular matrix and cytoskeleton, apical-basal polarity, planar cell polarity and growth factor signaling (Grusche et al., 2010; Boggiano and Fehon, 2012; Staley and Irvine, 2012; Yu and Guan, 2013). Hyperactivation of the Hippo pathway (e.g. by overexpression of Hpo or Ex) induces apoptosis, leading to the formation of smaller organs and inactivation of Yki (Harvey et al., 2003; Udan et al., 2003; Wu et al., 2003; Verghese et al., 2012, 2013); however, its downregulation (e.g. by loss of function of ex, hpo, sav, wts and mats) results in overgrowth and activation of Yki. Activated Yki binds to its cognate transcription factor(s) [Scalloped (Sd), Homothorax (Hth), Teashirt (Tsh)] and translocates to the nucleus to induce transcriptional expression of its target genes (Harvey et al., 2003; Jia et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003, 2008; Goulev et al., 2008; Zhang et al., 2008; Peng et al., 2009). Hippo pathway target genes include genes that regulate cell proliferation, cell survival or cell growth to limit organ size, e.g. cyclin E, cyclin A, cyclin B, E2f1, Drosophila inhibitor of apoptosis 1 (Diap1), bantam microRNA and Myc. In addition, several upstream components such as kibra, ex, crumbs (crb) and four-jointed (fj) are regulated via a feedback mechanism to maintain steady-state Hippo signaling (Verghese et al., 2013). Thus, the Hippo pathway responds to multiple upstream regulatory inputs by transcriptionally regulating a battery of genes in a tissue- and context-dependent manner.
Drosophila imaginal discs are an excellent model with which to study the role of signaling pathways in growth and patterning. Drosophila eyes are derived from a set of eye imaginal discs (Poulson, 1950; Cohen, 1993) that grow during larval stages, and each give rise to an adult compound eye comprising 800 unit eyes or ommatidia (Ready et al., 1976; Wolff and Ready, 1993; Kumar, 2011; Singh et al., 2012; Tare et al., 2013a). Each ommatidium is made up of ∼20 cells, including eight photoreceptor neurons and non-neuronal cells such as pigment cells, cone cells and bristles (Ready et al., 1976; Held, 2002; Roignant and Treisman, 2009; Kumar, 2011, 2013; Singh et al., 2012). Cell fate specification and differentiation in the developing eye field are regulated by a group of genes referred to as retinal determination (RD) genes (Pappu and Mardon, 2004; Roignant and Treisman, 2009; Kumar, 2011; Singh et al., 2012; Burgy-Roukala et al., 2013). These are twin of eyeless (toy), eyeless (ey), eyegone (eyg), twin of eyegone (toe), Optix, eyes absent (eya), sine oculis (so), dachshund (dac) and ophthalmosa (opt) (Bonini et al., 1993; Hanson et al., 1993; Cheyette et al., 1994; Mardon et al., 1994; Quiring et al., 1994; Seimiya and Gehring, 2000; Jang et al., 2003). Among these, the Drosophila Pax6 homolog Ey is required for eye field specification, whereas downstream genes eya, so and dac are required for retinal determination and differentiation (Kumar, 2011; Burgy-Roukala et al., 2013). Loss of function of RD genes blocks early eye development, and misexpression of these genes can reprogram other tissues to form ectopic eyes (Pappu and Mardon, 2004; Burgy-Roukala et al., 2013).
During early third instar, following a period of proliferation, a synchronous wave of differentiation is initiated at the posterior margin of the eye imaginal disc and is referred to as the morphogenetic furrow (MF) (Ready et al., 1976; Wolff and Ready, 1993). The MF moves from posterior margin of the eye imaginal disc to anterior and results in differentiation of retinal precursor cells into photoreceptor (PR) neurons (Ready et al., 1976; Wolff and Ready, 1993; Kumar, 2013). The PR clusters are regularly spaced and comprise eight (R1-R8) photoreceptor neurons. The proneural genes of the achaete-scute complex (AS-C)- and atonal (ato)-encoding basic HLH proteins, play important roles in PR differentiation (Jarman et al., 1994; Bertrand et al., 2002; Tanaka-Matakatsu and Du, 2008). The PR cluster formation involves the selection of R8 founder neuron and subsequent recruitment of additional photoreceptor precursors in the order R2/5, R3/4 and R1/6/7 (Ready et al., 1976; Wolff and Ready, 1993).
The initiation and progression of the MF also requires signaling morphogens [e.g. hedgehog (hh), decapentaplegic (dpp)] and other transcription factors. In the developing eye, dpp expression is restricted to a stripe straddling the MF as it traverses across the eye disc, and serves as an excellent marker for the MF (Chanut and Heberlein, 1997; Kumar, 2013). An important function of Dpp is to repress the expression of Wingless (Wg), a negative regulator of the MF (Ma and Moses, 1995; Treisman and Rubin, 1995; Burke and Basler, 1996). Wg, a signaling morphogen, serves as a ligand for the highly conserved Wg/WNT signaling pathway. Wg is required for nuclear localization of β-catenin homolog Armadillo (Arm) (Aberle et al., 1997; Seto and Bellen, 2004; Geissler and Zach, 2012; Swarup and Verheyen, 2012). In the absence of Wg, Armadillo (Arm) is phosphorylated by Shaggy kinase (Sgg), which leads to its retention in the cytoplasm and its eventual degradation, which prevents spatial expression of Wg target genes (Swarup and Verheyen, 2012). In the developing eye imaginal disc, wg is involved in several diverse functions of cell proliferation, differentiation and cell death. Wg regulates expression of Hth, a homeodomain-containing transcription factor (Moskow et al., 1995; Rieckhof et al., 1997; Kurant et al., 1998; Pai et al., 1998; Bessa et al., 2002), to suppress eye fate and thereby define the boundary of the developing eye. Hth is expressed anterior to the MF and acts as a negative regulator of retinal differentiation: loss of hth results in eye enlargement, whereas gain-of-function of hth suppresses the eye development (Pai et al., 1998; Pichaud and Casares, 2000). tsh encodes a nuclear protein with zinc-finger motifs and plays diverse functions during development (Fasano et al., 1991; Roder et al., 1992; de Zulueta et al., 1994; Mathies et al., 1994; Erkner et al., 1999; Wu and Cohen, 2000; Singh et al., 2002, 2004). During eye development, tsh requires Wg signaling to suppress eye fate by induction of hth (Singh et al., 2002, 2004, 2012; Tare et al., 2013a). Thus, eye differentiation is orchestrated by the concerted activities of signaling morphogens and their pathways that regulate MF progression.
Several components of the Hippo pathway show general growth defects but also specific defects in eye development, for example, loss of ex causes reduction in the eye size and duplication of antennae (Boedigheimer and Laughon, 1993). Similarly, loss of ft is reported to cause loss of neurons in the eye and a marked delay in MF progression (Silva et al., 2006; Tyler and Baker, 2007; Napoletano et al., 2011). We, therefore, tested the effects of loss of Hippo signaling on eye development and differentiation to investigate whether differentiation defects occur in all or certain components of the Hippo pathway. We found that Yki activation caused separable effects on growth and differentiation of the eye. Expression of Yki and its activated forms (YkiS168A and YkiS3A) results in increased proliferation and suppression of retinal differentiation or delayed differentiation in the eye disc. Here, we present many pieces of evidence to show that Yki activation regulates retinal differentiation in the developing fly retina. Activation of Yki can suppress eye fate by blocking MF progression and by downregulation of expression of RD genes. Furthermore, Wg signaling is ectopically activated during this process. Thus, our data suggest a role for the Hippo pathway effector Yki not only in the proliferative phase, but also later in patterning and differentiation during organogenesis.
RESULTS
Downregulation of the Hippo pathway (e.g. by loss of function of wts) throughout the developing imaginal discs, or in somatic clones causes defects in growth and differentiation. Loss-of-function clones of wts, a core kinase of the Hippo signaling pathway, exhibit loss of retinal differentiation on both dorsal (D) and ventral (V) eye margins (supplementary material Fig. S1). However, the mechanism by which Hippo signaling is involved in eye development is yet to be understood. Therefore, to test the role of Hippo signaling in the developing Drosophila eye, we used bi-Gal4 to misexpress transgenes that either downregulate or upregulate the Hippo pathway. The bi-Gal4 expression is limited to the DV margins (both anterior and posterior to the MF) of the developing eye imaginal disc (Calleja et al., 1996; Tare et al., 2013b). This expression domain of bi-Gal4 allows assessment of effects of misexpression on both the differentiation of photoreceptor neurons (posterior to the MF), as well as in undifferentiated retinal precursor cells (anterior to the MF) (Tare et al., 2013b).
Yki activation regulates eye development
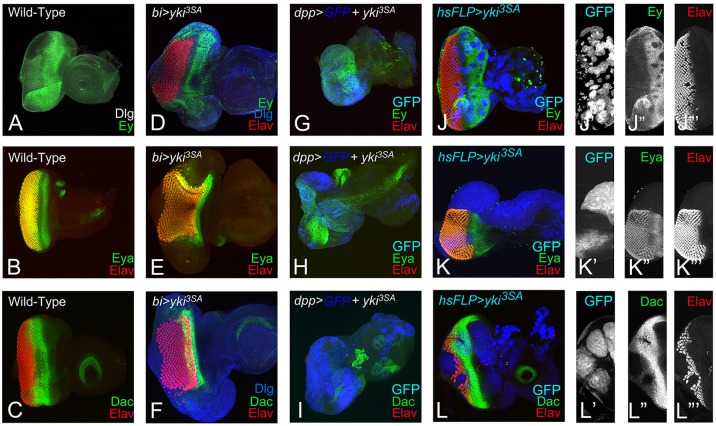
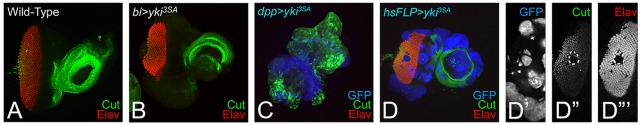
We first blocked Hippo signaling by misexpression of three different yki transgenes: full-length yki tagged with GFP (UAS-yki GFP) and two constitutively active yki transgenes (UAS-ykiS168A and UAS-yki3SA) (Oh and Irvine, 2008, 2009), to test its role in retinal differentiation. In the wild-type eye imaginal disc, cell outlines were identified by the expression of Discs large (Dlg), and all photoreceptor neurons were detected by the expression of pan-neural protein Elav (Fig. 1A). Misexpression of full-length yki GFP (bi>yki GFP) resulted in a significant suppression of eye fate on the DV margins of the developing eye field (Fig. 1C,D; supplementary material Table S1). A few GFP-positive cells showed Elav expression in the bi region (Fig. 1C), and the corresponding adult eye was highly reduced along the DV margins (Fig. 1D) when compared with the wild-type eye (Fig. 1B). Misexpression of ykiS168A (bi>ykiS168A) or UAS-yki3SA (bi>yki3SA) at the DV margin resulted in a significantly stronger suppression of eye-specific fate in the eye imaginal disc (Fig. 1E,G; supplementary material Table S1) and the adult eye (Fig. 1F,H) respectively. By contrast, misexpression of UAS-ykiRNAi (N+C) (bi>ykiRNAi (N+C)) resulted in the enlargement of eye field at DV margins (Fig. 1I,J). Thus, transgenes expressing full-length or activated forms of Yki showed significant overgrowth and problems in the MF progression and differentiation of the photoreceptors (Fig. 1C,E,G).
Fig. 1.
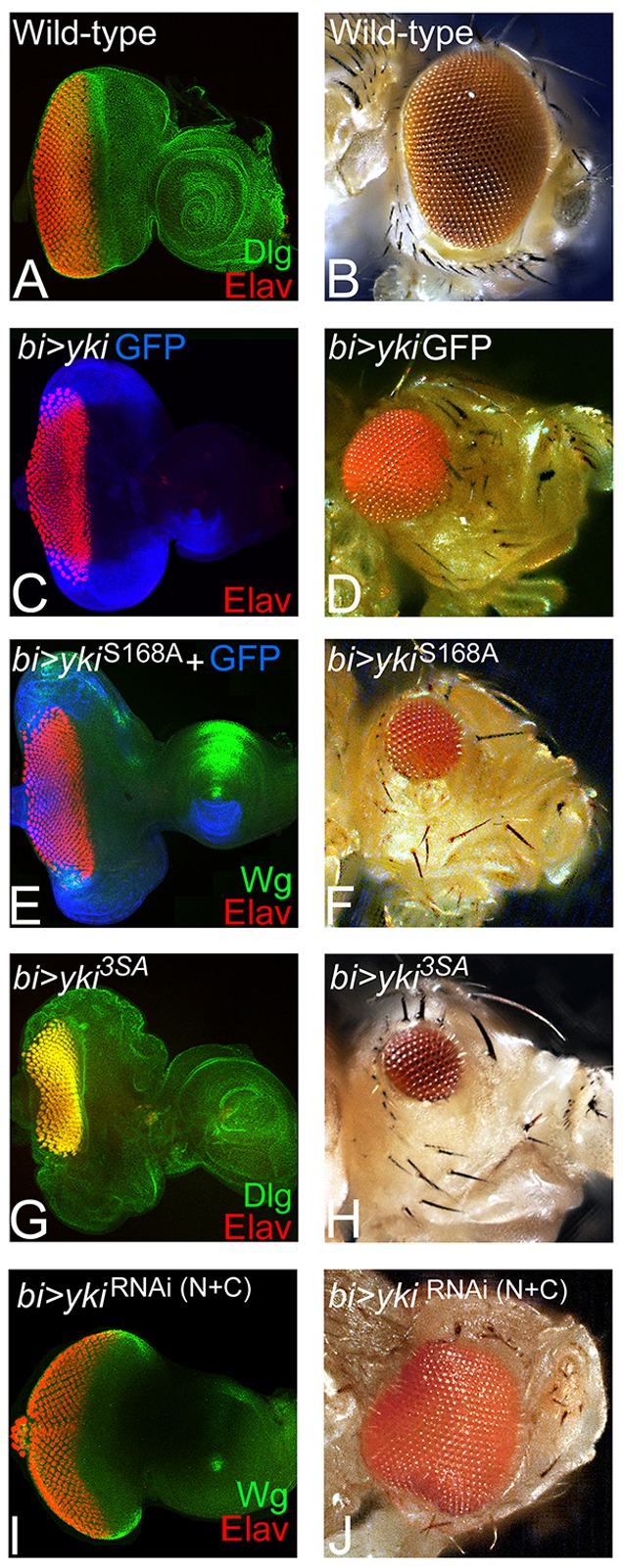
Hippo signaling is required for eye development. (A,B) Wild-type eye imaginal disc and compound eye of adult fly are shown. (A) Eye imaginal disc is stained for the membrane-specific marker Discs large (Dlg, green) and the pan-neural marker Elav (red), which marks the retinal neuron-specific fate. (C,E,G) Eye discs in which bi-Gal4 drives misexpression of (C) full-length yki (bi>yki-GFP), and its hyper activated forms (E) ykiS168A (bi>ykiS168A) and (G) bi>yki3SA (bi>yki3SA). The resulting adult eyes are shown in D,F,H respectively. Hyperactivated Yki resulted in suppression of the eye fate on both dorsal and ventral margins of (C,E,G) the eye disc and (D,F,H) the adult eye. (C,E) GFP (blue) marks the bi-Gal4 driver domain in the eye disc. (I,J) Misexpression of ykiRNAi on DV margins (bi>dicer+ykiRNAi(N+C)) resulted in enlargement of the eye field at both dorsal and ventral margins of the (I) eye disc and in the (J) adult. The orientation of all imaginal discs is identical with posterior towards the left and dorsal upwards.
We then tested phenotypes of modulating Hippo (Hpo) levels on photoreceptor differentiation (Fig. 2). Misexpression of hpoRNAi using bi-Gal4 (bi>hpoRNAi) resulted in the enlargement of eye field along the DV margins, as evident from the extension of Elav expression domains (Fig. 2A; supplementary material Table S1). In comparison with the wild-type adult eye (Fig. 1B), bi>hpoRNAi formed a significantly larger adult eye (Fig. 2B). By contrast, misexpression of hpo on the DV margin (bi>hpo) resulted in a highly reduced or ‘no-eye’ phenotype as seen in the eye imaginal disc (Fig. 2C) as well as the adult eye (Fig. 2D). This decrease in size and number of photoreceptors may be attributed to the induction of cell death by hyperactivation of the Hippo pathway (Tapon et al., 2002; Udan et al., 2003; Verghese et al., 2012). Therefore, we analyzed gain-of-function ‘flp-out’ clones of hpo in the eye that were generated during the late second instar stage. We could not detect hpo gain-of-function clones 24 h after clone induction, as these clones were eliminated (supplementary material Table S1). Therefore, we analyzed them 12 h after induction, and found that the GFP-positive hpo gain-of-function clones fail to grow (two or three cells in size), did not affect the photoreceptor neurons and resulted in a highly reduced adult eye (Fig. 2F). These data further validate other studies demonstrating that gain of function of hpo primarily induces cell death (Verghese et al., 2012). However, our data also show that Yki affects eye differentiation; therefore, we tested the expression of core RD genes when Yki is activated.
Fig. 2.
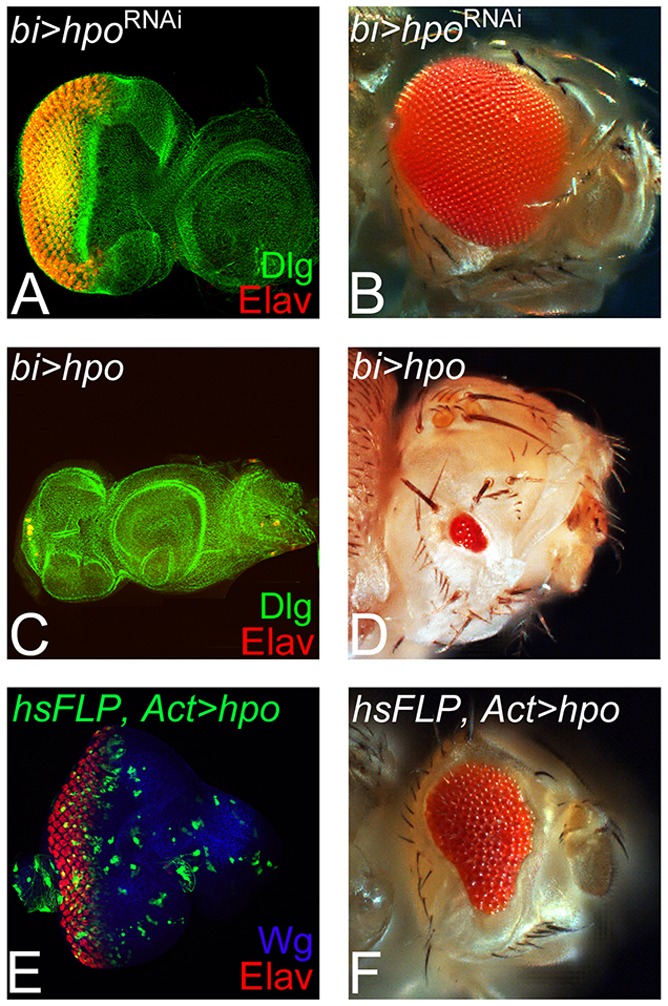
Modulating Hippo levels affects cell survival in the developing eye. (A,B) Blocking Hippo (Hpo) signaling by misexpression of hpoRNAi (bi>hpoRNAi) using a bi-Gal4 driver, resulted in dorsoventral (DV) enlargement of (A) the eye imaginal disc and (B) the adult eye. (C,D) Misexpression of hpo at the DV margin (bi>hpo) resulted in dramatic reduction of the eye fate, as evidenced from Elav expression in (C) the eye imaginal disc and in (D) the adult eye. (E,F) Gain-of-function ‘flp out’ clones of hpo (hsFLP>hpo), marked by GFP generated at late second instar and analyzed within 12 h show a few cell size clones that do affect eye fate, as seen in the (E) eye disc and (F) the adult eye.
Yki activity is required for RD gene expression
The human Pax6 homolog, Ey, is required for eye-field specification, and is one of the earliest genes expressed throughout the early first instar larval eye imaginal disc (Halder et al., 1995; Pappu and Mardon, 2004; Burgy-Roukala et al., 2013). During third instar larval stage, Ey is downregulated to allow eye differentiation to proceed; Ey expression is therefore restricted anterior to the MF in the cells that develop into head cuticle (Fig. 3A), (Halder et al., 1998). The RD gene eya, which acts downstream of ey, is expressed in a broad stripe in the differentiated cells posterior to the MF (Fig. 3B) (Bonini et al., 1993) and dac is expressed in two stripes – directly anterior and posterior to the MF (Fig. 3C) (Mardon et al., 1994). Misexpression of yki3SA (bi>yki3SA) resulted in an upregulation of Ey (Fig. 3D), and strong suppression of Eya (Fig. 3E) and Dac (Fig. 3F) at the DV margin. It is expected that Ey should be present if there is no retinal differentiation on the DV margin (Fig. 3D). To test whether these results could be extrapolated to other domains of the eye, we misexpressed yki3SA using dpp-Gal4 (dpp>yki3SA) and generated ‘flp-out’ clones (Act>yki3SA+GFP) in the eye imaginal disc. Misexpression of yki3SA using dpp-Gal4 (dpp>yki3SA), which drives expression on the posterior margin of the developing eye imaginal disc (marked by GFP reporter), resulted in the complete suppression of eye fate, as evidenced by the absence of Elav expression. In dpp>GFP+ yki3SA eye imaginal disc, Ey (Fig. 3G), Eya (Fig. 3H) and Dac are downregulated (Fig. 3I). Gain-of-function ‘flp-out’ clones of yki (GFP-positive) resulted in the suppression of eye fate, as evidenced by the lack of Elav positive nuclei in the clones (Fig. 3J,J‴,K′,K‴,L,L‴). Anterior to the MF, Ey was downregulated in the clones (Fig. 3J,J″).The gain-of-function clones of yki3SA strongly suppressed Eya (Fig. 3K,K″) and Dac (Fig. 3L,L″) expression. We observed that stochastic overexpression of yki3SA in the cells at disc margin using Gal4 drivers that induce expression very early during disc development, e.g. dpp-Gal4 or bi-Gal4, resulted in the induction of Ey but suppression of photoreceptor differentiation. However, overexpression of yki3SA later in the early to mid-second instar in ‘flp-out’ clones, resulted in the downregulation of Ey and in suppression of differentiation. Furthermore, the clone sizes ranged from large to small, reflecting whether the clone was induced during the late first or late second instar of larval development. Thus, suppression of differentiation by downregulation of RD genes (eya, dac) is a consistent phenotype of yki3SA overexpression. It is possible that hyperactivation of yki suppresses the R8 specification, and thus results in the observed defects in differentiation.
Fig. 3.
Activation of yki suppresses eye fate by downregulating the retinal determination genes. (A-C) Wild-type expression of (A) Eyeless (Ey, green), (B) Eyes absent (Eya, green) and (C) Dachshund (Dac, green) in the third instar eye imaginal disc. (A) Ey expression is restricted anterior to the morphogenetic furrow (MF). (B) Eya is expressed both posterior as well as anterior to the MF. (C) Dac is expressed in a wide band anterior to the MF. (D-F) Misexpression of yki3SA (bi>yki 3SA) at the dorsoventral (DV) margin of the developing eye disc resulted in (D) Ey (green) expression and suppression of Elav (red), (E) Eya (green) and (F) Dac (green) at the DV margin. (D,F) Dlg (blue) marks the outline of the eye disc. (G-I) Misexpression of yki3SA at the posterior margin of the developing eye disc using dpp-Gal4 driver (dpp>yki3SA) resulted in complete loss of eye field, as evidenced by (G-I) loss of Elav (red), (G) mild downregulation of Ey (green) near the posterior margin, (H) loss of Eya (green) and (I) loss of Dac (green). dpp>yki3SA resulted in a highly reduced eye field. (J-L‴) Random gain-of-function clones of yki3SA (hsFLP>yki3SA) marked by (J′,K′,L′) GFP (blue) reporter resulted in suppression of (J,J″) Ey (green), (K,K″) Eya (green) and (L,L″) Dac (green). (J‴,K‴,L‴) Elav expression (red) is downregulated in yki3SA clones. Orientation and magnification of all imaginal discs are identical.
Activated Yki blocks retinal differentiation
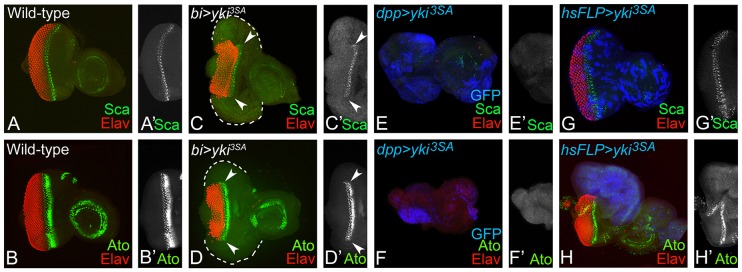
Expression of Scabrous (Sca) and Atonal (Ato) – the two early markers for R8 specification (Fig. 4A,B) (Baker et al., 1990; Mlodzik et al., 1990; Jarman et al., 1994) were analyzed when Hippo signaling was downregulated or Yki was hyperactivated. Misexpression of yki3SA using bi-Gal4 driver (bi>yki3SA) resulted in the complete loss of Sca (Fig. 4C), Ato (Fig. 4D) and Elav expression at the DV margin. It suggests that the cells expressing hyperactivated yki not only suppress the RD genes, but also downregulate the markers for R8 photoreceptor specification and differentiation. These effects were confirmed by misexpression of yki using dpp-Gal4 driver (dpp>yki3SA; Fig. 4E,F) and in random ‘flp-out’ gain-of-function clones of yki3SA (marked by GFP reporter) (Fig. 4G,H) in the eye imaginal disc. Thus, hyperactivation of yki blocks differentiation of the R8 cells. As R8 specification and differentiation are associated with morphogenetic furrow (MF) progression, the requirement for yki3SA in MF progression was tested.
Fig. 4.
Activation of yki blocks retinal differentiation in the developing eye disc. Panels show the expression pattern of (A) Scaborous (Sca, green) and (B) Atonal (Ato, green), the two markers for R8 specification in the wild-type developing eye disc. Misexpression of yki3SA by (C,D) bi-Gal4 (bi>yki3SA), (E,F) dpp-Gal4 and (G,H) ‘flp out’ clone of yki3SA (hsFLP>yki3SA) marked by GFP (blue) suppressed the (C,C′,E,E′,G,G′) Sca (green) and (D,D′,F,F′,H,H′) Ato (green) expression in the eye disc. (C,D) White dotted line marks the outline of the eye imaginal disc. The orientation and magnification are identical for all imaginal discs. Arrowheads in C-D′ indicate the region of R8 specification in eye imaginal disc to show that R8 specification does not extend into the bi domain.
Yki is required for MF progression
In the developing eye, Dpp and Hh signaling is required for normal development and MF progression (Ready et al., 1976; Wolff and Ready, 1993; Schlichting and Dahmann, 2008; Kumar, 2013). To test the involvement of Hippo signaling pathway in MF progression, dpp-lacZ reporter expression was examined. In the wild-type eye imaginal discs, dpp-lacZ, a transcriptional reporter of dpp gene (Blackman et al., 1991) is expressed in a thin stripe that overlays the apical constrictions caused by the MF cells, and marks the anterior extent of Elav positive differentiated cells (Fig. 5A,A″). Misexpression of hpoRNAi (bi>hpoRNAi) resulted in the extension of dpp-lacZ expression anteriorly, along the DV margins due to extension of MF, leading to generation of enlarged eyes caused by ectopic Elav expression (Fig. 5B,B′). However, misexpression of yki3SA led to the suppression of eye fate and MF progression causing the furrow to bend posteriorly (Fig. 5C,C′, arrowheads). Large overgrowths are observed in the bi-Gal4 domains in bi>yki3SA discs despite suppression of retinal differentiation and suppression of MF progression (Fig. 5C,C′). It suggests that Yki is involved in regulating MF progression. A comparison of effects of misexpression of hpoRNAi and yki3SA revealed that unlike Yki, loss of hpo does not affect MF progression, suggesting that the Hippo pathway regulates growth and differentiation via separable mechanisms.
Fig. 5.
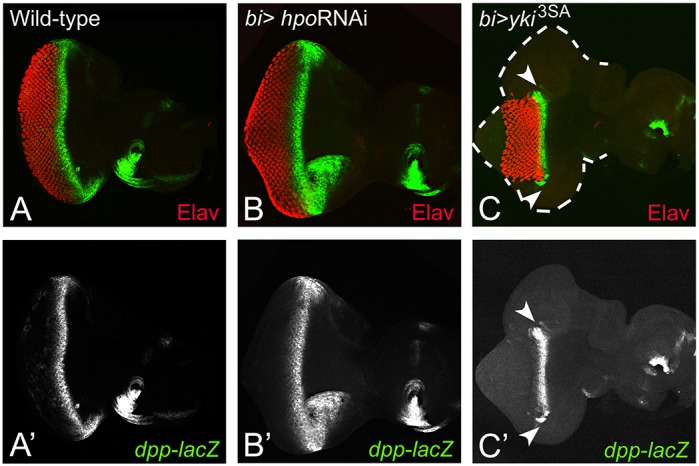
Activation of yki blocks morphogenetic furrow (MF) progression. A comparison of dpp-lacZ expression (green), a MF marker, is shown in the (A) wild-type, (B) bi>hpoRNAi and (C) bi>yki3SA eye imaginal discs. (A-C) Differentiated photoreceptor cells are marked by Elav (red). (C,C′) Misexpression of yki3SA resulted in the suppression of MF at both the dorsal and ventral margins (white arrowheads). The eye field (boundary marked by a white dotted line) is formed at the DV margin but retinal differentiation is suppressed. The orientation and magnification are identical for all imaginal discs.
Yki activation induces Wingless signaling to regulate MF progression
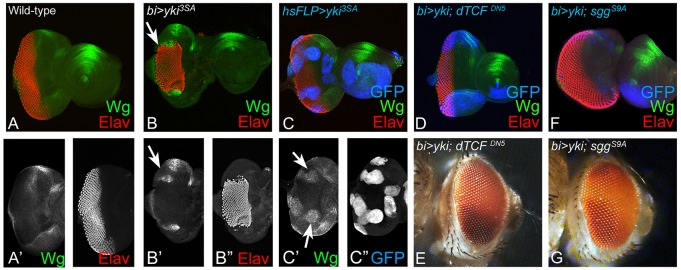
In the developing eye, Wg serves as a negative regulator of eye fate, and is known to block MF progression (Ma and Moses, 1995; Treisman and Rubin, 1995; Kumar, 2011). Therefore, we tested whether downregulation of Hippo signaling affected Wg levels in regions where retinal differentiation was suppressed. Misexpression of hyperactivated yki3SA using bi-Gal4 (bi>yki3SA) (Fig. 6B,B′) or in ‘flp-out’ clones (Fig. 6C,C′; supplementary material Table S1) induced ectopic Wg expression. These observations suggest that upregulation of Wg signaling may be the mechanism by which misexpression of yki3SA suppresses MF progression. We therefore tested effects of modulating levels of Wg signaling in the cells misexpressing yki3SA. Activation of Wg signaling ultimately activates the downstream transcription factor dTCF, which in turn induces Wg target genes (Swarup and Verheyen, 2012). Therefore, we tested two antagonists of Wg signaling pathway using transgenes that expressed either a dominant-negative form of Drosophila T-cell Factor (UAS-dTCFDN5) (van de Wetering et al., 1997) or the activated form of shaggy (UAS-sggS9A), the negative regulator of the pathway (Hazelett et al., 1998). Co-expression of yki with dTCFDN5 (bi>yki+dTCFDN5) (Fig. 6D,E) or with Sgg (bi>yki+sggS9A) (Fig. 6F,G) resulted in a significant rescue of retinal differentiation (marked by Elav) in the bi domain of the eye disc (supplementary material Table S1). The resulting eye imaginal disc (Fig. 6D,F) and the associated adult phenotype displayed a wild-type eye (Fig. 6E,G). The eye disc associated with bi>yki+sggS9A displayed ectopic Elav expression due to the extension of the Elav-positive cells along the DV margin (Fig. 6F), in comparison with the wild-type eye (Fig. 1A). These results demonstrate that, normally, Hippo signaling negatively regulates Wg signaling during retinal differentiation in the developing eye imaginal disc.
Fig. 6.
Activation of yki suppresses the eye fate by ectopically inducing Wg signaling. (A,A′) Panels show wild-type Wg (green) expression in the third instar eye imaginal disc. Wg (green) is expressed antero-laterally on dorsal and ventral eye margins of the eye imaginal disc. (B,C) Misexpression of yki3SA, under (B-B″) bi-Gal4 (bi>yki3SA) at DV margins or in ‘FLP-out’ random clones (marked by GFP, blue) under (C-C″) hsFLP>yki3SA in the eye disc, results in suppression of eye fate (marked by Elav, red) and ectopic induction of Wg expression (green, marked by white arrows in B,B′,C′). Blocking Wg signaling in bi>yki eye disc by misexpression of antagonists of Wg signaling such as (D,E) dominant-negative dTCFDN (bi>yki+dTCFDN5) and (F,G) Shaggy (Sgg) (bi>yki+SggS9A), resulted in the rescue of eye suppression by yki (bi>yki) in the (D,F) eye disc and (E,G) the adult eye. The orientation and magnification of imaginal discs and adult heads are identical in all panels.
Yki activation leads to ectopic induction of Hth and Tsh
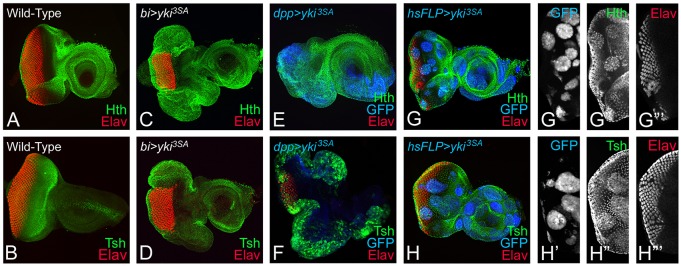
Wg is known to activate hth – a negative regulator known to suppress eye fate in the developing eye imaginal disc (Pichaud and Casares, 2000; Singh et al., 2002; Tare et al., 2013a). Furthermore, Tsh can induce Wg and Hth to suppress the eye fate (Singh et al., 2002; Tare et al., 2013a). We tested Hth and Tsh expression levels in the bi>yki3SA eye discs. In wild-type eye disc, Hth is expressed in the cells anterior to the MF that are not destined to form the head cuticle, in the peripodial membrane of the eye disc, and in a proximal ring in the antennal region of the eye-antennal imaginal disc (Fig. 7A). Gain-of-function of hth blocks differentiation of the photoreceptor neurons (van de Wetering et al., 1997; Pai et al., 1998; Bessa et al., 2002; Singh et al., 2002). Wild-type Tsh expression is also seen directly anterior to the MF (Fig. 7B). Misexpression of activated yki either by using bi-Gal4 (bi>yki3SA) (Fig. 7C,D) or dpp-Gal4 (dpp>yki3SA) (Fig. 7E,F) caused ectopic induction of Hth and Tsh along with complete loss of Elav (Fig. 7C,D). These results were further verified in ‘flp-out’ clones misexpressing UAS-yki3SA, which showed a loss of Elav-positive photoreceptors (Fig. 7G,H), as well as an upregulation of Hth (Fig. 7G,G″) and Tsh expression (Fig. 7H,H″). Overall, these data showed that activated Yki induced ectopic expression of Hth and Tsh to suppress retinal differentiation in the eye.
Fig. 7.
Activation of yki induces Homothorax (Hth) and Teashirt (Tsh) expression. Eye imaginal discs stained for (A,C,E,G,G′) Hth (green) and (B,D,F,H,H′) Tsh (green) are shown from (A,B) wild-type, (C,D) bi>yki3SA, (E,F) dpp>yki3SA and (G-H‴) hsFLP; Act>y+>Gal4 UASyki3SA (hsFLP>yki3SA) larvae. (A) Normally, Hth and (B) Tsh expression is restricted anterior to the MF in the eye. Both (C,E,G,G″) Hth and (D,F,H,H″) Tsh are upregulated, and (C-H,G‴,H‴) eye differentiation (Elav, red) is suppressed when yki3SA is overexpressed under (C,D) bi-Gal4, (E,F) dpp-Gal4 or in ‘FLP-out’ clones using (G,H) hsFLP>yki3SA. (G-H′) ‘Flp-out’ clones of yki3SA are marked by GFP (blue).
Activated Yki suppresses eye fate by a Wg-dependent mechanism
To test whether activated Yki suppresses the eye fate by Wg-mediated induction of Hth and Tsh, we blocked Wg signaling in the bi domain where Yki is activated (bi>yki+sgg) and then tested expression of Wg, Hth and Tsh. We found that blocking Wg signaling along with Yki activation (bi>yki+sgg) not only suppressed Wg (Fig. 8A,A′), but also blocked activation of Hth (Fig. 8B,B′) and Tsh (Fig. 8C,C′) on the DV margins. It suggests that Yki activation triggers Wg signaling, which in turn induces Hth and Tsh to block retinal determination and differentiation in the eye.
Fig. 8.
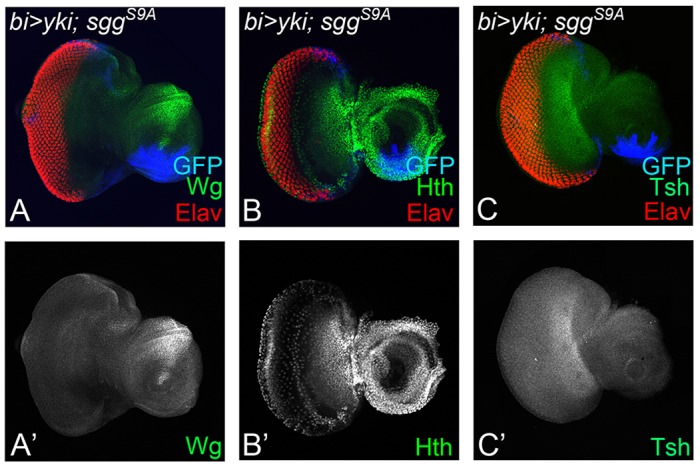
Activation of Yki suppresses eye fate through Wg signaling-mediated induction of Hth. Panels show effects of co-expression of activated yki and sgg with bi-Gal4 (bi>yki-GFP, sggS9A) on expression of (A,A′) Wg, (B,B′) Hth and (C,C′) Tsh in the DV margin of eye imaginal disc. The orientation and magnification of imaginal discs are identical in all panels.
Yki activation does not change eye fate to antenna
The ectopic expression of Hth or Tsh in yki3SA-expressing cells presented the alternate possibility that the cells overexpressing yki3SA have undergone a change in cell fate from eye disc to antenna or head cuticle. This idea was based on the fact that the larval eye imaginal disc gives rise to not only the compound eye but also to the antenna and the head of the adult fly. In developing eye discs, Cut (Ct) serves as a marker for the antennal specific fate (Kenyon et al., 2003; Duong et al., 2008; Wang and Sun, 2012; Weasner and Kumar, 2013). In the wild-type eye-antennal imaginal discs, Ct expression is found in a circular expression domain in the antenna region, and marks cone cells within the developing eye field (Fig. 9A). In the cells overexpressing yki3SA (bi>yki3SA), the cone cell-specific Ct expression was lost (Fig. 9B,D,D″). Together, the suppression of Cut (Fig. 9B-D) and Elav (Fig. 9D,D″) in bi>yki3SA and in ‘flp-out’ yki3SA clones suggests that hyperactivation of Yki strongly suppresses differentiation in the developing eye. Furthermore, as Ct expression is not induced in the ‘flp-out’ clones, it suggests that yki3SA misexpression does not cause a change in cell fate. Overall, these experiments show that the primary influence of yki3SA misexpression is linked to cell differentiation.
Fig. 9.
Activation of Yki does not change the cell fate from eye to antenna. (A) The wild-type expression of Cut (Ct, green), a marker for antennal fate, is restricted to a circular domain in the antennal region, as well as in the cone cells of the eye field. (B) Misexpression of yki3SA using a bi-Gal4 driver (bi>yki3SA) at the DV margin suppresses the retinal differentiation, as evident from Elav (red) expression, but does not induce ectopic Cut expression. (C) Misexpression of yki3SA on the posterior margin of the eye disc using a dpp-Gal4 driver (dpp>yki3SA) results in suppression of Elav (red) as well as Ct (green) expression. (D-D‴) Random gain-of-function clones of yki3SA (hsFLP>yki3SA) marked by (D,D′) GFP (blue) reporter results in suppression (D,D″) Ct (green). (D,D‴) Elav expression (red) is downregulated in yki3SA clones. The orientation and magnification of imaginal discs and adult heads are identical in all panels.
Activated Yki regulates retinal differentiation through Ft-Wts branch
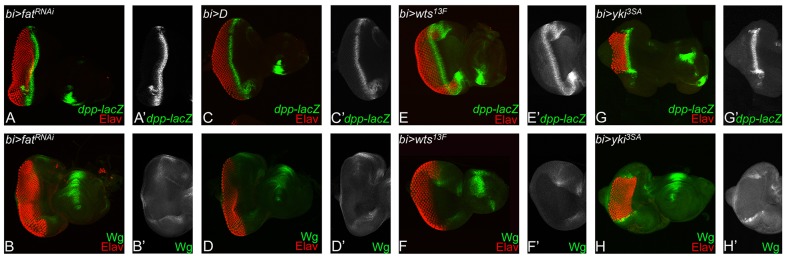
Our studies suggested that loss of Hippo signaling by downregulation of wts or by misexpression of activated forms of Yki affects growth and differentiation likely by separable independent mechanisms. Therefore, we investigated other Hippo pathway components, to test whether all or only specific upstream regulators of the pathway affect differentiation and growth. One of the upstream regulators is the Fat branch of the pathway that regulates Wts stability via the atypical myosin Dachs (D) (Cho et al., 2006). In addition, loss of function of the Fat branch components also shows broad DV and PD defects, and defects in MF progression (Cho and Irvine, 2004; Mao et al., 2006; Matakatsu and Blair, 2012). Misexpression of UAS-ftRNAi (bi>ftRNAi) at the DV margin shows mild suppression of the retinal differentiation and MF progression, marked by the loss of Elav-positive cells (Fig. 10A,A′). These defects coincide with ectopic Wg expression at the DV margins (Fig. 10B,B′), suggesting a similar mechanism of suppression of differentiation and MF progression as yki3SA (Fig. 10G,H). Interestingly, overexpression of D, which is negatively regulated by Ft, showed weak overgrowth and extension of MF progression (Fig. 10C,C′), and downregulation of Wg (Fig. 10D,D′). Within the Ft branch, D negatively regulates Wts, and overexpression of Wts throughout the eye using ey-Gal4 (data not shown) or GMR-Gal4 (Tapon et al., 2002; Wu et al., 2003; Lai et al., 2005) is shown to cause mild cell death. However, misexpression of Wts using bi-Gal4 resulted in an unexpected phenotype in which, although the overall eye size was not overly overgrown, the MF progression appeared extended at both the dorsal and ventral margins in the bi-Gal4 domain (Fig. 10E,E′). Wg expression was downregulated in these discs (Fig. 10F,F′). Overall, these data revealed a shared defect in MF progression in several components of the Ft branch of the pathway that is not shared by other upstream regulators like Hpo.
Fig. 10.
Modulating the Fat branch of the Hippo signaling pathway affects retinal differentiation in the developing eye. Panels show comparison of dpp-lacZ (green) and Elav (red) expression in the eye imaginal discs from larvae of the following genotypes: (A-B′) bi-Gal4 driver UAS-ftRNAi (bi>ftRNAi), (C-D′) bi-Gal4 driver UAS-D (bi>D), (E-F′) bi-Gal4 driver UAS-wts13F (bi>wts13F) and (G-H′) bi-Gal4 driver UAS-yki3SA (bi>yki3SA). The magnification of all images is identical.
DISCUSSION
During development, patterning and growth are tightly controlled by highly conserved signaling pathways to determine the size and shape of an animal. In Drosophila eye, an initial proliferative phase (growth spurt) occurs during early larval development (under the control of several growth regulatory pathways), which is followed by retinal determination and differentiation (Tare et al., 2013a). Together, these processes direct the formation of the fly retina and other associated cells (Wolff and Ready, 1993). Interestingly, the expression of growth regulatory pathway genes continues when cells are undergoing differentiation, suggesting that these genes may play an important role during retinal differentiation.
The Hippo pathway regulates both growth and retinal differentiation in the developing eye
In Drosophila, the Hippo signaling pathway members regulate the activity of the transcriptional co-activator Yki to control tissue growth. Activation of Yki by downregulation of the Hippo pathway, leads to its nuclear localization, where it activates target genes that trigger cell proliferation and inhibit apoptosis (Huang et al., 2005; Edgar, 2006; Pan, 2010; Halder and Johnson, 2011; Verghese et al., 2013; Yu and Guan, 2013). The Yki homolog YAP/TAZ function as oncogenes in the context of many cancers. Recently, other functions of the Hippo signaling pathway have been described, including stem cell renewal and maintenance, regeneration, wound healing and axial patterning (Halder and Camargo, 2013; Yu and Guan, 2013). Earlier data have shown that the prominent effect of downregulation of the Hippo pathway is excess growth due to increased proliferation and reduced cell death (Fig. 2; supplementary material Fig. S1). By contrast, hyperactivation of the pathway results in the reduction of developing eye field (Fig. 2) by hpo-mediated upregulation of the pro-apoptotic gene head involution defective (hid) (Udan et al., 2003). Interestingly, misexpression of yki (bi>yki), which also results in downregulation of the pathway, showed overgrowth of the eye disc but suppression of photoreceptor differentiation in the eye (Fig. 1). Taken together, these data suggest that the Hippo pathway regulates the growth of eye field and eye fate specification/differentiation by separable mechanisms. These phenotypes of Yki misexpression suggested that Yki may regulate the expression of the retinal differentiation genes or other negative regulators of eye development. Previously it has been suggested that Yki and Sd regulate tissue specification (Zhang et al., 2011).
There are several signaling pathways involved in eye development but the RD genes represent the core transcription factor cascade, which is required to specify and differentiate epithelial cells into retinal neurons (Bonini et al., 1993; Cheyette et al., 1994; Mardon et al., 1994; Quiring et al., 1994; Halder et al., 1995, 1998; Chen et al., 1997, 1999; Shen and Mardon, 1997). We tested expression of three RD network genes (ey, eya and dac) in cells where yki was hyperactivated. Misexpression of yki (bi>yki3SA) blocked the eye fate by suppressing RD gene expression (Fig. 3). These data suggest that Yki levels and activity need to be regulated during eye growth and differentiation, as inappropriate upregulation of Yki activity leads to suppression of retinal differentiation.
Yki is required for retinal differentiation and MF progression
Retinal differentiation is marked by onset of expression of Ato, which marks the R8 photoreceptor fate and initiates the process of retinal differentiation behind the MF (Baker et al., 1990; Mlodzik et al., 1990; Jarman et al., 1994). Misexpression of yki on the posterior margin of the eye disc by the dpp-Gal4 (dpp>yki3SA) driver resulted in a ‘no-eye’ phenotype (Fig. 3J-L and Fig. 4G,H). Lack of retinal photoreceptor neurons raised an interesting possibility that retinal differentiation process may not have initiated at all in dpp>yki3SA eye imaginal disc (Fig. 3J-L and Fig. 4G,H). Initiation and progression of MF depends on the function of Dpp and Hh signaling (Dominguez and Hafen, 1997; Borod and Heberlein, 1998; Kumar, 2013). Activation of Yki in a subset of cells at the DV margin can prevent initiation of the MF, as expression of dpp-lacZ (a key gene in maintaining MF progression) and downstream RD genes is suppressed (Figs 4 and 5). Furthermore, MF initiation and R8 specification was completely blocked when Yki was activated at the posterior margin of the developing eye imaginal disc (dpp >yki3SA). Thus, activated Yki suppressed retinal differentiation. Taken together, these studies suggest that Yki is required for the MF progression.
Yki regulates Wg signaling to promote MF progression
The highly conserved Wg signaling pathway is involved in various developmental functions, including eye development and retinal differentiation. Wg, a signaling molecule, is expressed at the antero-lateral margin of the developing eye disc and its expression recedes as the MF progresses anteriorly from the posterior margin. Wg is known to be a negative regulator of retinal development, and blocks MF progression in the developing eye (Ma and Moses, 1995; Treisman and Rubin, 1995). Interestingly, Wg is one of the downstream targets of the Hippo pathway effector Yki (Cho et al., 2006). Misexpression of yki caused ectopic expression of Wg, suggesting that Wg signaling is activated by Yki on the DV margins to block MF progression, as well as to prevent retinal differentiation in the developing eye (Fig. 6). Furthermore, downregulation of Wg signaling caused ectopic differentiation to occur when yki was misexpressed (Fig. 6). Thus, Wg acts downstream of Yki to suppress MF progression and retinal differentiation (Fig. 7). When Wg signaling was blocked by co-expressing antagonists of Wg signaling such as SggS9A or dTCFDN5, the loss of retinal differentiation due to misexpression of Yki was restored to near wild type (Fig. 6). These results further substantiate our earlier conclusions that the Hippo pathway affects multiple steps during differentiation, e.g. MF progression, regulation of expression of RD genes and activation of negative regulators of eye development.
In addition to its role in MF progression, Wg is also essential for delimiting the border between the compound eye and the adult head cuticle (Royet and Finkelstein, 1996, 1997). Similarly, expression of the head-specific fate markers such as Hth and Tsh also becomes restricted to anterior to the MF in the presumptive head cuticle region (Bessa et al., 2002; Singh et al., 2002). Tsh is responsible for Wg-mediated induction of Hth in the ventral eye to define the boundary between the head capsule and the eye field (Bessa et al., 2002; Singh et al., 2002; Tare et al., 2013a). Our studies showed that Yki-mediated suppression of retinal differentiation causes ectopic induction of Wg, Hth and Tsh (Fig. 6). Both tsh and hth have been shown in previous studies to be targets of yki and wg (Rieckhof et al., 1997; Gallet et al., 1998; Peng et al., 2009). Our studies demonstrate that Yki uses its downstream target, Wg, to regulate expression of the negative regulator Hth in order to regulate retinal fate (Figs 8 and 11). It is possible that cells misexpressing yki undergo transformation from eye to antennal fate. However, the antennal fate marker Ct was not induced in the cells where retinal fate was suppressed by Yki misexpression (Fig. 9). Thus, the Yki misexpressing cells do not undergo transformation from eye to antenna-specific cells.
Fig. 11.
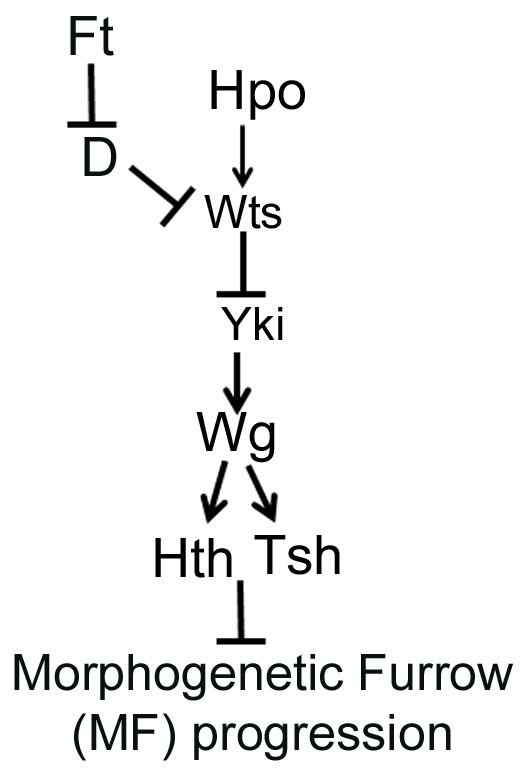
Model to demonstrate the role of Hippo signaling in regulating retinal differentiation and MF progression. The Ft-Wts branch of Hippo signaling downregulates Wts-mediated phosphorylation of Yki, which triggers Wg, a downstream target of Yki, to induce Hth- and Tsh-mediated block of retinal differentiation.
Mechanism of Hippo-mediated regulation of retinal differentiation
Our results uncovered a role for the Hippo pathway in the regulation of expression of RD genes, by controlling MF progression. These findings also raised the issue of whether all or only some components of the Hippo pathway were involved in the regulation of retinal differentiation. As Hippo gain of function resulted in reduced eye due to cell death (Fig. 2), we tested the effects of upstream components of the Fat branch specifically because prior reports suggested that ft mutant cells show marked delay in MF progression. Indeed, all major components of the Fat branch, e.g. ft, D and wts, showed defects in MF progression that were separate from the defects in growth regulation. Other upstream regulators (e.g. Sav, Mats, Kibra) did not cause an obvious change in MF progression. However, as previously reported, Ex, which also acts downstream of Ft, shows reduced eye size, and defects in MF progression and differentiation (data not shown) (Boedigheimer and Laughon, 1993; Tyler and Baker, 2007). Taken together, our studies show that Ft-wts branch of the Hippo signaling pathway affect MF progression via Yki-mediated regulation of its downstream target Wg (Fig. 11). Wg, in turn, then induces Hth and Tsh to suppress the eye fate and MF progression (Fig. 11). These functions of the Hippo pathway are separable from its role in the regulation of overall tissue size.
Thus, identification of Ft, D, Wts and Yki as key components involved in retinal differentiation and MF progression provides a striking parallel with the recently discovered involvement of the Wts-Hpo pathway and Yki/YAP in regulating primordial cell populations in vertebrates, notably the segregation of trophoectoderm and inner cell mass in early mammalian embryos (Nishioka et al., 2009; Zecca and Struhl, 2010), patterning and differentiation of airway epithelial progenitors (Mahoney et al., 2014), and patterning of neural and endodermal progenitor cells into spinal cord neurons and gut (Camargo et al., 2007; Cao et al., 2008). Our findings are also supported by recent reports from mammalian model organisms where the Hippo pathway is proposed to play a role in differentiation (Asaoka et al., 2014; Chen et al., 2014). We suggest that this novel employment of the pathway constitutes a new, and potentially general, mechanism for regulating tissue and organ size.
MATERIALS AND METHODS
The stocks used in this study are listed in FlyBase. The fly stocks used were UAS-fatRNAi (Dietzl et al., 2007), UAS-wtsRNAi (Fernandez et al., 2011; Rauskolb et al., 2011), UAS-ykiRNAi(N+C) (Zhang et al., 2008), UAS-D (Mao et al., 2006), UAS-hpo (Udan et al., 2003), UAS-wts13F (Kwon et al., 2015), y w; hsFLP; UAS-hpoRNAisymp19/ SM6-TM6B, Tb (Pantalacci et al., 2003), dpp-Gal4 (Staehling-Hampton et al., 1994), bi-Gal4 (Calleja et al., 1996), UAS-Dicer (Misquitta et al., 2008), dpp-lacZ (Blackman et al., 1991), UAS-Sgg (Hazelett et al., 1998), UAS-dTCFDN5 (van de Wetering et al., 1991), w; P(Act>y+>Gal4)25 P(UAS-GFPS65T)/CyO (Ito et al., 1997), y w hsFLP122 (Struhl and Basler, 1993), w; wtsx1FRT82B/TM6B, Tb (Xu et al., 1995) and Canton-S. For activation of Yki, we used the following transgenes: UAS-yki GFP (full length) (Oh and Irvine, 2008), UAS-ykiS168A (Oh and Irvine, 2008) and UAS-yki3SA (Oh and Irvine, 2008).
We used the Gal4/UAS system for targeted misexpression studies (Brand and Perrimon, 1993). All Gal4/UAS crosses were maintained at 18°C, 25°C and 29°C to sample different induction levels. Genetic epistasis experiments were carried out (at 25°C) in order to determine the genetic hierarchy.
Genetic mosaic analysis
Gain-of-function clones of UAS-yki GFP, UAS-yki3SA and UAS-hpo were generated using ‘flp-out’ technique, by crossing y w hsFLP122; P(Act>y+>Gal4)25 P(UAS-GFPS65T)/CyO (Struhl and Basler, 1993) virgins with males from UAS-yki GFP or UAS-yki3SA or UAS-hpo to generate GFP-positive clones. Eggs were collected at 6 h intervals at 25°C from a synchronous culture, and subjected to a single heat shock (20 min for UAS-hpo and 45 min for UAS-yki) at 37°C at about 24 h after egg laying (AEL) or as indicated. The larvae were transferred to 25°C for recovery and further development. Loss-of-function clones of wts were generated by crossing w; wtsx1FRT82B/TM6B, Tb males with eyFLP; FRT82B Ubi-GFP virgins.
Immunohistochemistry
Eye-antennal discs from wandering third instar larvae were dissected and stained following the standard protocol (Singh et al., 2002). The primary antibodies used were rabbit anti-Dlg (1:200, a gift from K. Cho, KAIST, Korea), mouse anti-Dlg (1:50, DSHB, 4F3), rat anti-Elav (1:100, DSHB, 7E8A10), mouse anti-Wg (1:50, DSHB, 4D4), mouse anti-Ey (1:100, DSHB, Ey), mouse anti-Eya (1:100, DSHB, eya10H6), mouse anti-Dac (1:100, DSHB, mABdac2-3), mouse anti-Cut (1:20, DSHB, 2B10), mouse anti-Sca (1:100), rabbit anti-Hth (1:100, a gift from Henry Sun, Institute of Molecular Biology, Taiwan), rabbit anti-Tsh (1:150, a gift from Stephen Cohen, Institute of Molecular and cell Biology, Singapore), rabbit anti-β galactosidase (1:100, Promega, Z3781) and guinea pig anti-Atonal (1:500, a gift from Daniel Marenda, Drexel University, PA, USA). The secondary antibodies were: donkey anti-rat IgG conjugated with Cy5 (1:250, Jackson, 712-175-153), donkey anti-mouse IgG conjugated to Cy3 (1:300, Jackson, 715-165-150), donkey anti-mouse IgG conjugated to FITC (1:200, Jackson, 715-095-150), donkey anti-rat IgG conjugated to Cy3 (1:300, Jackson, 715-165-150), goat anti-rabbit IgG conjugated to Cy5 (1:250, Jackson, 111-175-144), donkey anti-guinea pig IgG conjugated to Cy3 (1:250, Jackson, 706-165-152), donkey anti-rabbit IgG conjugated to Cy3 (1:250, Jackson, 711-165-152) and goat anti-rabbit IgG conjugated to FITC (1:200, Jackson, 711-165-152). Images were captured using the Olympus Fluoview 1000 Laser Scanning Confocal Microscope. The adult eye imaging was carried out using Zeiss Apotome Imager.Z1 microscope. The images were processed and analyzed using the Adobe Photoshop CS4 software.
Acknowledgements
We thank Justin Kumar, J. Treisman, Y. Henry Sun and the Bloomington Stock Center for the Drosophila strains; U. Walldorf, Patrick Callaerts, Kyung Ok Cho and the Developmental Studies Hybridoma Bank (DSHB) for the antibodies; and members of Singh and Kango-Singh labs for critical comments on the manuscript. Confocal microscopy was supported by a Biology Department central core facility.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
M.K.-S. is supported by Knight's Templar Eye Foundation and start-up support from the University of Dayton. This work is supported by start-up support from the University of Dayton and Ohio Cancer Research Associates to A. Singh.
Author contributions
E.W. and A. Sarkar performed experiments and data analysis; K.G. performed experiments; M.K.-S. carried out data analysis and co-wrote the manuscript; A. Singh developed the concept, carried out data analysis and co-wrote the manuscript.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.117358/-/DC1
References
- Aberle H., Bauer A., Stappert J., Kispert A. and Kemler R. (1997). beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797-3804. 10.1093/emboj/16.13.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka Y., Hata S., Namae M., Furutani-Seiki M. and Nishina H. (2014). The Hippo pathway controls a switch between retinal progenitor cell proliferation and photoreceptor cell differentiation in zebrafish. PLoS ONE 9, e97365 10.1371/journal.pone.0097365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E. (2001). Cell proliferation, survival, and death in the Drosophila eye. Semin. Cell Dev. Biol. 12, 499-507. 10.1006/scdb.2001.0274 [DOI] [PubMed] [Google Scholar]
- Baker N. E., Mlodzik M. and Rubin G. M. (1990). Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science 250, 1370-1377. 10.1126/science.2175046 [DOI] [PubMed] [Google Scholar]
- Bertrand N., Castro D. S. and Guillemot F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517-530. 10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- Bessa J., Gebelein B., Pichaud F., Casares F. and Mann R. S. (2002). Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev. 16, 2415-2427. 10.1101/gad.1009002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman R. K., Sanicola M., Raftery L. A., Gillevet T. and Gelbart W. M. (1991). An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development 111, 657-666. [DOI] [PubMed] [Google Scholar]
- Boedigheimer M. and Laughon A. (1993). Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development 118, 1291-1301. [DOI] [PubMed] [Google Scholar]
- Boggiano J. C. and Fehon R. G. (2012). Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev. Cell 22, 695-702. 10.1016/j.devcel.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Leiserson W. M. and Benzer S. (1993). The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72, 379-395. 10.1016/0092-8674(93)90115-7 [DOI] [PubMed] [Google Scholar]
- Borod E. R. and Heberlein U. (1998). Mutual regulation of decapentaplegic and hedgehog during the initiation of differentiation in the Drosophila retina. Dev. Biol. 197, 187-197. 10.1006/dbio.1998.8888 [DOI] [PubMed] [Google Scholar]
- Brand A. H. and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Burgy-Roukala E., Miellet S., Mishra A. K. and Sprecher S. G. (2013). Early eye development: specification and deteremination. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 1-36. NewYork; Heidelberg; Dordrecht; London: Springer. [Google Scholar]
- Burke R. and Basler K. (1996). Hedgehog-dependent patterning in the Drosophila eye can occur in the absence of Dpp signaling. Dev. Biol. 179, 360-368. 10.1006/dbio.1996.0267 [DOI] [PubMed] [Google Scholar]
- Calleja M., Moreno E., Pelaz S. and Morata G. (1996). Visualization of gene expression in living adult Drosophila. Science 274, 252-255. 10.1126/science.274.5285.252 [DOI] [PubMed] [Google Scholar]
- Camargo F. D., Gokhale S., Johnnidis J. B., Fu D., Bell G. W., Jaenisch R. and Brummelkamp T. R. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054-2060. 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- Cao X., Pfaff S. L. and Gage F. H. (2008). YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 22, 3320-3334. 10.1101/gad.1726608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut F. and Heberlein U. (1997). Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development 124, 559-567. [DOI] [PubMed] [Google Scholar]
- Chen R., Amoui M., Zhang Z. and Mardon G. (1997). Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell 91, 893-903. 10.1016/S0092-8674(00)80481-X [DOI] [PubMed] [Google Scholar]
- Chen R., Halder G., Zhang Z. and Mardon G. (1999). Signaling by the TGF-beta homolog decapentaplegic functions reiteratively within the network of genes controlling retinal cell fate determination in Drosophila. Development 126, 935-943. [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhang N., Gray R. S., Li H., Ewald A. J., Zahnow C. A. and Pan D. (2014). A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 28, 432-437. 10.1101/gad.233676.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette B. N. R., Green P. J., Martin K., Garren H., Hartenstein V. and Zipursky S. L. (1994). The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977-996. 10.1016/0896-6273(94)90308-5 [DOI] [PubMed] [Google Scholar]
- Cho E. and Irvine K. D. (2004). Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131, 4489-4500. 10.1242/dev.01315 [DOI] [PubMed] [Google Scholar]
- Cho E., Feng Y., Rauskolb C., Maitra S., Fehon R. and Irvine K. D. (2006). Delineation of a Fat tumor suppressor pathway. Nat. Genet. 38, 1142-1150. 10.1038/ng1887 [DOI] [PubMed] [Google Scholar]
- Cohen S. M. (1993). Imaginal disc development. In The Development of Drosophila melanogaster (ed. Bate M. and Martinez Arias A.), Vol. 2, pp. 747-841. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- de Zulueta P., Alexandre E., Jacq B. and Kerridge S. (1994). Homeotic complex and teashirt genes co-operate to establish trunk segmental identities in Drosophila. Development 120, 2287-2296. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dominguez M. and Hafen E. (1997). Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye. Genes Dev. 11, 3254-3264. 10.1101/gad.11.23.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong H. A., Wang C. W., Sun Y. H. and Courey A. J. (2008). Transformation of eye to antenna by misexpression of a single gene. Mech. Dev. 125, 130-141. 10.1016/j.mod.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A. (2006). From cell structure to transcription: Hippo forges a new path. Cell 124, 267-273. 10.1016/j.cell.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Erkner A., Gallet A., Angelats C., Fasano L. and Kerridge S. (1999). The role of Teashirt in proximal leg development in Drosophila: ectopic Teashirt expression reveals different cell behaviours in ventral and dorsal domains. Dev. Biol. 215, 221-232. 10.1006/dbio.1999.9452 [DOI] [PubMed] [Google Scholar]
- Fasano L., Röder L., Coré N., Alexandre E., Vola C., Jacq B. and Kerridge S. (1991). The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell 64, 63-79. 10.1016/0092-8674(91)90209-H [DOI] [PubMed] [Google Scholar]
- Fernandez B. G., Gaspar P., Bras-Pereira C., Jezowska B., Rebelo S. R. and Janody F. (2011). Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138, 2337-2346. 10.1242/dev.063545 [DOI] [PubMed] [Google Scholar]
- Gallet A., Erkner A., Charroux B., Fasano L. and Kerridge S. (1998). Trunk-specific modulation of wingless signalling in Drosophila by teashirt binding to armadillo. Curr. Biol. 8, 893-902. 10.1016/S0960-9822(07)00369-7 [DOI] [PubMed] [Google Scholar]
- Geissler K. and Zach O. (2012). Pathways involved in Drosophila and human cancer development: the Notch, Hedgehog, Wingless, Runt, and Trithorax pathway. Ann. Hematol. 91, 645-669. 10.1007/s00277-012-1435-0 [DOI] [PubMed] [Google Scholar]
- Goulev Y., Fauny J. D., Gonzalez-Marti B., Flagiello D., Silber J. and Zider A. (2008). SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 18, 435-441. 10.1016/j.cub.2008.02.034 [DOI] [PubMed] [Google Scholar]
- Grusche F. A., Richardson H. E. and Harvey K. F. (2010). Upstream regulation of the hippo size control pathway. Curr. Biol. 20, R574-R582. 10.1016/j.cub.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Halder G. and Camargo F. D. (2013). The hippo tumor suppressor network: from organ size control to stem cells and cancer. Cancer Res. 73, 6389-6392. 10.1158/0008-5472.CAN-13-2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G. and Johnson R. L. (2011). Hippo signaling: growth control and beyond. Development 138, 9-22. 10.1242/dev.045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Callaerts P. and Gehring W. J. (1995). Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788-1792. 10.1126/science.7892602 [DOI] [PubMed] [Google Scholar]
- Halder G., Callaerts P., Flister S., Walldorf U., Kloter U. and Gehring W. J. (1998). Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development 125, 2181-2191. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Seawright A., Hardman K., Hodgson S., Zaletayev D., Fekete G. and van Heyningen V. (1993). PAX6 mutations in aniridia. Hum. Mol. Genet. 2, 915-920. 10.1093/hmg/2.7.915 [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Pfleger C. M. and Hariharan I. K. (2003). The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457-467. 10.1016/S0092-8674(03)00557-9 [DOI] [PubMed] [Google Scholar]
- Hazelett D., Bourouis M., Walldorf U. and Treisman J. (1998). decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125, 3741-3751. [DOI] [PubMed] [Google Scholar]
- Held L. I. J. (2002). The eye disc. In Imaginal Disc (ed. Held L. I.), pp. 197-236. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Huang J., Wu S., Barrera J., Matthews K. and Pan D. (2005). The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421-434. 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y. and Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761-771. [DOI] [PubMed] [Google Scholar]
- Jang C.-C., Chao J.-L., Jones N., Yao L.-C., Bessarab D. A., Kuo Y. M., Jun S., Desplan C., Beckendorf S. K. and Sun Y. H. (2003). Two Pax genes, eye gone and eyeless, act cooperatively in promoting Drosophila eye development. Development 130, 2939-2951. 10.1242/dev.00522 [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Grell E. H., Ackerman L., Jan L. Y. and Jan Y. N. (1994). Atonal is the proneural gene for Drosophila photoreceptors. Nature 369, 398-400. 10.1038/369398a0 [DOI] [PubMed] [Google Scholar]
- Jia J., Zhang W., Wang B., Trinko R. and Jiang J. (2003). The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 17, 2514-2519. 10.1101/gad.1134003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D. and Desplan C. (2011). Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev. Cell 21, 874-887. 10.1016/j.devcel.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D., Xie B., Rister J., Terrell D., Charlton-Perkins M., Pistillo D., Gebelein B., Desplan C. and Cook T. (2013). Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science 342, 1238016 10.1126/science.1238016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice R. W., Zilian O., Woods D. F., Noll M. and Bryant P. J. (1995). The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534-546. 10.1101/gad.9.5.534 [DOI] [PubMed] [Google Scholar]
- Kango-Singh M. and Singh A. (2009). Regulation of organ size: insights from the Drosophila Hippo signaling pathway. Dev. Dyn. 238, 1627-1637. 10.1002/dvdy.21996 [DOI] [PubMed] [Google Scholar]
- Kango-Singh M., Nolo R., Tao C., Verstreken P., Hiesinger P. R., Bellen H. J. and Halder G. (2002). Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719-5730. 10.1242/dev.00168 [DOI] [PubMed] [Google Scholar]
- Kenyon K. L., Ranade S. S., Curtiss J., Mlodzik M. and Pignoni F. (2003). Coordinating proliferation and tissue specification to promote regional identity in the Drosophila head. Dev. Cell 5, 403-414. 10.1016/S1534-5807(03)00243-0 [DOI] [PubMed] [Google Scholar]
- Kumar J. P. (2011). My what big eyes you have: how the Drosophila retina grows. Dev. Neurobiol. 71, 1133–1152. 10.1002/dneu.20921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J. (2013). Catching the next wave: patterning of the Drosophila eye by the morphogenetic furrow. In In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Madhuri K.-S.), pp. 75-97. NewYork: Springer. [Google Scholar]
- Kurant E., Pai C. Y., Sharf R., Halachmi N., Sun Y. H. and Salzberg A. (1998). Dorsotonals/homothorax, the Drosophila homologue of meis1, interacts with extradenticle in patterning of the embryonic PNS. Development 125, 1037-1048. [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Waghmare I., Verghese S., Singh A., Singh A. and Kango-Singh M. (2015). Drosophila C-terminal Src kinase regulates growth via the Hippo signaling pathway. Dev. Biol. 397, 67-76. 10.1016/j.ydbio.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Lai Z.-C., Wei X., Shimizu T., Ramos E., Rohrbaugh M., Nikolaidis N., Ho L.-L. and Li Y. (2005). Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120, 675-685. 10.1016/j.cell.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Ma C. and Moses K. (1995). Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development 121, 2279-2289. [DOI] [PubMed] [Google Scholar]
- Mahoney J. E., Mori M., Szymaniak A. D., Varelas X. and Cardoso W. V. (2014). The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137-150. 10.1016/j.devcel.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Rauskolb C., Cho E., Hu W.-L., Hayter H., Minihan G., Katz F. N. and Irvine K. D. (2006). Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 133, 2539-2551. 10.1242/dev.02427 [DOI] [PubMed] [Google Scholar]
- Mardon G., Solomon N. M. and Rubin G. M. (1994). dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473-3486. [DOI] [PubMed] [Google Scholar]
- Matakatsu H. and Blair S. S. (2012). Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development 139, 1498-1508. 10.1242/dev.070367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies L. D., Kerridge S. and Scott M. P. (1994). Role of the teashirt gene in Drosophila midgut morphogenesis: secreted proteins mediate the action of homeotic genes. Development 120, 2799-2809. [DOI] [PubMed] [Google Scholar]
- Misquitta L., Wei Q. and Paterson B. M. (2008). Drosophila RNA interference (RNAi) using a Gal-4 inducible transgene vector. CSH Protoc. pdb ip51 10.1101/pdb.ip51. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Baker N. E. and Rubin G. M. (1990). Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 4, 1848-1861. 10.1101/gad.4.11.1848 [DOI] [PubMed] [Google Scholar]
- Moskow J. J., Bullrich F., Huebner K., Daar I. O. and Buchberg A. M. (1995). Meis1, a PBX1-related homeobox gene involved in myeloid leukemia in BXH-2 mice. Mol. Cell. Biol. 15, 5434-5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoletano F., Occhi S., Calamita P., Volpi V., Blanc E., Charroux B., Royet J. and Fanto M. (2011). Polyglutamine Atrophin provokes neurodegeneration in Drosophila by repressing fat. EMBO J. 30, 945-958. 10.1038/emboj.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N., Inoue K.-i., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R. O., Ogonuki N. et al. (2009). The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398-410. 10.1016/j.devcel.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Oh H. and Irvine K. D. (2008). In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081-1088. 10.1242/dev.015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H. and Irvine K. D. (2009). In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916-1927. 10.1038/onc.2009.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H. and Irvine K. D. (2010). Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 20, 410-417. 10.1016/j.tcb.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C.-Y., Kuo T.-S., Jaw T. J., Kurant E., Chen C.-T., Bessarab D. A., Salzberg A. and Sun Y. H. (1998). The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 12, 435-446. 10.1101/gad.12.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. (2010). The hippo signaling pathway in development and cancer. Dev. Cell 19, 491-505. 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S., Tapon N. and Léopold P. (2003). The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921-927. 10.1038/ncb1051 [DOI] [PubMed] [Google Scholar]
- Pappu K. S. and Mardon G. (2004). Genetic control of retinal specification and determination in Drosophila. Int. J. Dev. Biol. 48, 913-924. 10.1387/ijdb.041875kp [DOI] [PubMed] [Google Scholar]
- Peng H. W., Slattery M. and Mann R. S. (2009). Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 23, 2307-2319. 10.1101/gad.1820009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud F. (2014). Transcriptional regulation of tissue organization and cell morphogenesis: the fly retina as a case study. Dev. Biol. 385, 168-178. 10.1016/j.ydbio.2013.09.031 [DOI] [PubMed] [Google Scholar]
- Pichaud F. and Casares F. (2000). homothorax and iroquois-C genes are required for the establishment of territories within the developing eye disc. Mech. Dev. 96, 15-25. 10.1016/S0925-4773(00)00372-5 [DOI] [PubMed] [Google Scholar]
- Pires-daSilva A. and Sommer R. J. (2003). The evolution of signalling pathways in animal development. Nat. Rev. Genet. 4, 39-49. 10.1038/nrg977 [DOI] [PubMed] [Google Scholar]
- Poulson D. F. (1950). Histogenesis, oogenesis, and differentiation in the embryo of Drosophila melanogaster meigen. In Biology of Drosophila (ed. Demerec M.), pp. 168-274. New York: Wiley. [Google Scholar]
- Quiring R., Walldorf U., Kloter U. and Gehring W. J. (1994). Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265, 785-789. 10.1126/science.7914031 [DOI] [PubMed] [Google Scholar]
- Raff M. C. (1996). Size control: the regulation of cell numbers in animal development. Cell 86, 173-175. 10.1016/S0092-8674(00)80087-2 [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Pan G., Reddy B. V. V. G., Oh H. and Irvine K. D. (2011). Zyxin links fat signaling to the hippo pathway. PLoS Biol. 9, e1000624 10.1371/journal.pbio.1000624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E. and Benzer S. (1976). Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53, 217-240. 10.1016/0012-1606(76)90225-6 [DOI] [PubMed] [Google Scholar]
- Rieckhof G. E., Casares F., Ryoo H. D., Abu-Shaar M. and Mann R. S. (1997). Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171-183. 10.1016/S0092-8674(00)80400-6 [DOI] [PubMed] [Google Scholar]
- Roder L., Vola C. and Kerridge S. (1992). The role of the teashirt gene in trunk segmental identity in Drosophila. Development 115, 1017-1033. [DOI] [PubMed] [Google Scholar]
- Roignant J.-Y. and Treisman J. E. (2009). Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 53, 795-804. 10.1387/ijdb.072483jr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J. and Finkelstein R. (1996). hedgehog, wingless and orthodenticle specify adult head development in Drosophila. Development 122, 1849-1858. [DOI] [PubMed] [Google Scholar]
- Royet J. and Finkelstein R. (1997). Establishing primordia in the Drosophila eye-antennal imaginal disc: the roles of decapentaplegic, wingless and hedgehog. Development 124, 4793-4800. [DOI] [PubMed] [Google Scholar]
- Schlichting K. and Dahmann C. (2008). Hedgehog and Dpp signaling induce cadherin Cad86C expression in the morphogenetic furrow during Drosophila eye development. Mech. Dev. 125, 712-728. 10.1016/j.mod.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Seimiya M. and Gehring W. J. (2000). The Drosophila homeobox gene optix is capable of inducing ectopic eyes by an eyeless-independent mechanism. Development 127, 1879-1886. [DOI] [PubMed] [Google Scholar]
- Seto E. S. and Bellen H. J. (2004). The ins and outs of Wingless signaling. Trends Cell Biol. 14, 45-53. 10.1016/j.tcb.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Shen W. and Mardon G. (1997). Ectopic eye development in Drosophila induced by directed dachshund expression. Development 124, 45-52. [DOI] [PubMed] [Google Scholar]
- Silva E., Tsatskis Y., Gardano L., Tapon N. and McNeill H. (2006). The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr. Biol. 16, 2081-2089. 10.1016/j.cub.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Singh A., Kango-Singh M. and Sun Y. H. (2002). Eye suppression, a novel function of teashirt, requires Wingless signaling. Development 129, 4271-4280. [DOI] [PubMed] [Google Scholar]
- Singh A., Kango-Singh M., Choi K.-W. and Sun Y. H. (2004). Dorso-ventral asymmetric functions of teashirt in Drosophila eye development depend on spatial cues provided by early DV patterning genes. Mech. Dev. 121, 365-370. 10.1016/j.mod.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Singh A., Tare M., Puli O. R. and Kango-Singh M. (2012). A glimpse into dorso-ventral patterning of the Drosophila eye. Dev. Dyn. 241, 69-84. 10.1002/dvdy.22764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehling-Hampton K., Hoffmann F. M., Baylies M. K., Rushtont E. and Bate M. (1994). dpp induces mesodermal gene expression in Drosophila. Nature 372, 783-786. 10.1038/372783a0 [DOI] [PubMed] [Google Scholar]
- Staley B. K. and Irvine K. D. (2012). Hippo signaling in Drosophila: recent advances and insights. Dev. Dyn. 241, 3-15. 10.1002/dvdy.22723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G. and Basler K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527-540. 10.1016/0092-8674(93)90072-X [DOI] [PubMed] [Google Scholar]
- Swarup S. and Verheyen E. M. (2012). Wnt/Wingless signaling in Drosophila. Cold Spring Harb. Perspect. Biol. 4, a007930 10.1101/cshperspect.a007930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M. and Du W. (2008). Direct control of the proneural gene atonal by retinal determination factors during Drosophila eye development. Dev. Biol. 313, 787-801. 10.1016/j.ydbio.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N., Harvey K., Bell D. W., Wahrer D. C. R., Schiripo T., Haber D. A. and Hariharan I. K. (2002). salvador promotes both cell cycle exit and apoptosis in drosophila and is mutated in human cancer cell lines. Cell 110, 467–478. 10.1016/S0092-8674(02)00824-3 [DOI] [PubMed] [Google Scholar]
- Tare M., Puli O. R. and Singh A. (2013a). Molecular genetic mechanisms of axial patterning: mechanistic insights into generation of axes in the developing eye. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 37-75. NewYork; Heidelberg; Dordrecht; London: Springer. [Google Scholar]
- Tare M., Puli O. R., Moran M. T., Kango-Singh M. and Singh A. (2013b). Domain specific genetic mosaic system in the Drosophila eye. Genesis 51, 68-74. 10.1002/dvg.22355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J. E. and Rubin G. M. (1995). wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121, 3519-3527. [DOI] [PubMed] [Google Scholar]
- Tumaneng K., Russell R. C. and Guan K.-L. (2012). Organ size control by Hippo and TOR pathways. Curr. Biol. 22, R368-R379. 10.1016/j.cub.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. M. and Baker N. E. (2007). Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev. Biol. 305, 187-201. 10.1016/j.ydbio.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan R. S., Kango-Singh M., Nolo R., Tao C. and Halder G. (2003). Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914-920. 10.1038/ncb1050 [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oosterwegel M., Dooijes D. and Clevers H. (1991). Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 10, 123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., Loureiro J., Ypma A., Hursh D., Jones T., Bejsovec A. et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789-799. 10.1016/S0092-8674(00)81925-X [DOI] [PubMed] [Google Scholar]
- Verghese S., Bedi S. and Kango-Singh M. (2012). Hippo signalling controls Dronc activity to regulate organ size in Drosophila. Cell Death Differ. 19, 1664-1676. 10.1038/cdd.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese S., Waghmare I., Singh S. R. and Kango-Singh M. (2013). Drosophila eye as a model to study regulation of growth control: the discovery of size control pathways. In Molecular Genetics of Axial Patterning, Growth and Disease in the Drosophila Eye (ed. Singh A. and Kango-Singh M.), pp. 229-270. NewYork; Heidelberg; Dordrecht; London: Springer. [Google Scholar]
- Wang C.-W. and Sun Y. H. (2012). Segregation of eye and antenna fates maintained by mutual antagonism in Drosophila. Development 139, 3413-3421. 10.1242/dev.078857 [DOI] [PubMed] [Google Scholar]
- Weasner B. M. and Kumar J. P. (2013). Competition among gene regulatory networks imposes order within the eye-antennal disc of Drosophila. Development 140, 205-215. 10.1242/dev.085423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T. and Ready D. F. (1993). Pattern formation in the Drosophila retina. In The Development of Drosophila melanogaster (ed. Bate M. and Martinez Arias A.), Vol. 2, pp. 1277-1325. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Wu J. and Cohen S. M. (2000). Proximal distal axis formation in the Drosophila leg: distinct functions of teashirt and homothorax in the proximal leg. Mech. Dev. 94, 47-56. 10.1016/S0925-4773(00)00311-7 [DOI] [PubMed] [Google Scholar]
- Wu S., Huang J., Dong J. and Pan D. (2003). hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445-456. 10.1016/S0092-8674(03)00549-X [DOI] [PubMed] [Google Scholar]
- Wu S., Liu Y., Zheng Y., Dong J. and Pan D. (2008). The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14, 388-398. 10.1016/j.devcel.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Xu T., Wang W., Zhang S., Stewart R. A. and Yu W. (1995). Identifying tumour suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053-1063. [DOI] [PubMed] [Google Scholar]
- Yu F.-X. and Guan K.-L. (2013). The Hippo pathway: regulators and regulations. Genes Dev. 27, 355-371. 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M. and Struhl G. (2010). A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 8, e1000386 10.1371/journal.pbio.1000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ren F., Zhang Q., Chen Y., Wang B. and Jiang J. (2008). The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14, 377-387. 10.1016/j.devcel.2008.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhou Q. and Pignoni F. (2011). Yki/YAP, Sd/TEAD and Hth/MEIS control tissue specification in the Drosophila eye disc epithelium. PLoS ONE 6, e22278 10.1371/journal.pone.0022278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Tumaneng K. and Guan K.-L. (2011). The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877-883. 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]