Abstract
Background
Bone marrow edema (BME) is a radiological term which can be found in many conditions with varied pathogenesis and histopathological images. It usually presents with pain in the affected joint and is diagnosed with MRI. Subchondroplasty (SCP) and core decompression (CD) are the surgical methods that are available to achieve pain relief and functional improvement. Both surgical methods have their own indications and are used depending on the patient's history. The aim of this literature review article is to discuss the surgical modalities for the management of bone marrow edema focusing on the knee joint. Such topic which analyzes both surgical methods for treatment of bone marrow edema of the knee joint has never been described in a review article before.
Materials and methods
For the purpose of our manuscript we thoroughly searched electronic databases such as Pubmed and Medline to acquire the appropriate material for our review paper. Only English articles were used in this review. In our study we included every article that had described the surgical management of BME of the knee by CD and SCP. In the discussion we included 18 studies (9 CD and 9 SCP) with a total number of patients equal to 397, while 206/397 had undergone surgical intervention (169 underwent SCP and 37 CD).
Results
Follow-up of 180 patients out of 206 were available for our review. A total number of 166 patients (92.2%) were successfully treated. Specifically, 29 (100%) patients were treated by CD and 137 (90.7%) by SCP. In a study, 10 patients who underwent SCP for BME secondary to advanced osteoarthritis (OA) yielded poor results. In other studies, pain persistency was observed in 2 patients, 1 patient had postoperative infection and another patient eventually underwent total knee arthroplasty (TKA). 70% prevention of TKA was achieved by SCP in a study of 66 patients with BME secondary to advanced OA. Thus, a total number of 166 patients were considered as clinical success and 14 patients as clinical failure.
Conclusions
The included studies that have been published referred to the surgical methods of CD or SCP for the management of BME of the knee but none of that summarizes all current studies on both methods. Those studies seem that CD is a surgical technique that is proposed to perform in patients without findings of OA that usually fail to respond to conservative treatment. On the other hand, the option of SCP technique is carried out in patients with varied stage of OA associated with subchondral BME. Both methods aim to reduce the pain and to improve function in the setting of subchondral BME. Nevertheless it is not clear in literature which method is the best according to the criteria of the use. This literature review shows a lack of standardized guidelines with respect to diagnosis and surgical treatment.
Keywords: Knee, Bone marrow edema, Surgical treatment, Subchondroplasty, Core decompression
1. Introduction
Bone marrow is considered one of the largest tissues in the human body.1,2 It is composed of the yellow marrow and the red marrow, a haemopoietically inactive and active component, respectively. Red marrow is gradually converted to fatty yellow marrow until the adulthood (age 25), when it is found only in the proximal metaphyses of the long bones.1, 2, 3, 4 Bone marrow edema (BME) describes an area of increased-signal intensity on T1-weighed MR images and intermediate or increased-signal intensity on T2-weighed, fat suppressed and short tau inversion recovery sequences, which can only be detected by MRI.5, 6, 7, 8 In 1988, Wilson et al.9 was the first author who referred to the term of ‘bone marrow edema’, describing it as a regional increase of water contents in the bone marrow. The descriptive term of BME is a non-specific MRI finding, which could be found in several pathological conditions with varied histopathological pictures and often caused by miscellaneous mechanisms including ischemic BME, mechanical BME and reactive BME.5,6,8,10 The cardinal symptom of BME of the knee joint is pain caused by high intraosseous pressure, which is aggravated during weight loading and at night, while there is a spectrum of asymptomatic patients as well.10, 11, 12 Histopathologically, the meaning of BME is still unknown. A variety of histopathological pictures can be found in BME, depending on the clinical condition, including inflammatory infiltration, edema, fibrosis, abnormal vascularization, demineralization and osteonecrosis or a mixed pattern.1,8,10,13 In the literature, there are several terms that are used to describe BME, such as transient osteoporosis (TOP), transient bone marrow edema (TBME), regional migratory osteoporosis (RMO), reflex sympathetic dystrophy (RSD), bone marrow edema syndrome (BMES), bone bruise or contusion or bone marrow edema-like lesions (BMEL) and the most recent term of bone marrow lesions (BML). The term ‘bone bruise’ reflects to the traumatic origin of the BME, while the term transient and BMES refers to clinical entities of BME that are self-limited.4,8,14, 15, 16, 17 BME most commonly affects the hip, knee, ankle and foot joint.18 Treatment options for the management of BME consists of symptomatic treatment, prostacyclins, bisphosphonates, pulsed electromagnetic fields, vitamin D, polysulfated polysacharides and surgical treatments such as core decompression (CD) and subchondroplasty (SCP).18 Taking into consideration the fact that BME is clinically challenging entity with uncertain clinical course and with no standardized guidelines with respect to diagnosis and surgical treatment we decided to write a literature review article that summarizes the surgical modalities for the management of BME with focus on the knee joint. Such a topic, which analyzes both CD and SCP for the treatment of BME of the knee joint has never been described in a literature review article before.
2. Material and methods
2.1. Search strategy
For the purpose of our manuscript we thoroughly searched electronic databases such as Pubmed and Medline to acquire the appropriate material for our review paper. Only English articles were used in this review. Keywords that have been utilized in our manuscript include “Knee”, “Bone Marrow Edema”, “Surgical Treatment”, “Subchondroplasty”, “Core Decompression”.
2.2. Study selection
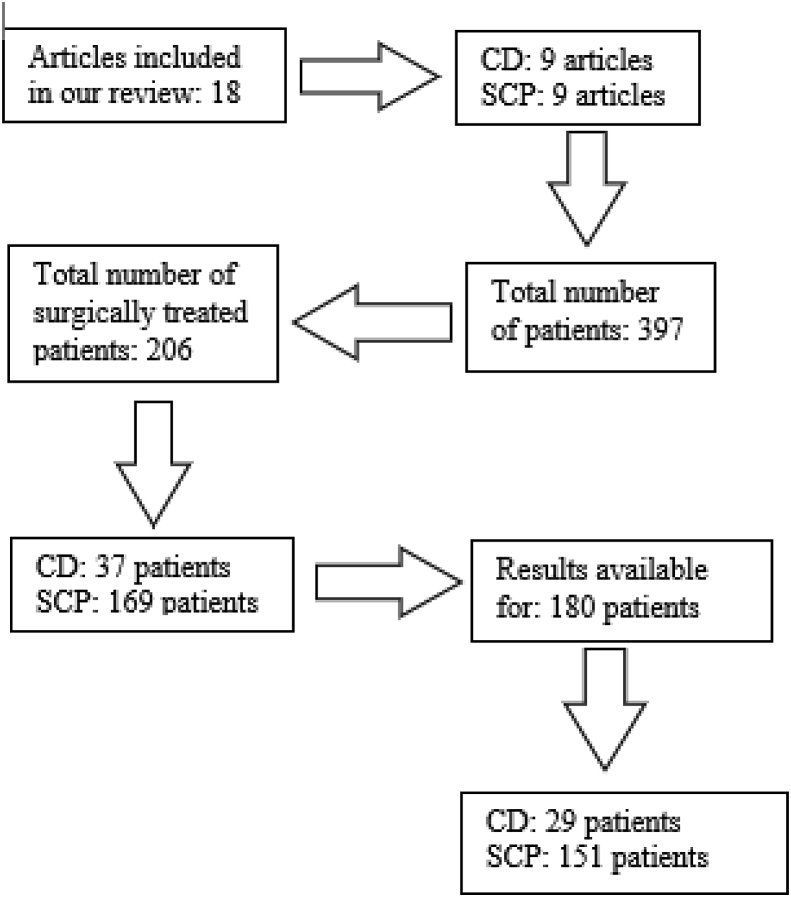
In our study we included every article that had described the surgical management of BME of the knee by CD and SCP (Flowchart 1).
Flowchart 1.
Study flow diagram.
2.3. Number of patients
We included 18 studies (9 CD and 9 SCP) with a total number of patients 206 out of 397 that had undergone surgical intervention (169 underwent SCP and 37 CD). Features of patients, results and scores are analyzed in (Table 1, Table 2).
Table 1.
Features of the patients including number of surgically treated patients, site, method of treatment, follow-up, method of Assessment and results.
| Study | N | Site | Treatment | Follow-up | Methods of assessment | Results |
|---|---|---|---|---|---|---|
| Berger et al.(2006)42 | 18 | MFC: 15 knees LFC: 7 knees IM: 2 knees BKI: 6 patients |
CD | 12 months (18 N) | MRI | All patients clinically & radiologically recovered |
| Berkem et al.(2013)41 | 4 | – | CD | 14 months (1 N) | Lysholm, VAS, MRI | 1 clinically & radiologically recovered, 3 are not referred in the article |
| McCarthy(1998)45 | 5 | PT: 3 patients DF: 2 patients |
CD | 2 years (3 N) | – | 3 were pain free, 2 lost on follow-up |
| Karantanas et al.(2008)44 | 2 | – | CD | 2 years (2 N) | MRI | All patients were clinically & radiologically recovered |
| Karantanas et al.(2008)43 | 3 | – | CD | – | – | – |
| Arjonilla et al.(2004)47 | 2 | MFC: 2 patients | CD | 15.4 months (2 N) | – | In both patients symptoms resolved gradually |
| Miranian et al.(2015)40 | 1 | R&L MFC, R LFC&R TP |
CD | 3 months (R knee) 9 months (L knee) |
Radiograph | Both knees had a full range of motion, no effusion, and no tenderness |
| Papadopoulos et al.(2002)48 | 1 | MFC: 1 patient | CD | 3 years (1 N) | MRI | The patient was asymptomatic & no functional limitation, MRI revealed complete resolution |
| Aigner et al.(2008)46 | 1 | LFC&MFC: 1 patient | CD | 9 months (1 N) | VAS | Patient became asymptomatic |
| Cohen and Sharkey (2016)49 | 66 | – | SCP | 2 years (59 N-VAS) (48 N-IKDC) |
VAS, IKDC | Significant improvement in both pain and function (59 patients VAS & 48 patients IKDC) 70% nonconversion to TKA |
| Farr and Cohen37 (2013) |
59 | T: 22 patients F: 15 patients KL: 22 patients MC: 48 patients LC: 8 patients MC&LC: 3 patients |
SCP | 14.7 months (59 N) | VAS, IKDC, SF-12 | Brisk pain improvement in the immediate post-operative period with a less pronounced but still progressive improvement afterwards. |
| Chatterjee et al.(2015)35 | 33 | MTC: 15 patients LFC: 2 patients MFC: 5 patients |
SCP | 12 months (22 N) | KOOS, Lysholm | 12 patients-clinical success & 10 patients- clinical failure. 11 lost on follow-up |
| Bonadio et al.(2016)50 | 5 | MFC: 4 patients MTP: 1 patient |
SCP | 6 months (5 N) | KOOS, VAS | Significant improvement in functional capacity & significant quick pain improvement |
| Sharkey et al.(2012)54 | 1 | MTP: 1 patient | SCP | 31 months (1 N) | VAS, MRI | Early pain relief was achieved |
| Fodor et al.(2016)55 | 1 | MFC: 1 patient | SCP | 1 year (1 N) | IKDC, KOOS, Lysholm | Improvement: pain, function & quality of life |
| Conaway et al.(2019)51 | 1 | MTP: 1 patient | SCP | 2 years (1 N) | VAS | Continued knee pain |
| Dold et al.(2017)53 | 1 | – | SCP | – | – | S. aureus osteomyelitis |
| Nevalainen et al.(2015)52 | 2 | MTP: 2 patients | SCP | 17 months (1 N) 6 months (1 N) |
MRI | The pain persisted on one patient and TKA was performed 17 months after subchondroplasty on the other |
MFC = medial femoral condyle; LFC = lateral femoral condyle; IM = intraarticular migrating; BKI = bilateral knee involvement; PT = proximal tibia; DF = distal femur; TP = tibial plateau; KL = kissing lesions; MC = medial compartment; LC = lateral compartment; L = left; R = right; T = tibial; F = femoral; MTC = medial tibial condyle; MTP = medial tibial plateau; SCP = subchondroplasty; CD = core decompression; VAS = visual analogue pain scale; IKDC = international knee documentation committee; KOOS = knee injury osteoarthritis outcome score; SF-12 = 12-item short form health survey; TKA; total knee arthroplasty; N = number of patients; MRI = magnetic resonance imaging; IV = intravenous; TKA = total knee arthroplasty.
Table 2.
Features of the patients treated surgically including gender, age, indications, contraindications.
| Study | Gender | Age | Indications | Contraindications |
|---|---|---|---|---|
| Berger et al.(2006)42 | 13 M, 5F | mean 53.7+-5.4 range 35–64 |
Pain during mechanical loading BME on knee MRI |
Secondary causes of BME such as neoplasm, inflammatory disease, subchondral fracture, history of trauma, medication that would affect bone turnover. Radiographic findings of avascular necrosis |
| Berkem et al.(2013)41 | 3 M, 1F | – | Knee pain within a 2-y period BME on knee MRI Fail to respond to conservative treatment and did not have a 50% decrease in BME on MRI |
Patients who did not return for the second or third evaluations, patients who were revealed via MRI to have osteoarthritis with subchondral BME, history of acute trauma (stress fractures), osteochondral defects |
| McCarthy (1998)45 | 3 M, 2F | range 47–65 | Stiffness and pain ranging from 3 to 8 m duration BME on knee MRI Rule out infection or neoplasia. |
– |
| Karantanas et al.(2008)44 | – | – | Lower extremity arthralgia BME on knee MRI |
No medical history of significant trauma or other factors, which could predispose to osteonecrosis or bone marrow infarction. |
| Karantanas et al.(2008)43 | 2 M, 1F | range 53–72 | Acute pain in the knee BME on MRI Coexisting osteoarthritis defined as articular cartilage thinning and the presence of osteophytes, were included only when the craniocaudal diameter of the BME was longer than 2 cm in the absence of any subchondral cysts |
No history of trauma, previous surgery in the knee, fatigue fractures with typical location away from the joint regardless of the history, excessive alcohol consumption, previous treatment of any kind for osteoporosis, clinical and/or laboratory signs of an infectious or inflammatory arthritis, “band-like” lesions on MRI, predisposing factors for osteonecrosis and/or history of corticosteroid administration |
| Arjonilla et al.(2004)47 | – | – | Severe knee pain, tenderness and slightly limited range of motion. BME on knee MRI |
– |
| Miranian et al.(2015)40 | 1 M | 44 | 2-month history of right medial knee pain and a mild effusion without inciting injury. BME on knee MRI No response to conservative treatment |
– |
| Papadopoulos et al.(2002)48 | 1 M | 45 | Pain of an abrupt onset associated with walking and strenuous activities. No history of recent trauma, locking or effusions of the knee. The patient had no identifiable risk factors for osteonecrosis. No response to conservative treatment |
– |
| Aigner et al.(2008)46 | – | – | Intra-articular migratory BMES On the routinely performed MRI investigation 3 months after the initial diagnosis, the location of the BME in the knee was found to have shifted to a different part of the knee. Pain 10 w average |
Avascular necrosis in the knee and other joints, and osteochondritis dissecans in a demarcated bone area BME secondary to osteoarthritis, mechanical stress or trauma, and patients with algodystrophy and corticosteroid medication at initial presentation |
| Cohen and Sharkey (2016)49 | 32 M, 34F | mean 55.9 range 35–76 |
Moderate to severe pain >2 m Failure of symptom relief with corticosteroid injections, hyaluronic acid injections, NSAIDs, physical therapy, and/or unloader bracing Presence of BML(s) on MRI in a weight-bearing region of the knee (medial/lateral femoral condyle or tibial plateau) Patient pain confined to the same compartment as the BML Pain in compartment of BML at least 4/10 Moderate to severe joint disease confined to the same compartment as the BML Advanced knee OA |
Primary cause of patient pain and loss of function due to pathology other than BML, by patient history and clinical evaluation Presence of gross instability >8° of varus or valgus Tricompartmental radiographic kellgren-lawrence grade 4 OA |
| Farr and Cohen (2013)37 | 30 M, 29F | mean 55.6 range 35–76 |
Knee pain for a mean period of 22.5 w BME on knee MRI |
– |
| Chatterjee et al. (2015)35 | 13 M, 9F | mean 53.5 range 38–70 |
Our general indications for use of this procedure were the presence of subchondral BMELs observed on MRI involving weightbearing regions of the knee associated with localized pain on weightbearing and palpation Failure to respond to conservative therapy for at least 3 m. Osteoarthritis |
Baker's cysts, large chondral defects, fractures, dislocations, and Kellgren-Lawrence grade greater than 3, pain secondary to extensive nondegenerative meniscal tears with a flipped displaced component at the level of the BME or with mechanical axis deviation greater than 8 |
| Bonadio et al.(2016)50 | 1 M, 4F | mean 67.7+-9.67 range 58–85 |
Knee pain at least 6 m MRI BML located in the subchondral region of the tibial or femoral condyle Kellgren-Lawrence grade 1–2 OA. Pain in one knee for at least 6 m, after a minimum of 3 m of conservative treatment without improvement. |
Autoimmune diseases, renal disease requiring dialysis, osteoarthritis radiographically classified in the Kellgren–Lawrence system as greater than grade 3, diversion of the mechanical alignment of the lower limbs larger than 8°, or radiographic alterations of the patellofemoral joint associated with symptoms of anterior pain in the knee. |
| Sharkey et al.(2012)54 | 1F | 51 | Severe knee pain, which had worsened over 8 m. No response to conservative treatment. |
– |
| Fodor et al.(2016)55 | 1 M | 51 | Knee pain >3 m MRI with BML Kellgren-Lawrence grade 2 knee osteoarthritis. |
– |
| Conaway et al.(2019)51 | 1F | 54 | Tenderness and pain/stiffness with activity Osteoarthritis |
– |
| Dold et al.(2017)53 | 1 M | 64 | – | – |
| Nevalainen et al.(2015)52 | 2 M | range 42–58 | Knee pain with duration average of 22 m Fail to conservative treatment |
– |
M = male; F = female; BME = bone marrow edema; MRI = magnetic resonance imaging; m = months; OA = osteoarthritis; BML = bone marrow lesion; NSAIDs = nonsteroidal anti-inflammatory drugs; BMELs = bone marrow edema-like lesions; BMES = bone marrow edema syndrome; y = year; w = weeks.
3. Mechanical BME of the knee
Trauma remains the primary cause of BME.1 Bone bruise or contusion are terms that have been used indistinguishable to describe the traumatic origin of these lesions.15 Osseous injury caused by direct strike, applied shear forces, multiple bones impacting one another or from traction forces in the context of avulsion injury may result in bone bruise.3 They may be acute, following a recent injury, or chronic, due to recurring microtrauma.1 T2 weighted MR images could facilitate to determine the acuteness of bone bruise.19 MRI findings include, diffuse BME with no signs of fracture or osteonecrosis in the subcortical bone, making it the gold standard for the diagnosis of bone bruise.10 Histopathologically, diffuse BME, disruption of trabeculae, osteocytes necrosis and hemorrhage in the bone marrow are seen.1,10,20 These edema-like lesions are reversible and subside within 2–4 months.6,21 Separation between bone bruise and BMES is achieved by the presence of trauma in the patient's history.22 The mechanism of the injury and the associated soft-tissue injuries can be revealed through the study of the distribution of marrow edema.22,23 According to Sanders et al.,23 there are five patterns of BME as well as soft-tissue injury around the knee joint, including pivot shift injury, dashboard injury, hyperextension injury, clip injury and lateral patellar dislocation. Pivot shift injuries constitute the most common pattern that is associated with subchondral contusion of the knee and may coexist with anterior cruciate ligament (ACL) rupture. The edema pattern following pivot shift injuries (non-contact injuries) is observed in the midportion of the lateral femoral condyle, in the posterior aspect of the lateral tibial plateau and in the posterior aspect of medial femoral condyle.15,21, 22, 23, 24 Dashboard injuries are usually associated with BME of the anterior aspect of the proximal tibia. Hyperextension injuries may lead to posterior cruciate ligament (PCL) rupture and to subsequent BME of the anterior aspect of the tibia and femur.15,22,23 Clip injuries cause BME of the lateral femoral condyle and the medial femoral condyle and finally lateral patellar dislocations result in BME of the anterior aspect of the lateral femoral condyle.15,22,23
4. Ischemic BME of the knee
There are three transient conditions, including TOP, RMO, complex regional pain syndrome (CRPS) or previously referred to as RSD or algodystrophy. Those transient entities have identical MRI findings of diffuse subchondral-bone marrow high signal with indistinct margins, reaching the articular surface but maintaining it intact. Differential diagnosis between these conditions can be achieved by taking into account the patient's gender, age and clinical history: TOP classically affects the femoral head of pregnant or peripartum women. RMO is characterized by migration in different joints including hips, knees or metatarsal head in middle-aged men over a period of weeks to years, but on the other hand there may be a transition of TOP to RMO. CPRS supervenes secondary to an initiator (major or minor trauma).21,24 BMES most frequently affects the hip, knee, foot and ankle joint, and rarely affects the upper extremities and children.3 Yet, it is unclear whether BMES constitutes a separate pathological condition or is a potential form of osteonecrosis. There are no abnormal findings on plain radiographs in the first 4–6 weeks, until findings of demineralization occur, so BMES is commonly called as TOP. MRI depicts areas of diffuse BME that may be associated with joint effusion.1,10 The nomenclature of TBMES has been utilized to characterize the abrupt onset of knee pain and associated MRI features corresponding to a BML pattern of uncertain cause.20 Moreover, TBMES has been described as generic nomenclature with clinical and radiological features indiscernible of TOP.1 Although, TBMES is presented with symptomatology, BME on radiological evaluation and positive bone scans identical of TOP, on the other side it is not associated with radiological findings of osteopenia.5,25 In clinical examination, the knee may be tender and tappering test may be positive on the femoral condyle that is afflicted. Pregnancy, alcohol or steroids consumption and hypothyroidism are considered as predisposing factors for the development of BMES.13 Many hypothesis for the possible pathogenesis of BMES have been noted including neurogenic compression,26 local venous obstruction and secondary hyperaemia,27 ischemia that occurs in small vessels proximal to nerve roots,14 or even synovial pathology.7
5. Reactive BME of the knee
Earlier, it was postulated that osteoarthritis (OA) was a degenerative disease which primarily caused cartilage loss. Nowadays, it is well-known that all the joint parts are involved in the pathogenesis, as well as synovium and subchondral bone.8 The knee is a commonly affected joint, due to the high weight it is subjected to.1 OA is associated with radiological findings of joint space narrowing, subchondral cysts-sclerosis and osteophytes.1,5 Diagnosis of chronic degenerative joint disease is established without the use of MRI, although in some complicated circumstances it may be valuable for the evaluation of concomitant alterations such as regional effusions, subchondral lesions and reactive synovitis.10 The application of MRI contributed to the enlargement of our knowledge concerning the pathogenesis and clinical course of OA related to subchondral alterations.8 OA is an arthropathy that is usually associated with BME patterns.5 Those patterns may be present before cartilage loss.24 OA–related BME is restricted to areas of subsequent stress and trabecular remodeling, and also appear as an area of hemispheric-like lesion at the knee joint.5,28 The presence of BME of the knee joint in the context of OA varies between 57 and 82%.28 The frequency and severity of BME correlates directly with the severity and extent of cartilage degeneration, leading to the conclusion that the greater the loss of cartilage, the more extensive and frequent the presence of BME is.3,20 It has been observed that increase of BME serves as negative prognostic factor for cartilage deterioration, development of pain, as well as predictive for arthroplasty.20,23 Felson et al.,29 in a study of 401 patients with OA of the knee joint reported a strong correlation between the prevalence of BMLs and the presence of pain, compared to asymptomatic group. OA-related overloading on the knee joint, especially in the medial compartment in case of varus knees and in the lateral compartment in case of valgus knees has led to a higher incidence of BML, an assumption that is strongly advocated by the potential contribution of micro-injury in the genesis of such lesions.21,24 Histological evaluation of BME in the setting of OA corresponds to various unrelated findings such as small areas of osteonecrosis, large areas of fibrosis, microfractures, edema, hemorrhage, fibrovascular ingrowth and areas of trabecular remodeling.3,5,24,28 Nevertheless, clinical experience has shown that the natural course of BML is unpredictable, and may vary with either aggravation, regression or even complete resolution.20,30
6. Surgical management of BME of the knee - surgical options
6.1. Subchondroplasty
The SCP procedure is performed with the patient laid on a radiolucent (not required) operative table in the supine position.1,37,31,32 If radiolucent table is not utilized then the patient can be positioned on the edge of the table so that it can be easier to use manipulations including leg abduction to acquire appropriate fluoroscopic images.31 The operative knee is placed appropriately in order to allow anteroposterior (AP) and lateral radiographic views throughout the procedure while the contralateral knee is padded and positioned flat on the operative table.32, 33, 34 A thigh tourniquet is placed and inflated at 250 mmHg and the leg is draped and prepared under the traditional sterile way.31,33 Anesthetic options that can be used are general, regional or spinal anesthesia.34 MRI should be performed before the initiation of procedure, in order to mark the volume, site and depth of the BML since it is important for the evaluation of the amount of calcium phosphate injected.32,35, 36, 37 The MRI Osteoarthritis Knee Score scoring system (MOAKS) may potentially help to quantify the extent of the BML relative to the compartment.37 Standard arthroscopy is performed in all 3 compartments of the knee (medial, lateral, patellafemoral) before the injection of calcium phosphate to assess the intra-articular space and subsequent status of the adjacent to BML structures.31,32,34,37 Any concomitant lesions that are found should be treated as well, with additional arthroscopical interventions including debridement chondroplasty, meniscal repair or meniscectomies, synovectomy, cyst drainage and loose body removal.32,34,35,37 Before the incision is made (2 cm incision), the targeted area should be localized by a fluoroscopy.33 AP and lateral fluoroscopical images are obtained, which are cross-referenced with the preoperative MRI study and the area is then marked on the overlying skin to assist surgeon to determine the cannula entry point for the injection of calcium phosphate.33,34 The location of guide pin can be established either by fluoroscopic guidance during the procedure or tibial navigation guide.34 A 11 × 120mm fenestrated cannula is then inserted over the guide pin, while the pin is removed.31,34,35 The injectable material is prepared until reaching a liquid/pasty viscosity.6 A syringe is inserted through the cannula for the injection of the calcium phosphate in the designated area.31,34 Calcium phosphate (ranges between 5 and 16 mL) is then under fluoroscopic guidance injected in the injured area and cannula is left in place for approximately 7–10 min for the cement to harden and after the injection, a trocar is placed through the cannula to push towards the remaining material.33, 34, 35, 36 Injection of the calcium phosphate is continued until a darkened blush is detectable on the fluoroscopic image, which represents the pattern of BML on MRI. An intraoperative radiological depiction of the area is performed to evaluate the possible leakage of the material in the joint spaces or soft tissue.34 The cannula is drawn out and the incision site is closed.33 Finally, arthroscopic procedure is carried out after the end of operation to remove possible fragments of calcium phosphate.32,33
6.2. Core decompression
The surgical technique is similar to that of the hip joint.38 The patient is lied on the operative table in the supine position without the use of tourniquet.39 Under fluoroscopic guidance a 3 mm rigid Ficar trocar is used to measure the intra osseous pressure. Pressure stress test is performed to assess the pressure, which is positive if baseline pressure is more than 30 mmHg or fails to fall under 30 mmHg after 5 min of the test.38 The approach for the decompression is made through a 2.5 cm distal femoral incision on the side of the pathology or medial incision if both sides are affected.38 The muscles of the area are carefully retracted anteriorly to expose the bone, but without entering the joint space.38 The pin is advanced carefully in order not to breach the subchondral bone and leave the cartilage intact.38,39 When the drilling is finished, the trocar is removed and the site is closed.39 Any bleeding occurring in the intraosseous space should be controlled by application of pressure for 5 min.39 On the second postoperative day, the patient is allowed to passively move the joint but only 50% of weight bearing is allowed by the utilization of crutches or cane for 4–6 weeks.38,39 Deep venous thrombosis medicines are not always administered, depending on the mobilization status of the patient.39 Full weight bearing is usually achieved over another 8 weeks or as tolerated.38 One year of no symptoms and no clinical and radiological signs of collapse, should give the permission to return to all postsurgical activities.39
7. Results
Follow-up of 180 patients out of 206 were available for our review. A total number of 166 patients (92.2%) were successfully treated. Specifically, 29 (100%) patients were treated by CD and 137 (90.7%) by SCP. In a study, 10 patients who underwent SCP for BME secondary to advanced OA yielded poor results. In other studies, pain persistency was observed in 2 patients, 1 patient had postoperative infection and another patient eventually underwent TKA. 70% prevention of TKA was achieved by SCP in a study of 66 patients with BME secondary to advanced OA. Thus, a total number of 166 patients were considered as clinical success and 14 patients as clinical failure (Table 1).
8. Discussion
Although our knowledge concerning the pathophysiology to development of BME is limited, many treatment options have been suggested.40) Due to the fact that BME frequently has a self-limited course and conservative treatment may be adequate, the utilization of surgical intervention such as CD and SCP is not thoroughly described in the literature.18,41 Only a handful of cases of BME of the knee joint which were treated by CD have been reported through the literature.42 The aforementioned surgical procedure may be an alternative to achieve rapid pain relief and resolution of MRI changes. Drilling a hole within the bone marrow at the site of the BME leads to subsequent pain relief, a finding which supports the speculation that the high intraosseous pressure is the causative factor that induces pain.18 In a study by Karantanas et al.,43 were 98 patients included with findings of acutely presented BME in the knee all patients were treated conservatively by administration of NSAIDs, oral biphosphonates (alendronate), oral calcium and total restriction of weight-loading except 3 patients that underwent CD. In one patient (72 year aged male) the intervention aimed at diagnostic biopsy, while the other two (male aged 55 and female aged 53) the procedure aimed to relieve from pain. In a retrospective study that was carried out by the same author,44 22 patients with findings of BME of the knee joint were analyzed. The BME was either primary located in the knee joint or migrated by another joint or even from the contralateral knee. Pain was the cardinal symptom in all patients. History of severe trauma was excluded. 2 patients were treated with the utilization of CD, in order to relieve from persistent pain. In another study by McCarthy,45 19 patients with transient osteoporosis were examined. The presenting symptom was pain in all cases. Involvement of knee joint was observed in 5 patients, all of who underwent CD as the therapeutic option. Pain relief was seen in 3 of them and the other 2 were lost at follow-up. Aigner et al.,46 in a retrospective study, reviewed eight patients with intra-articular BME. Three months after the diagnosis, MRI depicted a shift of the site of BME within the knee joint. 7 patients were treated conservatively with iloprost in off-label use. Only one patient was treated surgically by CD twice (first on the medial femoral condyle and 5 months later on the lateral femoral condyle). Resolution of symptoms was succeeded in all patients with a mean period of 9 months. In a study by Arjonilla et al.,47 8 patients with MRI signs of TBME of the knee were included. On clinical examination all the patients were symptomatic. 6 of them were treated conservatively and the remaining ones underwent surgical CD. All patients were asymptomatic on the follow-up. (mean-period 4.6 months). In another study which was conducted by Berger et al.,42 24 knees of 18 patients undergone surgical CD for the treatment of BMES. 6 patients had bilateral BMES. On clinical evaluation, all patients were symptomatic, with pain as their predominant symptom. All patients had radiological confirmation of BMES and had free history of trauma. Pre-surgical evaluation of possible intra-articular comorbidities were found and treated accordingly. On follow-up, all patients were asymptomatic and with normal radiological findings, 6 weeks and 12 weeks post-surgery, respectively, and after 5 years of follow-up they still remained free of disease. In a clinical study conducted by Berkem et al.,41 67 patients were examined. Follow-up mean-period was 13 months. Patients were diagnosed with BME of the knee with the use of MRI, more commonly found in the medial compartment. All patients started conservative treatment immediately, with NSAIDs and restriction of weight-bearing, and reassessed every two months with MRI. If any patient had an at least 50% reduction of the edema then conservative approach was continued and re-evaluation at 4 months was required. 4 patients (3 male and 1 female) were treated surgically by CD, since reduction of edema above 50% was not achieved. There are few case reports referring to the method of CD as possible management of patients with BME of the knee.40,48
In addition to surgical decompression, a novel surgical technique which is called ‘subchondroplasty’ has been utilized by orthopedic surgeons as a surgical modality for the management of painful BME related to OA.34 There are few articles referring to the treatment of BME by the use of SCP with focus on the knee joint. Two main indications of SCP procedure are management of symptomatic subchondral BMLs of the knee and management of BME which is associated with increased osseous stress or early insufficiency fractures.32,34,37 In a study by Chatterjee et al.,35a total of 22 patients were included. All patients had received SCP (percutaneous injection of calcium phosphate) for the management of painful BME of the knee. Indications for SCP in this study were symptomatic BME of the knee joint and failure to respond to conservative treatment for at least 3 months. Patients were evaluated postoperatively (mean follow-up period of 6 months). Of 22 patients, 12 (55%) were treated successfully and the remaining 10 (45%) were failed to respond. Moreover, Agten et al.,34 in a radiological study attempted to evaluate the pre- and post-operative radiological findings of SCP that was performed in patients of the aforementioned study. Cohen and Sharkey,49 in a retrospective study, including 66 patients aimed to find the efficacy of SCP on pain and function improvement in patients with BMLs in the setting of advanced OA. Results were promising with significant improvement of pain and function, suggesting the method as possible future treatment of BMLs in the setting of OA. In another study by Bonadio et al.,50 5 patients were prospectively analyzed. Presenting symptom was pain located on the knee joint. Patients failed to respond to conservative treatment of at least 3 months. According to VAS score, 24 weeks postoperatively, pain reduction was achieved, and after first postoperative day all patients walked without the need of any support. In an additional study conducted by Farr and Cohen,37 59 individuals were included, who underwent SCP for the treatment of knee pain associated to radiologically confirmed BMLs. The results of this study were the following: 15 patients had undergone either TKA or UKA. The remaining ones had experienced pain and function improvement on the follow-up. In the literature, except of the aforementioned articles, there are a handful of articles including case reports,51, 52, 53, 54, 55 technical notes,32,33 cadaveric study,56 animal study,57 and a recent review article36 that describe the procedure of SCP on the knee joint for the management of BMLs.
9. Limitations
The weaknesses of this literature review included low levels of evidence (all studies were level III-V) presented in this review result in the same major limitations found in retrospective therapeutic case series. In addition, the number of patients (14 studies included less than 5 patients) was too small to show the result in studies. Furthermore significant sources of selection bias were often presented for the included studies, including significant heterogeneity in patient populations, techniques, and measures of clinical outcomes, which also prevented a direct quantitative comparison across studies.
10. Conclusion
To our knowledge this is the first literature review showing a complete overview of treatment and outcome of surgical BME management of the knee, from 1998 until 2019. A literature search has been performed, including 18 articles describing mostly retrospective series of surgical treatment in 206 patients. Subchondral BME of the knee joint can be treated surgically either by CD or SCP. The included studies that have been published referred to the surgical methods of CD or SCP for the management of BME of the knee but none of that summarizes all current studies on both methods. Those studies seem that CD is a surgical technique that is proposed to perform in patients without findings of OA that usually fail to respond to conservative treatment. On the other hand the option of SCP technique is carried out in patients with varied stage of OA associated with subchondral BMLs. Both methods aim to reduce the pain and to improve function in the setting of subchondral BME. Nevertheless it is not clear in literature which method is the best according to the criteria of the use. This literature review shows a lack of standardized guidelines with respect to diagnosis and surgical treatment.
Conflicts of interest
we would like to declare we have no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2019.08.025.
Contributor Information
Athanasios N. Ververidis, Email: averver@otenet.gr.
Konstantinos Paraskevopoulos, Email: paraskevopouloskonstantinos@hotmail.com.
Konstantinos Tilkeridis, Email: tilkerorth@yahoo.com.
Georgios Riziotis, Email: georgeriz78@gmail.com.
Stylianos Tottas, Email: stottasdoc@hotmail.com.
Georgios I. Drosos, Email: drosos@otenet.gr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fowkes L.A., Toms A.P. Bone marrow oedema of the knee. The Knee. 2010;17(1):1–6. doi: 10.1016/j.knee.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Kung J.W., Yablon C.M., Eisenberg R.L. Bone marrow signal alteration in the extremities. AJR Am J Roentgenol. 2011;196(5):W492–W510. doi: 10.2214/AJR.10.4961. [DOI] [PubMed] [Google Scholar]
- 3.Starr A.M., Wessely M.A., Albastaki U., Pierre-Jerome C., Kettner N.W. Bone marrow edema: pathophysiology, differential diagnosis, and imaging. Acta Radiol. 2008;49(7):771–786. doi: 10.1080/02841850802161023. [DOI] [PubMed] [Google Scholar]
- 4.Thiryayi W.A., Thiryayi S.A., Freemont A.J. Histopathological perspective on bone marrow oedema, reactive bone change and haemorrhage. Eur J Radiol. 2008;67(1):62–67. doi: 10.1016/j.ejrad.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach L.S., Suh K.J. Bone marrow edema pattern around the knee on magnetic resonance imaging excluding acute traumatic lesions. Semin Musculoskelet Radiol. 2011;15(3):208–220. doi: 10.1055/s-0031-1278421. [DOI] [PubMed] [Google Scholar]
- 6.Bonadio M.B., Filho A.G.O., Helito C.P., Stump X.M., Demange M.K. Bone marrow lesion: image, clinical presentation, and treatment. Magn Reson Insights. 2017;10 doi: 10.1177/1178623x17703382. 1178623X17703382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil H.C., Levine S.M., Zoga A.C. MRI findings in the subchondral bone marrow: a discussion of conditions including transient osteoporosis, transient bone marrow edema syndrome, SONK, and shifting bone marrow edema of the knee. Semin Musculoskelet Radiol. 2006;10(3):177–186. doi: 10.1055/s-2006-957171. [DOI] [PubMed] [Google Scholar]
- 8.Manara M., Varenna M. A clinical overview of bone marrow edema. Reumatismo. 2014;66(2):184–196. doi: 10.4081/reumatismo.2014.790. [DOI] [PubMed] [Google Scholar]
- 9.Wilson A.J., Murphy W.A., Hardy D.C., Totty W.G. Transient osteoporosis: transient bone marrow edema. Radiology. 1988;167(3):757–760. doi: 10.1148/radiology.167.3.3363136. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann S., Kramer J., Vakil-Adli A., Aigner N., Breitenseher M. Painful bone marrow edema of the knee: differential diagnosis and therapeutic concepts. Orthop Clin N Am. 2004;35(3):321–333. doi: 10.1016/j.ocl.2004.04.005. (ix) [DOI] [PubMed] [Google Scholar]
- 11.Soder R.B., Simões J.D., Soder J.B., Baldisserotto M. MRI of the knee joint in asymptomatic adolescent soccer players: a controlled study. AJR Am J Roentgenol. 2011;196(1):W61–W65. doi: 10.2214/AJR.10.4928. [DOI] [PubMed] [Google Scholar]
- 12.Pappas G.P., Vogelsong M.A., Staroswiecki E., Gold G.E., Safran M.R. Magnetic resonance imaging of asymptomatic knees in collegiate basketball players: the effect of one season of play. Clin J Sport Med. 2016;26(6):483–489. doi: 10.1097/JSM.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S.A., Hageman J., Quatman C.E., Wordeman S.C., Hewett T.E. Prevalence and location of bone bruises associated with anterior cruciate ligament injury and implications for mechanism of injury: a systematic review. Sport Med. 2014;44(2):281–293. doi: 10.1007/s40279-013-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korompilias A.V., Karantanas A.H., Lykissas M.G., Beris A.E. Bone marrow edema syndrome. Skelet Radiol. 2009;38(5):425–436. doi: 10.1007/s00256-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 15.Vanhoenacker F.M., Snoeckx A. Bone marrow edema in sports: general concepts. Eur J Radiol. 2007;62(1):6–15. doi: 10.1016/j.ejrad.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Kornaat P.R., de Jonge M.C., Maas M. Bone marrow edema-like signal in the athlete. Eur J Radiol. 2008;67(1):49–53. doi: 10.1016/j.ejrad.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 17.Papalia R., Torre G., Vasta S. Bone bruises in anterior cruciate ligament injured knee and long-term outcomes. A review of the evidence. Open Access J Sport Med. 2015;6:37–48. doi: 10.2147/OAJSM.S75345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghasemi R.A., Sadeghi S., Rahimee N., Tahmasebi M. Technologies in the treatment of bone marrow edema syndrome. Orthop Clin N Am. 2019;50(1):131–138. doi: 10.1016/j.ocl.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Mathis C.E., Noonan K., Kayes K. "Bone bruises" of the knee: a review. Iowa Orthop J. 1998;18:112–117. [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer F.W., Frobell R., Hunter D.J. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthr Cartil. 2009;17(9):1115–1131. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Kon E., Ronga M., Filardo G. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sport Traumatol Arthrosc. 2016;24(6):1797–1814. doi: 10.1007/s00167-016-4113-2. [DOI] [PubMed] [Google Scholar]
- 22.Mandalia V., Fogg A.J., Chari R., Murray J., Beale A., Henson J.H. Bone bruising of the knee. Clin Radiol. 2005;60(6):627–636. doi: 10.1016/j.crad.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Sanders T.G., Medynski M.A., Feller J.F., Lawhorn K.W. Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. RadioGraphics. 2000:S135–S151. doi: 10.1148/radiographics.20.suppl_1.g00oc19s135. 20 Spec. [DOI] [PubMed] [Google Scholar]
- 24.Marcacci M., Andriolo L., Kon E., Shabshin N., Filardo G. Aetiology and pathogenesis of bone marrow lesions and osteonecrosis of the knee. EFORT Open Rev. 2016;1(5):219–224. doi: 10.1302/2058-5241.1.000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecouvet F.E., Malghem J., Maldague B.E., Vande Berg B.C. MR imaging of epiphyseal lesions of the knee: current concepts, challenges, and controversies. Radiol Clin N Am. 2005;43(4):655–672. doi: 10.1016/j.rcl.2005.02.002. (vii-viii) [DOI] [PubMed] [Google Scholar]
- 26.CURTISS P.H., KINCAID W.E. Transitory demineralization of the hip in pregnancy. A report of three cases. J Bone Joint Surg Am. 1959;41-A:1327–1333. [PubMed] [Google Scholar]
- 27.Rosen R.A. Transitory demineralization of the femoral head. Radiology. 1970;94(3):509–512. doi: 10.1148/94.3.509. [DOI] [PubMed] [Google Scholar]
- 28.McQueen F.M. A vital clue to deciphering bone pathology: MRI bone oedema in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2007;66(12):1549–1552. doi: 10.1136/ard.2007.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felson D.T., Chaisson C.E., Hill C.L. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 30.Felson D.T., McLaughlin S., Goggins J. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 31.Abrams G.D., Alentorn-Geli E., Harris J.D., Cole B.J. Treatment of a lateral tibial plateau osteochondritis dissecans lesion with subchondral injection of calcium phosphate. Arthrosc Tech. 2013;2(3):e271–e274. doi: 10.1016/j.eats.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen S.B., Sharkey P.F. Surgical treatment of osteoarthritis pain related to subchondral bone defects or bone marrow lesions: subchondroplasty. Tech Knee Surg. 2012;11:170–175. [Google Scholar]
- 33.Rebolledo B.J., Smith K.M., Dragoo J.L. Hitting the mark: optimizing the use of calcium phosphate injections for the treatment of bone marrow lesions of the proximal tibia and distal femur. Arthrosc Tech. 2018;7(10):e1013–e1018. doi: 10.1016/j.eats.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agten C.A., Kaplan D.J., Jazrawi L.M., Burke C.J. Subchondroplasty: what the radiologist needs to know. AJR Am J Roentgenol. 2016;207(6):1257–1262. doi: 10.2214/AJR.16.16521. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee D., McGee A., Strauss E., Youm T., Jazrawi L. Subchondral calcium phosphate is ineffective for bone marrow edema lesions in adults with advanced osteoarthritis. Clin Orthop Relat Res. 2015;473(7):2334–2342. doi: 10.1007/s11999-015-4311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astur DC, de Freitas EV, Cabral PB, Morais CC, Pavei BS, Kaleka CC, et al. Evaluation and Management of Subchondral Calcium Phosphate Injection Technique to Treat Bone Marrow Lesion.Cartilage 10.1177/1947603518770249. [DOI] [PMC free article] [PubMed]
- 37.Farr J., Cohen S.B. Expanding applications of the subchondroplasty procedure for the treatment of bone marrow lesions observed on magnetic resonance imaging. Oper Tech Sport Med. 2013;21(2):138–143. [Google Scholar]
- 38.Jacobs M.A., Loeb P.E., Hungerford D.S. Core decompression of the distal femur for avascular necrosis of the knee. J Bone Joint Surg Br. 1989;71(4):583–587. doi: 10.1302/0301-620X.71B4.2768301. [DOI] [PubMed] [Google Scholar]
- 39.Marulanda G., Seyler T.M., Sheikh N.H., Mont M.A. Percutaneous drilling for the treatment of secondary osteonecrosis of the knee. J Bone Joint Surg Br. 2006;88(6):740–746. doi: 10.1302/0301-620X.88B6.17459. [DOI] [PubMed] [Google Scholar]
- 40.Miranian D., Lanham N., Stensby D.J., Diduch D. Progression and treatment of bilateral knee bone marrow edema syndrome. JBJS Case Connect. 2015;5(2):e391–e397. doi: 10.2106/JBJS.CC.N.00077. [DOI] [PubMed] [Google Scholar]
- 41.Berkem L., Turkmen I., Unay K., Akcal M.A., Aydemir N. Factors influencing outcome of knee bone marrow oedema: a clinical study. Acta Orthop Belg. 2013;79(5):572–577. [PubMed] [Google Scholar]
- 42.Berger C.E., Kröner A.H., Kristen K.H. Transient bone marrow edema syndrome of the knee: clinical and magnetic resonance imaging results at 5 years after core decompression. Arthroscopy. 2006;22(8):866–871. doi: 10.1016/j.arthro.2006.04.095. [DOI] [PubMed] [Google Scholar]
- 43.Karantanas A.H., Drakonaki E., Karachalios T., Korompilias A.V., Malizos K. Acute non-traumatic marrow edema syndrome in the knee: MRI findings at presentation, correlation with spinal DEXA and outcome. Eur J Radiol. 2008;67(1):22–33. doi: 10.1016/j.ejrad.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Karantanas A.H., Nikolakopoulos I., Korompilias A.V., Apostolaki E., Skoulikaris N., Eracleous E. Regional migratory osteoporosis in the knee: MRI findings in 22 patients and review of the literature. Eur J Radiol. 2008;67(1):34–41. doi: 10.1016/j.ejrad.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy E.F. The pathology of transient regional osteoporosis. Iowa Orthop J. 1998;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 46.Aigner N., Meizer R., Petje G., Meizer E., Abdelkafy A., Landsiedl F. Natural course of intra-articular shifting bone marrow edema syndrome of the knee. BMC Muscoskelet Disord. 2008;9:45. doi: 10.1186/1471-2474-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arjonilla A., Calvo E., Alvarez L., Fernández Yruegas D. Transient bone marrow oedema of the knee. The Knee. 2005;12(4):267–269. doi: 10.1016/j.knee.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Papadopoulos ECh, Papagelopoulos P.J., Kaseta M., Themistocleous G.S., Korres D.S. Bone marrow edema syndrome of the knee: a case report and review of the literature. The Knee. 2003;10(3):295–302. doi: 10.1016/s0968-0160(02)00105-9. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S.B., Sharkey P.F. Subchondroplasty for treating bone marrow lesions. J Knee Surg. 2016;29(7):555–563. doi: 10.1055/s-0035-1568988. [DOI] [PubMed] [Google Scholar]
- 50.Bonadio M.B., Giglio P.N., Helito C.P., Pécora J.R., Camanho G.L., Demange M.K. Subchondroplasty for treating bone marrow lesions in the knee - initial experience. Rev Bras Ortop. 2017;52(3):325–330. doi: 10.1016/j.rboe.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conaway WK, Agrawal R, Nazal MR, Stelzer JW, Martin SD. Changing MRI after subchondroplasty with partial meniscectomy for knee osteoarthritis. Clin Imaging 10.1016/j.clinimag.2019.02.016. [DOI] [PubMed]
- 52.Nevalainen M.T., Sharkey P.F., Cohen S.B., Roedl J.B., Zoga A.C., Morrison W.B. MRI findings of subchondroplasty of the knee: a two-case report. Clin Imaging. 2016;40(2):241–243. doi: 10.1016/j.clinimag.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Dold A., Perretta D., Youm T. Osteomyelitis after calcium phosphate subchondroplasty a case report. Bull Hosp Jt Dis. 2013;75(4):282–285. 2017. [PubMed] [Google Scholar]
- 54.Sharkey P.F., Cohen S.B., Leinberry C.F., Parvizi J. Subchondral bone marrow lesions associated with knee osteoarthritis. Am J Orthop. 2012;41(9):413–417. [PubMed] [Google Scholar]
- 55.Fodor P., Prejbeanu R., Predescu V. Novel surgical technique for bone marrow lesion—case report. J Interdiscipl Med. 2016;1(S2):27–30. [Google Scholar]
- 56.Colon D.A., Yoon B.V., Russell T.A., Cammisa F.P., Abjornson C. Assessment of the injection behavior of commercially available bone BSMs for Subchondroplasty® procedures. The Knee. 2015;22(6):597–603. doi: 10.1016/j.knee.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Brimmo O.A., Bozynski C.C., Cook C.R. Subchondroplasty for the treatment of post-traumatic bone marrow lesions of the medial femoral condyle in a pre-clinical canine model. J Orthop Res. 2018;36(10):2709–2717. doi: 10.1002/jor.24046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.